Abstract
By analyzing the exome sequences of 2,536 schizophrenia cases and 2,543 controls, we have demonstrated a polygenic burden primarily arising from rare (<1/10,000), disruptive mutations distributed across many genes. Especially enriched genesets included the voltage-gated calcium ion channel and the signaling complex formed by the activity-regulated cytoskeleton-associated (ARC) scaffold protein of the postsynaptic density (PSD), sets previously implicated by genome-wide association studies (GWAS) and copy-number variation (CNV) studies. Similar to reports in autism, targets of the fragile × mental retardation protein (FMRP, product of FMR1) were enriched for case mutations. No individual gene-based test achieved significance after correction for multiple testing and we did not detect any alleles of moderately low frequency (~0.5-1%) and moderately large effect. Taken together, these data suggest that population-based exome sequencing can discover risk alleles and complements established gene mapping paradigms in neuropsychiatric disease.
Genetic studies of schizophrenia (MIM: 181500) have demonstrated a substantial heritability (Sullivan et al., 2003; Lichtenstein et al., 2009) that reflects common and rare alleles at many loci. Genome-wide association studies (GWAS) continue to uncover common single nucleotide polymorphisms (SNPs) at novel loci (Ripke et al., 2013). Rare or de novo genic deletions and duplications (copy number variants, CNV) have been firmly established, including risk variants at 22q11.2, 15q13.3, 1q21.1 (Levinson et al, 2011; Sullivan et al., 2012). One striking outcome of these large-scale, genome-wide investigations is the degree of polygenicity, consistent with thousands of genes and non-coding loci harboring risk alleles (International Schizophrenia Consortium, 2008, 2009; Lee et al., 2012; Ripke et al, 2013; Malhotra & Sebat, 2012).
Nonetheless, progress has been made in implicating biological systems and quantifying shared genetics among related psychiatric disorders (e.g. Psychiatric Genomics Bipolar Disorder Working Group, 2011; Moreno-De-Luca et al., 2010); for example, in pointing to common variants in calcium ion channel genes impacting schizophrenia and bipolar disorder (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013) and to de novo CNVs impacting genes encoding members of the postsynaptic density (PSD) proteome (Kirov et al., 2012), in particular members of the neuronal ARC protein and N-methyl-D-aspartate receptor (NMDAR) postsynaptic signaling complexes.
Here we apply massively parallel short-read sequencing to assay a substantial portion of variation that previously was effectively invisible: rare coding point mutations (single nucleotide variants, SNVs) and small insertions and deletions (indels). Although previous schizophrenia studies have applied sequencing, the results have been inconclusive, reflecting limited sample sizes or a focus on small numbers of candidate genes (Need et al., 2012; Crowley et al., 2013; Takata et al., 2013; Timms et al., 2013). Exome-sequencing studies of de novo mutation published to date have neither demonstrated an increased rate in schizophrenia, nor conclusively implicated individual genes (Girard et al., 2011, Xu et al., 2012), although some data suggest a link with particular classes of gene, such as those with higher brain expression in early fetal life (Xu et al., 2012). De novo studies in intellectual disability (ID, de Ligt et al., 2012; Rauch et al., 2012) and autism (Iossifov et al., 2012; Neale et al., 2012; O’Roak et al., 2012; Sanders et al., 2012) have made significant progress in identifying large-effect alleles and the underlying gene networks, however.
In this study, we sought to identify the alleles, genes or gene networks that harbor rare coding variants of moderate or large effect on risk for schizophrenia by exome-sequencing 5,079 individuals, selected from a Swedish sample of more than 11,000 individuals. Previous analyses of the full sample (SI section 1) have demonstrated an enriched burden of rare CNVs and a polygenic common variant component (Ripke et al., 2013). We generated high coverage exome sequence to ensure sufficient sensitivity to detect and genotype alleles observed in only one heterozygous individual (singletons, implying an allele frequency of ~1/10,000 although the true population frequency will typically be rarer).
The high baseline rate of rare, neutral mutations (e.g. Keinan & Clark, 2012) makes it difficult to detect rare alleles that increase risk for common diseases. Although power can be increased by jointly testing groups of variants in a gene (Wu et al., 2011), association testing across all genes is likely to be under-powered at current sample sizes. Indeed, a recent application of population-based exome sequencing in autism did not identify genes (Liu et al., 2013), despite moderately large sample size and the success of the de novo paradigm. Furthermore, many confirmed results from candidate gene sequencing studies of non-psychiatric disease still fall short of exome-wide significance (Kiezun et al., 2012).
We therefore adopted a top-down strategy in which we studied a large set of genes with a higher likelihood of playing a role in schizophrenia, based on existing genetic evidence (SI section 7). We focused on ~2,500 genes implicated by unbiased, large-scale genome-wide screens, including GWAS, CNV and de novo SNV studies, testing for enrichment of rare case alleles. To prioritize individual genes, we characterized emerging signals with respect to the genes and frequency and type of mutations. We coordinated analysis with an independent trio exome sequencing study (Fromer et al., this issue) and note key points of convergence below.
After alignment and variant calling of all samples jointly, we removed 11 subjects with low quality data along with likely spurious sites and genotypes (SI sections 2 & 3). Per individual, 93% (81%) of targeted bases were covered at ≥ 10-fold (30-fold). The final dataset comprised 2,536 cases and 2,543 controls (ED Table 1a and ED Figure 2a). Cases and controls had similar technical sequencing metrics, including total coverage, proportion of deeply covered targets, and overall proportion of non-reference alleles (ED Table 1b). We observed 635,944 coding and splice-site passing variants of which 56% were singletons. Using Sanger sequencing and ExomeChip data on these samples, we determined high specificity and sensitivity for singletons (SI section 3).
We annotated variants with respect to RefSeq and combined five in silico algorithms to predict missense deleteriousness (ED Table 1c and SI section 4). As expected, allelic types more likely to impact protein function showed greater constraint: 69% of nonsense variants were singletons, compared to 58% of missense and 51% of silent variants. Primary analyses tested (1) disruptive variants (nonsense, essential splice site and frameshifts, N = 15,972 alleles with MAF < 0.1%), (2) disruptive plus missense variants predicted to be damaging by all five algorithms (N = 50,369) and (3) disruptive plus missense variants predicted to be damaging by at least one algorithm (N = 233,575). These groups are labeled (where NS indicates nonsynonymous): disruptive, NSstrict and NSbroad. We also stratified most analyses by allele frequency: (1) singletons; (2) up to 0.1% (10 or fewer minor alleles); (3) up to 0.5% (50 or fewer minor alleles). In the main geneset analyses, we empirically corrected for multiple testing over the nine combinations of these factors (SI section 7).
The most significant SNV or indel association (P = 5×10-8) was for a common missense allele in CCHCR1, in the MHC, a known risk locus; this top SNP was in LD with many other schizophrenia-associated SNPs in the MHC. All P<10-5 variants were for either common alleles or a few instances of likely aberrant variants that had escaped earlier filtering (SI section 5). We performed two series of gene-based tests: a one-sided burden test of an increased rare allele rate in cases, and the SKAT (Wu et al., 2011), which allows for risk and protective effects. For both tests, the distribution of gene-based statistics broadly followed a global null (ED Figure 2b).
Considering only disruptive variants, the genic test yielding the lowest nominal P-value was for KYNU (kynureninase), showing 10 variants in cases and 0 in controls (ED Table 3 and Supplementary Table 1); one novel nonsense mutation at chr2:143713804 (g.468T>A; p.Y156*) was observed in 7 cases and not present in either the Exome Variant Server or 1000 Genomes Project. Although previous studies have suggested links between the kynurenine pathway and schizophrenia (e.g. Linderholm et al., 2012), our P-value of 1.7×10-3 does not withstand correction for multiple testing, even if considering only the 246 genes with ≥ 10 rare disruptive mutations capable of achieving a nominally significant result.
A polygenic burden of rare coding variants
We evaluated a polygenic burden of rare coding variants in cases, first selecting 2,546 genes (~10% of the exome) based on prior genetic studies that we hypothesized to be enriched for schizophrenia-associated mutations (SI section 6). Sources included genome-wide CNV studies (Sullivan et al., 2012; Kirov et al., 2012), GWAS (Schizophrenia Psychiatric Genome-Wide Association Study Consortium, 2011; Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013; Ripke et al., 2013) and exome sequencing of de novo mutation (Girard et al., 2011; Xu et al., 2012; Fromer et al., current issue). In our sample, these genes had a significantly higher rate of rare (MAF < 0.1%) disruptive mutations in cases compared to controls (P = 10-4 for 1,547 versus 1,383 mutations). The enrichment was unlikely to represent technical or ancestry-related artifact because the P-values controlled for potential differences in exome-wide burden in cases and controls, and because we observed no differences exome-wide (P = 0.24). Furthermore, enrichment P-values were empirically derived by permuting phenotypes within subgroups of m:n cases to controls, matched on exome-wide identity-by-state, experimental batch and sex; the above result withstood correction for multiple testing (Table 1). We observed similar results for rarer (singletons P = 8×10-4) and more frequent alleles (MAF < 0.5%, P = 2×10-4). We also observed case enrichment for the strictly defined set of damaging mutations (NSstrict P = 1.5×10-3) but not the broader set (NSbroad P = 0.13).
Table 1. Geneset analysis of primary schizophrenia candidate genesets.
Enrichment test empirical P-values for rare (singleton; minor allele frequency (MAF) <0.1%; MAF < 0.5%) variants from disruptive, NSstrict and NSbroad sets. P-values represent the relative case enrichment compared to average exome-wide case/control difference. Bolded values are significant at Pcorrected<0.05. Initial comparison corrects (based on the empirical distribution of minimum P-values) for the 9 correlated tests (top panel). The lower panel focuses on the 12 subsets of the primary geneset, for disruptive variants only as they showed the greatest enrichment for the entire primary set. Again, bold values are significant after correcting for the 36 tests performed.
| Variant type | Geneset/subset | N genes | Singletons | MAF < 0.1% | MAF < 0.5% |
|---|---|---|---|---|---|
| Disruptive | 0.0008 | 0.0001 | 0.0002 | ||
| Nonsyn (strict) | Primary | 2,546 | 0.0059 | 0.0015 | 0.0110 |
| Nonsyn (broad) | 0.0986 | 0.1295 | 0.1126 | ||
|
| |||||
| Disruptive | SCZ de novo genes | ||||
| Exome sequencing (disruptive) | 87 | 0.0319 | 0.0007 | 0.0003 | |
| Exome sequencing (nonsyn) | 611 | 0.0053 | 0.0011 | 0.0055 | |
| Copy number variants | |||||
| de novo CNV genes (Kirov et al, 2012) | 234 | 0.0234 | 0.0039 | 0.0124 | |
| SCZ-associated CNV genes | 345 | 0.3308 | 0.4596 | 0.4376 | |
| GWAS | |||||
| Voltage-gated calcium channel genes | 26 | 0.0019 | 0.0214 | 0.0212 | |
| Common SNPs (P < 1e-4 intervals) | 479 | 0.1794 | 0.0368 | 0.0037 | |
| miRNA-137 targets | 446 | 0.6573 | 0.5609 | 0.4747 | |
| Synaptic genes | |||||
| PSD (human core) | 685 | 0.0808 | 0.1154 | 0.1256 | |
| ARC | 28 | 0.0016 | 0.0014 | 0.0014 | |
| NMDAR network | 61 | 0.0158 | 0.0251 | 0.0252 | |
| PSD-95 | 65 | 0.0017 | 0.0009 | 0.0010 | |
| mGluRS | 39 | 0.1327 | 0.0900 | 0.0902 | |
This enrichment suggests a polygenic burden of rare variants. Although not so marked as to be detectable at the exome-wide level given the sample size, it is relatively concentrated in genes that were found to be associated with schizophrenia by other methods. The mean allelic effect was not large: in the primary comparison, the odds ratio was 1.12 (1.04 – 1.20 95% CI) for each MAF<0.1% disruptive mutation; 46% of cases carried one or more allele in this primary set (0.62 per case) compared to 41% of controls (0.55 per control). At two extremes, the modest mean effect could represent either that a subset of mutations are fully penetrant or that every allele is associated but increases risk by only 12%, similar to common alleles from GWAS. To extract subsets of potentially stronger-effect alleles, we individually tested the constituent gene sources (Table 1, ED Figure 2c), focusing on disruptive variants as they showed the strongest omnibus enrichment. For disruptive mutations, 8 of 12 sets were nominally significant (P < 0.05), indicating that the initial observation was not driven by a single category.
