Abstract
Purpose
To establish a lymph-cannulated mouse model, and use the model to investigate the impact of lipid dose on exogenous and endogenous lipid recruitment, and drug transport, into the lymph of males versus females. Finally, lymphatic transport and drug absorption in the mouse were compared to other pre-clinical models (rats/dogs).
Methods
Animals were orally or intraduodenally administered 1.6 mg/kg halofantrine in low or high 14C-lipid doses. For bioavailability calculation, animals were intravenuosly administered halofantrine. Lymph or blood samples were taken and halofantrine, triglyceride, phospholipid and 14C-lipid concentrations measured.
Results
Lymphatic lipid transport increased linearly with lipid dose, was similar across species and in male/female animals. In contrast, lymphatic transport of halofantrine differed markedly across species (dogs>rats>mice) and plateaued at higher lipid doses. Lower bioavailability appeared responsible for some species differences in halofantrine lymphatic transport; however other systematic differences were involved.
Conclusions
A contemporary lymph-cannulated mouse model was established which will enable investigation of lymphatic transport in transgenic and disease models. The current study found halofantrine absorption and lymphatic transport are reduced in small animals. Future analyses will investigate mechanisms involved, and if similar trends occur for other drugs, to establish the most relevant model(s) to predict lymphatic transport in humans.
Keywords: lipid, lymphatic drug transport, mouse, sex, species
INTRODUCTION
Recent estimates suggest that 40% of currently marketed drugs and up to 75% of drugs in development may be regarded as ‘poorly water soluble’ (1). These drug candidates are a challenge to deliver via the oral route as their absorption is often poor and unpredictable when administered in conventional (tablet/capsule) dosage forms (1). A range of delivery technologies have been developed to address the issues of low water solubility and slow dissolution and these include manipulation of crystal form (polymorphs, salts, cocrystals) and particle size, the use of complexation agents such as cyclodextrins and the application of formulation approaches such as solid dispersions (1). For poorly water soluble lipophilic drugs, lipid based formulations have particular appeal and may enhance absorption, and for a subset of extremely lipophilic drugs (typically those with log Ds >5 and long chain lipid solubilities >50 mg/g (2), although exceptions are apparent (3–5)), also promote intestinal lymphatic transport (6,7).
Intestinal lymphatic transport has the additional benefits of the potential to enhance bioavailability through avoidance of first pass metabolism in the enterocyte (8) as well as the liver (9–12), and may influence drug activity and toxicity by altering drug clearance (13) and distribution (13–15). Indeed, recent studies suggest that selection of drug design and delivery strategies to promote uptake via the lymphatics may, in some therapeutic classes, improve drug selectivity (and thus efficacy and toxicity profiles) by increasing delivery to active sites (e.g. immunomodulators, anti-cancer, antivirals etc.) (15–17).
Pre-clinical assessment of drug exposure during discovery can identify candidates that carry the risk of poor oral bioavailability and thus prevent progression of compounds with unworkable biopharmaceutical properties. These pharmacokinetic studies are commonly performed in rats and dogs with rat studies being more common, at least in early discovery, as smaller animals are cheaper to house and maintain. Animal studies can also be employed to better understand the fundamental processes that drive (and/or limit) absorption and bioavailability, including, for example, lymphatic drug transport. Intestinal lymphatic drug transport studies are most commonly conducted in rats due to the presence of well-established surgical procedures for lymphatic cannulation and the availability of a plethora of comparative data (18–22). Intestinal lymphatic drug transport has also been evaluated in dogs, and this enables the measurement of lymphatic drug transport after administration of full sized human dosage forms (9,23). However, dog studies are not performed routinely as experimental logistics are complicated due to the terminal nature of lymph cannulation studies. The availability of mutant and knockout mouse strains, including mice with altered expression profiles for transporters and enzymes that impact on drug bioavailability makes the conduct of drug absorption studies in murine models attractive. However, the surgical complexities of lymphatic cannulation have, to this point, precluded application in drug transport studies, although a lymph cannulated mouse model has been used previously to examine intestinal lymphatic lipid transport (24,25).
The primary aim of the present study was therefore to establish and validate a contemporary lymph-cannulated mouse model to examine lymphatic drug transport. The surgery to cannulate the mesenteric lymph duct in mice is more challenging and time consuming than the equivalent surgery in rats, however, the model provides an opportunity to conduct studies in transgenic animals and disease models that are commonly not available in rats. In the current study, the lymph cannulated mouse model was used to probe the impact of lipid dose on the recruitment of exogenous and endogenous lipids into the lymph of male and female mice, and to examine the impact of changes in lymphatic lipid transport on the lymphatic transport of a model drug, halofantrine. Finally, the generation of a lymphatic transport model in the mouse has allowed a comparison of trends in lymphatic transport and drug absorption data across common pre-clinical models (mouse – rat – dog). The data suggest that lymphatic lipid transport is remarkably similar (on a mg/kg basis) across species, but that for halofantrine, at least, lymphatic drug transport becomes less efficient in smaller animal models. The data provide an important comparison of lymphatic transport data across preclinical species.
MATERIALS AND METHODS
Halofantrine (Hf) base (1,3-Dichloro-alpha-[2-(dibutylami-no)ethyl]-6-(trifluoromethyl)-9- phenanthrenemethanol), 2,4-dichloro-6-trifluoromethyl-9-{1-[2-(dibutylamino) ethyl] phenanthrenemethanol HCl, and N, N-dibutyl-3-(1,3-dichloro-6-trigluoromethyl9-phenanthyl-3-hydroxypropionamide (the internal standards for HPLC and HPLC-MS assays, respectively) were kind gifts from GlaxoSmithKline, King of Prussia, PA. Oleic acid [1–14C] (Perkin Elmer Life Sciences, Boston, MA), oleic acid (OA), sodium taurocho-late, sodium chloride, ammonium sulfate, soybean oil, cremophor EL, triacetin (Sigma Chemicals, Australia), Tween 80 (BDH Chemicals, Australia), Maisine 35–1 (Gattefosse, France), Miglyol 812 (Sasol, Germany), Intralipid and normal saline for injection (Baxter Healthcare, Australia) were used as received. Acetonitrile, sodium dodecyl sulphate, glacial acetic acid and ammonium hydroxide were analytical grade. Water was obtained from a Milli-Q (Millipore, Milford, MA) purification system. Ketamine and Xylazine (Ilium Ketamil®, and Xylazil>#x000AE;, Troy laboratories) and Acepromazine (ACP-10 injection, Ceva animal health, Australia) were used for anaesthesia. Triglyceride kit® (Roche diagnostics GmbH, Mannheim, Germany) Control serum II® and Wako Phospholipids kit (Wako Chemicals, Richmond, VA) were used for analysis of triglyceride (TG) and phospholipid (PL) levels. Irgasafe plus® (Packard Bioscience, Meriden, CT) liquid scintillation cocktail was used for scintillation counting of radioactivity levels. Polyethylene (PE) and polyvinyl chloride (PVC) cannulas with 0.96 and 0.58 mm, 0.8 and 0.5 mm and 0.5 and 0.2 mm external and internal diameters were obtained from Microtube Extrusions, NSW, Australia. All other chemicals were analytical reagent grade.
Experiment Design
In part 1, a mouse model of lymphatic drug transport was developed and used to assess the lymphatic transport of lipids (exogenously administered fatty acid and endogenous triglyceride and phospholipid) and halofantrine in mesenteric lymph duct cannulated, anaesthetised male and female mice after intraduodenal administration of 0, 18.1, 250 or 1000 mg/kg oleic acid or 250 mg/kg capric acid. This enabled examination of the impact of lipid dose, lipid type and sex on lymphatic lipid and drug transport.
In Part 2, the lymphatic transport of triglyceride and halofantrine in male mice was compared to previous data collected in mesenteric lymph duct cannulated rats (20,21,26–28) and thoracic lymph duct cannulated dogs (9,29) administered various long chain lipid (oleic acid, olive oil, soybean oil) doses. Additional data was also obtained after administration of 18.1 mg/kg long chain lipid to mesenteric lymph duct cannulated anaesthetised male and female rats, and conscious male rats to provide a complete set of data across species at this dose. The data for lymphatic transport in conscious rats (Supplementary Material Fig. S1) was similar to that obtained in anaesthetised animals verifying that surgical anaesthesia was not a major contributor to inter-species differences in lymphatic transport.
In part 3, differences in halofantrine oral bioavailability in non-lymph duct cannulated animals was explored across species and as a function of conscious state (anaesthetised vs conscious) to explore the potential for differences in total absorption to explain species differences in lymphatic transport. The oral bioavailability of halofantrine was calculated after administration to anaesthetised or conscious mice and rats via comparison of plasma AUCs after oral dosing with 18.1 mg/kg long chain lipid and after IV dosing. The mouse and rat data was compared to previous data obtained after administration of halofantrine with 18.1 mg/kg long chain lipid to thoracic lymph duct cannulated conscious greyhound dogs (9,29).
Formulation Preparation
For experiments in anaesthetised mice and rats, halofantrine was administered in an oleic acid emulsion. Previous studies have demonstrated that the digestive capability of rodents is reduced when anaesthetised, but that absorption and lymphatic transport is as efficient as it is in conscious animals when anaesthetised animals are administered well dispersed and pre-digested lipids (ie fatty acids and/or monoglycerides rather than triglycerides) (27). The data in Supplementary Material Fig. S1 showing equivalent lymphatic transport of halofantrine in anaesthetised rats administered halofantrine in dispersed and pre-digested lipids, and conscious rats administered halofantrine in dispersed but undigested lipids, is also consistent with this suggestion. The components of the emulsions administered intraduodenally to mice are listed in Table I. Table I also lists the formulation administered intraduodenally to rats which comprised 1.6 mg/kg halofantrine and 18.1 mg/kg oleic acid (containing a trace of 0.5 µCi of 14C-oleic acid) dispersed in 5.6 ml of 0.2% Tween 80 in normal saline. The oleic acid emulsions were prepared as described previously (21) and the drug and 14C-fatty acid content were verified by HPLC and liquid scintillation counting, respectively, on the day of dosing.
Table I.
Experimental Groups Including Species, Studies Conducted, Method of Drug Administration, Animal Conscious State and Formulation Details
| Species | Study | Method of administration |
Conscious state |
Formulation | Halofantrine dose | Lipid dose | Radiolabel | Surfactant/cosolvent | Aqueous | Total volume |
|---|---|---|---|---|---|---|---|---|---|---|
| Mouse | Lymphatic transport and bioavailability |
Intraduodenal | Anaesthetised | Low long-chain lipid dose |
1.6 mg/kg | 18.1 mg/kg Oleic acid |
0.2 µCi l4C oleic acid |
0.2% Tween 80 | Normal saline | 0.5 ml |
| Mouse | Lymphatic transport | Intraduodenal | Anaesthetised | Intermediate long-chain lipid |
1.6 mg/kg | 250 mg/kg Oleic acid |
0.2 µCi l4C oleic acid |
0.2% Tween 80 | Normal saline | 0.5 ml |
| Mouse | Lymphatic transport | Intraduodenal | Anaesthetised | High long-chain lipid dose |
1.6 mg/kg | 1000 mg/kg Oleic acid |
0.2 µCi l4C oleic acid |
0.2% Tween 80 | Normal saline | 0.5 ml |
| Mouse | Lymphatic transport | Intraduodenal | Anaesthetised | Medium-chain lipid dose |
1.6 mg/kg | 250 mg/kg Capric acid |
0.2 µCi l4C capric acid |
5 mM sodium taurocholate |
Phosphate buffered saline pH6.9 |
0.5 ml |
| Rat | Lymphatic transport and bioavailability |
htraduodenal | Anaesthetised | Low long-chain lipid dose |
1.6 mg/kg | 18.1 mg/kg Oleic acid |
0.5 µCi l4C oleic acid |
0.2% Tween 80 | Normal saline | 5.6 ml |
| Mouse | Bioavailability | Oral gavage | Conscious | Low long-chain lipid dose |
1.6 mg/kg | 18.1 mg/kg soybean oil: maisine 35-1 (50:50 w/w) |
– | 9.37 mg/kg cremophor EL, 2.19 mg/kg ethanol |
Milli-Q water | 0.1 ml |
| Rat | Lymphatic transport and bioavailability |
Oral gavage | Conscious | Low long-chain lipid dose |
1.6 mg/kg | 18.1 mg/kg soybean oil: maisine 35-1 (50:50 w/w) |
– | 9.37 mg/kg cremophor EL, 2.19 mg/kg ethanol |
Normal saline | 1 ml |
| Dog (29) |
Lymphatic transport and bioavailability |
Oral | Conscious | Low long-chain lipid dose |
1.6 mg/kg | 18.1 mg/kg soybean oil: maisine 35-1 (50:50 w/w) |
– | 9.37 mg/kg cremophor EL, 2.19 mg/kg ethanol |
Normal saline | 1 g in capsule |
For experiments in conscious animals, 1.6 mg/kg halo-fantrine was prepared in a long-chain lipid based self-emulsifying drug delivery systems (SEDDS) according to previously established methods (9). The SEDDS formulation consisted of 30.5% w/w soybean oil, 30.5% w/w Maisine 35–1, 31.6% w/w Cremophor EL and 7.4% w/w ethanol and was administered such that animals received 18.1 mg/kg long chain lipid (soybean oil and maisine 35–1 combined) (approximately 31.25 mg/kg of SEDDS formulation). In mice and rats, the appropriate volume of SEDDS was pre-dispersed in 0.1 ml or 1 ml water, respectively, prior to dosing. In dog studies, the SEDDS formulation was incorporated into a capsule as described (29). The dispersion and emulsification characteristics of the formulations were assessed as previously described (30) and the halofantrine content of the SEDDS and pre-dispersed SEDDS were verified by HPLC or LC-MS on the day of dosing.
