Abstract
Objective
The link between a reduced capacity for skeletal muscle mitochondrial fatty acid oxidation (FAO) and lipotoxicity in human insulin resistance has been the subject of intense debate. The objective of this study was to investigate whether reduced FAO is associated with elevated acyl CoA, ceramide, and diacylglycerol (DAG) in severely obese insulin resistant subjects.
Design and Methods
Muscle biopsies were conducted in lean (L, 22.6 ± 0.5 kg/m2, n = 8), Class I (CI, 32.1 ± 0.4 kg/m2, n = 7) and Class II&III obese (CII&III, 45.6 ± 1.1 kg/m2, n = 15) women for acyl CoA, sphingolipid and DAG profiling. Intramyocellular triglyceride (IMTG) content was determined by histology. FAO was assessed by incubating muscle homogenates with [1–14C]palmitate and measuring 14CO2 production. Cardiolipin content was quantified as an index of mitochondrial content. Lipid metabolism proteins, DGAT1, PLIN5, and PNPLA2 were quantified in biopsy samples by western blot.
Results
CII&III were more insulin resistant (HOMA-IR: 4.5 ± 0.5 vs. 1.1 ± 0.1, P < 0.001), and had lower FAO (~58%, P = 0.007) and cardiolipin content (~31%, P = 0.013) compared to L. IMTG was elevated in CI (P = 0.04) and CII&III (P = 0.04) compared to L. Sphingolipid content was higher in CII&III compared to L (13.6 ± 1.1 vs. 10.3 ± 0.5 pmol/mg, P = 0.031) whereas DAG content was not different among groups. DGAT1 was elevated in CII&III, and PLIN5 was elevated in CI compared to L.
Conclusions
Severe obesity is associated with reduced muscle oxidative capacity and occurs concomitantly with elevated IMTG, ceramide and insulin resistance.
Introduction
Obesity predisposes the development of insulin resistance and type 2 diabetes (T2DM) and is a principal cause of morbidity in the United States. While the exact mechanisms that underlie insulin resistance in obese humans remain elusive, efforts have been made to further understand the role of dysregulated fatty acid metabolism (1,2). Moreover, impairments in skeletal muscle fatty acid oxidation and reduced mitochondrial content/function have been implicated in insulin resistance (3). It has been hypothesized that a reduced capacity to oxidize fatty acids leads to storage of excess intramyocellular triglyceride (IMTG) in lipid droplets and accumulation of long chain acyl CoA, sphingolipids and diacylglycerol (DAG).
Sphingolipids and DAG have been identified in cell culture and animal models as key mediators of insulin resistance through inhibition of AKT and IRS-1/2 signaling (4,5). Studies in human muscle are equivocal, with some showing that muscle ceramide content is elevated in obesity (6), while others report no difference in muscle ceramide content between subjects with a wide range of insulin sensitivity (7), or following acute lipid infusion-induced insulin resistance (8). The extent to which muscle DAG content is elevated in obesity is also unclear. Muscle DAG content was elevated in obese and T2DM subjects (9) and increased following acute lipid infusion-induced insulin resistance (8). In contrast, muscle DAG content was not elevated in obese diabetics (10), or in insulin resistant obese subjects (11), and was reported to be elevated in insulin sensitive athletes (12). Accumulation of long chain acyl CoA has also been reported in obesity (1), although this lipid species has received less attention.
Thus, there is a lack of consensus in the literature regarding the role of sphingolipid and DAG in insulin resistance in human obesity. Previous literature on mitochondrial dysfunction, lipid accumulation, and insulin resistance in obesity have perhaps been inconsistent because there is a range of metabolic health within obesity. Importantly, there is a paucity of data in the severely obese in whom the associations of muscle mitochondrial dysfunction, DAG, ceramide and long chain acyl-CoA accumulation, and insulin resistance would perhaps be expected to be more evident. In addition, given that the prevalence of severe obesity is increasing at a greater rate than moderate obesity, it is clear that the etiology of insulin resistance in human severe obesity requires further study.
