Abstract
Catalysis of covalent modification of aliphatic amine groups, such as the lysine (Lys) side chain, by nucleic acids has been challenging to achieve. Such catalysis will be valuable, e.g., for practical preparation of Lys-modified proteins. We previously reported DNA-catalyzed modification of the tyrosine and serine hydroxyl side chains, but Lys modification has been elusive. In this study, we show that increasing the reactivity of the electrophilic reaction partner by using 5′-phosphorimidazolide (5′-Imp) rather than 5′-triphosphate (5′-ppp) enables DNA-catalyzed modification of Lys in a DNA-anchored peptide substrate. DNA-catalyzed reaction of Lys + 5′-Imp is observed in an architecture in which the nucleophile and electrophile are not preorganized, whereas catalysis was not observed in our prior efforts that used Lys + 5′-ppp in a preorganized arrangement. Therefore, substrate reactivity is more important than preorganization in this context. These findings will assist ongoing efforts to identify DNA catalysts for reactions of protein substrates at lysine side chains.
Keywords: deoxyribozymes, DNA, in vitro selection, peptides, lysine modification
Deoxyribozymes are specific DNA sequences that have catalytic activity.[1] We have focused on expanding deoxyribozyme catalysis to include reactions of peptide side chains,[2] with the longer-term goal of achieving DNA-catalyzed covalent modification of large proteins. Our initial report demonstrated robust DNA catalysis (>70% yield in 1 h) of nucleopeptide formation between the nucleophilic tyrosine (Tyr) phenolic OH side chain and an electrophilic 5′-triphosphate RNA (5′-pppRNA; Fig. 1a).[2a] In parallel, however, catalysis by separate new deoxyribozymes involving the serine (Ser) aliphatic hydroxyl side chain was extremely poor (only ∼0.2% yield), and reactivity of the lysine (Lys) amine side chain was not observed. That initial study presented each single amino acid residue in a highly preorganized three-helix-junction (3HJ) architecture, in which the nucleophilic side chain and the electrophilic 5′-ppp are closely juxtaposed (Fig. 1b).[3] We later found that retaining the 3HJ architecture but expanding the substrate to include a tripeptide rather than a single amino acid enabled much more robust Ser reactivity.[2b] In contrast, when Lys was similarly presented, reaction was instead observed at the oxygen atom of a nearby phosphoramidate linkage, whereas Lys reactivity was still absent.[2d] This finding suggested that we should examine more reactive electrophiles than 5′-ppp for DNA-catalyzed reactions of amine nucleophiles.
Figure 1.
Electrophilic substrates and architectures for DNA-catalyzed nucleophilic reactivity of amino acid side chains. a) Electrophilic substrate structures, showing attack by a nucleophile (Nu:) such as an hydroxyl or amino group. X = OH (RNA) or H (DNA). b) Three-helix-junction (3HJ) architecture for juxtaposing nucleophile and electrophile, illustrated with Tyr as nucleophile and 5′-triphosphate (5′-ppp) as electrophile. Base pairing creates the third helix and preorganizes the nucleophile and electrophile; see previous studies for origin and analysis of the 3HJ.[3] (C) Open architecture, with amine as nucleophile and 5′-phosphorimidazolide (5′-Imp) as electrophile. Note the lack of preorganization of nucleophile and electrophile in the open architecture relative to the 3HJ architecture.
Nature provides significant precedent for protein-catalyzed reaction of the Lys side chain with various electrophiles, including activated acyl groups.[4] Lys also reacts with the 5′-triphosphate moiety of ATP during reactions catalyzed by RNA and DNA ligase enzymes[5] and in protein adenylylation reactions.[6] In nonbiological contexts, more reactive phosphorus electrophiles than 5′-ppp can be employed. Prebiotically focused nucleotide oligomerization experiments have typically used more reactive nucleotide analogues such as 5′-phosphorimidazolide (5′-Imp) rather than 5′-ppp,[7] for which the reactivity difference is on the order of 102-fold. In this study, we evaluated 5′-Imp as a more reactive electrophile (Fig. 1a). In all of these efforts, we used the less structurally constrained architecture of Fig. 1c, because our long-term goal is to identify deoxyribozymes that function with free peptide and protein substrates,[8] and the 3HJ architecture of Fig. 1b is incompatible with this goal. DNA-catalyzed nucleophilic Lys reaction was achieved by using 5′-Imp DNA in the less preorganized architecture. From these results, we conclude that DNA can catalyze covalent modification of the nucleophilic Lys side chain, and a high degree of preorganization is dispensable when the electrophile is sufficiently reactive.