For intravenous administration, halofantrine was incorporated into intralipid emulsions as described previously (4,27). Fasted mice received 0.2 ml intralipid emulsion, fasted rats 1 ml intralipid emulsion and dogs were fed ~30 g of lipid in food and received 5 ml of intralipid formulation. Total lipid doses administered to the animals were thus kept relatively similar across species (~1 g/kg, 0.33 g/kg and 1 g/kg in mice, rats and dogs, respectively) as a recent study has suggested that the clearance, distribution and thus area under the plasma concentration vs time profile and oral bioavailability calculations for halofantrine may be influenced by systemic lipid levels (13). The intravenous formulation administered to the fed dogs contained 50 mg (~1.6 mg/kg) halofantrine in 5 ml intralipid (9). For administration to fasted mice and rats, 5 mg or 10 mg halofantrine were dissolved in 750 µl and 400 µl (respectively) of 3:5 dimethylacetamide:triacetin and 100 µl and 400 µl of the resultant mixture added dropwise (5 µL per addition) to 4 ml or 15 ml of intralipid. Mice received 0.2 ml of the resultant mixture whereas rats received 1 ml. The intralipid formulation was ultrasonicated between each dropwise addition of drug in cosolvent using a Misonix XL 2020 ultrasonic processor (Misonix, Farmingdale, NY) equipped with a 3.2 mm microprobe which was pulsed (1 min on/20 sec off) at an amplitude of 240 µm and a frequency of 20 kHz. During ultrasonication, the emulsion was cooled on ice. The emulsion was stored at 4°C overnight prior to dosing. Immediately prior to dosing, the emulsion was passed through a 0.2 µm filter (Minisart CE, Santorius, UK). An aliquot of the filtered emulsion was subsequently assayed for drug content by HPLC to accurately determine the dose administered.
Animal Experiments
All surgical and experimental procedures were approved by the local institutional animal ethics committee. Data from the dog studies (9,29) and some of the rat studies (20,21,26–28) has been reported previously and is reported here for comparative purposes. The lymphatic transport and bioavailability studies described here were performed in male Sprague–Dawley rats (280–320 g) and male (22– 26 g) or female (20–24 g) C57BL/6 mice. The rats were anaesthetised using a previously described combination of ketamine, xylazine and acepromazine (31). In mice, anaesthesia was initiated by subcutaneous injection of 133 mg/kg ketamine and 10 mg/kg xylazine and anaesthesia was maintained throughout the experiment with top up doses of 40 mg/kg ketamine and 3 mg/kg xylazine every 20– 60 min. Whilst anaesthetised, the body temperature of the mice and rats was maintained by placing them on a heated pad at 37°C (Ratek, Australia). At the conclusion of the experiments, mice and rats were killed via a lethal IP dose of 0.1 or 1 ml, respectively, of sodium pentobarbitone (100 mg/ml).
Lymphatic Transport Studies in Mice
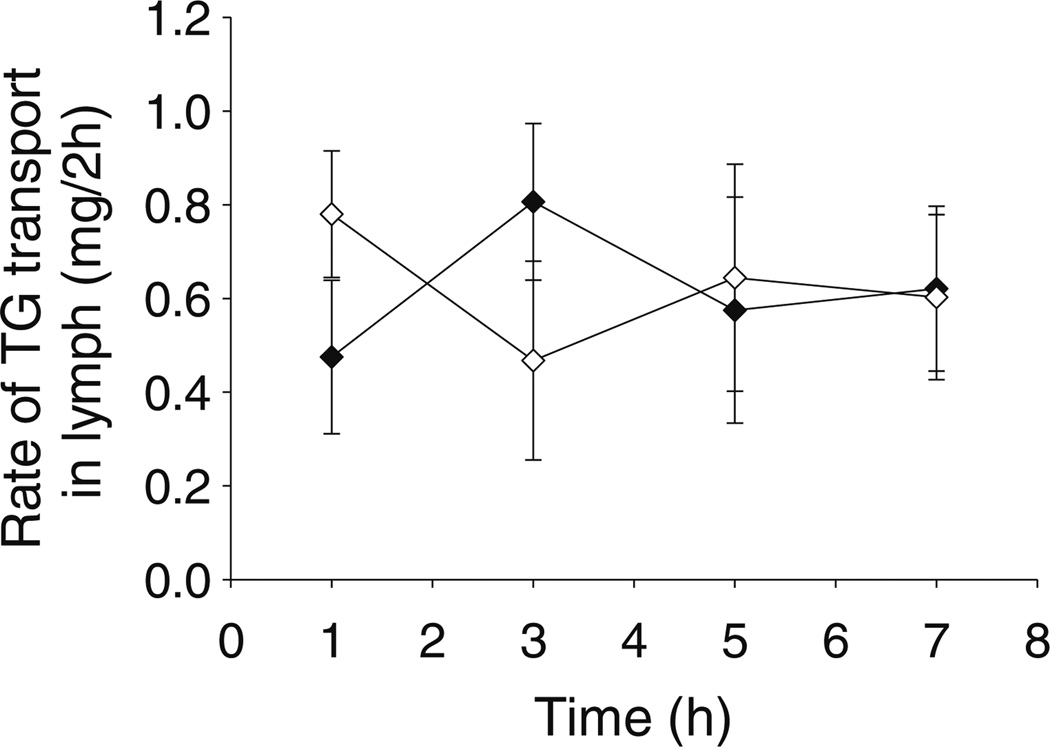
Mice were fasted for 2 h prior to studies as the mesenteric lymph duct was not visible, even when viewed through a microscope, in overnight fasted animals. However, the relatively short period of fasting (plus the surgical period and a 0.5 h recovery period post-surgery) was sufficient to return lymph lipid transport to baseline, fasted levels, as the lymphatic transport of lipids was consistent in the samples collected from 0–2, 2–4, 4–6 to 6–8 h in mice infused with saline (Fig. 1).
Fig. 1.
Rate of triglyceride (TG) transport into the lymph (mg per 2 h period) over 8 h during intestinal administration of 0.3 ml/h saline to mesenteric lymph duct cannulated male (black diamond) or female (white diamond) mice. Data represent mean ± SEM for n=4–5 animals.
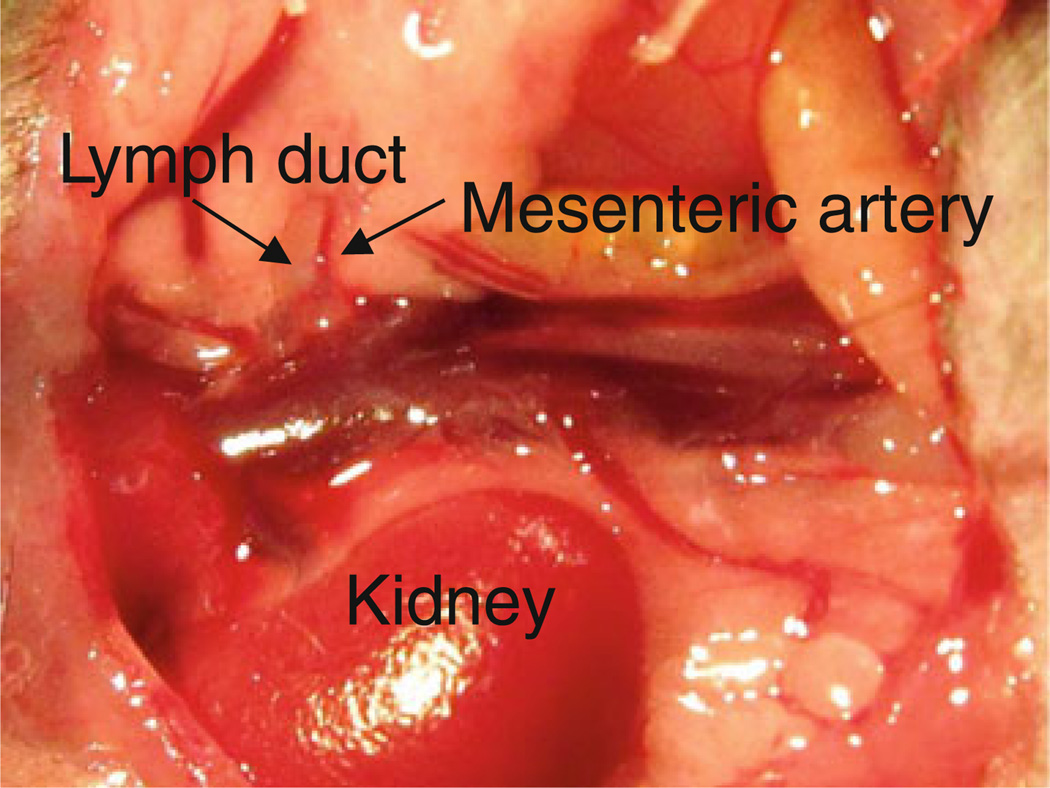
To cannulate the mesenteric lymph duct, the abdomen was shaved and cleaned aseptically with chlorhexidine solution and the abdominal muscle wall was opened with a straight ~1 cm incision extending from the midline to the right hand side approximately 0.3 cm down from the ribcage. The small intestine was exteriorised, placed on the left hand side of the animal and wrapped in a piece of sterile gauze soaked in normal saline so that the mesenteric lymph duct could be accessed. Surgery was performed under a surgical microscope and the mesenteric lymph duct was recognised as a small, white, opaque vessel running perpendicular to the right kidney and beside a similar diameter, pulsating dark red blood vessel (the mesenteric artery). Figure 2 shows a photograph of the mesenteric lymph duct in mice. The mesenteric lymph duct was carefully cleaned using a pair of fine forceps to remove any adherent layers of tissue or fat. A small hole was made in the mesenteric lymph duct using a 25 g needle. A cannula (the tip of which consisted of a 0.2 mm i.d. and 0.5 mm o.d. polyethylene cannula and was connected to a larger polyethylene cannula with 0.5 mm i.d. and 0.8 mm o.d.) was subsequently inserted through the hole into the mesenteric lymph duct. The cannula was secured with a drop of instant cyanoacrylate adhesive (Selleys Supa Glue®, Australia) and the integrity of cannulation confirmed by the appearance of lymph in the cannula.
Fig. 2.
Mesenteric lymph duct, mesenteric artery and right kidney in a mouse.
The intestine was cannulated by placing a hole in the duodenum ~0.3 cm below the pylorus with a 25 G needle, introducing a polyethylene cannula (0.5 mm i.d. and 0.8 mm o.d., which was prepared by heat moulding the tip into a ‘J’ shape that hooked into the intestine) and securing the cannula with a drop of instant cyanoacrylate adhesive. Once the duodenum was cannulated, the mice were rehydrated with a 0.3 ml/h infusion of normal saline. The abdominal wound was subsequently closed with sterile sutures and the mice were rehydrated for 0.5 h prior to infusion of either saline (as a control) or the halofantrine formulations (Table I) at a rate of 0.5 ml/h for 1 h. After 1 h, the intraduodenal infusion was changed back to 0.3 ml/h normal saline. Lymph was collected continuously into tared tubes containing 15 µl of 1,000 IU/ml heparin that were changed every 2 h for 8 h after initiation of formulation administration. Triglyceride, phospholipid, 14C oleic acid and halofantrine concentrations in lymph were measured as described below.