The aim of the present study was to address the uncertainty surrounding the role for specific intramyocellular lipid metabolites in human obesity and particularly in severe obesity. We conducted comprehensive mass spectrometry-based determination of content and molecular species of long chain acyl CoA, DAG and sphingolipid in muscle of lean, class I and class II&III obese subjects to test the primary hypotheses that long chain acyl CoA, sphingolipid and DAG content is higher in severe obesity along with a reduced capacity for mitochondrial fatty acid oxidation. We also determined the expression of several lipid droplet associated proteins including PNPLA2 (ATGL), PLIN5 (OXPAT) and DGAT1, as they are proteins involved in lipolysis, lipid oxidation and triglyceride formation, respectively. We hypothesized that alterations in expression of these proteins may play a role in altered muscle lipid profile and reduced oxidative capacity in obesity. These data provide novel insight to the role of intramyocellular lipid species and muscle oxidative capacity in obese and severely obese humans.
Methods
Study population
Subjects for this study were recruited from an ongoing clinical investigation of obesity. Only female subjects were included to provide a gender-controlled analysis as previous studies have described gender differences in muscle lipid metabolism and insulin sensitivity in obesity (9,13). Women aged 30–55 years were recruited though advertisements in the Pittsburgh, PA area. Subjects who were determined to be sedentary (exercise ≤2 day/week), weight stable (±5% over the previous 6 months), with a body mass index (BMI) 20–40+ kg/m2, and were further evaluated at the Clinical Translational Research Center (CTRC) at the University of Pittsburgh. Degree of obesity was defined by the following BMI ranges: Lean (BMI < 24.9 kg/m2), Class I obese (30–34.9 kg/m2), Class II&III obese (>35 kg/m2). Exclusionary criteria included uncontrolled hypertension (>150/95 mmHg), anemia (Hct < 34%), elevated liver enzymes (25% above normal), proteinuria, or hypothyroidism (TSH > 8 mIU L−1), poor kidney function (serum creatinine > 1.5). Other exclusion criteria were: Type 1 and 2 Diabetes Mellitus, chronic diseases (cancer, cardiovascular disease), a history of gastric bypass or other bariatric surgery, pregnancy/lactating during the previous 6 months, smoking or taking medications known to affect glucose homeostasis. Fasting blood samples were drawn for the determination of glucose and insulin. All subjects gave written consent to the protocol, which was approved by the University of Pittsburgh’s Institutional Review Board.
Body composition and peak aerobic capacity
Body weight was determined using a calibrated digital scale (BWB-800, Tanita Corporation, Tokyo, Japan). Height was measured with a wall-mounted stadiometer. Total body fat (FM) and fat free mass (FFM) were assessed by whole body dual-energy X-ray absorptiometry (Lunar Prodigy and Encore 2005 software v9.3, General Electric Healthcare, Milwaukee, MI). For subjects whose limbs fell outside the scanning area, a “mirrored image” analysis was used. Subjects performed a VO2 peak test on an electronically braked cycle ergometer to determine aerobic fitness (Ergoline 800S, Sensor Medics, Yorba Linda, CA) (14). Oxygen consumption and carbon dioxide production was determined by indirect calorimetry (Moxus, AEI Technologies, Pittsburgh, PA).