We used in vitro selection[9] to identify deoxyribozymes that catalyze covalent modification of amino groups, where in vitro selection is a process by which random-sequence populations are iteratively enriched through multiple rounds to identify those particular sequences that have catalytic activity. Two amine substrates were used in these selection experiments (Fig. 2a). The first substrate, DNA-C3-NH2, presents an aliphatic amino group on a short C3 tether at the 3′-terminus of a DNA oligonucleotide anchor. The second substrate, DNA-HEG-CKA, presents Lys as part of a Cys-Lys-Ala (CKA) tripeptide substrate covalently attached via a disulfide linkage to a hexa(ethylene glycol), or HEG, tether at the DNA 3′-terminus. For both amine substrates and particularly for the HEG-tethered Lys, the degree of preorganization is low, especially in comparison to the highly preorganized 3HJ architecture (compare Fig. 1c to Fig. 1b).
Figure 2.
In vitro selection design for DNA-catalyzed amine reactivity with a 5′-phosphorimidazolide (5′-ImpDNA), using the open architecture of Fig. 1c. a) Structures of the aliphatic amine and Lys nucleophiles. b) Key in vitro selection step. N40 denotes a 40-nucleotide random region. The dashed loop enables separation of catalytically active DNA sequences from the random N40 population by PAGE shift but is dispensable for catalysis and was absent in all single-turnover kinetic assays. The PAGE-shifted products were separated and amplified by PCR to enter the next selection round. See Fig. S1 for selection details.
Each of Mg2+, Mn2+, and Zn2+ has been an effective catalytic cofactor for DNA in many previous in vitro selection experiments.[1b-g] Using the two amine substrates of Fig. 2a, parallel selection experiments were performed using an N40 random region (i.e., 40 random nucleotides). For each experiment, incubation conditions in the key selection step of Fig. 2b were either (A) 70 mM HEPES, pH 7.5 with 40 mM Mg2+, 20 mM Mn2+, 1 mM Zn2+, and 150 mM Na+ at 37 °C for 14 h, or (B) 50 mM CHES, pH 9.0 with 40 mM Mg2+ and 150 mM Na+ at 37 °C for 14 h (all metal ions provided as chloride salts). Neither Mn2+ nor Zn2+ could be included at the higher pH due to oxidation or precipitation, respectively. Thus, in total, four selection experiments were performed.
In both selection experiments that used the DNA-C3-NH2 substrate, DNA-catalyzed activity was observed. After 8 rounds (conditions A; final round only 2 h incubation) or 7 rounds (conditions B), 20% or 13% ligation activity was observed (see all selection progressions in Fig. S2). Individual deoxyribozymes were cloned (see Fig. S3 for sequences) and observed to catalyze amine-DNA conjugation by reaction of the amino group with the 5′-Imp.