Bioavailability Studies in Mice
Bioavailability was calculated, as described below, by comparing the area under the plasma concentration versus time profiles following intestinal or oral administration of halofantrine with data obtained after IV administration. Studies were conducted in both anaesthetised mice (for direct comparison to lymph transport data) and conscious mice (to enable comparison to conscious dog studies and to evaluate the levels of consistency between anaesthetised and conscious mice).
In anaesthetised and conscious mice, oral bioavailability studies were conducted following a 2 hour fast using a population approach where each mouse was able to provide blood samples at 2 time points only. Anaesthetised animals were intraduodenally dosed with 1.6 mg/kg halofantrine in 18.1 mg/kg long chain lipid as an emulsion whereas conscious animals were dosed via oral gavage with 1.6 mg/kg halofantrine in 18.1 mg/kg long chain lipid contained in a SEDDS formulation pre-dispersed in 0.1 ml of milliQ water (see Table I for formulation details). IV administration of halofantrine in intralipid was achieved via bolus administration into the tail vein of conscious, restrained animals. In oral/intestinal and IV dosing studies, two 100 µl blood samples were taken from each mouse via submandibular bleeding from 2 sites on opposite sides of the jaw. Replicate experiments were performed to provide n=3–6 blood samples at pre-set time points following dosing (between –5 min and 26 h post-dosing). Plasma was separated from blood via centrifugation and plasma halofantrine concentrations were measured by LC-MS.
Lymphatic Transport Studies in Rats
For lymphatic studies in anaesthetised rats, rats were fasted overnight prior to initiation of anaesthesia. The duodenum and mesenteric lymph duct were then cannulated as described previously (21,32). After surgery the rats were allowed to rehydrate for 0.5 h with an intraduodenal infusion of 2.8 ml/h normal saline. Following the rehydration period, rats were infused with the halofantrine emulsion (see Table I) at a rate of 2.8 ml/h for 2 h. Following the 2 h dosing period, normal saline was infused intraduodenally at 2.8 ml/h for the remainder of the experiment.
For studies in conscious rats, rats were anaesthetised, the jugular vein and mesenteric lymph duct cannulated and cannulas exteriorised to the back of the neck and through a spring and swivel system, as described previously (20,26). The rats were allowed to recover overnight with free access to water but food was withheld. During the overnight recovery period and for the remainder of the experiment, saline was infused into the jugular vein at a rate of 0.5 ml/h. The next morning, rats were administered 1.6 mg/kg halofantrine in 18.1 mg/kg long chain lipid contained in a SEDDS formulation (Table I) pre-dispersed in 1 ml milliQ water. The dispersed formulation was administered by oral gavage. Food was returned to the animals 8 h after dosing.
In both anaesthetised and conscious animals, lymph was continuously collected for 8 h following initiation of dosing. Lymph was collected into tared polyethylene tubes containing 10 µL of 1,000 IU/ml heparin. Collection tubes were changed hourly. Triglyceride, phospholipid, 14C oleic acid and halofantrine concentrations in lymph were determined as described below.
Bioavailability Studies in Rats
Bioavailability was calculated, as described below, by comparing the area under the plasma concentration versus time profiles following oral and IV administration of halofantrine. Studies were conducted in both anaesthetised rats (for direct comparison to lymph transport data) and conscious rats (to enable comparison to conscious dog studies and to evaluate the level of consistency between anaesthetised and conscious rats).
For bioavailability studies in anaesthetised rats, rats were fasted overnight prior to initiation of anaesthesia and the duodenum and carotid artery were cannulated as described previously (21,26,32). After surgery the rats were allowed to rehydrate for 0.5 h with an intraduodenal infusion of 2.8 ml/h normal saline. Following the rehydration period, rats were infused with the halofantrine emulsion (see Table I) at a rate of 2.8 ml/h for 2 h. Following the 2 h dosing period, normal saline was infused intraduodenally at 2.8 ml/h for the remainder of the experiment. Blood samples (300 µl) were taken from the carotid artery at pre-set time points between −5 min and 8 h after initiation of formulation dosing and plasma was separated from blood via centrifugation. Plasma drug concentrations were measured by LC-MS.
For studies in conscious animals, rats were anaesthetised prior to surgery and after surgery were allowed to regain consciousness and recover overnight (with free access to water but not food) prior to drug administration and sampling. In the oral administration studies, the carotid artery was cannulated as described previously (20,26) and the next day animals were administered 1.6 mg/kg halofantrine in long chain SEDDS via oral gavage. In the IV administration studies, the jugular vein and carotid artery were cannulated as described previously (20,26) and the next day animals were administered halofantrine in intralipid via slow IV infusion over 5 min. Blood samples (300 µl) were taken from the carotid artery at pre-set time points between −5 min and 28 h after initiation of formulation dosing and plasma was separated from blood via centrifugation. Plasma drug concentrations were measured by LC-MS.
Bioavailability and Lymphatic Transport Studies in Dogs
Bioavailability and lymphatic transport data in greyhound dogs have been reported previously (9,29,30) and are reproduced here for comparative purposes. Oral and IV dosing studies were performed in conscious, thoracic lymph duct cannulated dogs with a catheter also placed in the cephalic vein to enable blood sampling. In oral dosing studies, dogs were fasted overnight and for 12 h post-dose, and were administered a single (1 g) soft gelatin capsule containing 1.6 mg/kg halofantrine in 18.1 mg/kg long chain lipid in a SEDDs formulation (see Table I). Lymph was collected for 10 h post-dose (data reported here is up to 8 h post-dose) into tared tubes changed at least hourly. Blood samples were collected at pre-set times between 0 and 10 h post-dose. In IV dosing studies, dogs were administered 2 mg/kg halofantrine in 5 ml intralipid and blood samples were collected at pre-set times between 0 and 24 h post-dose. Plasma was separated from all blood samples via centrifugation and halofantrine concentrations in lymph and plasma measured via a validated HPLC method.
Analytical Methods
HPLC
The lymph concentrations of halofantrine in rats and mice were measured using previously described sample preparation procedures followed by HPLC (9,21,26,33). A Shimadzu HPLC system (comprising an online DGU-20A5 degasser, two LC20AD pumps, an SIL-20A autosampler and a SPD-20A UV/Vis detector connected via a CBM-20A communications bus module and controlled via LC Solutions software) was used for analysis (Shimadzu, Kyoto, Japan). Recovery of Hf spiked into blank rat or mouse lymph (at low, medium and high concentrations of 0.5, 1 and 2 µg/ml) was > 95% (n=5 analyses at each concentration) and the assay was accurate and precise (to within ± 10% of nominal concentrations) at concentrations of 25–1,000 ng/ml.
LCMS
Halofantrine concentrations in mouse and rat plasma were assayed by LC-MS. Plasma samples (50 µl) were prepared for LC-MS via addition of 1,000 ng/ml of the internal standard (N, N-dibutyl-3-(1,3-dichloro-6-trigluoromethyl9-phenanthyl-3-hydroxypropionamide) followed by a 1 min vortex, addition of 50 µl saturated ammonium sulphate solution and a further 30 s vortex. 150 µl of acetonitrile was then added followed by a 1 min vortex and samples were left to stand at room temperature for 20 min prior to centrifugation at 3,500 g for 5 min. The supernatant (25 µl) was pipetted into vials for LC-MS analysis.
The LC-MS system consisted of two LC-20 AD pumps, an on-line DGU-20A5 solvent degasser, CTO-20A column oven and a single quadrupole mass spectrometer with an electrospray ionization (ESI) interface (Shimadzu, Kyoto, Japan). Data acquisition and processing were performed using LCMS Solutions software (Shimadzu, Kyoto, Japan). 5 µL samples were injected onto a Phenomenex Gemini C18 110A column (3 µm particle size, 50 mm×2.00 mm i.d. Phenomenex, CA) at 29°C and the mobile phase flow rate was 0.4 mL/min. Mobile phase A was 60:40 (v/v) acetonitrile:water with 0.2% ammonium hydroxide and mobile phase B was 95:5 (v/v) acetonitrile:water with 0.2% ammonium hydroxide. The mobile phase gradient sequence was as follows: mobile phase B was initially held at 50% for 0.5 min, then linearly increased to 100% over the next 2 min, prior to holding at 100% for 4.5 min, and return to 50% over 0.5 min. Mobile phase B was then held at 50% for 4 min prior to injection of the subsequent sample. The total run time was 11.5 min per injection with the flow to the MS redirected to waste for the first 2 min using a flow diverter positioned after the column. Halofantrine and the internal standard eluted at 5.6 and 3.2 min.
LC-MS detection of halofantrine and the internal standard was performed with the single quadrupole mass spectrometer ESI interface in positive ion mode. Halofantrine and the internal standard were detected by selective ion monitoring (SIM) of the 499.80 and 513.95 mass/charge ion peaks (m/z) ([M+H]+). The heat block and curved desolvation line (CDL) were maintained at 200°C, interface and CDL voltages were 4.5 kV and −50.0 V, the nebulising gas flow rate was 1.5 L/min, and the drying gas flow rate 10 L/min.
The LC-MS method was validated by assay of replicate (n=4) quality control samples at low, medium and high concentrations (10, 50, 500 and 1,000 ng/ml) on three separate days. The assays were found to be accurate (within 10% of target concentration) and precise (co-efficient of variation <10%) for concentrations of halofantrine between 10 and 1,000 ng/ml. Inter-day variability in precision and accuracy was < 10% and the limit of quantitation was 10 ng/ml.
Lipid Analysis
Lymph concentrations of triglyceride (TG) and phospholipid (PL) were determined using commercial enzymatic colorimetric methods, as described previously (9).
The concentration of exogenous, radiolabelled fatty acid (FA) in each lymph sample was measured by scintillation counting following addition of 2 ml Irgasafe plus® to 10 µl (mouse) or 30 µl (rat) lymph samples. The scintillation method was validated by spiking blank lymph samples with low, medium and high concentrations of 14C-oleic acid and the measured concentrations were within 5% of the nominal concentration.
Calculations
The mass of triglyceride, phospholipid, exogenous 14C-oleic acid and halofantrine transported in lymph was calculated from the product of the measured concentration and the lymph flow rate during each time period. These values were in some cases, further normalised across species by dividing by body weight (where the weight of individual rats or dogs was not available from previous studies, average weights of 300 g and 32 kg, respectively, were used for calculations since these values were recorded). Total, exogenous and endogenous FA transport into the lymph were calculated as described previously for rats (21). Total FA transport in lymph (expressed in terms of the total moles of FA) was calculated from the mass of TG and PL transported in lymph by assuming that each mole of lymph TG and PL comprised 3 and 2 moles of FA, respectively. Endogenous FA transport in intestinal lymph was calculated by subtracting the mass of exogenous (radiolabelled) FA recovered in each lymph sample from the mass of total FA (from TG and PL) recovered.
Noncompartmental Pharmacokinetic Analysis of Data from Rats and Dogs
Pharmacokinetic parameters following intravenous, oral or intraduodenal administration of halofantrine to rats and dogs were calculated according to standard noncompartmental methods. The first order terminal elimination rate constant (k) was determined from the slope of the terminal log-linear phase of the individual plasma drug concentration-time profiles. The area under the plasma concentration-time profiles from time zero (0 h) to infinity (∞) in dogs and rats were calculated using the linear trapezoidal method from time zero until the last measured time point and addition of the area for the terminal phase (ie the last measured plasma concentration divided by k).
The bioavailability (Fapparent) of halofantrine in rats was calculated as the ratio of the AUC values following oral and IV administration, ie:
| (1) |
where DPO and DIV are the total doses administered via the oral and intravenous routes, respectively, and and represent the plasma AUC from time zero to infinity following oral and IV dosing, respectively.