Muscle biopsy procedure
Percutaneous muscle biopsy samples were obtained following an overnight fast as described previously (15,16). Briefly, muscle biopsies were obtained under local anesthesia (2% buffered lidocaine) from the vastus lateralis, 15 cm above the patella using a 5 mm muscle biopsy Bergstrom cannula (Stille Surgical instruments, Eskilstuna, Sweden). Immediately after the biopsy procedure the specimen was blotted dry and trimmed of visible adipose tissue with a standard dissecting microscope (Leica EZ4, Leica Microsystems, Switzerland). Three portions of the specimen (~30 mg each) were snap frozen in liquid nitrogen and stored at −80 °C for sphingolipid, diacylglycerol, long chain acyl CoA, and western blot analysis. A fourth portion (~60 mg) was placed in ice-cold sucrose-EDTA medium for the palmitate oxidation assay. For immuno-histochemistry, a fifth portion was mounted on a small piece of cork with mounting medium (Shandon Cryochrome; Thermo Electron, Pittsburgh, PA), frozen in isopentane cooled with liquid nitrogen for 2–3 min (−160°C), and then placed into liquid nitrogen. All samples were stored at −80°C until analysis.
Analysis of sphingolipid and diacylglycerol (DAG) species
Intramuscular sphingolipids and DAGs were quantified by high pressure liquid chromatography (HPLC)-tandem mass spectrometry as described previously (17) (Lipidomics Core, Medical University of South Carolina). Briefly, liquid nitrogen-frozen samples (~30 mg) were homogenized in ice-cold buffer (250 mM sucrose, 25 mM KCl, 50 mM Tris, and 0.5 mM EDTA, pH 7.4) and were fortified with internal standards and extracted into a one-phase neutral organic solvent system (ethyl acetate/isopropyl alcohol/water; 60:30:10 v/v/v), evaporated and reconstituted in methanol and quantified by a surveyor/TSQ 7000 liquid chromatography/mass spectrometry system (Thermo Finigan, Thermo Fisher Scientific, Waltham, MA). Quantitative analysis was performed in a positive multiple-reaction monitoring mode, based on calibration curves generated by adding to an artificial matrix known amounts of target analytes, synthetic standards, and an equal amount of internal standard. Intramuscular DAG and ceramide content was normalized to tissue wet weight (pmol/mg tissue).
Analysis of long chain acyl CoA species
Quantification of acyl CoA species was carried out in a subset of subjects from each group, based on availability of biopsy specimens (CII&III; n = 6, CI; n = 6; L; n = 8). Analysis of long chain fatty acyl CoA esters was conducted by HPLC-mass spectrometry methods essentially as described by Sun et al., (18) with minor modifications. Briefly, a spectrum Lab pulverizer (Rancho Dominguez, CA) cooled in liquid nitrogen was employed to prepare frozen tissue powder. The pulverized material prepared from ~30 mg sample of frozen vastus lateralis biopsy was suspended in extraction medium (100 mM β-glycerophosphate/histidine buffer (pH 7.3 at 21°C), methanol, 2-popanol, and acetonitrile (1:1:1:1)), containing 25 ng of heptadecanoyl-CoA (C17-CoA) as an internal standard. The tissue suspension was additionally dispersed by Polytron homogenizer equipped with 7 mm shaft. The suspension was centrifuged at 3000 RPM (1,800 g) for 20 min at 4°C and dried by vacuum centrifugation. The residue was reconstituted in ddH2O, extracted by chloroform and the ddH2O extract was desalted by mixing with 2-propanol:ddH2O (1:4 v/v). Sample analysis was performed by a HPLC (C18 column) system equipped with single-quadrupole mass spectrometer LC-2010 (Shimadzu, USA) (18).