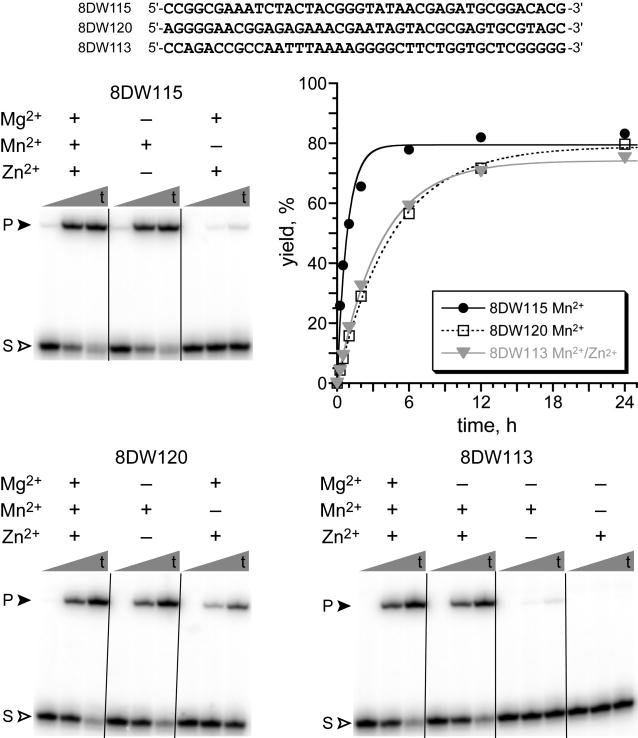
Seven deoxyribozymes were found for conditions A, with kobs values of 0.2–1.2 h–1 and up to 85% yield (Fig. 3 and Fig. S4). Among these seven DNA enzymes, three different metal dependences were observed. 8DW115 and four other deoxyribozymes each require Mn2+, with little or no activity (<2%) in the presence of only Mg2+ and Zn2+. 8DW120 has optimal activity with Mn2+ but separately has some catalytic activity with the combination of Mg2+ and Zn2+. Finally, 8DW113 requires both Mn2+ and Zn2+, whereas Mg2+ is dispensable for catalysis. All seven deoxyribozymes were assayed at pH 7.2, 7.5, or 7.8; all but 8DW120 had optimal yield at pH 7.5 (but still generally substantial yield at pH 7.2 and 7.8), whereas 8DW120 had slightly higher yield at pH 7.8 (Fig. S5). Separately, seven Mg2+-dependent deoxyribozymes were identified for conditions B, with kobs of ∼0.03 h–1 and 30–40% yield in 48 h (Fig. S6). The optimal Mg2+ concentration for each of these deoxyribozymes was ∼30 mM (Fig. S7). Each deoxyribozyme was assayed at pH values of 8.0 through 10.0 in 0.5-unit increments, and in each instance, optimal activity was observed at either pH 8.5 or 9.0 (Fig. S6).
Figure 3.
Deoxyribozymes for reaction of the DNA-C3-NH2 substrate with 5′-ImpDNA. Representative PAGE images are shown for single-turnover assays of deoxyribozymes identified from conditions A (pH 7.5, Mg/Mn/Zn, i.e., the “8DW1” deoxyribozymes.[10] S = DNA-C3-NH2 substrate; P = ligation product. The initially random (N40) region sequence of each deoxyribozyme is shown. t = 30 s, 2 h, and 24 h. Incubation conditions: 50 mM (–Zn2+) or 70 mM (+Zn2+) HEPES, pH 7.5 with 150 mM Na+ and the indicated combinations of 40 mM Mg2+, 20 mM Mn2+, and 1 mM Zn2+ at 37 °C. See Fig. S4 for comprehensive metal ion dependence and kobs values and Fig. S5 for kinetic plots at various pH values for all seven 8DW deoxyribozymes.
The two selection experiments that used the DNA-HEG-CKA substrate also led to substantial DNA-catalyzed activity. After 9 rounds (conditions A) or 14 rounds (conditions B), 19% or 14% ligation activity was observed. A single deoxyribozyme, 9DT105, emerged from conditions A to catalyze reaction of the Lys amino group of DNA-HEG-CKA with 5′-Imp, with kobs 0.10 h–1 and 50% yield (Fig. 4). 9DT105 requires both Mn2+ and Zn2+, whereas Mg2+ is dispensable. The yield of 9DT105 was substantially reduced at pH values below 7.5, e.g., 2% yield in 48 h at pH 7.2 (Fig. S8). A second deoxyribozyme from this selection, 9DT114, catalyzed a reaction with kobs 0.17 h–1 (Fig. S9). However, the DNA anchor alone (lacking both HEG tether and CKA peptide) was sufficient for reactivity, surprisingly indicating that the nucleophile for 9DT114-catalyzed reaction with 5′-Imp is on the DNA anchor itself. More detailed investigation revealed that the nucleophile is the C4-NH2 group of a particular deoxycytidine (Fig. S9). Separately, six deoxyribozymes were found for reactivity of DNA-HEG-CKA under conditions B, with kobs of ∼0.05 h–1, yields up to 50% in 48 h (Fig. S10), and optimal Mg2+ concentration of >50 mM (Fig. S11). For all six deoxyribozymes, yield increased with pH between 8.0 and 10.0 (Fig. S10).