The bioavailability of halofantrine in thoracic lymph duct cannulated dogs was calculated via the addition of the proportion of the dose recovered in lymph, and the proportion presented to the systemic circulation following absorption via the portal vein (FPortal). Although data were taken from published papers (9,29), Fportal was not previously calculated. Fportal was calculated as the ratio of dose normalised values following oral versus IV administration to lymph cannulated dogs:
| (2) |
where DPO and DIV are the total doses administered via the oral and intravenous routes, respectively, and and represent the plasma AUC from time zero to infinity following oral dosing and IV dosing to lymph cannulated dogs, respectively.
Population Pharmacokinetic Modelling of Mice Data
The bioavailability after oral dosing in mice was calculated via population pharmacokinetic modelling using the importance sampling algorithm (pmethod=4) in S-ADAPT (version 1.57) (34) and the SADAPT-TRAN facilitator tool (35). Data from 39 mice each contributing two plasma concentrations at two of eleven different time points over 24 h were simultaneously modelled. Nineteen mice received an intravenous bolus dose and 20 mice were dosed orally. Linear pharmacokinetic models with one, two, or three disposition compartments, and an oral absorption compartment with or without an absorption lag compartment, as described previously (36), were considered. Between animal variability was described by log-normal distributions using a major diagonal variance-covariance matrix. A combined additive and proportional residual error model was used. Model evaluation was performed using the objective function and a series of diagnostic plots as described previously (37). Population pharmacokinetic modelling provides the advantage that it can estimate both the mean bioavailability and its between animal variability. This variability estimate is not provided by Bailer’s method (38).
The halofantrine concentrations in mice after oral and intravenous dosing were best described by a linear three-compartment model. The oral absorption was excellently fitted by an absorption compartment and an absorption lag compartment linking the former with the central compartment. This model yielded unbiased and precise curve fits (fitted vs. observed concentrations: r>0.999 for individual fits and r=0.872 for population fits) and provided highly sufficient predictive performance assessed via visual predictive checks and normalised prediction distribution error plots (results not shown). The estimated bioavailability had a geometric mean of 15.5% and a small between animal variability (3.6% coefficient of variation) suggesting consistent oral absorption in mice.
Statistical Analysis
Statistically significant differences among group means for lymph transport data in mice and for lymphatic transport across species were assessed by one-way analysis of variance (ANOVA), using a Tukey HSD post hoc test where variances were homogenous or Dunnett T3 post hoc test where variances were not homogenous. These analyses were performed using SPSS for Windows versions 15.0. (SPSS Inc, Chicago, Il). Significant differences in oral bioavailability and data across species were assessed using the z test. Differences with values of p<0.05 were considered significant.
RESULTS
Validation of Mesenteric Lymph Cannulated Mouse Model
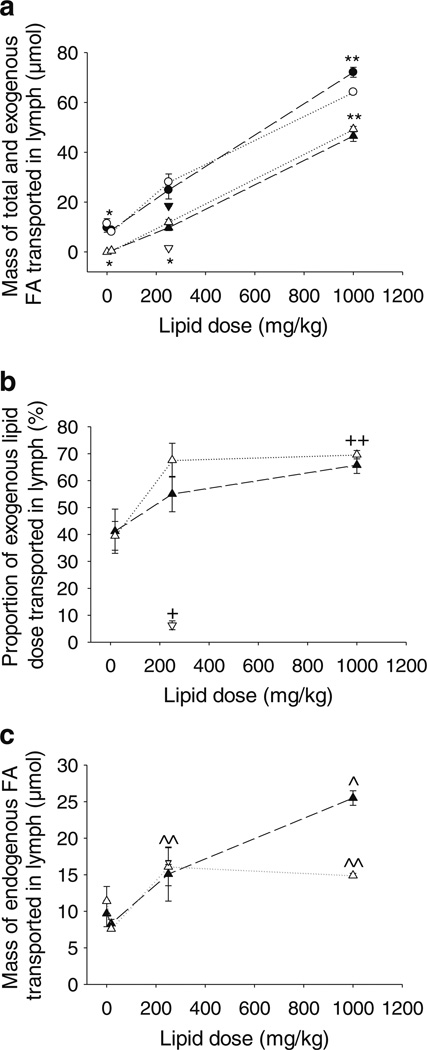
The mesenteric lymph flow rates after administration of the different lipid based formulations to mice ranged from 55 to 200 µl/h. Mean lymph flow rates ranging from 140 to 250 µl/h have previously been reported for conscious mice administered continuous lipid infusions into the duodenum at a rate of 0.3 ml/h for 6 h (24). The flow rates in the current study are consistent with this although they appear related to the mass of lipid dosed. The average mesenteric lymph flow rate in rats ranges from 400 to 3,000 µl/h (20,27,32,39) and since mice weigh approximately 10–12 fold less than rats the flow rate of mesenteric lymph is comparable between the two species on a µl/h/kg basis, providing further confidence in the lymph cannulated mouse model. Irrespective of flow rate, Fig. 3b and Table II show that between 40% and 70% of the administered dose of exogenous oleic acid (doses ranged from 18.1 mg/kg to 1,000 mg/kg) was recovered in the lymph of male and female mice with recovery related to the mass of lipid dosed. This data is consistent with studies describing the recovery of 40–80% of a dose of administered, exogenous fatty acid or triglyceride in the mesenteric lymph of conscious or anaesthetised rats (26,40–42). In mice, Nauli et al. found that 21–70% (mean 50%) of a 4 µmol/h (3.5 mg/h or ~175 mg/h/kg) dose of triolein was transported into the mesenteric lymph of conscious male mice whereas female mice had a distinct bimodal distribution with 12 mice falling in the range of 0–30%, and 20 mice falling in the range of 31–70% (25,43,44).
Fig. 3.
Mass transport of lipids into the lymph over 8 h following intra-duodenal administration of halofantrine (Hf) in 18.1, 250 or 1,000 mg/kg oleic acid or 250 mg/kg capric acid containing formulations to mesenteric lymph duct cannulated male or female mice. (a) Mass of total fatty acid (FA, males black circle, females white circle) and mass of exogenous FA (males black triangle, females white triangle) transported into lymph (µmol) of oleic acid dosed animals, and mass of total FA (males inverted black triangle) and mass of exogenous FA (males inverted white triangle) transported into lymph (µmol) of capric acid dosed animals. (b) Proportion (%) of the exogenous FA dose transported into lymph in oleic acid (males black triangle, females white triangle) or capric acid (males inverted white triangle) dosed animals. (c) Mass of endogenous FA transported into lymph (µmol) in oleic acid (males black triangle, females white triangle) or capric acid (males inverted white triangle) dosed animals. Data represent mean ± SEM, n=3–5 per group. * Significantly lower in animals of the same sex administered saline, 18.1 mg/kg oleic acid or 250 mg/kg capric acid when compared to 250 or 1,000 mg/kg oleic acid (p<0.05), **Significantly greater in animals of the same sex administered 1,000 mg/kg oleic acid when compared to saline, 18.1 or 250 mg/kg oleic acid or 250 mg/kg capric acid (p<0.05), + Significantly lower in male animals administered capric acid when compared to any dose of oleic acid (p<0.05), ++ Significantly greater in animals of the same sex administered 1,000 mg/kg oleic acid when compared to 18.1 mg/kg oleic acid or 250 mg/kg capric acid (p<0.05), ^ Significantly greater in male animals administered 1,000 mg/kg oleic acid when compared to saline or 18.1 mg/kg oleic acid (p<0.05), ^^Significantly greater in female animals administered 250 or 1,000 mg/kg oleic acid when compared to 18.1 mg/kg oleic acid (p<0.05).
Table II.
Mass of Total, Endogenous and Exogenous Fatty Acids (µmol), and % of the Administered Dose of Halofantrine, Transported into Lymph Over 8 h Following Intestinal Administration of 1.6 mg/kg Halofantrine and 18.1 mg/kg, 250 mg/kg or 1,000 mg/kg 14C Oleic Acid (OA) Dispersed in 0.5 ml 0.2% Tween 80 in Saline Over 1 h to Lymph Duct Cannulated Male or Female Mice
| Formulation | Sex | Halofantrine (% dose) |
Total fatty acid (µmol) |
Exogenous fatty acid (µmol) |
Endogenous fatty acid (µmol) |
Mass ratio exogenous: total fatty acid (as a%) |
|---|---|---|---|---|---|---|
| Saline | Male | 9.7 ± 1.8 | 9.7 ± 1.8 | |||
| 18.1 mg/kg oleic acid | Male | 1.9 ±0.4 | 8.8±0.6 | 0.5±0.1 | 8.3 ±0.6 | 5.7 |
| 250 mg/kg oleic acid | Male | 5.5 ± 1.7 | 24.8 ± 3.8a | 9.7 ± 1.2b | 15.1 ±3.7 | 39.1 |
| 1000 mg/kg oleic acid | Male | 5.8 ±0.4b | 72.1 ±2.0c | 46.6±2.2d | 25.5 ± 1.0a | 64.6 |
| Saline | Female | 11.4±2.0 | 11.4 ±2.0 | |||
| 18.1 mg/kg oleic acid | Female | 2.1 ±0.3 | 8.1 ±0.2 | 0.5±0.1 | 7.6 ±0.1 | 6.2 |
| 250 mg/kg oleic acid | Female | 6.5 ± 1.0b | 28.1 ±3.2a | 12.0± 1.1b | 16.1 ±2.6b | 42.3 |
| 1000 mg/kg oleic acid | Female | 5.2±0.8b | 64.2±0.8e,c | 49.3 ± 1.1d | 14.9±0.3e,b | 76.8 |
Data represent mean ± SEM for n=3–5 animals
Significantly greater than the same parameter following administration of saline or 18.1 mg/kg oleic acid to animals of the same sex (p<0.05)
Significantly greater than the same parameter following administration of 18.1 mg/kg oleic acid to animals of the same sex (p<0.05)
Significantly greater than the same parameter following administration of saline, 18.1 or 250 mg/kg oleic acid to animals of the same sex (p<0.05)
Significantly greater than the same parameter following administration of 18.1 or 250 mg/kg oleic acid to animals of the same sex (p<0.05)
Significantly lower than the same parameter in male mice (p<0.05)
Lymphatic Lipid Transport and Lymph Flow in Mice: Impact of Lipid Dose, Lipid Type and Sex
Lymphatic transport of endogenous and exogenous lipid was remarkably similar in male and female mice (Table II and Fig. 3a). One exception was the transport of endogenous fatty acid in the groups administered 1,000 mg/kg oleic acid, in which transport was significantly higher in male when compared to female mice (Table II). Lymphatic transport of both endogenous and exogenous lipids was also higher in male when compared to female rats after administration of 18.1 mg/kg long chain lipid (see Supplementary Material Fig. S2). As expected, lymphatic transport of triglyceride increased according to the mass of long chain lipid (oleic acid) administered to the mice (Fig. 3a and Table II). The increase in total lipid (fatty acid) transport versus the dose of lipid administered was relatively linear over the dose range of 0–1,000 mg/kg long chain lipid and was paralleled by increases in mass transport of exogenous oleic acid into the lymph (Fig. 3a and Table II). Interestingly, the proportion of the dose of oleic acid transported into the lymph increased with lipid dose (Fig. 3b and Table II), suggesting that the efficiency of lymphatic lipid transport increased at higher lipid loads.
As the mass of lipid dosed was increased, exogenous lipids accounted for an increasing proportion of the lipids in the lymph. In comparison, endogenous fatty acid transport into the lymph appeared to decrease (although not significantly) in animals administered the lowest long chain lipid dose (18.1 mg/kg) when compared to saline infused animals. In mice administered higher doses of long chain lipid (250– 1,000 mg/kg) lymphatic transport of endogenous lipid was slightly higher than that in fasted levels. Significant differences (p<0.05) were evident between the 1,000 mg/kg lipid and saline infused groups in male mice. The trend toward increased endogenous fatty acid transport in lymph (Fig. 3c) with increasing lipid dose from 18.1 to 1,000 mg/kg long chain lipid was consistent with the increase in the proportion of exogenous lipid transport into lymph under the same conditions (Fig. 3b) suggesting that similar driving forces may be responsible for partitioning of exogenous and endogenous lipid into the lymph at higher lipid doses.