Palmitate oxidation assay
The palmitate oxidation assay was conducted on a subset of samples, based on availability of biopsy specimens (CII&III; n = 10, CI; n = 5; NW; n = 7). The assay was carried out using muscle homogenates as previously described (19). Following percutaneous biopsy of the vastus lateralis, ~60 mg of the specimen was placed into ice-cold sucrose-EDTA medium (SETH buffer; 250 nM sucrose, 1 mM EDTA, 10 mM Tris-HCL, and 2mM ATP, pH 7.4). The sample was then blotted dry, weighed, and placed into 200 μL of SETH buffer, minced thoroughly with a scissors (~200 snips) and diluted 20-fold with additional SETH buffer. The minced tissue was then homogenized (12 passes) on ice using a teflon coated pestle and glass homogenizer (Kontes Duall, Kimble Chase, Vineland, NJ). Forty microliters of homogenate was then added to the incubation wells of a modified 48-well plate with a channel cut between the adjacent trap wells (Nunc, Thermo Fisher Scientific, Rochester, NY). The trap wells contained 200 μL of 1 N sodium hydroxide for the collection of liberated 14-CO2. To the homogenate, 160 μL of incubation buffer (0.2 mM palmitate ([1–14-C] palmitate at 0.5 Ci mL−1), 100 mM sucrose, 10 mM Tris HCL, 5 mM potassium phosphate, 80 mM potassium chloride, 1 mM magnesium chloride, 0.1 mM malate, 2 mM ATP, 1 mM dithiothreitol, 0.2 mM EDTA, 1 mM L-Carnitine, 0.05 mM coenzyme A, and 0.5% fatty acid free bovine serum albumin, pH 7.4) was added to initiate the reaction. The plate was quickly sealed and incubated in a shaking water bath at 37°C for 30 min. Following the incubation, 100 μL of 70% perchloric acid was added to terminate the reaction. One hundred and fifty microliters of the sodium hydroxide from the trap wells was counted for label incorporation into trapped 14-CO2 as an index of complete palmitate oxidation. This was determined by scintillation counting using 4 mL of EcoScint (National Diagnostics, Atlanta, GA). The reaction mix was then centrifuged (14,000 g, 20 min, 4°C) and 50 μL of supernatant was counted (acid soluble metabolites, ASM) as an index of incompletely oxidized products.
Cardiolipin assay
Cardiolipin was measured in muscle homogenates from the palmitate oxidation assay using a previously described method (20). The assay is based on the derivatization of cardiolipin with 1-pyrenyldiazomethane (PDAM) to form stable fluorescent 1-pyrenylmethyl esters. Cardiolipin species are then identified and quantified by reverse phase HPLC.
Western blot procedure
A portion of frozen muscle was homogenized (T8 Ultra Turrax; IKA, Wilmington, NC) in ice-cold cell lysis buffer (Cell signaling Technology, Danvers, MA) including a protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). The homogenates were incubated with gentle rocking (1 h, 4°C) and then centrifuged (10 min, 12,000g, 4°C) and then the supernatant was removed. Protein content of the supernatant was determined using the bicinchoninic acid assay (BCA) (Thermo Scientific, Rockford, IL). Aliquots of supernatant were mixed with 5× Laemmli buffer (10% SDS, 10% glycerol, 10 mM beta-mercaptoethanol, 0.05% bromophenol blue, 0.2 M Tris-HCl pH 6.8) and denatured by heating (5 min, 100°C). Samples were separated on a 12% SDS-PAGE gel followed by transfer onto polyvinylidine difluoride membranes (Bio-Rad Laboratories, Hercules, CA). Membranes were stained in 5% Ponceau S (Sigma Aldrich) and imaged (ChemiDoc XRS+; Bio-Rad, Hercules, CA). Membranes were then destained in PBS-Tween20, blocked in 5% nonfat milk, and incubated with the following primary antibodies: Anti-PLIN5 (OXPAT/Perilipin 5, American Research Products, Belmont, MA), anti-PNPLA2 (ATGL, Cell Signaling technologies, Danvers, MA), anti-DGAT1 (diacylglycerol O-acyltransferase 1, Abcam, Cambridge, MA), anti-OXPHOS antibody cocktail (Mitosciences, Eugene, OR), and anti-α tubulin (Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were then incubated in appropriate species-specific HRP-conjugated secondary antibodies (Cell signaling technologies, Danvers, MA). Protein bands were visualized using a chemiluminescence detection kit (Bio-Rad Laboratories, Hercules, CA) and gel documentation system (ChemiDoc XRS+; Bio-Rad, Hercules, CA). Protein bands of interest were quantified by image analysis using the software ImageJ 1.34K (NIH Image for the Macintosh, National institutes of Health, Bethesda, MD). Protein loading was controlled by normalizing bands of interest to α-tubulin and to Ponceau S staining for OXPHOS. The OXPHOS expression data for each of the obese groups was then normalized to the lean group (Figure 1D).