Figure 4.
9DT105 deoxyribozyme for reaction of Lys of the DNA-HEG-CKA substrate with 5′-ImpDNA, identified from conditions A (pH 7.5, Mg/Mn/Zn). The initially random (N40) region sequence of 9DT105 is shown. t = 30 s, 6 h, and 48 h. Incubation conditions: 50 mM (–Zn2+) or 70 mM (+Zn2+) HEPES, pH 7.5 with 150 mM Na+ and the indicated combinations of 40 mM Mg2+, 20 mM Mn2+, and 1 mM Zn2+ at 37 °C. See Fig. S8 for pH dependence.
MALDI mass spectrometry corroborated product structures for several representative deoxyribozymes (Fig. S12). The identity of each newly formed phosphoramidate (P–N) linkage was consistent with the observed acid sensitivity (Fig. S13).[2b,2d,11] Negative control experiments were consistent with nucleophilic reactivity of the amine and electrophilic reactivity of 5′-Imp (Fig. S14).
The 21 deoxyribozymes collectively obtained from the four different selection experiments (excluding 9DT114) were each separately assayed with four substrates, two of which were the selection substrates depicted in Fig. 2a (for simplicity now omitting the prefix “DNA-” for the DNA anchor): C3-NH2, HEG-NH2, C3-CKA, and HEG-CKA. (The C3-CKA and HEG-NH2 substrates have structures analogous to those in Fig. 2a. For C3-CKA, the C3 tether terminates in a thiol rather than an amine and is joined via a disulfide to CKA. For HEG-NH2, the HEG tether terminates in an amine rather than a thiol.) The purpose of these assays was to evaluate comprehensively the tether and peptide dependence of the various deoxyribozymes. The results reveal two distinct types of substrate preference, both of which are sensible based on the selection origins of the various deoxyribozymes (Fig. 5).[9b] The deoxyribozymes identified from selection using the C3-NH2 substrate under either incubations conditions A (deoxyribozymes designated 8DW1) or B (7DX1) all have activity in the order C3-NH2 > HEG-NH2 > C3-CKA and HEG-CKA. Conversely, the deoxyribozymes selected using the HEG-CKA substrate under conditions A (9DT105) or B (14DV1) all have higher activity with the Lys-containing substrates, HEG-CKA > HEG-NH2 and C3-CKA > C3-NH2. 9DT105 prefers the shorter-tethered peptide (C3-CKA > HEG-CKA), whereas each of the 14DV1 deoxyribozymes favors the longer-tethered peptide (HEG-CKA > C3-CKA). From these data, a key finding is that performing selection using the HEG-tethered substrate is necessary to achieve substantial DNA-catalyzed reactivity with that substrate.
Figure 5.
Dependence of catalysis on substrate structure. The assays used substrates that have different tether lengths and amine contexts. For the 8DW1, 7DX1, and 14DV1 deoxyribozymes, data for one representative catalyst is shown. See Fig. S15, Fig. S16, and Fig. S17 for comprehensive data. Incubation conditions as in Figs. 3 and 4.