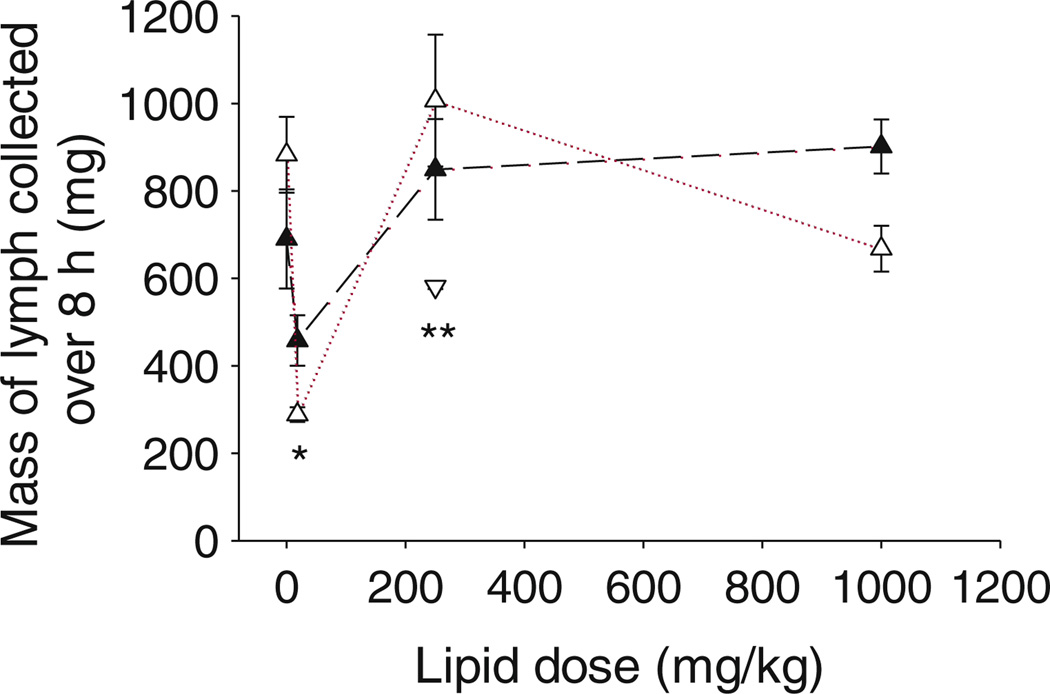
Lymph flow rates also showed similar trends to that seen for endogenous fatty acid transport, with a significant (p< 0.05) decrease in lymph flow noted for both male and female mice administered 18.1 mg/kg lipid when compared to saline (Fig. 4). Lymph flow rate increase to baseline fasted levels in mice administered 250 mg/kg and 1,000 mg/kg long chain lipid (Fig. 4).
Fig. 4.
Mass of lymph collected over 8 h (mg) versus the mass of oleic acid administered (males black triangle, females white triangle) or mass of capric acid administered (males inverted white triangle) intraduodenally with halofantrine to mesenteric lymph duct cannulated male or female mice. Data represent mean ± SEM, n=3–5 per group. *Significantly lower in animals of same sex administered 18.1 mg/kg oleic acid when compared to saline, 250 or 1,000 mg/kg oleic acid (p<0.05), **Significantly lower in male animals administered 250 mg/kg capric when compared to oleic acid (p<0.05).
After administration of 250 mg/kg of the medium chain fatty acid, capric acid, lymphatic transport of triglyceride (Fig. 3a) and exogenous fatty acid (Fig. 3a, b) was significantly lower when compared to administration of oleic acid. Only 6.3% of the dose of capric acid was transported in the lymph. Consistent with these results, the lymph flow rate was significantly (p<0.05) lower after administration of capric acid when compared to oleic acid (Fig. 4). In contrast, transport of endogenous fatty acids into the lymph was similar to that seen after administration of the same 250 mg/kg dose of medium or long chain fatty acid (Fig. 3c).
Lymphatic Drug Transport in Mice: Impact of Lipid Dose, Lipid Type and Sex
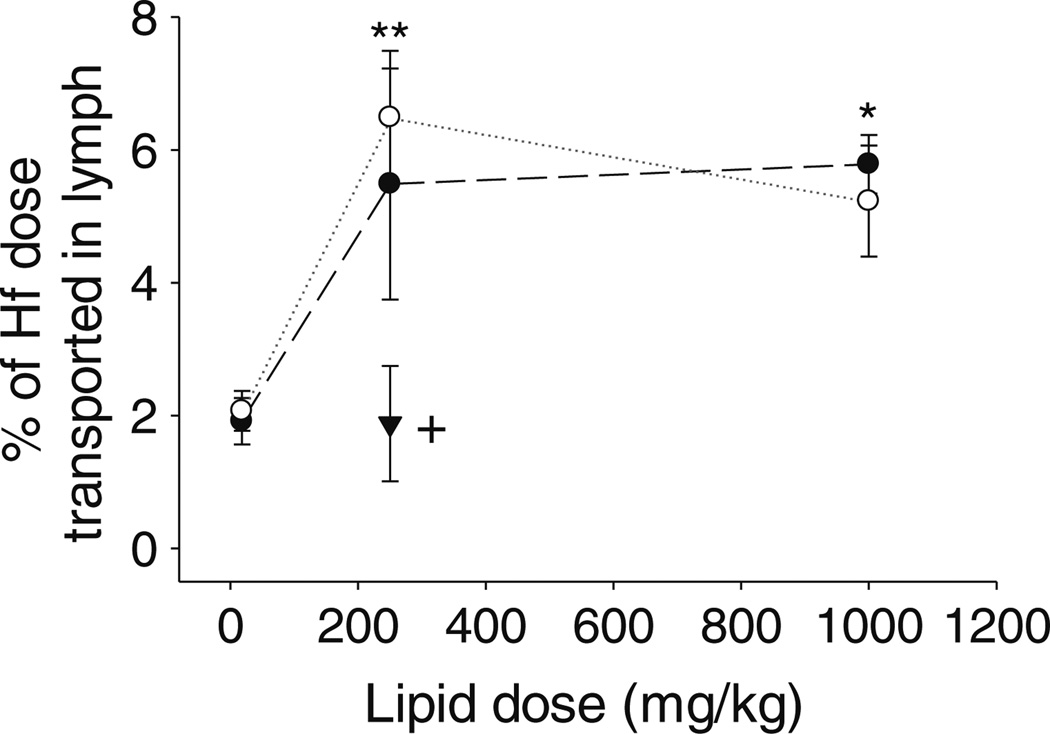
Consistent with the lipid transport data, lymphatic drug transport was similar in male and female mice (Fig. 5). In rats, however, lymphatic drug transport appeared slightly (but not significantly) lower in female when compared to male animals administered the 18.1 mg/kg lipid dose (Supplementary Material Fig. S2), consistent with the data for lymphatic lipid transport in the same groups. In the mice lymphatic transport of halofantrine increased as the long chain lipid dose was increased from 18.1 to 250 mg/kg but unlike the data for lipid transport which continued to increase as the lipid dose was increased from 250 to 1,000 mg/kg, drug transport plateaued at higher lipid doses and did not increase further on administration with 1,000 mg/kg long chain lipid (Fig. 5). Compared to the long chain lipid transport data (where 40–70% of the lipid dose was recovered in lymph (Fig. 3b)), recovery of halofantrine in the lymph was also lower (<6% of the dose after administration with all doses of oleic acid) (Fig. 5). Lymphatic transport of halofantrine was lower again after administration with 250 mg/kg capric acid (1.9% of dose) when compared to 250 mg/kg oleic acid (5.5% of dose) (Fig. 5) consistent with lower transport of medium chain fatty acid in the lymph (Fig. 3a, b).
Fig. 5.
Mass transport of halofantrine (Hf) into the lymph over 8 h (% of dose) versus the mass of oleic acid administered (males black circle, females white circle) or mass of capric acid administered (males inverted black triangle) to mesenteric lymph duct cannulated male or female mice. Data represent mean ± SEM, n=3–5 per group. * Significantly greater in animals of the same sex administered 1,000 mg/kg oleic acid when compared to 18.1 mg/kg oleic acid (p<0.05), ** significantly greater in female animals administered 250 mg/kg oleic acid when compared to 18.1 mg/kg oleic acid (p<0.05), + significantly lower in male animals administered 250 mg/kg capric acid when compared to 250 or 1,000 mg/ kg oleic acid (p<0.05).
Lymphatic Lipid and Drug Transport Across Species
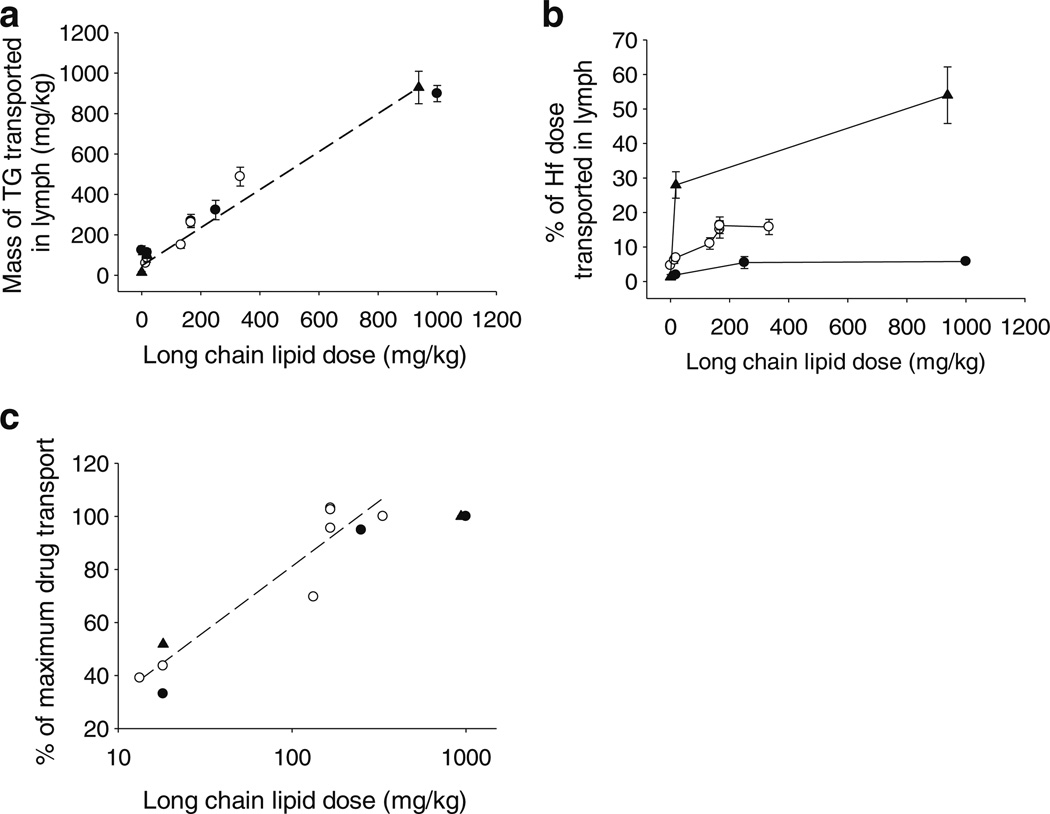
Cumulative transport of triglyceride in the lymph over 8 h after administration of different doses of long chain lipid was remarkably similar across species when normalised for body weight (mg/kg) (Fig. 6a). In all species, transport of triglyceride in the lymph increased approximately linearly as a function of increasing long chain lipid doses, consistent with the data described for mice (Fig. 3a). Linear regression analysis utilising the data in Fig. 6a from all species yielded an equation with an r2 value 0.996:
| (3) |
where MTG is the mass of triglyceride transported in lymph over 8 h (mg/kg) and MLL is the mass of long chain lipid dosed (mg/kg). The linear regression analysis suggests that in the fasted state the rate of triglyceride transport in lymph is approximately 47 mg/kg/8 h and thereafter that triglyceride transport increases by the equivalent of 94% of the mass of long chain lipid administered. This increase is not, however, simply due to transport of 94% of administered lipids into the lymph (the data in Fig. 3b show that between 40% and 60% of exogenously administered lipids are transported in lymph depending on lipid dose) but rather reflects a combination of enhanced endogenous lipid transport into the lymph, an increase in the mass of exogenous lipid administered and transport of increasing proportions of the exogenous lipid dose into the lymph with increasing lipid doses.
Fig. 6.