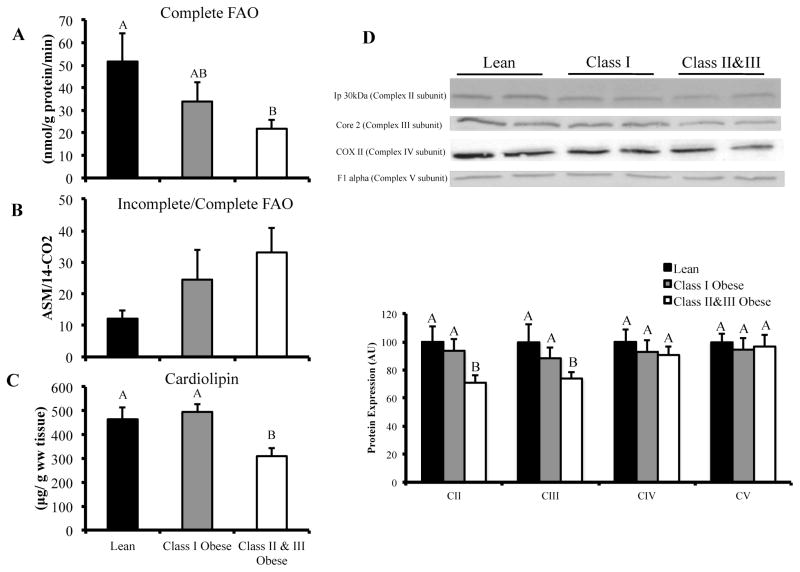
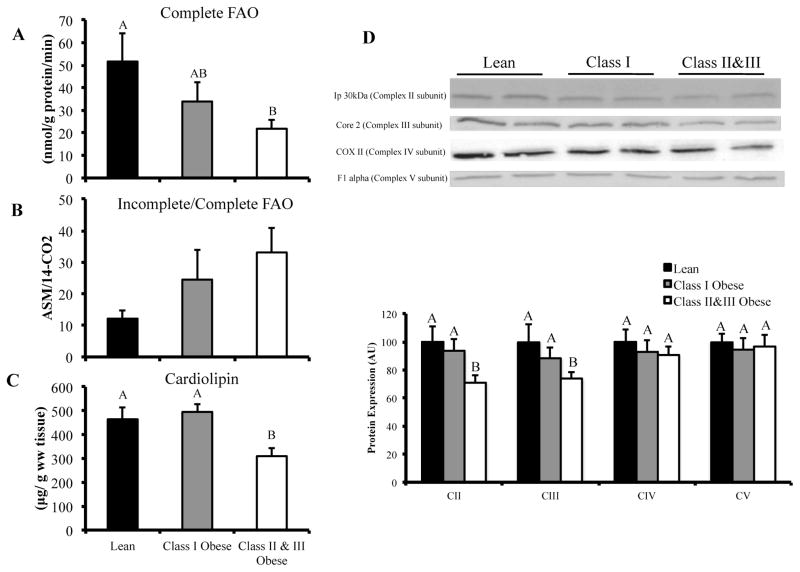
FIGURE 1.
Vastus lateralis palmitate oxidation and mitochondrial content in lean, class I and class II&III obese subjects. Panel A: Complete palmitate oxidation (14CO2 production) in vastus lateralis homogenate. Panel B: Ratio of incomplete-to-complete palmitate oxidation (ASM/14CO2) in vastus lateralis homogenate. Panel C: Cardiolipin content in homogenate of vastus lateralis. (CII&III, n = 10; CI, n = 5; L, n = 7). Panel D: Western blot data for electron transport chain subunit protein content in vastus lateralis. (CII&III; n = 15, CI; n = 7; L; n = 8). The letters A, B and C denote significant differences between groups (P < 0.05, ANOVA).