9DT105 and the six 14DV1 deoxyribozymes were each assayed with the free (non-DNA-anchored) CKA tripeptide at up to 1 mM concentration. The unattached DNA anchor oligonucleotide was included to occupy the corresponding deoxyribozyme binding arm. In all cases, no Lys reactivity was observed (<1%; data not shown). This observation is unsurprising because the peptide was tethered to the DNA anchor oligonucleotide throughout the selection process (Fig. 2). Therefore, the DNA sequences were never challenged to function in the absence of the tether. In other experiments, we have nevertheless identified deoxyribozymes that do have some activity with free peptides,[2e,8] although such activity was not always found.[12] Overall, the rules are unclear for emergence of free peptide reactivity, suggesting the need for a strategy aimed specifically at this outcome. In a parallel study, we have established a new selection approach that enables use of free, unanchored peptides directly during selection and thereby provides deoxyribozymes with useful yields and apparent Km values for the free peptide substrates.[13] We anticipate that this new approach will be successful with Lys side chain reactivity of free peptide substrates in future experiments.
Rate enhancements for the various deoxyribozymes were estimated by comparing their kobs values (Fig. 3 and Fig. 4) to the observed rate constants for appropriate background reactions (kbkgd; Fig. S18). Both conditions A (pH 7.5) and B (pH 9.0) were evaluated. Using the random N40 pool in place of a catalytically active deoxyribozyme in the background assay, kbkgd values were ∼10–4 h–1 (for A) and ∼2–3-fold higher (for B). For conditions A, the DNA-catalyzed rate enhancement was up to 104 with the DNA-C3-NH2 substrate and up to 103 with the DNA-HEG-CKA substrate. For conditions B, the rate enhancements were as high as 102 for both substrates, with these more modest values largely reflecting that the deoxyribozymes from conditions B have lower kobs than do their counterparts from conditions A.
The selections performed here at pH 7.5 each used a mixture of Mg2+, Mn2+, and Zn2+, and the resulting DNA catalysts each require either Mn2+ or a combination of Mn2+ and Zn2+ for optimal activity. We previously reported a DNA-hydrolyzing deoxyriboyzme that similarly requires a combination of Mn2+ and Zn2+, although mutations could remove the Mn2+ dependence.[14] Interestingly, none of the new deoxyribozymes found here at pH 7.5 requires Mg2+, although each of the deoxyribozymes identified by selection at pH 9.0 in the presence of Mg2+ alone (because Mn2+ and Zn2+ cannot be used) requires Mg2+. Understanding these various metal ion requirements, and indeed understanding all mechanistic aspects of the new deoxyribozymes, will require more detailed biochemical experiments, likely in the context of high-resolution structural information that is currently unavailable for any DNA catalyst.[15]
Lys reactivity has never been observed previously with either DNA or RNA enzymes, including in our previous studies that successfully led to Tyr- and Ser-modifying deoxyribozymes.[2a,2b] This relative unreactivity of Lys using nucleic acid catalysts has been a surprising challenge. The unprotonated aliphatic amino group of Lys is comparable in nucleophilicity to the deprotonated phenolic OH of Tyr, and an amine is many orders of magnitude more nucleophilic than is the nondeprotonated aliphatic OH of Ser;[16] both of these considerations suggest that Lys should be rather reactive. Several observations are consistent with the collective picture that nucleic acid-catalyzed nucleophilic reactions of nitrogen are difficult but achievable. Earlier we described DNA-catalyzed reductive amination involving a guanosine nucleobase N2-amine,[17] and others found RNA-catalyzed reaction of a peptide N-terminal α-amino group even when a Lys side chain was available as a competing nucleophile.