ARC, PSD-95 and calcium ion channel genes
Three of the smaller significantly enriched sets (the ARC and PSD-95 complexes and calcium ion channel genes) had odds ratios > 5. We observed enrichment (P = 1.6×10-3) of disruptive mutations among the 28 ARC complex genes: 9 mutations in 9 genes (all singletons) in cases, 0 in controls yielding an odds ratio of 19.2 (2.4 – 2471 95% confidence intervals, ED Table 3). Along with the NMDAR geneset (also significantly enriched), ARC genes largely accounted for the overall PSD enrichment (P = 4×10-8) in Kirov et al. (2012), in which four ARC genes had one or more de novo CNVs. Of note, in an independent exome-sequencing study in trios, Fromer et al. (current issue) found that the ARC geneset was enriched (P = 5×10-4) for nonsynonymous de novo SNVs and indels, with four genes harboring six mutations (ED Table 8). The other PSD geneset with strong enrichment (P = 9×10-4, odds ratio = 5.1, 1.8 – 19.2 95% CI) was the PSD-95 complex, which contains 65 genes and overlaps with ARC. PSD genes are very highly conserved and play critical roles in excitatory neural signaling components, as well as dendrite and spine plasticity. Further categorization of neuronal genes based on subcellular localization (Kirov et al., 2012; ED Table 4a) or associated mouse and human phenotypes (Bayés et al., 2011) did not yield further enrichment.
The other subset yielding a large odds ratio of 8.4 (2.03 – 77 95% CI) was the 26 voltage-gated calcium ion channel genes (12 cases, 1 control disruptive singletons, P=2×10-3, although the effect is attenuated when including recurrent alleles: 15/8, P = 0.021, see ED Table 3). The singleton enrichment was predominantly driven by the pore-forming α1 and auxiliary α2δ subunits; of the α1 subunits, the CaV1/L-type genes carried the most case mutations, including two in CACNA1C, a gene implicated by GWAS of bipolar disorder and schizophrenia (PGC Bipolar Disorder Group, 2012; Ripke et al., 2013). Calcium signaling is involved in many cell functions including regulating gene expression (Dolmetsch et al., 1998) and is critical for modulating synaptic plasticity (Yasuda et al., 2003). In a secondary analysis of proteins found in the nano-environment of the calcium channel (Müller et al., 2010), we observed independent enrichment for other ion channel transporters (Supplementary Table S1), odds ratio 9.1 (2.2 – 83) for disruptive singletons (P = 1×10-3; 13/1 alleles).
Convergence with de novo studies
A line of convergence across studies was that genes carrying nonsynonymous de novo mutations (Girard et al., 2011; Xu et al., 2012; Fromer et al., current issue) were enriched for rare disruptive mutations (P = 1×10-3; Table 1 & ED Tables 7a, 7b). We observed a similar result for the smaller class of genes carrying disruptive de novo mutations (P = 7×10-4, from 47 genes in our study); these genes included UFL1 (5/0 disruptive mutations, P = 0.03; 7/0 NSstrict, P = 0.008), SYNGAP1 (4/0 NSstrict, P = 0.04), and SZT2 (18/9 NSstrict, P = 0.049). SYNGAP1 (Synaptic Ras GTPase Activating Protein 1) is a component of the NMDAR PSD complex (Komiyama et al, 2002) and mutations in this gene are known to cause ID and autism (Berryer et al, 2013).
Genes under previously associated CNV regions did not show significant enrichment of rare disruptive mutations, although there was an enrichment of NSstrict mutations (P = 0.0044, ED Table 5). Of the eleven CNV regions, only the 3q29 locus, that contains multiple genes including DLG1 (Levinson et al., 2011), was significant (P = 0.0006) and withstood correction for multiple testing.
Autism/ID genes including targets of FMRP
We next tested, as a single set, the 2,507 genes representing autism and ID candidates (SI section 6), which yielded only nominal significance (P < 0.05) for disruptive and NSstrict variants and no test survived correction for multiple testing (Table 2). Considering the twelve constituent sets, genes from autism de novo studies showed no enrichment (ED Figure 2c), despite greater sample size and number of disruptive de novo mutations. There was no evidence for autism or ID genes curated from the literature (Betancur, 2011) or for genes in the protein-protein interaction-derived subnetworks built around autism de novos (O’Roak et al., 2012).
Table 2. Geneset analysis of secondary autism/ID candidate genesets.
Enrichment test empirical P-values for the secondary (autism/ID) geneset. As in Table 2, the top panel shows uncorrected P-values; tests significant after multiple test correction are in bold (i.e. all Pcorrected >0.05). Because no class of variant is significant after multiple test correction for the omnibus test (top panel), we applied and corrected for all 108 tests (9 conditions by 12 subsets) in the lower panel. The single category FMRP targets (Darnell et al.) mainly reflects disruptive and NSstrict singleton enrichment.
| Variant type | Geneset/subset | N genes | Singletons | MAF < 0.1% | MAF < 0.5% |
|---|---|---|---|---|---|
| Disruptive | 0.029 | 0.043 | 0.049 | ||
| Nonsyn (strict) | Autism/ID | 2,507 | 0.052 | 0.008 | 0.013 |
| Nonsyn (broad) | 0.532 | 0.619 | 0.287 | ||
|
| |||||
| N genes | Min. pcorrected (for 9×12=108 tests) | ||||
| Disruptive, nonsyn(strict), & nonsyn(broad) | De novo genes (exome sequencing) | ||||
| Autism (disruptive) | 128 | 1.000 | |||
| Autism (nonsyn) | 743 | 1.000 | |||
| ID (disruptive) | 30 | 0.070 | |||
| ID (nonsyn) | 132 | 0.995 | |||
| Neurodevelopmental candidates | |||||
| Betancur (2011), ASD candidates | 112 | 1.000 | |||
| Betancur (2011), ID candidates | 196 | 1.000 | |||
| Autism PPI networks | |||||
| O’Roak et al (2012), CHD8 network | 6 | 1.000 | |||
| O’Roak et al (2011), 49-gene network | 49 | 1.000 | |||
| O’Roak et al (2012), 74-gene network | 74 | 1.000 | |||
| Fragile × mental retardation protein targets | |||||
| Darnell et al. (2012) targets | 788 | 0.010 | |||
| Ascano et al. (2012) targets | 939 | 0.997 | |||
| Ascano et al. (2012) FMRP/autism overlap | 93 | 0.993 | |||
The nominal omnibus signals arose largely from the Darnell et al. (2011) list of fragile X mental retardation protein (FMRP) targets. FMRP is encoded by the gene FMR1 (the locus of the Mendelian fragile X syndrome repeat mutation) and is an RNA-binding protein that regulates translation and is needed at synapses for normal glutamate receptor signaling and neurogenesis (Callan & Zarnescu, 2011). Targets of FMRP are enriched for de novos in autism (Darnell et al., 2011; Ascano et al., 2012; Iossifov et al., 2012); here we find significant enrichment of disruptive singletons (P = 1.4×10-3, 289/223 case/control count, OR = 1.3). These FMRP targets overlap with PSD genes (ED Table 4b), although were still enriched independently (SI section 6). In addition, these genes were enriched in GWAS of this sample (P < 10-3, SI section 9). Whereas the Darnell list is derived from mouse brain, a second recently reported FMRP target list (Ascano et al., 2012) was generated from cultured human embryonic kidney cells, using a different experimental approach (SI section 6). This list has relatively little overlap with Darnell targets and, in contrast to the Darnell list, does not show any enrichment for rare case mutations, for GWAS loci, or comparable overlap with PSD genes (ED Table 4b).
Our results are perhaps surprising: unlike Fromer et al., we did not observe direct evidence for overlap at the individual gene level with autism and ID, despite CNV studies showing pleiotropic effects of individual loci. Nonetheless, at the broader level of genesets, all three disorders showed enrichment for FMRP targets; autism and ID de novos also showed strong enrichment in several PSD complexes enriched in our study, including NMDAR and PSD-95, and (for ID) ARC (Fromer et al., current issue). At the least, our results suggest that any overlap is far from complete, although more refined analyses in larger samples will be needed before a clearer picture can emerge of which genes and pathways are shared and which are specific to one disease.
Characterizing enrichment by variant type
To further characterize the observed enrichment with respect to mutational function and frequency, we created a single “composite” set of 1,796 genes comprising all members of the most prominently enriched sets (Supplementary Table S2). Rare disruptive mutations in this set were present in 990 cases and 877 controls (for singletons, 645 to 530). Cases carrying rare disruptive mutations did not appear to be phenotypically or clinically unusual in terms of sex, ancestry, history of drug abuse, general medical conditions plausibly etiologically related to psychosis, or epilepsy, although they did have a higher rate of admissions noting co-morbid intellectual disability compared to other cases (P = 0.009, ED Table 9b).
Figure 1 shows composite set enrichment across a range of conditions. As this set merges other sets showing enrichment, it necessarily shows enrichment; it was not, however, due to confounding effects of ancestry, sex or experimental wave (SI section 8). It was primarily driven by singleton nonsense mutations across a large number of genes, as it was removed or greatly attenuated when either singleton or nonsense mutations were excluded. Considered alone, neither splice-site, frameshift, missense, silent nor noncoding mutations showed enrichment at P<0.01. Different ways of defining damaging missense mutations did not substantively impact results. Considering only nonsynonymous coding variants present on ExomeChip, we did not observe enrichment. Rather, enrichment mainly reflected novel variants (ED Table 6b), which is expected as most rare variants in our study are novel. We also took an alternative approach, whereby instead of filtering variants on frequency, we excluded genes with any control disruptive variants before calculating the burden of case alleles; the composite set was still highly enriched (“case-unique” in Figure 1; see ED Table 6b & SI section 7). Finally, the enrichment could not be attributed to only a small number of variants or genes (ED Figure 9a).
Figure 1. Composite set geneset analysis, stratified by mutation type.
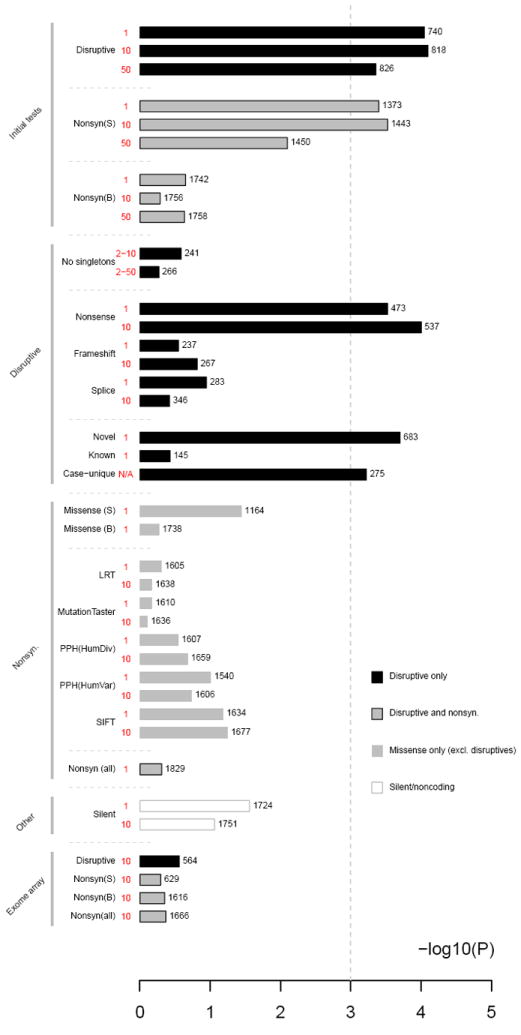
Statistical significance (x-axis) for the composite gene set stratified by type and frequency of mutation and other variables. Numbers to the right of each bar represent the number of genes with at least one mutation in that category for the composite set. (S) represents strictly-defined damaging missenses; (B) broadly-defined group. For the exome array contrasts (in which ExomeChip sites were tested using the exome sequence calls), D represents disruptive mutations, NS all nonsynonymous mutations.
These findings do not preclude potentially important effects from other classes of rare variation in specific genes or other genesets, although exploratory analyses of generic genesets (e.g. based on Gene Ontology terms) did not unambiguously identify novel signals after correction for multiple testing (SI section 7). We found preferential enrichment in genes with high brain expression, but not for genes with a prenatally-biased developmental trajectory (ED Figure 10). In fact, greater enrichment came from postnatally-biased genes. Finally, while greatly attenuated compared to disruptive mutations, other categories displayed nominal (0.01 < P < 0.05) enrichment in Figure 1 and strictly-defined damaging missense mutations alone showed enrichment for ARC and NMDAR genesets (32/15 for ARC, P = 0.007; ED Tables 6a & 8). Although rare coding alleles other than ultra-rare nonsense mutations will undoubtedly contribute to risk, it will likely prove harder still to elucidate such effects.