Histological analysis
Determination of IMTG content was performed using a modified version of methods previously used in our laboratory (16). Briefly, serial transverse sections (10 μm) of mounted biopsy samples were generated using a cryostat (Cryotome E; Thermo Shandon, Pittsburgh, PA) at −20°C and placed on a cleaned glass slide (Fisherfinest, Fischer Scientific, Pittsburgh, PA). Sections were then stained in a filtered solution of Oil Red O (300 mg/ml in 36% triethylphosphate) for 30 min at room temperature. Thereafter, sections were incubated with primary antibodies for anti-human myosin heavy chain (MYH)7 (type I myocytes) and MYH2 (type IIa myocytes) overnight at room temperature and subsequently incubated with fluorescein (FITC) (type IIa myocytes) and Rhodamine (type I myocytes) conjugated secondary antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA). Type IIx fibers remained unstained. Images were visualized using a Leica microscope (Leica DM 4000B; Leica Microsystems, Bannockburn, IL), digitally captured (Retiga 2000R camera; Q Imaging, Surrey, Canada), and semi-quantitative image analysis was analyzed using specialized software (Northern Eclipse, v6.0; Empix Imaging, Cheektowaga, NY). Oil red O staining intensity, cross sectional area and proportion were determined for type I, type IIa, and type 2× myocytes. Analysis is based on ~200 fibers per section (range 130–350).
Statistical analysis
Group comparisons were made with a 1×3 ANOVA with a protected Fishers least significant difference post hoc test. Prior to ANOVA, data was tested for assumptions of normality (Shapiro-Wilk test and Q-Q plot) and equality of variance (Levene test). Non-normal data were transformed. Correlations between variables were determined by Person’s correlation coefficient. All data are means ± SEM unless otherwise stated. Statistical significance was set at P < 0.05.
Results
Subject characteristics, aerobic capacity, and body composition
Thirty women were recruited to lean (n = 8), class I obese (n = 7), and class II&III obese (n = 15) groups (Table 1). There was a greater proportion of African American subjects in the CII&III group (9AA, 6C). However, there were no statistically significant differences between Caucasian and African American CII&III obese subjects for any of the primary variables. The average age was slightly greater for CI and CII&III groups compared to the lean group but was not anticipated to be a confounding influence based on previously reported IMTG differences between young and old (21). As per study design, BMI, weight, fat mass and fat free mass were greater in the obese groups compared to the lean group. CII&III had elevated fasting insulin, HOMA-IR, and tended to have elevated glucose (P = 0.073) compared to L, indicative of insulin resistance in that group. There was a trend toward elevated insulin (P = 0.060), and HOMA-IR (P = 0.087) in CI compared to L. Aerobic fitness (VO2 peak) was lower in the CII&III group (P < 0.001) compared to L.
TABLE 1.
Subject characteristics, aerobic capacity and body composition.