(a) Mass of triglyceride (TG) transported into lymph over 8 h (mg/kg), (b) % of the halofantrine (Hf) dose transported into lymph over 8 h, and (c) % of the maximum drug transport achieved in each species versus the mass of long chain lipid (mg/ kg) with which halofantrine was administered to lymph-duct cannulated mice (black circle), rats (white circle) or dogs (black triangle). Data represent mean ± SEM, n=3–5 per group. Rat data from the current study and previous rat (20,21,26–28) and dog data (9,29) from referenced publications included.
In comparison to the consistency in lipid transport across species, lymphatic transport of halofantrine differed markedly. In all species, lymphatic transport of halofantrine increased with lipid dose until it reached a plateau at long chain lipid doses greater than approximately 200 mg/kg. At all lipid doses, however, lymphatic transport of halofantrine was significantly (p<0.05) higher in dogs when compared to rats and in rats when compared to mice. In dogs, up to 54% of the dose of halofantrine was recovered in lymph which was significantly greater than the maximum lymphatic drug transport in rats (16% of dose) and mice (5.8% of dose). The ratio of lymphatic transport after administration of a given lipid dose to maximum lymphatic transport in each species (ie the % of maximum lymphatic transport achieved at each lipid dose) was, however, relatively similar (Fig. 6c). A correlation was also evident between the % of maximum lymphatic transport and the logarithm of lipid dose, with an r2 value of 0.82, y intercept and gradient of −0.3 and 16.6 in Fig. 6c.
Lymphatic Transport and Bioavailability Comparison Across Species After Administration of 18.1 mg/kg Long Chain Lipid
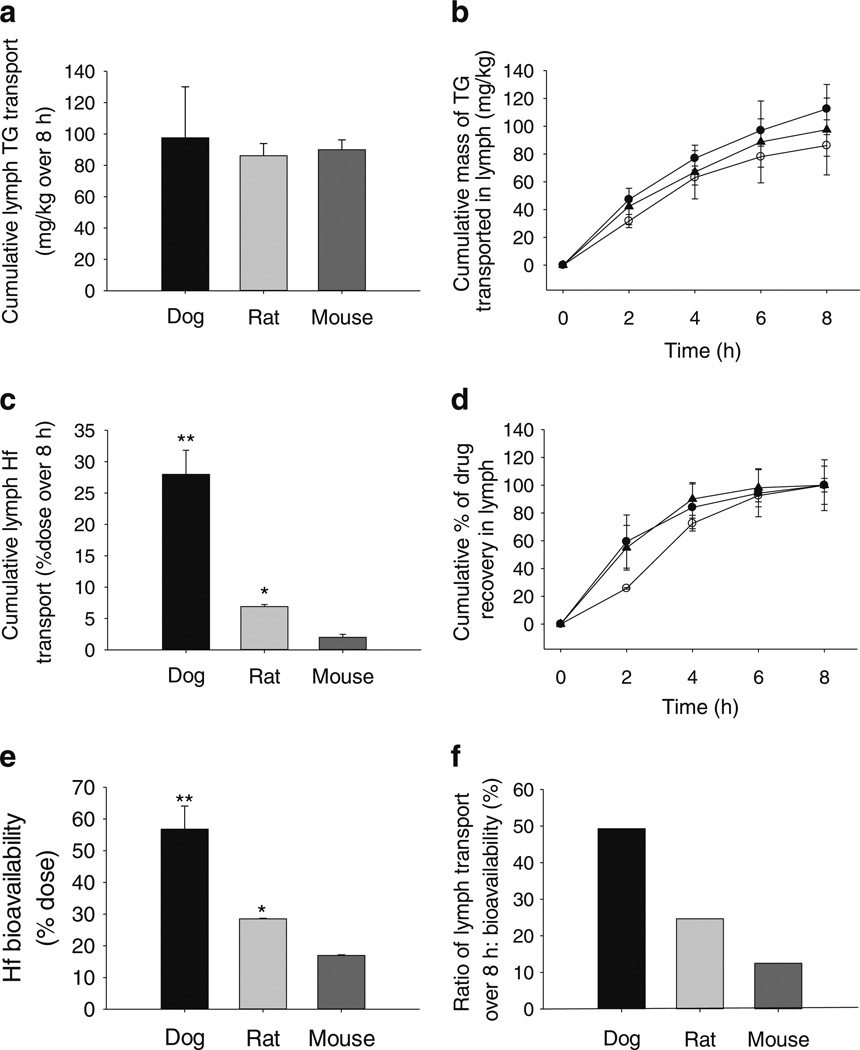
Figure 7 shows a comparison of halofantrine lymphatic transport and bioavailability in dogs, rats and mice after administration of 18.1 mg/kg long chain lipid (i.e. a mass of lipid that when administered to a dog is contained within a single unit capsule). Consistent with the data for triglyceride transport as a function of lipid dose in Fig. 6a, lymphatic triglyceride transport was not significantly different across species at a fixed lipid dose of 18.1 mg/kg (Fig. 7a). The rate of triglyceride transport into the lymph (Fig. 7b) was also similar across species and in conscious and anaesthetised rats (Supplementary Material Fig. S1). In contrast, lymphatic transport of halofantrine was greatest in dogs (28% of the dose over 8 h) when compared with anaesthetised or conscious rats (6–7% of dose over 8 h) and significantly higher in the rats when compared to the mice (2% of dose over 8 h) (Fig. 7c). The mass ratio of halofantrine:triglyceride (mg/g) transported into the lymph was therefore significantly higher in dogs (5.4) when compared to rats (1.2) and mice (0.3). The rate of halofantrine transport in lymph (Fig. 7d) was similar in dogs, mice and conscious rats (Supplementary Material Fig. S1) but slower in anaesthetised rats, perhaps because formulations were administered over 2 h to the anaesthetised rats, but via bolus to conscious rats and dogs, and over 1 h to mice. The similarity in lymphatic transport in conscious and anaesthetised rats (Supplementary Material Fig. S1) confirmed that differences in mass transport of halofantrine into the lymph across species are unlikely a result of differences in the method of drug administration (oral versus intraduodenal) or the conduct of studies in different conscious states (conscious versus anaesthetised).
Fig. 7.
(a) Mass of triglyceride (TG) transported into the lymph over 8 h per body weight (mg/ kg), (b) Cumulative mass of triglyceride transported into the lymph (per body weight, mg/kg) versus time, (c) Total % of the halofantrine (Hf) dose transported into the lymph over 8 h, (d) Cumulative % transport of the total mass of drug recovered in lymph over 8 h versus time, (e) Apparent oral bioavailability (% of dose) and (f) Ratio of lymphatic transport to oral bioavailability of Hf (as a %) following oral or intestinal administration of 1.6 mg/kg halofantrine and 18.1 mg/kg long chain lipid to male dogs, rats and mice. Data represent mean ± SEM, n=3–5 animals. *significantly greater than the same parameter in mice (p < 0.05), **significantly greater than the same parameter in mice and rats (p<0.05).
Similar to the data for lymphatic transport, the apparent bioavailability of halofantrine was significantly higher in the conscious dogs (57%) when compared to rats (29%) and mice (16%) (Table III, Fig. 7e). Bioavailability could not be accurately calculated in the anaesthetised rats and mice due to the difficulty in estimating a terminal elimination phase in the plasma profiles that are necessarily abbreviated in anaesthetised studies that can run for only ~10 h. However, estimates using truncated plasma data suggested that the bioavailability was approximately 28% in the anaesthetised rats and 9% in the anaesthetised mice. Halofantrine thus appeared to be more efficiently absorbed in the dogs when compared to rats and mice. Importantly, however, even taking into account the differences in bioavailability, the proportion of the dose transported via the lymph varied across species and was greatest in dogs (49%), and lower in rats (25%) and mice (12.5% when comparing lymphatic transport to bioavailability in conscious animals, and 23% when comparing lymphatic transport to bioavailability in anaesthetised animals) suggesting that differences in drug absorption/bioavailability do not completely explain the species differences in lymphatic transport of halofantrine.
Table III.
Oral Bioavailability (% of Dose), % of the Drug Dose Transported in Lymph Over 8 h and Proportion of the Bioavailable Fraction Transported via the Lymph (as a %) Following Oral or Intestinal Administration of 1.6 mg/kg Halofantrine and 18.1 mg/kg Long Chain Lipid to Male Dogs, Rats and Mice. Data represent mean ± SEM for n=3–5 animals
| Mice | Rats | Dogs | |
|---|---|---|---|
| Bioavailability (%) | 15.5±3.6a | 28.5±0.2b | 56.8 ± 7.3c |
| Lymphatic transport (%)d | 2.0 ±0.5 | 6.9 ±0.3b | 28.0±3.9c |
| Proportion transported via lymph (%) | 12.9 | 24.2 | 49 |
Co-efficient of variation shown for mouse bioavailability data
Significantly greater than the same parameter in mice (p<0.05)
Significantly greater than the same parameter in mice and rats (p<0.05)
Studies were conducted in conscious animals administered halofantrine via the oral route, except for the lymphatic transport studies in mice and rats which were conducted in anaesthetised animals administered halofantrine via intraduodenal infusion. Lymphatic transport of halofantrine was similar in conscious and anaesthetised rats (Supplementary Material Fig. S1) providing support for the use of data in anaesthetised rats and mice in the current comparison.
DISCUSSION
A mesenteric lymph duct cannulated murine model, previously employed to evaluate lymphatic lipid transport (24,25) has been successfully established and applied to the investigation of lymphatic drug transport. This model will ultimately allow access to transgenic animals and therefore more effective exploration of the role of, for example, specific membrane transporters, binding proteins and enzymatic pathways in the lymphatic transport of lipids and drugs. In the first instance, however, the mouse model has been utilised to examine similarities or differences in lymphatic lipid and drug transport in male and female animals, in different species (mouse versus rat versus dog) and in response to the administration of different quantities of lipid.
Lymphatic lipid and drug transport patterns were, in general, highly consistent in male and female animals in the current study (Fig. 3). Exceptions include slightly lower lipid transport in female when compared to male mice administered 1,000 mg/kg lipid, and slightly lower lipid and drug transport in female rats seen here after administration of 18.1 mg/kg lipid (Supplementary Material Fig. S2). As far as we are aware, lymphatic drug transport has not previously been explicitly compared in male and female animals. Lipid transport has, however, been examined in a couple of studies. Vahouny et al. noted subtle differences in the lymph lipoprotein (chylomicron vs VLDL) distribution of exogenous oleic acid and cholesterol in male and female rats after bolus administration of an emulsion containing 25 mg cholesterol and 110 mg (366– 550 mg/kg) oleic acid but did not describe large changes to the total recovery of oleic acid and cholesterol in lymph (45). More recently, lymph flow rate, lymphatic lipid transport and lymph lipoproteins were compared in male and female C57BL/6 mice administered a continuous lipid infusion for 6 h containing a 4 µmol/h (3.5 mg/h or ~175 mg/h/kg) dose of triolein. In this study, Nauli et al. found that in male mice the mean lymph flow rate was 0.25 ml/h and that 21– 70% (mean 50%) of the triolein dose was transported into the mesenteric lymph at steady state. In contrast, female mice had a distinct bimodal distribution in lymph transport with 20 mice falling into a group with lymph flow rates (mean 0.25 ml/h) and lipid transport (31–70% of triolen dose) similar to male mice, whereas a separate group of 12 mice had notably lower lymph flow rates (~0.14 ml/h) and triolein transport (0–30% of dose) (44). In Nauli et al., and consistent with the findings of Vahouny et al., more lipid was transported in the form of VLDL than chylomicrons in female animals when compared to the male mice and this occurred in both the high and low transport group. Differences in lipid transport in the females appeared unrelated to estrous cycle in this study, whereas others have found that different levels of sex hormones may influence lymphatic transport in males versus females. For example, administration of ethinyl estradiol (an estrogen), to male rats reduced the lymphatic transport of triglyceride and apolipoproteins leading to reduced plasma lipid and apolipoprotein concentrations (46). In contrast, administration of estrogen to ovariectomized female rats significantly increased lymphatic transport of linoleic acid (47). A clear role for sex hormones in determining patterns of lymphatic lipid transport is therefore not apparent. Consistent with this suggestion, the current data did not find substantial differences in lymphatic lipid and drug transport in male versus female animals. Subtle differences were, however, apparent and may be related to the mass of lipid dosed. The data of Nauli et al. provide convincing evidence that lymph transport may exhibit a bimodal distribution in female animals, at least after administration of relatively high lipid doses (175 mg/kg/h for 6 h ie 1,050 mg/kg). It is possible that this was not observed in the current study due to the lower number of animals per experimental group. Nonetheless the current data suggest that animals of either sex may be suitable for lymphatic drug transport studies as they yield quantitatively similar results.