Rare variants, CNVs and common GWAS variants
We quantified the relative impact of common SNPs (indexed by a genome-wide polygene score from independent GWAS samples from the Schizophrenia Psychiatric Genome-Wide Association Study Consortium (2011)), rare CNVs (the burden of genic deletions) and disruptive mutations in the composite set. Considering the same 5,079 individuals, all three classes of variation were uncorrelated and significantly, independently and additively enriched in cases compared to controls. From logistic regression, the relative effect sizes (reduction in model R2) were 5.7%, 0.2% and 0.4% for GWAS, rare CNV and rare coding variants (SI section 8). Although not a complete assessment, it indicates that for the current sets of identifiably enriched alleles, common GWAS variants account for an order-of-magnitude more heritability than this set of rare variants does. However, these estimates will be diluted to varying degrees, due to unassociated variants being included. As a consequence of this, and also the fact that true risk variants outside of composite set genes were not considered here, this estimate represents a conservative lower bound on the contribution of rare coding variation.
DISCUSSION
We have demonstrated a polygenic burden that increases risk for schizophrenia, primarily comprised of many ultra-rare nonsense mutations distributed across many genes. Implicating individual genes remains challenging, as genes that contributed to the highest-ranked sets typically had unremarkable P-values, often around 0.5 with the gene containing only one or two rare mutations. Nonetheless, we were able to detect several small and highly enriched sets, notably of genes related to calcium channels and the postsynaptic ARC complex. Across these ~50 genes, approximately 1% of cases carried a rare disruptive mutation likely to have a considerable impact on risk. However, reported effect sizes will have a tendency to over-estimate true population values (SI section 5).
We add to previous work that has implicated disruption of synaptic processes in schizophrenia (Kirov et al., 2012). The PSD is comprised of supramolecular multiprotein complexes that detect and discriminate patterns of neuronal activity and regulate plasticity processes responsible for learning (Migaud et al. 1998). Members of the membrane-associated guanylate kinase (MAGUK) family of scaffold proteins, such as PSD-95, play a key role in assembling ~2MDa complexes comprising calcium channels, including the glutamate-gated NMDAR, voltage-gated calcium channels and ARC (Husi et al., 2000; Husi & Grant, 2001; Fernandez et al., 2009; Müller et al., 2010). The genetic disruption of MAGUKs and their associated components result in specific cognitive impairments in mice and humans (Nithiantharajah et al., 2013). One possibility is that the genetic risk identified here reflects altered tuning in calcium-dependent signaling cascades, triggered by NMDAR (Steward & Worley, 2001) and L-type calcium channels (Waltereit et al., 2001), mediated by postsynaptic MAGUK signaling complexes driving ARC synthesis.
Although we cannot yet use rare mutations to partition patients into more homogeneous clinical subgroups, this will remain a central goal for future sequencing studies. The few population-based common disease exome sequencing studies published to date, in psychiatric (e.g. Liu et al., 2013) and non-psychiatric (e.g. Albrechtsen et al., 2012) diseases, have not been successful in finding individual genes showing significant enrichment. Our study yields similar findings for individual genes, but yields positive results when considering gene sets. These current findings likely foreshadow the definitive identification of individual genes in larger cohorts, following the trajectory of GWAS and other genetic studies of complex disease.
METHODS SUMMARY
Sample ascertainment
Cases with schizophrenia were identified via the Swedish Hospital Discharge Register (Ripke et al., 2013). Case inclusion criteria: ≥2 hospitalizations with a discharge diagnosis of schizophrenia, both parents born in Scandinavia, age ≥18 years. Case exclusion criteria: hospital register diagnosis of any disorder mitigating a confident diagnosis of schizophrenia. Controls were randomly selected from Swedish population registers. Control inclusion criteria: never hospitalized for schizophrenia or bipolar disorder, both parents born in Scandinavia, age ≥18 years. All subjects provided informed consent; institutional human subject committees approved the research.
Sequencing
The samples (2,536 cases, 2,543 controls) were sequenced using either the Agilent SureSelect Human All Exon Kit (29Mb, n=132) or the Agilent SureSelect Human All Exon v.2 Kit (33Mb). Sequencing was performed by IlluminaGAII or Illumina HiSeq2000. Sequence data were aligned and variants called by the Picard (http://picard.sourceforge.net) zBWA (Li & Durbin, 2009)/GATK (de Pristo et al., 2011) pipeline. Validation of selected variants used Sanger sequencing. Based on validation and ExomeChip data, we estimated high sensitivity and specificity of singleton calls. BAM and VCF files are available in the dbGaP study phs000473.v1 Sweden-Schizophrenia Population-Based Case-Control Exome Sequencing.
Analysis
We used PLINK/Seq (http://atgu.mgh.harvard.edu/plinkseq/) to annotate variants according to RefSeq gene transcripts (UCSC Genome Browser, http://genome.ucsc.edu). Single site association used Fisher’s exact test; primary gene-based association used a burden test and the sequence kernel association test, SKAT (Wu et al., 2011). Analyses controlled for ancestry and QC metrics. Genesets used were from recent literature. Genesets were evaluated on the empirical distribution of the sum of individual gene burden statistics, and incorporated an empirical correction for multiple testing. Odd ratios with 95% confidence intervals used penalized maximum likelihood (Firth’s method) for low cell counts. See the SI for further details. Summary results are posted at http://research.mssm.edu/statgen/sweden/
Extended Data
Extended Data Table 1. Sample and detected variant properties.
a. Numbers of individuals in the final dataset, after individual-level QC. Finnish ancestry was inferred by multidimensional scaling. P-values from Fisher’s exact test. b. Technical metrics for the cases and controls (after individual-level QC); P-values for two-sided test of case/control differences (t-test). c. Properties of variants detected by exome sequencing. Counts (N) and minor allele counts (MAC) for various classes of variant in the main exome dataset, following all QC. Missense deleteriousness prediction algorithms and how they were combined described in SI section 4.
| a
| ||||
|---|---|---|---|---|
| Case/control status by sex (P = 5e-11) | Status | Male | Female | Female (%) |
| Control | 1291 | 1252 | 49% | |
| Case | 1520 | 1016 | 40% | |
|
| ||||
| Case/control status by ancestry (P = 2e-12) | Ancestry | Control | Case | Case (%) |
|
| ||||
| Swedish | 2356 | 2197 | 48% | |
| Finnish | 139 | 274 | 66% | |
| b
| |||
|---|---|---|---|
| Sample and sequencing metrics | Cases | Controls | P (case vs. control) |
| N | 2536 | 2543 | - |
| N (pre-QC) | 2546 | 2545 | - |
| Total number of reads | 100,532,755 | 100,079,333 | 0.62 |
| Filtered, unique reads aligned | 68,940,753 | 68,339,964 | 0.26 |
| Filtered, unique bases aligned | 5,106,614,996 | 5,070,497,844 | 0.34 |
| Mean target coverage | 89.98 | 89.55 | 0.53 |
| Percentage of target bases covered > 10x | 92.83 | 92.85 | 0.55 |
| Percentage of target bases covered > 20x | 87.30 | 87.30 | 0.93 |
| Percentage of target bases covered > 30x | 81.13 | 81.07 | 0.63 |
| Percentage of targets w/out any bases covered at 2x | 1.72 | 1.72 | 0.60 |
| Mean number of non-reference genotypes per individual (unfiltered) | 18772.9 | 18786.6 | 0.13 |
| Mean number of on-target singletons per individual (unfiltered) | 49.6 | 49.0 | 0.38 |
| Mean dbSNP % per individual | 98.3970% | 98.3969% | 1.00 |
| c
| ||||
|---|---|---|---|---|
| Property | Variant type | N | Mean MAC | % singleton |
| All alternate alleles | 635,944 | 103.37 | 56% | |
| Functional class | ||||
| Noncoding | 61,416 | 142.03 | 53% | |
| Silent | 185,336 | 152.85 | 51% | |
| Missense | 342,561 | 69.52 | 58% | |
| Non-essential splice site | 25,450 | 127.04 | 54% | |
| Nonsense | 9,022 | 20.68 | 69% | |
| Essential splice-site | 4,394 | 16.18 | 70% | |
| Frameshifting indel | 3,461 | 9.46 | 79% | |
| In silico annotation of missenses | ||||
| LRT | 168,437 | 34.55 | 62% | |
| Mutation Taster | 167,316 | 19.90 | 63% | |
| PolyPhen2 (HumDiv) | 130,719 | 28.84 | 62% | |
| PolyPhen2 (HumVar) | 91,156 | 24.74 | 64% | |
| SIFT | 140,345 | 43.85 | 61% | |
| Primary variant groupings for analysis | ||||
| Singletons | Gene disruptive | 12,047 | 1.00 | 100% |
| Nonsyn (strict) | 36,542 | 1.00 | 100% | |
| Nonsyn (broad) | 160,229 | 1.00 | 100% | |
| <0.1% MAF (1-10 alleles) | Gene disruptive | 15,972 | 1.56 | 75.4% |
| Nonsyn (strict) | 50,369 | 1.65 | 72.5% | |
| Nonsyn (broad) | 233,575 | 1.78 | 68.6% | |
| <0.5% MAF (1-50 alleles) | Gene disruptive | 16,523 | 2.24 | 72.9% |
| Nonsyn (strict) | 52,545 | 2.51 | 69.5% | |
| Nonsyn (broad) | 248,217 | 3.04 | 64.6% | |
Extended Data Figure 10. Stratified enrichment analysis P-values by developmental trajectory of expression in brain (BrainSpan & Human Brain Transcriptome (HBT) datasets).
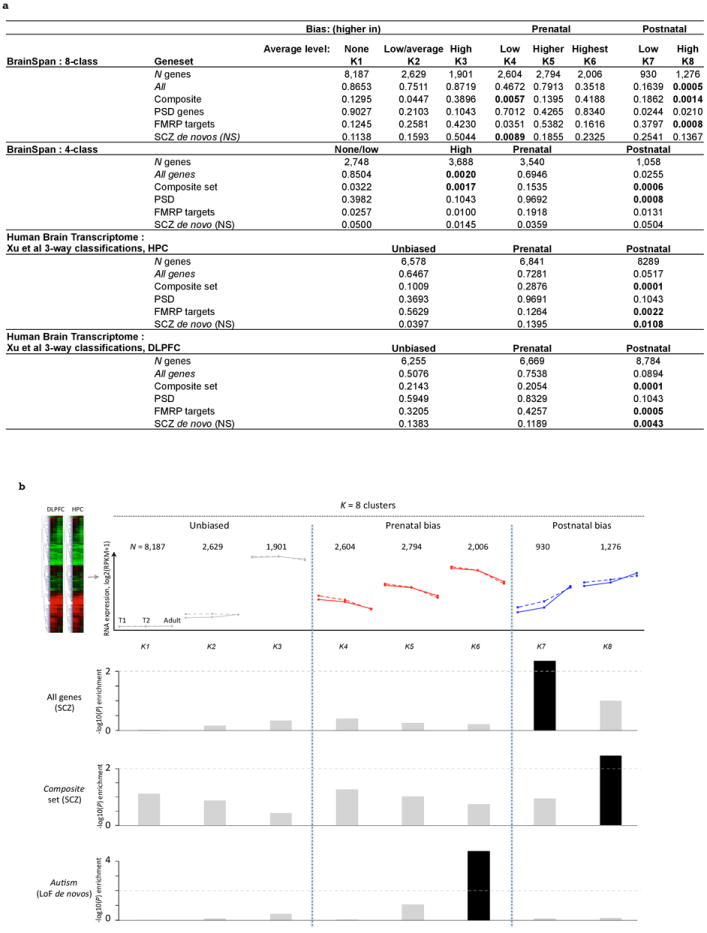
a. Uncorrected P-values for a set of exploratory analyses in which we stratified genes in the enrichment analyses by their developmental profile of brain expression. We used four schemes to classify genes as “brain expressed” and/or “biased” with respect to prenatal or postnatal expression (see SI section 6 and 10b below for details). We merged data on the hippocampus and dorsolateral prefrontal cortex for the BrainSpan classifications; to mirror the classification of Xu et al. (2012), we kept separate these two groupings for the HBT dataset. Results presented for MAF<0.1% disruptive variants; similar results are obtained for singletons with the exception that the “K4” prenatal enrichment signals are no longer significant. In general, the most consistent enrichment across variant classes, classification schemes and brain regions emerge for postnatally biased genes with high brain expression. b. Analysis of exome variants by developmental expression trajectory in human brain. Genes are grouped by cluster analysis of human postmortem brain expression into eight developmental trajectories, using RNA-sequencing data from the BrainSpan project. The top row gives the number of genes per cluster and the cluster centers in log2-scaled RPKM (reads per kilobase per million) values; solid and dotted solid lines indicate dorsolateral prefrontal cortex (DLPFC) and hippocampus (HPC) respectively. The bottom two rows show enrichment in the current study, relative to the exome-wide average, for singleton disruptive mutations in cases compared to controls, either subsetting all genes by expression profile (first row), or considering only genes in the composite set (second row). In both cases, we only observed nominally (P < 0.01) significant enrichment for genes that are postnatally biased. In contrast, a list of genes with loss-of-function (LoF) de novo mutations (compiled and reported in Fromer et al.) shows strong enrichment for prenatal bias (see Fromer et al. for details on how de novo enrichment was calculated). Alternative approaches to classifying genes as prenatally or postnatally biased led to similar conclusions (SI section 6).