[18] In the present study, the emergence of both 9DT105 and 9DT114 from the same selection experiment indicates that DNA-catalyzed amine reactivity for the less preorganized DNA-anchored HEG-CKA substrate is sufficiently difficult to achieve, such that reactivity of an alternative nucleophile on the DNA anchor itself (a C4-NH2 group)[19] can instead be observed. In this context, we reemphasize our prior finding that with 5′-pppRNA as the electrophile, a phosphoramidate functional group reacted in preference to a Lys amino group,[2d] highlighting the relative unreactivity of an aliphatic amine when using a nucleic acid catalyst. We also note that uncatalyzed, DNA-templated polymerization of 5′-ImpDNA monomers by reaction with 3′-NH2 groups is quite rapid (complete reaction in <1 h; pH 7.5, 4 °C).[20]
In summary, the key to successful DNA-catalyzed Lys reactivity was providing the more reactive 5′-phosphorimidazolide (5′-Imp) electrophile, which was attacked by the Lys nucleophile despite the less preorganized, open selection architecture. These results reveal that the degree of deoxyribozyme-substrate preorganization is a less important design consideration for DNA catalysts than is the inherent reactivity of the electrophilic reaction partner, at least for nucleophilic amine reactions. The findings in this study provide important fundamental information to enable ongoing identification of DNA catalysts for covalent modification of peptide and protein substrates. Our efforts are particularly focused on biologically relevant modifications such as acylation at Lys residues.[4]
Experimental Section
DNA oligonucleotides were obtained from Integrated DNA Technologies (Coralville, IA) or prepared by solid-phase synthesis on an ABI 394 instrument using reagents from Glen Research. All oligonucleotides and conjugates were purified by 7 M urea denaturing 20% or 8% PAGE with running buffer 1× TBE (89 mM each Tris and boric acid and 2 mM EDTA, pH 8.3), extracted from the polyacrylamide with TEN buffer (10 mM Tris, pH 8.0, 1 mM EDTA, 300 mM NaCl), and precipitated with ethanol. Peptides were prepared by solid-phase synthesis using Fmoc Rink amide MBHA resin as described.[2e] Each peptide was coupled to the DNA anchor oligonucleotide via a disulfide bond with the N-terminal cysteine side chain as described.[2e]
Full procedures for selection, cloning, and initial analysis of individual clones are provided in the Supporting Information.
The general single-turnover assay procedure for each deoxyribozyme was as follows. The DNA-anchored amine substrate was 5′-32P-radiolabeled using γ-32P-ATP and T4 polynucleotide kinase (Fermentas), using 10× kinase buffer that lacks DTT (500 mM Tris, pH 7.6, 100 mM MgCl2, and 1 mM spermidine) for disulfide-linked oligonucleotide-peptide conjugates. A 10 μL sample containing 0.2 pmol of 5′-32P radiolabeled substrate, 10 pmol of deoxyribozyme, and 30 pmol of 5′-Imp substrate were annealed in (for conditions A) 5 mM HEPES, pH 7.5, 15 mM NaCl, and 0.1 mM EDTA or (for conditions B) 5 mM CHES, pH 9.0, 15 mM NaCl, and 0.1 mM EDTA by heating at 95 °C for 3 min and cooling on ice 5 min. The DNA-catalyzed reaction was initiated by bringing the sample to 20 μL total volume containing (conditions A) 70 mM HEPES, pH 7.5, 1 mM ZnCl2, 20 mM MnCl2, 40 mM MgCl2 and 150 mM NaCl or (conditions B) 50 mM CHES, pH 9.0, 40 mM MgCl2, and 150 mM NaCl. The sample was incubated at 37 °C. At appropriate time points, 2 μL aliquots were quenched with 5 μL of stop solution (80% formamide, 1× TBE [89 mM each Tris and boric acid and 2 mM EDTA, pH 8.3], 50 mM EDTA, 0.025% bromophenol blue, 0.025% xylene cyanol). Samples were separated by 20% PAGE and quantified using a Phosphorimager. Values of kobs were obtained by fitting the yield versus time data directly to first-order kinetics; i.e., yield = Y•(1 – e–kt), where k = kobs and Y is the final yield. Each kobs value is reported with error calculated as the standard deviation from the indicated number of independent determinations. When kobs was sufficiently low such that an exponential fit was not meaningful, the initial points were fit to a straight line, and kobs was taken as the slope of the line.