Comparison of lymphatic lipid and drug transport patterns after administration of long and medium chain lipids in mice, revealed that lipid and drug transport was greater in the group administered long chain lipid (oleic acid) when compared to medium chain lipid (capric acid) (Fig. 3). The increased transport resulted from increased lymphatic transport of exogenous oleic acid (55% of dose) when compared to exogenous capric acid (6.3% of dose) as lymphatic transport of endogenous lipid was similar in both cohorts. The data are consistent with a number of published studies in rats where longer chain lipids (>14 carbons) were more highly lymphatically transported when compared to shorter chain (and more water soluble) fatty acids, that were primarily absorbed via the portal blood (48–50). The data are also consistent with published studies in rats and dogs that describe preferential lymphatic transport of long chain lipids and more effective lymphatic drug transport after co-administration with long rather than medium or short chain lipid (26,29). The current study validates that the lipid chain length effects previously seen in rats and dogs are also evident in mice.
Lymphatic transport of triglyceride increased linearly with lipid dose and was remarkably similar across species on a mg/kg basis (Fig. 6a). Indeed, regression analysis of all data sets yields a single relationship to quantify lymphatic lipid transport as a function of long chain lipid dose and is seemingly applicable to all the examined animal species (Eq. 3). This may find utility in predicting lipid transport after administration of different lipid doses in different species. In contrast, lymphatic transport of drug was not linearly related to lipid dose and differed markedly across species. Whilst mass transport of drug differed across species, however, the proportion of maximum drug transport achieved via administration of different lipid doses appeared to be the same in all species and related to the logarithm of lipid dose (Fig. 6c). The similarities in triglyceride transport across species and the relationship between lipid transport and the proportion of maximum drug transport suggest that results for lipid (and potentially drug) transport into lymph may be extrapolated across species with some certainty.
Analysis of the contribution of exogenous and endogenous lipids to total lymph lipid transport in mice administered long chain lipid (oleic acid) revealed that lymphatic transport of exogenous lipids increased and became a more important contributor to total lipid transport at increasing exogenous lipids doses (from 18.1 to 250 to 1,000 mg/kg). The increase in lymphatic transport of exogenous lipid was a function of administration of higher exogenous lipid doses (as expected), and also an increase in the proportion of the exogenous lipid dose that was lymphatically transported at higher lipid doses (Fig. 3a, b). These results in mice are consistent with previous studies in rats that describe the recovery of 40–80% of the dose of oleic acid in mesenteric lymph, with greater lymphatic recovery after administration of higher lipid doses (21,26,28,40–42). The underlying mechanisms involved in the increase in efficiency of lymphatic lipid transport with increasing lipid dose are unknown, but may involve the upregulation of transport proteins and metabolic enzymes that direct lipid into the lymph. A positive feedback loop such as this is consistent with previous findings showing that increases in systemic chylomicron concentrations stimulate enhanced lymph lipid transport (51), as well as likely energy needs. Thus, at lower lipid doses, a greater proportion of lipid is required as an immediate energy source and is therefore transported into the blood. In contrast, at higher lipid loads, the quantity of lipid is in excess of immediate energy requirements and is diverted to storage via resynthesis to triglyceride, assembly into lipoproteins and transport into the lymph. Lipoprotein associated triglyceride may subsequently be directed to storage sites such as adipose tissue. This hypothesis will be addressed in future studies.
The impact of exogenous lipid on endogenous lipid transport into lymph in mice was markedly dependent on lipid dose. Thus, at higher exogenous lipid doses (250 and 1,000 mg/kg) the entry of endogenous lipids into the lymph of mice was enhanced (Fig. 3c and Table II), and at the lowest lipid dose (18.1 mg/kg oleic acid) lymphatic transport of endogenous lipid was reduced. This pattern is consistent with previous studies in rats where administration of 100– 1,000 mg/kg of oleic acid to rats increased endogenous lipid entry into the lymph (21,28,52). In greyhound dogs administration of exogenous lipids has also been shown to stimulate lymphatic transport of endogenous lipids although in this case endogenous lipid recruitment appeared to occur after administration of even lower lipid doses (18.1 mg/kg long chain lipid) (9,29).
Despite subtle differences in endogenous lipid recruitment into lymph, total lymphatic lipid transport was similar across species after administration of 18.1 mg/kg exogenous lipid (Fig. 7). In contrast, the lymphatic transport of halofantrine after administration of the same lipid dose was markedly different and increased in the order: mice (~2% dose), rats (~7%) and dogs (~28%) (Fig. 7). Similarly, after administration with higher lipid doses or food, the maximum lymphatic transport of halofantrine in mice (6% of the dose) was significantly lower than that seen previously in rats (16– 20% (20,21,26)) and dogs (54% (9)). The primary drivers of lymphatic drug transport are expected to be the quantity of lymph lipoproteins in the enterocyte (as defined by the lipid dose and the efficiency of lymphatic lipid transport); the quantity of drug in the enterocyte (as defined by the drug dose, the fraction absorbed and the fraction that escapes gut based metabolism) and the affinity of drug for developing lymph lipoproteins in the enterocyte (often predicted based on log D and lipid solubility). As lymphatic lipid transport was similar across species, the differences in lymphatic transport of halofantrine do not appear to have resulted from reduced lymphatic lipid transport in the mice and rats when compared to dogs. The bioavailability of halofantrine did increase in dogs (~57%) when compared to rats (~29%) and mice (~16%) (Fig. 7), however, the differences in lymphatic drug transport were greater than the differences in bioavailability and the proportion of the bioavailable dose that was lymphatically transported was greater in dogs (49%) when compared to rats (25%) and mice (12.5%) (Fig. 7). As such changes in bioavailability appear to explain some, but not all of the differences seen. Drug affinity for mouse versus rat versus dog lymph lipoproteins is a function of the physicochemical properties of the drug (which are constant) and the nature of the lymph lipoproteins. It is possible that intestinal lipoproteins in the dog differ from that in the rat and mouse and provide increased affinity for halofantrine. However other factors may also affect lipoprotein partitioning including differences in enterocyte-based metabolism, intestinal blood and lymph flow and plasma (and lymph) protein binding. The species differences in lymphatic transport therefore appear to be a composite function of differences in absorption, and other factors. Ongoing studies aim to examine further the mechanism(s) responsible for the species differences in lymphatic transport of halofantrine.
The current data also raises the question as to whether absorption and lymphatic transport of highly lipophilic compounds is, in general, more efficient in dogs than in rats and mice. Although no previous studies have explicitly compared the absorption and lymphatic transport of highly lipophilic drugs across species, our limited experience suggests that lymphatic transport of lipophilic drugs is often more efficient in dogs when compared to smaller species such as rats. For instance, the maximum lymphatic transport of the CETP inhibitors CP532,623 and CP524,515 was 28 and 22% of the dose in rats and 36 and 56% of the dose in dogs, respectively. After administration with 13.3 mg/kg long chain lipid to rats and 18.1 mg/kg long chain lipid to dogs, the lymphatic transport of CP532,623 increased from 12 to 26% and of CP524,515 increased from 16 to 25% (4,5). Similarly, the maximum lymphatic transport of methylnortestosterone was 3% of the dose in rats and 6% of the dose in dogs (12). Further studies are obviously required to provide additional data to support these suggestions.
CONCLUSION
In summary, lymphatic lipid and drug transport has been compared as a function of lipid dose, species (mice, rats and dogs) and sex (male versus female animals). In particular a detailed analysis of the impact of lipid dose on endogenous lipid, exogenous lipid and drug transport into the mesenteric lymph has been presented across species. Lymphatic lipid transport was remarkably similar across species, and in male versus female mice, suggesting that data collected in animals of different species and sex are likely to be comparable. Surprisingly, despite similar lymphatic lipid transport profiles, lymphatic transport of halofantrine was substantially lower in mice < rats < dogs. Some of this difference appeared to result from lower bioavailability, however this was unable to explain all of the differences observed and more systematic differences in lymphatic drug transport across species appear evident. Future analyses will determine if similar trends in lymphatic transport are seen for other drugs and will focus on determining the mechanisms behind differences in lymphatic transport of halofantrine across species, in order to establish the most relevant model(s) to predict lymphatic transport in humans.
Supplementary Material
ACKNOWLEDGMENTS
DISCLOSURES
The authors would like to thank Luojuan Hu for her technical assistance in the mouse bioavailability experiments and Dr Juergen Bulitta for assistance in calculating bioavailability in the mice.
ABBREVIATIONS
- AUC
Area under the plasma concentration-time curve
- FA
Fatty acid
- Hf
Halofantrine
- HPLC
High Performance Liquid Chromatography
- LC-MS
HPLC-mass spectrometry
- PL
Phospholipid
- SEDDS
Self-Emulsifying Drug Delivery System
- TG
Triglyceride
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11095-013-1000-0) contains supplementary material, which is available to authorized users.
Contributor Information
Natalie L. Trevaskis, Email: Natalie.Trevaskis@monash.edu, Drug Delivery, Disposition and Dynamics, Monash Institute of Pharmaceutical Sciences, Monash University (Parkville Campus), 381 Royal Parade, Parkville, Victoria, Australia 3052.
Suzanne M. Caliph, Drug Delivery, Disposition and Dynamics, Monash Institute of Pharmaceutical Sciences, Monash University (Parkville Campus), 381 Royal Parade, Parkville, Victoria, Australia 3052
Gary Nguyen, Drug Delivery, Disposition and Dynamics, Monash Institute of Pharmaceutical Sciences, Monash University (Parkville Campus), 381 Royal Parade, Parkville, Victoria, Australia 3052.
Patrick Tso, Department of Pathology, Genome Research Institute, University of Cincinnati, 2120 East Galbraith Road, Cincinnati, Ohio, 45237, USA.
William N. Charman, Drug Delivery, Disposition and Dynamics, Monash Institute of Pharmaceutical Sciences, Monash University (Parkville Campus), 381 Royal Parade, Parkville, Victoria, Australia 3052
Christopher J. H. Porter, Email: Chris.Porter@monash.edu, Drug Delivery, Disposition and Dynamics, Monash Institute of Pharmaceutical Sciences, Monash University (Parkville Campus), 381 Royal Parade, Parkville, Victoria, Australia 3052.