Extended Data Figure 2. Ancestry and association summaries.
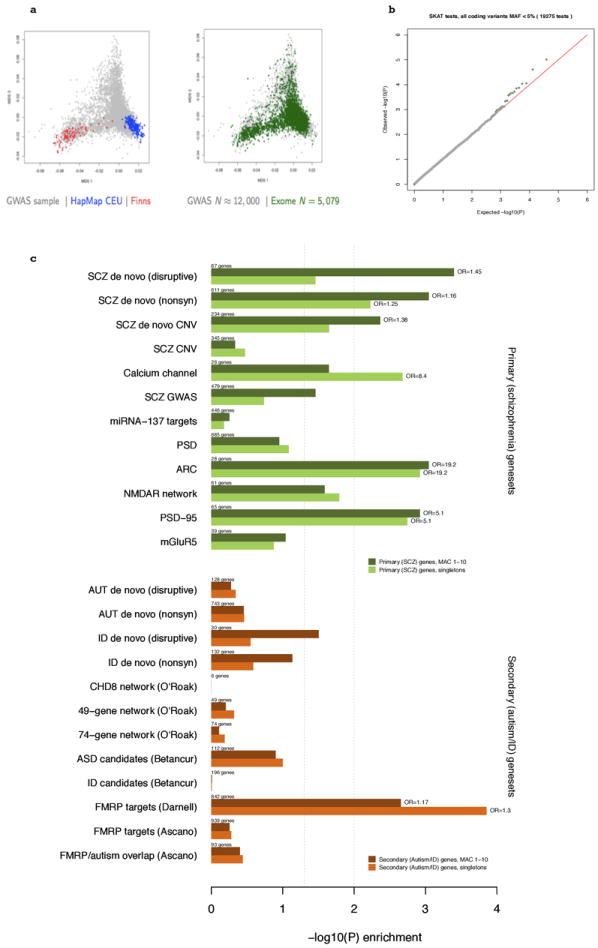
a. Multidimensional scaling plot of ancestry in the Swedish sample, including HapMap CEU and Finnish samples; showing sequenced individuals and the larger Swedish sample. b. Q-Q plot for gene-based SKAT results (MAF < 5% coding variants). Similar, or more conservative, profiles obtained for other subsets of variants. c Case enrichment of rare (MAF<0.1%) and singleton disruptive mutations for the constituent sets of the primary/schizophrenia gene set (top panel in green) and the secondary (autism/ID) geneset (bottom panel in orange). The primary set is enriched in cases (MAF<0.1% disruptive mutations P = 10-4; singletons P = 8×10-4, significant after correction for multiple testing) whereas the autism/ID shows only a modest trend (P = 0.04 and 0.03 for MAF<0.1% and singletons) and is not significant after correction. X-axis represents −log10(P); OR is odds ratio. Number of genes is for total in the set (whether or not they had a rare variant).
Extended Data Table 3. Genes prioritized as more likely to harbor large-effect alleles.
Individual gene case/control counts, odds ratios and P-values for genes from primary genesets with odds ratios > 5, and KYNU (top-ranked individual gene). Odds ratios are calculated using Firth’s method (penalized maximum likelihood logistic regression) and shown with 95% confidence intervals. P-values are empirical, uncorrected one-sided burden tests. FMRP target annotations are based on the Darnell et al. list only. Supplementary Table S1 lists singleton variant and genotype information for the genes listed here.
| Class | Gene | Singletons | MAF < 0.1% | Notes |
|---|---|---|---|---|
| ARC/PSD complex | ||||
| CYFIP1 | 1/0 | 1/0 | SCZ de novo (CNV) | |
| BAIAP2 | 1/0 | 1/0 | SCZ de novo (NS) | |
| DLG1 | 1/0 | 1/0 | SCZ de novo (NS), SCZ de novo (CNV) | |
| SLC25A3 | 1/0 | 1/0 | ||
| GLUD1 | 1/0 | 1/0 | ||
| CAMK2A | 1/0 | 1/0 | FMRP target | |
| ATP1B1 | 1/0 | 1/0 | AUT de novo (disruptive); FMRP target | |
| IQSEC2 | 1/0 | 1/0 | ID de novo (disruptive); FMRP target | |
| MBP | 1/0 | 1/0 | FMRP target | |
| Total | 9/0 | 9/0 | ||
| P = 0.0016 | P = 0.0014 | |||
| OR = 19.2 (2.4 - 2471) | OR = 19.2 (2.4 - 2471) | |||
| PSD-95 genes | ||||
| ABLIM1 | 1/0 | 1/0 | ||
| ACO2 | 1/0 | 1/0 | FMRP target | |
| ANKS1B | 3/1 | 3/1 | ||
| ATP1B1 | 1/0 | 1/0 | AUT de novo (disruptive); FMRP target | |
| ATP5A1 | 1/0 | 1/0 | FMRP target | |
| BAIAP2 | 1/0 | 1/0 | SCZ de novo (NS) | |
| CAMK2A | 1/0 | 1/0 | FMRP target | |
| CAMK2B | 2/0 | 2/0 | FMRP target | |
| DLG1 | 1/0 | 1/0 | SCZ de novo (NS), SCZ de novo (CNV) | |
| GAPDH | 1/0 | 1/0 | ||
| IQSEC2 | 1/0 | 1/0 | ID de novo (disruptive); FMRP target | |
| NRXN1 | 1/0 | 1/0 | SCZ de novo (NS); AUT de novo (disruptive); FMRP target | |
| PRDX1 | 0/1 | 0/1 | ||
| PRDX2 | 0/1 | 0/1 | ||
| SUCLA2 | 1/0 | 1/0 | AUT de novo (disruptive) | |
| SYNGAP1 | 1/0 | 1/0 | SCZ de novo (disruptive); ID de novo (disruptive); FMRP target | |
| Total | 17/3 | 17/3 | ||
| P = 0.0017 | P = 0.0009 | |||
| OR = 5.1 (1.8 - 19.2) | OR = 5.1 (1.8 - 19.2) | |||
| Voltage-gated calcium ion channel genes | ||||
| CACNA1B | 1/0 | 1/0 | FMRP target | |
| CACNA1C | 2/0 | 2/0 | SCZ & BP GWAS hit | |
| CACNA1H | 1/0 | 3/0 | ||
| CACNA1S | 2/0 | 2/3 | SCZ & AUT de novos (NS) | |
| CACNA2D1 | 1/0 | 1/0 | PSD | |
| CACNA2D2 | 3/0 | 3/0 | ||
| CACNA2D3 | 0/0 | 3/0 | AUT de novo (disruptive) | |
| CACNA2D4 | 1/0 | 2/4 | ||
| CACNB2 | 0/1 | 0/1 | ||
| CACNB4 | 1/0 | 1/0 | PSD | |
| Total | 12/1 | 15/8 | ||
| P = 0.0021 | P = 0.021 | |||
| OR = 8.4 (2.03 - 77) | OR = 2.1 (0.97 - 4.9) | |||
| Top disruptive gene-based test | ||||
| KYNU | 3/0 | 10/0 | ||
| P = 0.13 | P = 0.0017 | |||
| OR = 21.2 (2.7 - 2725) | ||||
Extended Data Table 4. Extended results for all PSD genesets.
a. Full PSD geneset association results. For all nine (3 annotation levels by 3 frequency levels), P-values for enrichment of all genesets described and tested in Kirov et al. (2012). In addition to the PSD genes (top five rows), enrichment statistics for presynaptic genes, and neuronal genes clustered on the basis of subcellular location are given. Although the P-values presented are uncorrected, we performed this analysis correcting for all 9×17=153 tests (by considering the distribution of the minimum empirical P-value across tests and sets, as described in the SI). The values in bold are significant (Pcorrected < 0.05) after correction for multiple testing. Both ARC and NMDAR network are significant after multiple test correction, for the singleton NSstrict category. (Note: for ARC the disruptive singleton category is, as reported in the primary test, highly significant and withstands correction for multiple testing in that context; in this broader, less focused analysis it yields Pcorrected=0.17; the majority of Pcorrected values (not shown in Table) are 1.00.) b. PSD and FMRP-target genesets: descriptive statistics and overlap. Overlap between Darnell et al. & Ascano et al. FMRP targets and PSD genes: for example, 57% (16/28) of ARC genes are in the Darnell FMRP list. In contrast, only 7% (2/28) are in the Ascano list. There is a similar trend across the three other major PSD subsets considered here: NMDAR network, PSD-95 and mGluR5 genes. Conversely, 22% of Darnell targets are in the PSD (human core) compared to only 9% of Ascano targets.
| a
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Set | N genes | Disruptive
|
Nonsyn. (strict)
|
Nonsyn. (broad)
|
||||||
| Singletons | MAF< 0.1% | MAF< 0.5% | Singletons | MAF< 0.1% | MAF< 0.5% | Singletons | MAF< 0.1% | MAF< 0.5% | ||
| PSD (human core) | 685 | 0.0729 | 0.1019 | 0.1083 | 0.0058 | 0.1045 | 0.1285 | 0.0866 | 0.5827 | 0.3743 |
| ARC | 28 | 0.0016 | 0.0013 | 0.0014 | 0.0004 | 0.0018 | 0.0047 | 0.2830 | 0.4607 | 0.3542 |
| NMDAR network | 61 | 0.0154 | 0.0229 | 0.0225 | 0.0001 | 0.0007 | 0.0005 | 0.0012 | 0.1426 | 0.0420 |
| mGluR5 | 39 | 0.1299 | 0.0861 | 0.0862 | 0.0628 | 0.0837 | 0.0900 | 0.0302 | 0.2192 | 0.1329 |
| PSD-95 | 65 | 0.0015 | 0.0008 | 0.0008 | 0.0027 | 0.0204 | 0.0393 | 0.2992 | 0.3722 | 0.1147 |
| Pre-synapse | 431 | 0.0187 | 0.0983 | 0.1458 | 0.2327 | 0.1811 | 0.3600 | 0.1306 | 0.7597 | 0.6515 |
| Pre-synaptic active zone | 173 | 0.0518 | 0.0487 | 0.0482 | 0.6162 | 0.6641 | 0.7082 | 0.8439 | 0.9918 | 0.9554 |
| Synaptic vesicle | 344 | 0.1030 | 0.3133 | 0.4151 | 0.1439 | 0.1093 | 0.2466 | 0.0375 | 0.3423 | 0.2718 |
| Cytoplasm | 271 | 0.5851 | 0.1793 | 0.1034 | 0.8983 | 0.5351 | 0.6007 | 0.1861 | 0.1084 | 0.1192 |
| Early Endosomes | 17 | 0.8917 | 0.7860 | 0.7826 | 0.2891 | 0.2420 | 0.2139 | 0.1472 | 0.4019 | 0.4123 |
| Endoplasmic Reticulum | 97 | 0.3005 | 0.1882 | 0.2612 | 0.6615 | 0.2805 | 0.5036 | 0.6194 | 0.5044 | 0.7878 |
| ER/Golgi-derived vesicles | 94 | 0.4258 | 0.2678 | 0.3644 | 0.3001 | 0.4977 | 0.6239 | 0.3063 | 0.6944 | 0.7649 |
| Golgi | 31 | 0.5130 | 0.5493 | 0.5481 | 0.1998 | 0.0921 | 0.1338 | 0.0074 | 0.1628 | 0.4178 |
| Mitochondrion | 197 | 0.0141 | 0.0259 | 0.0178 | 0.4351 | 0.0860 | 0.0671 | 0.6999 | 0.2112 | 0.6079 |
| Nucleus | 167 | 0.1790 | 0.3029 | 0.2900 | 0.0626 | 0.1512 | 0.2728 | 0.2006 | 0.3340 | 0.3196 |
| Plasma membrane | 50 | 0.7940 | 0.5659 | 0.5635 | 0.9416 | 0.8059 | 0.8091 | 0.8944 | 0.5531 | 0.3028 |
| Recycling Endosomes/trans-Golgi network | 68 | 0.1502 | 0.0944 | 0.1556 | 0.5349 | 0.4514 | 0.5359 | 0.0902 | 0.0862 | 0.1862 |
| b
| |||||||
|---|---|---|---|---|---|---|---|
| N | PSD (human core) | FMRP target (Darnell) | FMRP target (Ascano) | ||||
| N | % | N | % | N | % | ||
| PSD (human core) | 685 | - | - | 170 | 25% | 80 | 12% |
| ARC | 28 | - | - | 16 | 57% | 2 | 7% |
| NMDAR network | 61 | - | - | 32 | 52% | 5 | 8% |
| mGluR5 | 39 | - | - | 25 | 64% | 7 | 18% |
| PSD-95 | 65 | - | - | 30 | 46% | 4 | 6% |
| Pre-synapse | 431 | 213 | 49% | 87 | 20% | 31 | 7% |
| Pre-synaptic active zone | 173 | 121 | 70% | 50 | 29% | 10 | 6% |
| Synaptic vesicle | 344 | 162 | 47% | 72 | 21% | 27 | 8% |
| Cytoplasm | 271 | 77 | 28% | 16 | 6% | 23 | 8% |
| Early Endosomes | 17 | 6 | 35% | 2 | 12% | 1 | 6% |
| Endoplasmic Reticulum | 97 | 13 | 13% | 5 | 5% | 4 | 4% |
| ER/Golgi-derived vesicles | 94 | 24 | 26% | 7 | 7% | 4 | 4% |
| Golgi | 31 | 2 | 6% | 2 | 6% | 4 | 13% |
| Mitochondrion | 197 | 57 | 29% | 6 | 3% | 12 | 6% |
| Nucleus | 167 | 19 | 11% | 7 | 4% | 19 | 11% |
| Plasma membrane | 50 | 16 | 32% | 6 | 12% | 5 | 10% |
| Recycling Endosomes/trans-Golgi network | 68 | 19 | 28% | 3 | 4% | 7 | 10% |
| Total | 1509 | 685 | 45% | 170 | 11% | 80 | 5% |
Extended Data Table 5. Association results for individual CNV regions.