Supplementary Material
Footnotes
This research was supported by grants to S.K.S. from the National Institutes of Health (R01GM065966), the Defense Threat Reduction Agency (HDTRA1-09-1-0011), and the National Science Foundation (CHE0842534). B.M.B. was partially supported by NIH T32GM070421. We thank Yun Xie for initial selection experiments and Chih-Chi Chu for assistance with phosphorimidazolide preparation.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a Breaker RR, Joyce GF. Chem Biol. 1994;1:223–229. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]; b Peracchi A. ChemBioChem. 2005;6:1316–1322. doi: 10.1002/cbic.200500098. [DOI] [PubMed] [Google Scholar]; c Silverman SK. Chem Commun. 2008:3467–3485. doi: 10.1039/b807292m. [DOI] [PubMed] [Google Scholar]; d Schlosser K, Li Y. Chem Biol. 2009;16:311–322. doi: 10.1016/j.chembiol.2009.01.008. [DOI] [PubMed] [Google Scholar]; e Silverman SK. Angew Chem Int Ed. 2010;49:7180–7201. doi: 10.1002/anie.200906345. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Liu J, Cao Z, Lu Y. Chem Rev. 2009;109:1948–1998. doi: 10.1021/cr030183i. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Zhang XB, Kong RM, Lu Y. Annu Rev Anal Chem. 2011;4:105–128. doi: 10.1146/annurev.anchem.111808.073617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Pradeepkumar PI, Höbartner C, Baum DA, Silverman SK. Angew Chem Int Ed. 2008;47:1753–1757. doi: 10.1002/anie.200703676. [DOI] [PubMed] [Google Scholar]; b Sachdeva A, Silverman SK. Chem Commun. 2010;46:2215–2217. doi: 10.1039/b927317d. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sachdeva A, Chandra M, Chandrasekar J, Silverman SK. ChemBioChem. 2012;13:654–657. doi: 10.1002/cbic.201200048. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Sachdeva A, Silverman SK. Org Biomol Chem. 2012;10:122–125. doi: 10.1039/c1ob06088k. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Chandrasekar J, Silverman SK. Proc Natl Acad Sci USA. 2013;110:5315–5320. doi: 10.1073/pnas.1221946110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Coppins RL, Silverman SK. Nat Struct Mol Biol. 2004;11:270–274. doi: 10.1038/nsmb727. [DOI] [PubMed] [Google Scholar]; b Coppins RL, Silverman SK. J Am Chem Soc. 2005;127:2900–2907. doi: 10.1021/ja044881b. [DOI] [PubMed] [Google Scholar]
- 4.a Cole PA. Nat Chem Biol. 2008;4:590–597. doi: 10.1038/nchembio.111. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]; c Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning ZB, Zeng R, Xiong Y, Guan KL, Zhao S, Zhao GP. Science. 2010;327:1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Olsen CA. Angew Chem Int Ed. 2012;51:3755–3756. doi: 10.1002/anie.201200316. [DOI] [PubMed] [Google Scholar]; e Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, Lu Z, Ye Z, Zhu Q, Wysocka J, Ye Y, Khochbin S, Ren B, Zhao Y. Cell. 2011;146:1016–1028. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, Zwaans BM, Tishkoff D, Ho L, Lombard D, He TC, Dai J, Verdin E, Ye Y, Zhao Y. Mol Cell Proteomics. 2011;10:M111 012658. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Nat Chem Biol. 2011;7:58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Bao X, Zhao Q, Yang T, Fung YM, Li XD. Angew Chem Int Ed. 2013;52:4883–4886. doi: 10.1002/anie.201300252. [DOI] [PubMed] [Google Scholar]