REFERENCES
- 1.Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, et al. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65:315–499. doi: 10.1124/pr.112.005660. [DOI] [PubMed] [Google Scholar]
- 2.Charman WN, Stella VJ. Estimating the maximum potential for intestinal lymphatic transport of lipophilic drug molecules. Int J Pharm. 1986;34:175–178. [Google Scholar]
- 3.Gershkovich P, Hoffman A. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: linear correlation with intestinal lymphatic bioavailability. Eur J Pharm Sci. 2005;26:394–404. doi: 10.1016/j.ejps.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Trevaskis NL, McEvoy CL, McIntosh MP, Edwards GA, Shanker RM, Charman WN, et al. The role of the intestinal lymphatics in the absorption of two highly lipophilic cholesterol ester transfer protein inhibitors (CP524,515 and CP532,623) Pharm Res. 2010;27:878–893. doi: 10.1007/s11095-010-0083-0. [DOI] [PubMed] [Google Scholar]
- 5.Trevaskis NL, Shanker RM, Charman WN, Porter CJ. The mechanism of lymphatic access of two cholesteryl ester transfer protein inhibitors (CP524,515 and CP532,623) and evaluation of their impact on lymph lipoprotein profiles. Pharm Res. 2010;27:1949–1964. doi: 10.1007/s11095-010-0199-2. [DOI] [PubMed] [Google Scholar]
- 6.Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 7.Trevaskis NL, Charman WN, Porter CJ. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008;60:702–716. doi: 10.1016/j.addr.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trevaskis NL, Porter CJ, Charman WN. An examination of the interplay between enterocyte-based metabolism and lymphatic drug transport in the rat. Drug Metab Dispos. 2006;34:729–733. doi: 10.1124/dmd.105.008102. [DOI] [PubMed] [Google Scholar]
- 9.Khoo SM, Edwards GA, Porter CJ, Charman WN. A conscious dog model for assessing the absorption, enterocyte-based metabolism, and intestinal lymphatic transport of halofantrine. J Pharm Sci. 2001;90:1599–1607. doi: 10.1002/jps.1110. [DOI] [PubMed] [Google Scholar]
- 10.Trevaskis NL, Shackleford DM, Charman WN, Edwards GA, Gardin A, Appel-Dingemanse S, et al. Intestinal lymphatic transport enhances the post-prandial oral bioavailability of a novel cannabinoid receptor agonist via avoidance of first-pass metabolism. Pharm Res. 2009;26:1486–1495. doi: 10.1007/s11095-009-9860-z. [DOI] [PubMed] [Google Scholar]
- 11.Shackleford DM, Faassen WA, Houwing N, Lass H, Edwards GA, Porter CJ, et al. Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two andriol formulations in conscious lymph duct-cannulated dogs. J Pharmacol Exp Ther. 2003;306:925–933. doi: 10.1124/jpet.103.052522. [DOI] [PubMed] [Google Scholar]
- 12.White KL, Nguyen G, Charman WN, Edwards GA, Faassen WA, Porter CJ. Lymphatic transport of Methylnortestosterone undecanoate (MU) and the bioavailability of methylnortestosterone are highly sensitive to the mass of coadministered lipid after oral administration of MU. J Pharmacol Exp Ther. 2009;331:700–709. doi: 10.1124/jpet.109.154542. [DOI] [PubMed] [Google Scholar]
- 13.Caliph SM, Trevaskis NL, Charman WN, Porter CJ. Intravenous dosing conditions may affect systemic clearance for highly lipophilic drugs: implications for lymphatic transport and absolute bio-availability studies. J Pharm Sci. 2012;101:3540–3546. doi: 10.1002/jps.23211. [DOI] [PubMed] [Google Scholar]
- 14.Hauss DJ, Mehta SC, Radebaugh GW. Targeted lymphatic transport and modified systemic distribution of CI-976, a lipophilic lipid-regulator drug, via a formulation approach. Int J Pharm. 1994;108:85–93. [Google Scholar]
- 15.Trevaskis NL, Charman WN, Porter CJ. Targeted drug delivery to lymphocytes: a route to site-specific immunomodulation? Mol Pharm. 2010;7:2297–2309. doi: 10.1021/mp100259a. [DOI] [PubMed] [Google Scholar]
- 16.Dane KY, Nembrini C, Tomei AA, Eby JK, O’Neil CP, Velluto D, et al. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. J Control Release. 2011;156:154–160. doi: 10.1016/j.jconrel.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Kaminskas LM, Porter CJH. Targeting the lymphatics using dendritic polymers (dendrimers) Adv Drug Deliv Rev. 2011;63:890–900. doi: 10.1016/j.addr.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Caliph SM, Faassen WA, Vogel GM, Porter CJ. Oral bioavailability assessment and intestinal lymphatic transport of Org 45697 and Org 46035, two highly lipophilic novel immunomodulator analogues. Curr Drug Deliv. 2009;6:359–366. doi: 10.2174/156720109789000500. [DOI] [PubMed] [Google Scholar]
- 19.Holm R, Mullertz A, Christensen E, Hoy CE, Kristensen HG. Comparison of total oral bioavailability and the lymphatic transport of halofantrine from three different unsaturated triglycerides in lymph-cannulated conscious rats. Eur J Pharm Sci. 2001;14:331–337. doi: 10.1016/s0928-0987(01)00186-5. [DOI] [PubMed] [Google Scholar]
- 20.Porter CJ, Charman SA, Humberstone AJ, Charman WN. Lymphatic transport of halofantrine in the conscious rat when administered as either the free base or the hydrochloride salt: effect of lipid class and lipid vehicle dispersion. J Pharm Sci. 1996;85:357–361. doi: 10.1021/js9502229. [DOI] [PubMed] [Google Scholar]
- 21.Trevaskis NL, Porter CJ, Charman WN. Bile increases intestinal lymphatic drug transport in the fasted rat. Pharm Res. 2005;22:1863–1870. doi: 10.1007/s11095-005-6808-9. [DOI] [PubMed] [Google Scholar]
- 22.Dahan A, Mendelman A, Amsili S, Ezov N, Hoffman A. The effect of general anesthesia on the intestinal lymphatic transport of lipophilic drugs: comparison between anesthetized and freely moving conscious rat models. Eur J Pharm Sci. 2007;32:367–374. doi: 10.1016/j.ejps.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Lespine A, Chanoit G, Bousquet-Melou A, Lallemand E, Bassissi FM, Alvinerie M, et al. Contribution of lymphatic transport to the systemic exposure of orally administered moxidectin in conscious lymph duct-cannulated dogs. Eur J Pharm Sci. 2006;27:37–43. doi: 10.1016/j.ejps.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Lo CM, Nordskog BK, Nauli AM, Zheng S, Vonlehmden SB, Yang Q, et al. Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am J Physiol Gastrointest Liver Physiol. 2008;294:G344–G352. doi: 10.1152/ajpgi.00123.2007. [DOI] [PubMed] [Google Scholar]
- 25.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, et al. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197–1207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caliph SM, Charman WN, Porter CJ. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and noncannulated rats. J Pharm Sci. 2000;89:1073–1084. doi: 10.1002/1520-6017(200008)89:8<1073::aid-jps12>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Porter CJ, Charman SA, Charman WN. Lymphatic transport of halofantrine in the triple-cannulated anesthetized rat model: effect of lipid vehicle dispersion. J Pharm Sci. 1996;85:351–356. doi: 10.1021/js950221g. [DOI] [PubMed] [Google Scholar]
- 28.Trevaskis NL, Porter CJ, Charman WN. The lymph lipid precursor pool is a key determinant of intestinal lymphatic drug transport. J Pharmacol Exp Ther. 2006;316:881–891. doi: 10.1124/jpet.105.094094. [DOI] [PubMed] [Google Scholar]
- 29.Khoo SM, Shackleford DM, Porter CJ, Edwards GA, Charman WN. Intestinal lymphatic transport of halofantrine occurs after oral administration of a unit-dose lipid-based formulation to fasted dogs. Pharm Res. 2003;20:1460–1465. doi: 10.1023/a:1025718513246. [DOI] [PubMed] [Google Scholar]
- 30.Khoo SM, Humberstone AJ, Porter CJ, Edwards GA, Charman WN. Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of halofantrine. Int J Pharm. 1998;167:155–164. [Google Scholar]
- 31.Johnson BM, Chen W, Borchardt RT, Charman WN, Porter CJ. A kinetic evaluation of the absorption, efflux, and metabolism of verapamil in the autoperfused rat jejunum. J Pharmacol Exp Ther. 2003;305:151–158. doi: 10.1124/jpet.102.045328. [DOI] [PubMed] [Google Scholar]
- 32.Edwards GA, Porter CJ, Caliph SM, Khoo SM, Charman WN. Animal models for the study of intestinal lymphatic drug transport. Adv Drug Deliv Rev. 2001;50:45–60. doi: 10.1016/s0169-409x(01)00148-x. [DOI] [PubMed] [Google Scholar]
- 33.Humberstone AJ, Currie GJ, Porter CJ, Scanlon MJ, Charman WN. A simplified liquid chromatography assay for the quantitation of halofantrine and desbutylhalofantrine in plasma and identification of a degradation product of desbutylhalofantrine formed under alkaline conditions. J Pharm Biomed Anal. 1995;13:265–272. doi: 10.1016/0731-7085(95)01256-k. [DOI] [PubMed] [Google Scholar]
- 34.Bauer R, Guzy S, Ng C. A survey of population analysis methods and software for complex pharmacokinetic and pharmacodynamic models with examples. AAPS J. 2007;9:E60–E83. doi: 10.1208/aapsj0901007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bulitta JB, Bingolbali A, Shin BS, Landersdorfer CB. Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT. AAPS J. 2011;13:201–211. doi: 10.1208/s12248-011-9257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prokocimer P, Bien P, Surber J, Mehra P, DeAnda C, Bulitta JB, et al. Phase 2, randomized, double-blind, dose-ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral torezolid phosphate in patients with complicated skin and skin structure infections. Antimicrob Agents Chemother. 2011;55:583–592. doi: 10.1128/AAC.00076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bulitta JB, Kinzig M, Landersdorfer CB, Holzgrabe U, Stephan U, Sorgel F. Comparable population pharmacokinetics and pharmacodynamic breakpoints of cefpirome in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother. 2011;55:2927–2936. doi: 10.1128/AAC.01484-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailer AJ. Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm. 1988;16:303–309. doi: 10.1007/BF01062139. [DOI] [PubMed] [Google Scholar]
- 39.Kohan AB, Yoder SM, Tso P. Using the lymphatics to study nutrient absorption and the secretion of gastrointestinal hormones. Physiol Behav. 2011;105:82–88. doi: 10.1016/j.physbeh.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansbach CM, Arnold A. Steady-state kinetic analysis of triacylglycerol delivery into mesenteric lymph. Am J Physiol. 1986;251:G263–G269. doi: 10.1152/ajpgi.1986.251.2.G263. [DOI] [PubMed] [Google Scholar]
- 41.Mansbach IICM, Dowell RF, Pritchett D. Portal transport of absorbed lipids in rats. Am J Physiol. 1991;261:G530–G538. doi: 10.1152/ajpgi.1991.261.3.G530. [DOI] [PubMed] [Google Scholar]
- 42.McDonald GB, Weidman M. Partitioning of polar fatty acids into lymph and portal vein after intestinal absorption in the rat. Q J Exp Physiol. 1987;72:153–159. doi: 10.1113/expphysiol.1987.sp003059. [DOI] [PubMed] [Google Scholar]
- 43.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, et al. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115:1290–1297. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nauli AM. Ph D thesis. University of Cincinnati, Cincinnati: Department of Pathology and Laboratory Medicine; 2005. Intestinal lipid uptake and secretion of VLDL and chylo-micron; p. 170. [Google Scholar]
- 45.Vahouny GV, Blendermann EM, Gallo LL, Treadwell CR. Differential transport of cholesterol and oleic acid in lymph lip-oproteins: sex differences in puromycin sensitivity. J Lipid Res. 1980;21:415–424. [PubMed] [Google Scholar]
- 46.Krause BR, Sloop CH, Castle CK, Roheim PS. Mesenteric lymph apolipoproteins in control and ethinyl estradiol-treated rats: a model for studying apolipoproteins of intestinal origin. J Lipid Res. 1981;22:610–619. [PubMed] [Google Scholar]
- 47.Yang L, Koo SI, Jeon IJ. The lymphatic absorption of fatty acids and output of phospholipids are lowered by estrogen replacement in ovariectomized rats. Nutr Biochem. 1996;7:214–221. [Google Scholar]
- 48.Chaikoff IL, Bloom B, Stevens BP, Reinhardt WO, Dauben WG. Pentadecanoic acid-5-C14; its absorption and lymphatic transport. J Biol Chem. 1951;190:431–435. [PubMed] [Google Scholar]
- 49.Bloom B, Chaikoff IL, Reinhardt Intestinal lymph as pathway for transport of absorbed fatty acids of different chain lengths. Am J Physiol. 1951;166:451–455. doi: 10.1152/ajplegacy.1951.166.2.451. [DOI] [PubMed] [Google Scholar]
- 50.Kiyasu JY, Bloom B, Chaikoff IL. The portal transport of absorbed fatty acids. J Biol Chem. 1952;199:415–419. [PubMed] [Google Scholar]
- 51.Trevaskis NL, Charman WN, Porter CJ. Acute hypertriglyceridemia promotes intestinal lymphatic lipid and drug transport: a positive feedback mechanism in lipid and drug absorption. Mol Pharm. 2011;8:1132–1139. doi: 10.1021/mp100462d. [DOI] [PubMed] [Google Scholar]
- 52.Shiau YF, Popper DA, Reed M, Umstetter C, Capuzzi D, Levine GM. Intestinal triglycerides are derived from both endogenous and exogenous sources. Am J Physiol. 1985;248:G164–G169. doi: 10.1152/ajpgi.1985.248.2.G164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.