Focused enrichment analysis of genes under schizophrenia-associated CNV regions. The top panel presents omnibus P-values testing all genes/regions (bold indicates significance after correction for the four tests, Pcorrected = 0.016 for NSstrict MAF < 0.1% variants). This enrichment arises solely from the 3q29 locus (middle panel; bold indicates significance after correction of the 44 tests performed, Pcorrected = 0.024 for 3q29). Genes and NSstrict case/control counts for the 3q29 region (lower panel).
| Group | Genes | Disruptive | NSstrict | ||
|---|---|---|---|---|---|
| Singletons | MAF < 0.1% | Singletons | MAF < 0.1% | ||
| CNV loci | All | 0.3279 | 0.4557 | 0.0843 | 0.0044 |
|
| |||||
| 1q21.1 | 0.4533 | 0.6966 | 0.3205 | 0.1832 | |
| 2p16.3 | 0.4775 | 0.3580 | 0.4703 | 0.2750 | |
| 3q29 | 0.1054 | 0.0068 | 0.0123 | 0.0006 | |
| 7q36.3 | 0.8642 | 0.5750 | 0.6411 | 0.2688 | |
| 7q11.23 | 0.7199 | 0.6800 | 0.3329 | 0.2207 | |
| 15q11.2 | 0.3208 | 0.1616 | 0.5138 | 0.1362 | |
| 15q13.3 | 0.0883 | 0.3976 | 0.3672 | 0.2746 | |
| 16p13.11 | 0.4194 | 0.3775 | 0.8346 | 0.4124 | |
| 16p11.2 | 0.1613 | 0.1240 | 0.0655 | 0.0974 | |
| 17q12 | 0.7377 | 0.5205 | 0.4313 | 0.1385 | |
| 22q11.21 | 0.9386 | 0.9977 | 0.5456 | 0.8754 | |
|
| |||
|---|---|---|---|
| Gene | A/U | Gene name | |
| 3q29 genes | DLG1 | 5/0 | discs, large homolog 1 (Drosophila) |
| RNF168 | 5/1 | ring finger protein 168, E3 ubiquitin protein ligase | |
| CEP19 | 2/0 | centrosomal protein 19kDa | |
| LRRC33 | 2/0 | leucine rich repeat containing 33 | |
| PAK2 | 2/0 | p21 protein (Cdc42/Rac)-activated kinase 2 | |
| PCYT1A | 5/2 | phosphate cytidylyltransferase 1, choline, alpha | |
| PIGX | 6/3 | phosphatidylinositol glycan anchor biosynthesis, class X | |
| FBXO45 | 1/0 | F-box protein 45 | |
| NCBP2 | 1/0 | nuclear cap binding protein subunit 2, 20kDa | |
| PIGZ | 1/0 | phosphatidylinositol glycan anchor biosynthesis, class Z | |
| TFRC | 1/0 | transferrin receptor (p90, CD71) | |
| ZDHHC19 | 1/0 | zinc finger, DHHC-type containing 19 | |
| C3orf43 | 4/2 | chromosome 3 open reading frame 43 | |
| MFI2 | 15/12 | antigen p97 (melanoma associated) identified by monoclonal antibodies 133.2 and 96.5 | |
| SLC51A | 1/1 | solute carrier family 51, alpha subunit | |
| BDH1 | 0/1 | 3-hydroxybutyrate dehydrogenase, type 1 | |
| TCTEX1D2 | 0/1 | Tctex1 domain containing 2 | |
| WDR53 | 0/1 | WD repeat domain 53 | |
Extended Data Table 6. Further stratification of enrichment analyses by class of variant.
a. Geneset analyses for damaging missense mutations only. For the primary geneset and the 12 constituent subsets, a comparison of disruptive versus (strictlydefined) damaging missenses, i.e. an independent set of variants. The omnibus result for the primary test is modest (P=0.04) and did not withstand correction for multiple testing: as illustrated in Figure 1 and the main text, the bulk of the enrichment signal we observe comes from (singleton) disruptive mutations. Nonetheless, specific genesets such as ARC and the NMDAR network are highly and independently enriched for missense variants. N represents the number of genes with at least one mutation of this class observed in the sample. A/U represent case/control counts of non-reference genotypes. OR represents the odds ratio (not corrected for exome-wide rates) estimated by Firth’s method for sets with small cell counts. All tests are empirical and 1-sided (higher values expected in cases) as described in the main text and methods. b. Enrichment analyses of novel and case-unique disruptive mutations. For primary and secondary genesets (and constituent subsets) as well as the composite set: results of three alternative burden analyses. First, focusing only on genes without any control disruptive variants; no further frequency filter is imposed. Here “N genes(A/U)” indicates the number of genes with at least one disruptive variant, followed by the number of genes with case-only disruptive mutations and (for comparison) the number with control-only disruptive mutations. The “A(U)” column gives the number of case variants in the case-only genes: the test statistic is based on the empirical distribution of this count. The U in this field represents the similar quantity for controls (not explicitly used in the statistic). The second set of analyses represent standard burden/enrichment tests (i.e. as Tables 2 and 4) but stratified for novel versus known disruptive variants, according to dbSNP and the Exome Sequencing Project/Exome Variant Server (ESP/EVS) database. Novel variants show greater enrichment, although most rare variants observed in our study (both in cases and in controls) are novel, so tests of novel variants will have greater power.
| a
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Primary geneset | Disruptive singletons | Damaging missense (strict) singleton | ||||||
| P | N | A/U | OR | P | N | A/U | OR | |
| All primary genes | 0.0008 | 905 | 852/716 | 1.20 | 0.0393 | 1357 | 2080/2001 | 1.04 |
| SCZ de novo genes | ||||||||
| Exome sequencing (disruptive) | 0.0349 | 40 | 56/38 | 1.48 | 0.5613 | 53 | 121/120 | 1.01 |
| Exome sequencing (nonsyn) | 0.0059 | 332 | 384/309 | 1.25 | 0.2776 | 393 | 750/736 | 1.02 |
| Copy number variants | ||||||||
| de novo CNV genes (Kirov et al, 2012) | 0.0224 | 64 | 61/40 | 1.53 | 0.0593 | 90 | 125/112 | 1.12 |
| SCZ-associated CNV genes | 0.3378 | 72 | 65/55 | 1.19 | 0.0310 | 111 | 148/119 | 1.25 |
| GWAS | ||||||||
| Voltage-gated calcium channel genes | 0.0021 | 9 | 12/1 | 8.40 | 0.4629 | 18 | 37/35 | 1.06 |
| Common SNPs (P < 1e-4 intervals) | 0.1832 | 185 | 165/146 | 1.14 | 0.9246 | 268 | 359/395 | 0.91 |
| miRNA-137 targets | 0.6643 | 140 | 98/100 | 0.99 | 0.1415 | 263 | 376/361 | 1.05 |
| Synaptic genes | ||||||||
| PSD (human core) | 0.0824 | 219 | 172/145 | 1.19 | 0.0070 | 394 | 646/581 | 1.12 |
| ARC | 0.0012 | 9 | 9/0 | 19.20 | 0.0069 | 19 | 32/15 | 2.14 |
| NMDAR network | 0.0162 | 17 | 17/5 | 3.42 | 0.0003 | 34 | 76/45 | 1.70 |
| PSD-95 | 0.0018 | 16 | 17/3 | 5.10 | 0.0218 | 34 | 44/30 | 1.47 |
| mGluRS | 0.1335 | 10 | 9/3 | 3.02 | 0.1715 | 22 | 52/36 | 1.45 |
| b
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Geneset | Case-unique burden analysis | Known variants | Novel variants | ||||||||
| Singletons | MAF < 0.1% | Singletons | MAF < 0.1% | ||||||||
| P | N genes(A/U) | A(U) | P | N | P | N | P | N | P | N | |
| Composite | 0.0006 | 829(275/214) | 378(297) | 0.3733 | 145 | 0.2202 | 226 | 0.0002 | 683 | 0.0005 | 744 |
| Primary | 0.0022 | 1026(325/265) | 440(367) | 0.1003 | 191 | 0.0417 | 299 | 0.0074 | 831 | 0.0058 | 910 |
| SCZ de novo genes | |||||||||||
| Exome sequencing (disruptive) | 0.0018 | 47(16/6) | 29(12) | 0.5514 | 13 | 0.2196 | 24 | 0.0362 | 35 | 0.0010 | 40 |
| Exome sequencing (nonsyn) | 0.0037 | 371(108/80) | 159(116) | 0.3647 | 94 | 0.2064 | 144 | 0.0142 | 302 | 0.0071 | 326 |
| Copy number variants | |||||||||||
| de novo CNV genes | 0.1267 | 79(25/17) | 32(24) | 0.0156 | 13 | 0.0116 | 24 | 0.0819 | 59 | 0.0679 | 67 |
| SCZ-associated CNV genes | 0.7971 | 90(20/23) | 24(32) | 0.0355 | 23 | 0.0069 | 34 | 0.6081 | 63 | 0.9187 | 76 |
| GWAS | |||||||||||
| Voltage-gated calcium channel | 0.0129 | 9(5/1) | 10(1) | 0.4922 | 2 | 0.7077 | 3 | 0.0006 | 7 | 0.0022 | 8 |
| P < 1e-4 intervals | 0.1079 | 211(65/55) | 91 (78) | 0.0394 | 44 | 0.0409 | 69 | 0.4425 | 164 | 0.1882 | 180 |
| miRNA-137 targets | 0.4498 | 156(52/50) | 67(60) | 0.9939 | 14 | 0.9972 | 22 | 0.3846 | 133 | 0.2757 | 147 |
| Synaptic genes | |||||||||||
| PSD (human core) | 0.2234 | 244(92/79) | 113(109) | 0.5348 | 25 | 0.3072 | 40 | 0.1091 | 205 | 0.1629 | 226 |
| ARC | 0.0008 | 9(9/0) | 9(0) | 0 | 0 | 0.0016 | 9 | 0.0013 | 9 | ||
| NMDAR network | 0.0105 | 21(13/4) | 18(4) | 1.0000 | 1 | 0.6905 | 2 | 0.0075 | 16 | 0.0085 | 19 |
| PSD-95 | 0.0137 | 16(13/2) | 14(2) | 0.1218 | 1 | 0.1559 | 1 | 0.0034 | 15 | 0.0022 | 15 |
| mGluRS | 0.1363 | 11(7/1) | 8(1) | 0 | 0.1458 | 1 | 0.1427 | 10 | 0.1826 | 11 | |
| Secondary (autism/ID) | 0.0916 | 1249(348/314) | 479(471) | 0.1679 | 226 | 0.3543 | 352 | 0.1834 | 1041 | 0.0807 | 1143 |
| De novo genes (exome sequencing) | |||||||||||
| Autism (disruptive) | 0.662 | 65(17/17) | 20(26) | 0.4463 | 18 | 0.0781 | 23 | 0.6161 | 50 | 0.7009 | 56 |
| Autism (nonsyn) | 0.220 | 407(101/96) | 143(154) | 0.3198 | 89 | 0.4487 | 133 | 0.6656 | 336 | 0.4960 | 369 |
| ID (disruptive) | 0.262 | 8(4/1) | 4(2) | 1.0000 | 1 | 0.2747 | 3 | 0.3558 | 8 | 0.0578 | 8 |
| ID (nonsyn) | 0.052 | 69(22/18) | 35(28) | 0.1303 | 14 | 0.0934 | 26 | 0.5331 | 62 | 0.3368 | 66 |
| Neurodevelopmental candidates | |||||||||||
| Betancur (2011), ASD candidates | 0.110 | 37(12/6) | 16(7) | 0.5824 | 9 | 0.7543 | 14 | 0.0484 | 24 | 0.0429 | 29 |
| Betancur (2011), ID candidates | 0.994 | 88(14/28) | 16(38) | 0.6553 | 16 | 0.7056 | 24 | 0.9488 | 74 | 0.9556 | 82 |
| Autism PPI networks | |||||||||||
| CHD8 network | 1.000 | 1(0/1) | 0(1) | 0 | 0 | 1.0000 | 1 | 1.0000 | 1 | ||
| O’Roak et al. 49-gene network | 0.796 | 19(3/7) | 4(16) | 0.4755 | 5 | 0.7326 | 7 | 0.6081 | 30 | 0.7231 | 33 |
| O’Roak et al. 74-gene network | 0.654 | 33(6/13) | 10(28) | 0.6438 | 4 | 0.6667 | 4 | 0.7285 | 17 | 0.8411 | 19 |
| Fragile × mental retardation protein targets | |||||||||||
| Darnell et al. targets | 0.022 | 341(131/95) | 169(133) | 0.3048 | 39 | 0.3889 | 61 | 0.0007 | 288 | 0.0022 | 309 |
| Ascano et al. targets | 0.449 | 517(134/131) | 187(200) | 0.5571 | 83 | 0.7281 | 128 | 0.5261 | 439 | 0.4089 | 482 |
| Ascano et al. FMRP/autism | 0.423 | 33(10/6) | 12(12) | 0.0384 | 5 | 0.4624 | 11 | 0.6088 | 23 | 0.3954 | 28 |
Extended Data Table 7. Geneset analysis of de novo genes from schizophrenia exome-sequencing studies.