- 5.a Harvey CL, Gabriel TF, Wilt EM, Richardson CC. J Biol Chem. 1971;246:4523–4530. [PubMed] [Google Scholar]; b Lehman IR. Science. 1974;186:790–797. doi: 10.1126/science.186.4166.790. [DOI] [PubMed] [Google Scholar]; c Higgins NP, Cozzarelli NR. Methods Enzymol. 1979;68:50–71. doi: 10.1016/0076-6879(79)68006-0. [DOI] [PubMed] [Google Scholar]; d Ohtsuka E, Nishikawa S, Sugiura M, Ikehara M. Nucleic Acids Res. 1976;3:1613–1623. doi: 10.1093/nar/3.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Uhlenbeck OC, Gumport RI. In: The Enzymes. Boyer PD, editor. Vol. 15. Academic Press; New York: 1982. pp. 31–58. [Google Scholar]; f Ho CK, Wang LK, Lima CD, Shuman S. Structure. 2004;12:327–339. doi: 10.1016/j.str.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 6.a Itzen A, Blankenfeldt W, Goody RS. Trends Biochem Sci. 2011;36:221–228. doi: 10.1016/j.tibs.2010.12.004. [DOI] [PubMed] [Google Scholar]; b Müller MP, Albers MF, Itzen A, Hedberg C. ChemBioChem. 2014;15:19–26. doi: 10.1002/cbic.201300508. [DOI] [PubMed] [Google Scholar]
- 7.a Joyce GF. Cold Spring Harb Symp Quant Biol. 1987;52:41–51. doi: 10.1101/sqb.1987.052.01.008. [DOI] [PubMed] [Google Scholar]; b Ferris JP, Hill AR, Jr, Liu R, Orgel LE. Nature. 1996;381:59–61. doi: 10.1038/381059a0. [DOI] [PubMed] [Google Scholar]; c Orgel LE. Crit Rev Biochem Mol Biol. 2004;39:99–123. doi: 10.1080/10409230490460765. [DOI] [PubMed] [Google Scholar]
- 8.Wong O, Pradeepkumar PI, Silverman SK. Biochemistry. 2011;50:4741–4749. doi: 10.1021/bi200585n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a Wilson DS, Szostak JW. Annu Rev Biochem. 1999;68:611–647. doi: 10.1146/annurev.biochem.68.1.611. [DOI] [PubMed] [Google Scholar]; b Joyce GF. Annu Rev Biochem. 2004;73:791–836. doi: 10.1146/annurev.biochem.73.011303.073717. [DOI] [PubMed] [Google Scholar]; c Joyce GF. Angew Chem Int Ed. 2007;46:6420–6436. doi: 10.1002/anie.200701369. [DOI] [PubMed] [Google Scholar]
- 10.Each deoxyribozyme in this study was named as, for example, 8DW115, where 8 is the round number, DW1 is the systematic alphanumeric designation for the particular selection, and 15 is the clone number.
- 11.a Wada T, Moriguchi T, Sekine M. J Am Chem Soc. 1994;116:9901–9911. [Google Scholar]; b Chandra M, Sachdeva A, Silverman SK. Nat Chem Biol. 2009;5:718–720. doi: 10.1038/nchembio.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh SM, Sachdeva A, Silverman SK. J Am Chem Soc. 2013;135:14928–14931. doi: 10.1021/ja407586u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu C, Wong O, SIlverman SK. ChemBioChem. 2014;15 doi: 10.1002/cbic.201402255. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao Y, Allen EC, Silverman SK. Chem Commun. 2011;47:1749–1751. doi: 10.1039/c0cc04575f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowakowski J, Shim PJ, Prasad GS, Stout CD, Joyce GF. Nat Struct Biol. 1999;6:151–156. doi: 10.1038/5839. [DOI] [PubMed] [Google Scholar]
- 16.Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; Sausalito, CA: 2006. [Google Scholar]
- 17.Wong O, Mulcrone AE, Silverman SK. Angew Chem Int Ed. 2011;50:11679–11684. doi: 10.1002/anie.201104976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baskerville S, Bartel DP. Proc Natl Acad Sci USA. 2002;99:9154–9159. doi: 10.1073/pnas.142153799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.a Michelson AM, Todd AR. J Chem Soc. 1954 [Google Scholar]; b Tener GM. J Am Chem Soc. 1961;83:159–168. [Google Scholar]; c Sasaki T, Mizuno Y. Chem Pharm Bull. 1967;15:894–896. doi: 10.1248/cpb.15.894. [DOI] [PubMed] [Google Scholar]; d Uchiyama M, Aso Y, Noyori R, Hayakawa Y. J Org Chem. 1993;58:373–379. [Google Scholar]
- 20.Zhang S, Blain JC, Zielinska D, Gryaznov SM, Szostak JW. Proc Natl Acad Sci USA. 2013;110:17732–17737. doi: 10.1073/pnas.1312329110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.