a. Test of case enrichment of rare variants in cases compared to controls, for genes with one or more de novos in Fromer et al., Xu et al. and/or Girard et al. The P-values in bold are significant at Pcorrected < 0.05, correcting for all 3×3×6 = 54 tests reported. b. Genes nominally significant (no correction) that had an observed de novo in one of the schizophrenia studies.
| a
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Set | N genes | Disruptive
|
Nonsyn. (strict)
|
Nonsyn. (broad)
|
||||||
| Singletons | MAF < 0.1% | MAF < 0.5% | Singletons | MAF < 0.1% | MAF < 0.5% | Singletons | MAF < 0.1% | MAF < 0.5% | ||
| Fromer et al. (disruptive) | 63 | 0.1484 | 0.0075 | 0.0034 | 0.7401 | 0.7324 | 0.6264 | 0.3347 | 0.1660 | 0.2536 |
| Fromer et al. (nonsyn) | 464 | 0.0004 | 0.0003 | 0.0016 | 0.0341 | 0.0057 | 0.0892 | 0.4688 | 0.4842 | 0.6547 |
| Girard & Xu (disruptive) | 24 | 0.0342 | 0.0082 | 0.0082 | 0.0423 | 0.0412 | 0.0602 | 0.0774 | 0.1106 | 0.0891 |
| Girard & Xu (nonsyn) | 151 | 0.6916 | 0.4124 | 0.4186 | 0.1510 | 0.1326 | 0.1285 | 0.2258 | 0.3420 | 0.1790 |
| Combined SCZ (disruptive) | 87 | 0.0319 | 0.0007 | 0.0003 | 0.3355 | 0.3162 | 0.2692 | 0.1325 | 0.0757 | 0.1016 |
| Combined SCZ (nonsyn) | 611 | 0.0053 | 0.0011 | 0.0055 | 0.0192 | 0.0024 | 0.0379 | 0.3408 | 0.4064 | 0.4472 |
| b
| ||||||
|---|---|---|---|---|---|---|
| Gene | De novo study (type) | Test | N | A/U | P | Gene name |
|
| ||||||
| ALDH1L2 | Fromer et al. (Nonsyn) | disruptive | 6 | 10/3 | 0.028 | aldehyde dehydrogenase 1 family, member L2 |
| CACNA1S | Fromer et al. (Nonsyn) | ns-strict | 23 | 28/15 | 0.031 | calcium channel, voltage-dependent, Ltype, alpha 1S subunit |
| DLG1 | Fromer et al. (Nonsyn) | ns-strict | 4 | 5/0 | 0.021 | discs, large homolog 1 (Drosophila) |
| IGSF22 | Fromer et al. (Nonsyn) | disruptive | 5 | 5/0 | 0.043 | immunoglobulin superfamily, member 22 |
| JARID2 | Fromer et al. (Nonsyn) | ns-strict | 5 | 5/0 | 0.041 | jumonji, AT rich interactive domain 2 |
| LAMA4 | Fromer et al. (Nonsyn) | ns-strict | 8 | 12/4 | 0.041 | laminin, alpha 4 |
| NBEA | Fromer et al. (Nonsyn) | ns-strict | 5 | 5/0 | 0.025 | neurobeachin |
| POLL | Fromer et al. (Nonsyn) | disruptive | 4 | 4/0 | 0.042 | polymerase (DNA directed), lambda |
| PTK2B | Fromer et al. (Nonsyn) | ns-strict | 4 | 4/0 | 0.044 | PTK2B protein tyrosine kinase 2 beta |
| SHKBP1 | Fromer et al. (Nonsyn) | ns-strict | 9 | 15/4 | 0.018 | SH3KBP1 binding protein 1 |
| SULF2 | Fromer et al. (Nonsyn) | ns-strict | 5 | 7/0 | 0.007 | sulfatase 2 |
| SYNGAP1 | Xu et al. (2012) (LoF) | ns-strict | 4 | 4/0 | 0.043 | synaptic Ras GTPase activating protein 1 |
| SZT2 | Xu et al. (2012) (LoF) | ns-strict | 22 | 18/9 | 0.049 | seizure threshold 2 homolog (mouse) |
| TANC1 | Fromer et al. (Nonsyn) | ns-strict | 14 | 17/4 | 0.002 | tetratricopeptide repeat, ankyrin repeat and coiled-coil containing 1 |
| TEP1 | Xu et al. (2012) (Nonsyn) | ns-strict | 26 | 25/14 | 0.048 | telomerase-associated protein 1 |
| UFL1 | Fromer et al. (LoF) | ns-strict | 4 | 7/0 | 0.008 | UFM1-specific ligase 1 |
| UFL1 | Fromer et al. (LoF) | disruptive | 2 | 5/0 | 0.029 | UFM1-specific ligase 1 |
Extended Data Table 8. Summary of observed likely-deleterious variants in ARC genes across studies.
For the 28 ARC genes, a summary of which genes had singleton disruptive, or damaging missense, variants in the current study, compiled alongside the genes with de novo CNVs or SNVs observed in Kirov et al. (2012) or Fromer et al. as well as the intellectual disability (ID) de novo genes (compiled in Fromer et al.). The P-values at the bottom indicate that in each comparison, the ARC geneset was significantly enriched.
| ARC gene (N=28) | Current study | de novo CNV (Kirov et al.) | de novo SNV (Fromer et al.) | de novo SNV in ID | ||
|---|---|---|---|---|---|---|
| Disruptive | Damaging missense (strict) | |||||
| ACTN4 | 3/1 | |||||
|
| ||||||
| ARF5 | ||||||
|
| ||||||
| ATP1A1 | 3/0 | |||||
|
| ||||||
| ATP1A3 | 2/1 | |||||
|
| ||||||
| ATP1B1 | 1/0 | 1/0 | ||||
|
| ||||||
| BAIAP2 | 1/0 | NS(x2) | ||||
|
| ||||||
| CAMK2A | 1/0 | 1/0 | ||||
|
| ||||||
| CRMP1 | 1/3 | |||||
|
| ||||||
| CYFIP1 | 1/0 | 4/1 | 2 del; 2 dup | |||
|
| ||||||
| DLG1 | 1/0 | 2/0 | 1 del | NS | ||
|
| ||||||
| DLG2 | 2/3 | 2 del | LoF | |||
|
| ||||||
| DLG4 | 1/2 | NS | ||||
|
| ||||||
| DLGAP1 | 2/0 | 1 del | ||||
|
| ||||||
| DLGAP2 | 1/0 | |||||
|
| ||||||
| DPYSL2 | 0/1 | |||||
|
| ||||||
| GLUD1 | 1/0 | 1/0 | ||||
|
| ||||||
| GLUL | 2/0 | |||||
|
| ||||||
| GRIN1 | 2/0 | |||||
|
| ||||||
| HSPA8 | LoF & NS | |||||
|
| ||||||
| IQSEC1 | 4/1 | |||||
|
| ||||||
| IQSEC2 | 1/0 | 0/1 | LoF | |||
|
| ||||||
| MBP | 1/0 | 0/1 | ||||
|
| ||||||
| PKM2 | ||||||
|
| ||||||
| PLP1 | ||||||
|
| ||||||
| SLC25A3 | 1/0 | |||||
|
| ||||||
| SLC25A4 | ||||||
|
| ||||||
| SLC25A5 | ||||||
|
| ||||||
| STXBP1 | LoF, NS(x2) | |||||
|
| ||||||
| Counts: | 9/0 | 32/15 | 8 SCNVs | 6 SNVS | 5 SNVS | |
| P-value: | 0.0016 | 0.0069 | 0.00025 | 0.0005 | 0.00002 | |
Extended Data Figure 9. Genic and phenotypic subset analyses for the composite set.
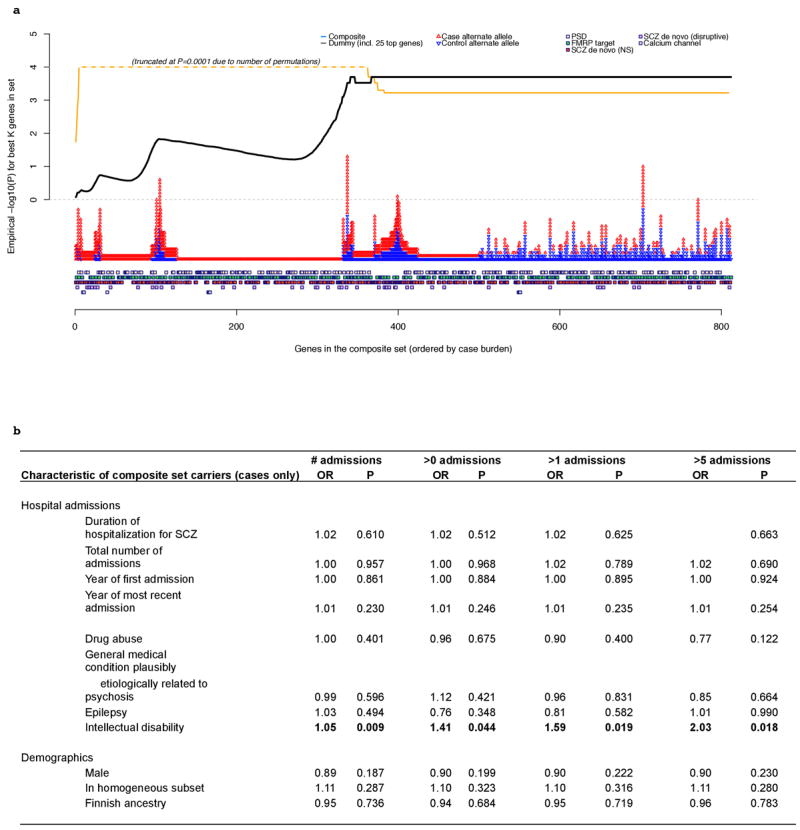
a. Individual gene-ranking of composite set genes. Genes are ranked by their case burden of rare disruptive mutations, from left to right, for the composite set. The squares along the bottom indicate to which sets each gene belongs. The red and blue triangles represent case and control counts for each gene. The lines above represent the statistical significance of the best test for this set: that is, the significance of the top K genes, evaluated by permutation. The black line represents results for the real data (disruptive MAF<0.1% composite set analysis). The orange line represents the dummy condition, in which we artificially constructed a set, where the number of genes, statistical enrichment, odds ratio and case/control counts where similar to the real composite set. However, this set included the 25 top-ranked genes from individual gene-based tests (disruptive MAF<0.1% variants), with the remainder selected at random. The profile of the best test line is markedly different between the real and dummy gene sets (note: truncated at P=0.0001 reflecting the number of permutations performed). Whereas the dummy P-value climbs quickly and then drops to the final aggregate result, the true composite set line continues to climb after 200 genes, indicating that many genes with a single disruptive mutation contribute to the observed set enrichment (rather than a relatively small proportion of the 1,796 genes accounting for the majority of the signal, as in the dummy set). b. Phenotypic characteristics of cases carrying mutations. Relationship between clinical and demographic measures in schizophrenia cases in relation to carrying one or more composite set disruptive risk alleles (MAF<0.1%). Hospital Discharge Registry data (ICD9 codes) were available on 979 of the 990 case carriers. All P-values (uncorrected) are two-sided from a case-only joint logistic regression of carrier status (one or more risk alleles) on all admission and demographic variables including year of first and last admissions. The four pairs of columns represent analyses in which we varied the way in which the HDR admission data were represented (for drug abuse, general medication condition, epilepsy and intellectual disability). “# admissions” = independent variables are the untransformed number of admissions; “>X admissions” = independent variable is binary 0/1 variable representing whether individuals had more than X admissions. Of all clinical/demographic measures considered, we observed a nominally-significant increased likelihood that cases carrying a disruptive allele in the composite set have increased rates of secondary diagnoses of intellectual disability compared to other cases (based on HDR ICD9 codes).
Supplementary Material
Supplementary Table 1 : Genes and variants in highly enriched genesets ∣ Details for specific variants for the genes in the small but highly enriched genesets, that showed large odds ratios (>5): ARC, PSD-95, calcium channel genes, Müller et al. Cav2 genes as well as disruptive KYNU variants. The variants from the four genesets are all singleton disruptive mutations, corresponding to the genes listed in ED Table 3. The (a) and (b) in the phenotype column indicate that the same individual carried two of these variants. (That the same cases carries two of these alleles is not necessarily surprising: assuming across cases equal and independent probabilities for carrying each allele, of the 49 case unique variants listed in this table, there is a 37% chance that at least one individual carries two of the 49 alleles, and a 7% chance that at least two individuals carry multiple alleles, based on 2,536 total cases.) The genotype meta-information fields are from GATK: AD = allele depth (number of reference, alternate reads); DP = total high quality reads used in calling; GQ = genotype quality, (max 99); PL = phredscaled genotype likelihoods (reference, heterozygote, homozygote).
Supplementary Table 2 : Genes selected for the composite set ∣ Source indicated: PSD = any PSD gene; FMRP-target = Darnell et al. FRMP target; SCZ de novo = nonsynonymous de novo mutation in Xu et al., Girard et al. or Fromer et al.; Calcium channel = voltage-gated calcium channel.
Acknowledgments
We are deeply grateful for the participation of all subjects contributing to this research, and to the collection team that worked to recruit them: Emma Flordal-Thelander, Ann-Britt Holmgren, Marie Hallin, Marie Lundin, Ann-Kristin Sundberg, Christina Pettersson, Radja Satgunanthan-Dawoud, Sonja Hassellund, Malin Rådstrom, Birgitta Ohlander, Leila Nyrén, and Isabelle Kizling. We gratefully acknowledge funding support from NIH/NIMH ARRA Grand Opportunity grant NIMH RC2 MH089905 (Purcell & Sklar), the Sylvan Herman Foundation, a philanthropic gift from Ted and Vada Stanley to the Stanley Center for Psychiatric Research, the Stanley Medical Research Institute, NIH/NHGRI grant U54HG003067 (Lander), NIH/NIMH R01 MH095088 (Haggarty), NIH/NIMH grant R01 MH091115 (Haggarty), the Tau Consortium (Haggarty), NIH/NIMH grant R01 MH099126 (Purcell), NIH/NHGRI grant R01HG005827 (Purcell), NIH/NIMH grant R01 MH077139 (Sullivan), NIH/NIMH grant R01 MH095034 (Sklar), the Friedman Brain Institute at Mount Sinai School of Medicine, the Karolinska Institutet, Karolinska University Hospital, the Swedish Research Council, an ALF grant from Swedish County Council, the Söderström Königska Foundation, the Netherlands Scientific Organization (NWO 645-000-003), the Wellcome Trust, Genes to Cognition Program, The Medical Research Council (MRC) and European Union projects GENCODYS no. 241995, EUROSPIN no. 242498 and SYNSYS no. 242167 (Fernández, Collins, Komiyama, Choudhary & Grant). Work at the Icahn School of Medicine at Mount Sinai was also supported by the Institute for Genomics and Multiscale Biology (including computational resources and staff expertise provided by the Department of Scientific Computing). The funders had no role in study design, execution, analysis, and manuscript preparation.
Footnotes
Author Contribution Statement.
Project leadership: SMP, JLM, PFS, SAM, CH, PS. Sample collection and phenotyping: AK, PKEM, PFS, KC, JLM, CH. Sample processing and data management: KC, DR, MF, JLM. Sequencing and variant calling: MDP, EB, KS, KG, TF, SG. Primary statistical analysis: DR, MF, SMP. Additional analyses: LD, ES, GG, SH, NS, PR, COD, SEB. Determination of synaptic genesets: SH, EF, MOC, NHK, JSC, SGNG. Interpretation of main findings: SMP, EMS, ESL, SH, MF, PFS, SAM, PS. Primary drafting of manuscript: SMP, PFS, SAM, ESL, PS. Production and approval of the final manuscript: all authors.
The authors declare no competing financial interests.
URLs
1000 Genomes Project : www.1000genomes.org/
ExomeChip : http://genome.sph.umich.edu/wiki/Exome_Chip_Design
Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP), Seattle, WA : http://evs.gs.washington.edu/EVS/ [accessed 5/2013]
PLINK/Seq : http://atgu.mgh.harvard.edu/plinkseq/
Summary results : http://research.mssm.edu/statgen/sweden/
References
- Albrechtsen A, Grarup N, Li Y, Sparsø T, Tian G, Cao H, Jiang T, Kim SY, Korneliussen T, Li Q, Nie C, Wu R, Skotte L, Morris AP, Ladenvall C, Cauchi S, Stančáková A, Andersen G, Astrup A, Banasik K, Bennett AJ, Bolund L, Charpentier G, Chen Y, Dekker JM, Doney AS, Dorkhan M, Forsen T, Frayling TM, Groves CJ, Gui Y, Hallmans G, Hattersley AT, He K, Hitman GA, Holmkvist J, Huang S, Jiang H, Jin X, Justesen JM, Kristiansen K, Kuusisto J, Lajer M, Lantieri O, Li W, Liang H, Liao Q, Liu X, Ma T, Ma X, Manijak MP, Marre M, Mokrosiński J, Morris AD, Mu B, Nielsen AA, Nijpels G, Nilsson P, Palmer CN, Rayner NW, Renström F, Ribel-Madsen R, Robertson N, Rolandsson O, Rossing P, Schwartz TW, Slagboom PE, Sterner M, Tang M, Tarnow L, Tuomi T, Van’t Riet E, van Leeuwen N, Varga TV, Vestmar MA, Walker M, Wang B, Wang Y, Wu H, Xi F, Yengo L, Yu C, Zhang X, Zhang J, Zhang Q, Zhang W, Zheng H, Zhou Y, Altshuler D, ‘t Hart LM, Franks PW, Balkau B, Froguel P, McCarthy MI, Laakso M, Groop L, Christensen C, Brandslund I, Lauritzen T, Witte DR, Linneberg A, Jørgensen T, Hansen T, Wang J, Nielsen R, Pedersen O D.E.S.I.R. Study Group, the DIAGRAM Consortium. Exome sequencing-driven discovery of coding polymorphisms associated with common metabolic phenotypes. Diabetologia. 2013;56(2):298–310. doi: 10.1007/s00125-012-2756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayés À, van de Lagemaat LN, Collins MO, Croning MDR, Whittle IR, Choudhary JS, Grant SGN. Characterisation of the proteome, diseases and evolution of the human postsynaptic density. Nature Neuroscience. 2011;14(1):19–21. doi: 10.1038/nn.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryer MH, Hamdan FF, Klitten LL, Møller RS, Carmant L, Schwartzentruber J, Patry L, Dobrzeniecka S, Rochefort D, Neugnot-Cerioli M, Lacaille JC, Niu Z, Eng CM, Yang Y, Palardy S, Belhumeur C, Rouleau GA, Tommerup N, Immken L, Beauchamp MH, Patel GS, Majewski J, Tarnopolsky MA, Scheffzek K, Hjalgrim H, Michaud JL, Di Cristo G. Mutations in SYNGAP1 cause intellectual disability, autism, and a specific form of epilepsy by inducing haploinsufficiency. Hum Mutat. 2013;34(2):385–94. doi: 10.1002/humu.22248. [DOI] [PubMed] [Google Scholar]
- Callan MA, Zarnescu DC. Heads-up: new roles for the fragile × mental retardation protein in neural stem and progenitor cells. Genesis. 2011 Jun;49(6):424–40. doi: 10.1002/dvg.20745. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Hilliard CE, Kim Y, Morgan MB, Lewis LR, Muzny DM, Hawes AC, Sabo A, Wheeler DA, Lieberman JA, Sullivan PF, Gibbs RA. Deep resequencing and association analysis of schizophrenia candidate genes. Molecular Psychiatry. 2013;18:138–140. doi: 10.1038/mp.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146(2):247–61. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, Vulto-van Silfhout AT, Koolen DA, de Vries P, Gilissen C, del Rosario M, Hoischen A, Scheffer H, de Vries BB, Brunner HG, Veltman JA, Vissers LE. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med. 2012;367(20):1921–9. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- de Pristo M, Banks E, Poplin R, Garimella K, Maguire J, Hartl C, Philippakis A, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell T, Kernytsky A, Sivachenko A, Cibulskis K, Gabriel S, Altshuler D, Daly M. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genetics. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Collins MO, Uren RT, Kopanitsa MV, Komiyama NH, Croning MD, Zografos L, Armstrong JD, Choudhary JS, Grant SG. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Molecular systems biology. 2009;5:269. doi: 10.1038/msb.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, Carrera N, Humphreys I, Johnson JS, Roussos P, Barker DB, Banks E, Milanova V, Grant SGN, Hannon E, Rose SA, Chambert K, Mahajan M, Scolnick EM, Moran JL, Kirov G, Palotie A, McCarroll SA, Holmans P, Sklar P, Owen MJ, Purcell SM, O’Donovan MC. De novo mutations in schizophrenia implicate synaptic networks. doi: 10.1038/nature12929. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard SL, Gauthier J, Noreau A, Xiong L, Zhou S, Jouan L, Dionne-Laporte A, Spiegelman D, Henrion E, Diallo O, et al. Increased exonic de novo mutation rate in individuals with schizophrenia. Nat Genetics. 2011;43:860–863. doi: 10.1038/ng.886. [DOI] [PubMed] [Google Scholar]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455(7210):237–41. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinan A, Clark AG. Recent explosive human population growth has resulted in an excess of rare genetic variants. Science. 2012;336:740–743. doi: 10.1126/science.1217283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiezun A, Garimella K, Do R, Stitziel NO, Neale BM, McLaren PJ, Gupta N, Sklar P, Sullivan PF, Moran JL, Hultman CM, Lichtenstein P, Magnusson P, Lehner T, Shugart YY, Price AL, de Bakker PI, Purcell SM, Sunyaev SR. Exome sequencing and the genetic basis of complex traits. Nat Genet. 2012 May 29;44(6):623–30. doi: 10.1038/ng.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, Moran J, Chambert K, Toncheva D, Georgieva L, Grozeva D, Fjodorova M, Wollerton R, Rees E, Nikolov I, van de Lagemaat LN, Bayés A, Fernandez E, Olason PI, Böttcher Y, Komiyama NH, Collins MO, Choudhary J, Stefansson K, Stefansson H, Grant SG, Purcell S, Sklar P, O’Donovan MC, Owen MJ. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17(2):142–53. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama NH, Watabe AM, Carlisle HJ, Porter K, Charlesworth P, Monti J, Strathdee DJ, O’Carroll CM, Martin SJ, Morris RG, O’Dell TJ, Grant SGN. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci. 2002;22(22):9721–9732. doi: 10.1523/JNEUROSCI.22-22-09721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, DeCandia TR, Ripke S, Yang J, Sullivan PF, Goddard ME, Keller MC, Visscher PM, Wray NR. Schizophrenia Psychiatric Genome-Wide Association Study Consortium (PGC-SCZ); International Schizophrenia Consortium (ISC); Molecular Genetics of Schizophrenia Collaboration (MGS) Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nature Genetics. 2012;44(3):247–50. doi: 10.1038/ng.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, Zhang N, Mowry BJ, Olincy A, Amin F, Cloninger CR, Silverman JM, Buccola NG, Byerley WF, Black DW, Kendler KS, Freedman R, Dudbridge F, Pe’er I, Hakonarson H, Bergen SE, Fanous AH, Holmans PA, Gejman PV. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168(3):302–16. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophrenia Bulletin. 2012;38:426–432. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Sabo A, Neale BM, Nagaswamy U, Stevens C, Lim E, Bodea CA, Muzny D, Reid JG, Banks E, Coon H, Depristo M, Dinh H, Fennel T, Flannick J, Gabriel S, Garimella K, Gross S, Hawes A, Lewis L, Makarov V, Maguire J, Newsham I, Poplin R, Ripke S, Shakir K, Samocha KE, Wu Y, Boerwinkle E, Buxbaum JD, Cook EH, Jr, Devlin B, Schellenberg GD, Sutcliffe JS, Daly MJ, Gibbs RA, Roeder K. Analysis of rare, exonic variation amongst subjects with autism spectrum disorders and population controls. PLoS Genet. 2013;9(4):e1003443. doi: 10.1371/journal.pgen.1003443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148(6):1223–41. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396(6710):433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Moreno-De-Luca D, Mulle JG, Kaminsky EB, Sanders SJ, Myers SM, Adam MP, Pakula AT, Eisenhauer NJ, Uhas K, Weik L, Guy L, Care ME, Morel CF, Boni C, Salbert BA, Chandrareddy A, Demmer LA, Chow EW, Surti U, Aradhya S, Pickering DL, Golden DM, Sanger WG, Aston E, Brothman AR, Gliem TJ, Thorland EC, Ackley T, Iyer R, Huang S, Barber JC, Crolla JA, Warren ST, Martin CL, Ledbetter DH SGENE Consortium, Simons Simplex Collection Genetics Consortium, GeneSTAR. Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am J Hum Genet. 2010;87(5):618–30. doi: 10.1016/j.ajhg.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CS, Haupt A, Bildl W, Schindler J, Knaus HG, Meissner M, Rammner B, Striessnig J, Flockerzi V, Fakler B, Schulte U. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc Natl Acad Sci U S A. 2010;107(34):14950–7. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need AC, McEvoy JP, Gennarelli M, Heinzen EL, Ge D, Maia JM, Shianna KV, He M, Cirulli ET, Gumbs CE, Zhao Q, Campbell CR, Hong L, Rosenquist P, Putkonen A, Hallikainen H, Repo-Tiihonen E, Tiihonen J, Levy DL, Meltzer HY, Goldstein DB. Exome Sequencing Followed by Large-Scale Genotyping Suggests a Limited Role for Moderately Rare Risk Factors of Strong Effect in Schizophrenia. American Journal of Human Genetics. 2012;91(2):303–312. doi: 10.1016/j.ajhg.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, Lin CF, Stevens C, Wang LS, Makarov V, Polak P, Yoon S, Maguire J, Crawford EL, Campbell NG, Geller ET, Valladares O, Schafer C, Liu H, Zhao T, Cai G, Lihm J, Dannenfelser R, Jabado O, Peralta Z, Nagaswamy U, Muzny D, Reid JG, Newsham I, Wu Y, Lewis L, Han Y, Voight BF, Lim E, Rossin E, Kirby A, Flannick J, Fromer M, Shakir K, Fennell T, Garimella K, Banks E, Poplin R, Gabriel S, DePristo M, Wimbish JR, Boone BE, Levy SE, Betancur C, Sunyaev S, Boerwinkle E, Buxbaum JD, Cook EH, Jr, Devlin B, Gibbs RA, Roeder K, Schellenberg GD, Sutcliffe JS, Daly MJ. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Komiyama NH, McKechanie A, Johnstone M, Blackwood DH, St Clair D, Emes RD, van de Lagemaat LN, Saksida LM, Bussey TJ, Grant SG. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nature Neuroscience. 2013;16(1):16–24. doi: 10.1038/nn.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nature Genetics. 2011;43(10):977–83. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, Albrecht B, Bartholdi D, Beygo J, Di Donato N, Dufke A, Cremer K, Hempel M, Horn D, Hoyer J, Joset P, Röpke A, Moog U, Riess A, Thiel CT, Tzschach A, Wiesener A, Wohlleber E, Zweier C, Ekici AB, Zink AM, Rump A, Meisinger C, Grallert H, Sticht H, Schenck A, Engels H, Rappold G, Schröck E, Wieacker P, Riess O, Meitinger T, Reis A, Strom TM. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380(9854):1674–82. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kähler AK, Akterin S, Bergen SE, Collins AL, Crowley JJ, Fromer M, Kim Y, Lee SH, Magnusson PK, Sanchez N, Stahl EA, Williams S, Wray NR, Xia K, Bettella F, Borglum AD, Bulik-Sullivan BK, Cormican P, Craddock N, de Leeuw C, Durmishi N, Gill M, Golimbet V, Hamshere ML, Holmans P, Hougaard DM, Kendler KS, Lin K, Morris DW, Mors O, Mortensen PB, Neale BM, O’Neill FA, Owen MJ, Milovancevic MP, Posthuma D, Powell J, Richards AL, Riley BP, Ruderfer D, Rujescu D, Sigurdsson E, Silagadze T, Smit AB, Stefansson H, Steinberg S, Suvisaari J, Tosato S, Verhage M, Walters JT, Levinson DF, Gejman PV, Kendler KS, Laurent C, Mowry BJ, O’Donovan MC, Owen MJ, Pulver AE, Riley BP, Schwab SG, Wildenauer DB, Dudbridge F, Holmans P, Shi J, Albus M, Alexander M, Campion D, Cohen D, Dikeos D, Duan J, Eichhammer P, Godard S, Hansen M, Lerer FB, Liang KY, Maier W, Mallet J, Nertney DA, Nestadt G, Norton N, O’Neill FA, Papadimitriou GN, Ribble R, Sanders AR, Silverman JM, Walsh D, Williams NM, Wormley B, Arranz MJ, Bakker S, Bender S, Bramon E, Collier D, Crespo-Facorro B, Hall J, Iyegbe C, Jablensky A, Kahn RS, Kalaydjieva L, Lawrie S, Lewis CM, Lin K, Linszen DH, Mata I, McIntosh A, Murray RM, Ophoff RA, Powell J, Rujescu D, Van Os J, Walshe M, Weisbrod M, Wiersma D, Donnelly P, Barroso I, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin AP, Deloukas P, Duncanson A, Jankowski J, Markus HS, Mathew CG, Palmer CN, Plomin R, Rautanen A, Sawcer SJ, Trembath RC, Viswanathan AC, Wood NW, Spencer CC, Band G, Bellenguez C, Freeman C, Hellenthal G, Giannoulatou E, Pirinen M, Pearson RD, Strange A, Su Z, Vukcevic D, Donnelly P, Langford C, Hunt SE, Edkins S, Gwilliam R, Blackburn H, Bumpstead SJ, Dronov S, Gillman M, Gray E, Hammond N, Jayakumar A, McCann OT, Liddle J, Potter SC, Ravindrarajah R, Ricketts M, Tashakkori-Ghanbaria A, Waller MJ, Weston P, Widaa S, Whittaker P, Barroso I, Deloukas P, Mathew CG, Blackwell JM, Brown MA, Corvin AP, McCarthy MI, Spencer CC, Bramon E, Corvin AP, O’Donovan MC, Stefansson K, Scolnick E, Purcell S, McCarroll SA, Sklar P, Hultman CM, Sullivan PF Multicenter Genetic Studies of Schizophrenia Consortium, Psychosis Endophenotypes International Consortium, Wellcome Trust Case Control Consortium 2. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nature Genetics. 2013;45(10):1150–9. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan-Sencicek AG, DiLullo NM, Parikshak NN, Stein JL, Walker MF, Ober GT, Teran NA, Song Y, El-Fishawy P, Murtha RC, Choi M, Overton JD, Bjornson RD, Carriero NJ, Meyer KA, Bilguvar K, Mane SM, Sestan N, Lifton RP, Günel M, Roeder K, Geschwind DH, Devlin B, State MW. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nature Genetics. 2011;43(10):969–76. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective Targeting of Newly Synthesized Arc mRNA to Active Synapses Requires NMDA Receptor Activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13(8):537–51. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60(12):1187–92. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Takata A, Iwayama Y, Fukuo Y, Ikeda M, Okochi T, Maekawa M, Toyota T, Yamada K, Hattori E, Ohnishi T, Toyoshima M, Ujike H, Inada T, Kunugi H, Ozaki N, Nanko S, Nakamura K, Mori N, Kanba S, Iwata N, Kato T, Yoshikawa T. A population-specific uncommon variant in GRIN3A associated with schizophrenia. Biol Psychiatry. 2013;73(6):532–9. doi: 10.1016/j.biopsych.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Timms AE, Dorschner MO, Wechsler J, Choi KY, Kirkwood R, Girirajan S, Baker C, Eichler EE, Korvatska O, Roche KW, Horwitz MS, Tsuang DW. Support for the N -Methyl-D-Aspartate Receptor Hypofunction Hypothesis of Schizophrenia From Exome Sequencing in Multiplex Families. JAMA Psychiatry. 2013;3:1–9. doi: 10.1001/jamapsychiatry.2013.1195. [DOI] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, Bundman M, Kuhl D. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J Neurosci. 2001;21(15):5484–93. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. American Journal of Human Genetics. 2011;89(1):82–93. doi: 10.1016/j.ajhg.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y, Levy S, Gogos JA, Karayiorgou M. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nature Genetics. 2012;44:1365–1369. doi: 10.1038/ng.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda R, Sabatini BL, Svoboda K. Plasticity of calcium channels in dendritic spines. Nat Neurosci. 2003 Sep;6(9):948–55. doi: 10.1038/nn1112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 : Genes and variants in highly enriched genesets ∣ Details for specific variants for the genes in the small but highly enriched genesets, that showed large odds ratios (>5): ARC, PSD-95, calcium channel genes, Müller et al. Cav2 genes as well as disruptive KYNU variants. The variants from the four genesets are all singleton disruptive mutations, corresponding to the genes listed in ED Table 3. The (a) and (b) in the phenotype column indicate that the same individual carried two of these variants. (That the same cases carries two of these alleles is not necessarily surprising: assuming across cases equal and independent probabilities for carrying each allele, of the 49 case unique variants listed in this table, there is a 37% chance that at least one individual carries two of the 49 alleles, and a 7% chance that at least two individuals carry multiple alleles, based on 2,536 total cases.) The genotype meta-information fields are from GATK: AD = allele depth (number of reference, alternate reads); DP = total high quality reads used in calling; GQ = genotype quality, (max 99); PL = phredscaled genotype likelihoods (reference, heterozygote, homozygote).
Supplementary Table 2 : Genes selected for the composite set ∣ Source indicated: PSD = any PSD gene; FMRP-target = Darnell et al. FRMP target; SCZ de novo = nonsynonymous de novo mutation in Xu et al., Girard et al. or Fromer et al.; Calcium channel = voltage-gated calcium channel.