| Lean | Class I Obese | Class II&III Obese | |
|---|---|---|---|
| N | 8 | 7 | 15 |
| Race (AA/C) | 1/7 | 1/6 | 9/6 |
| Age (yrs) | 41.6 ± 3.1A | 49.3 ± 1.5B | 48.3 ± 1.2B |
| Weight (Kg) | 61.5 ± 1.7A | 86.2 ± 2.8B | 118.2 ± 2.9C |
| BMI (Kg/m2) | 22.6 ± 0.5A | 32.1 ± 0.4B | 45.6 ± 1.1C |
| Plasma glucose (mg/dl) | 87.5 ± 1.8 | 92.3 ± 2.8 | 96.5 ± 3.6 |
| Plasma Insulin (mU/L) | 5.3 ± 0.5A | 11.5 ± 2.6A | 18.5 ± 1.8B |
| HOMA-IR | 1.2 ± 0.1A | 2.6 ± 0.6A | 4.5 ± 0.5B |
| Fat Mass | 19.4 ± 0.8A | 36.7 ± 1.1B | 61.4 ± 2.4C |
| Fat Free Mass | 38.7 ± 1.7A | 45.3 ± 1.8B | 52.5 ± 1.4C |
| VO2 Peak (ml/min) | 1936 ± 151AB | 2274 ± 70A | 1897 ± 78B |
| VO2 Peak (ml/KgBW/min) | 31.3 ± 1.8A | 26.5 ± 1.2A | 16.1 ± 0.6B |
Data are mean ± standard error. BW; body weight.
denote significant differences between groups (P < 0.05, ANOVA).
Muscle fiber distribution and cross sectional area
There was a higher percentage of type IIx myocytes in CII&III compared to L (Table 2, P = 0.035), with no difference in type I and type IIa myocyte proportion between groups. CII&III had greater type I (P = 0.018), type IIa (P = 0.023), and type IIx (P = 0.009) myocyte cross sectional area compared to L.
TABLE 2.
Muscle Fiber Distribution and Cross Sectional Area.
| Lean | Class I Obese | Class II&III Obese | |
|---|---|---|---|
| Type I myocyte content (%) | 46.6 ± 4.4 | 49.5 ± 2.4 | 44 ± 3.9 |
| Type IIA myocyte content (%) | 47.9 ± 2.9 | 41.9 ± 2.0 | 43.4 ± 2.9 |
| Type IIX myocyte content (%) | 5.5 ± 1.8A | 8.5 ± 2.9AB | 15.3 ± 3.2B |
| Type I myocyte area (μm2) | 3717 ± 145A | 4400 ± 302AB | 4897.5 ± 352B |
| Type IIA myocyte area (μm2) | 3339 ± 303A | 3399 ± 253A | 4587 ± 377B |
| Type IIX myocyte area (μm2) | 2421 ± 557A | 2473 ± 303AB | 3307 ± 330B |
Data are mean ± standard error. (CII&III; n=15, CI; n=7; L; n=8). BW; body weight.
denote significant differences between groups (P < 0.05, ANOVA).
Muscle fatty acid oxidation and content of mitochondria
Complete palmitate oxidation (14CO2 production) was significantly lower in CII&III (~58%, P = 0.007) compared to L (Figure 1A). While there was no difference in complete palmitate oxidation between CI and the other groups, this may have been due to the low number in this group (n = 5) and the inherent variability of the fatty acid oxidation assay. The ASM/14CO2 ratio (Incomplete/Complete palmitate oxidation) tended to be higher in CII&III compared to L, but the difference was not statistically significant (P = 0.116) (Figure 1B). Cardiolipin, an index of mitochondrial content, was lower in CII&III compared to L (P = 0.013, Figure 1C). When complete and incomplete oxidation were normalized to cardiolipin content (data not shown), there was no difference between groups, indicating that lower FAO in CII&III was due to lower mitochondrial content. Electron transport chain (ETC) subunits were also quantified (western blot) as an index of mitochondria content. We found lower levels of Ip 30 KDa (Complex II subunit) (P = 0.021) and core 2 (Complex III subunit) (P = 0.035) in CII&III compared to L (Figure 1D). There was no difference in expression of COX II (Complex IV subunit) or F1α (Complex V subunit) between groups. When data from all three groups were combined (CII&III, n = 10; CI, n = 5; L, n = 7), positive correlations were observed between cardiolipin content and Ip 30 KDa (R = 0.566, P = 0.022) and core 2 (R = 0.688, P = 0.003) protein content.
Intramyocellular triglyceride (IMTG) and sphingolipid content
IMTG and sphingolipid content is presented in Figure 2. Both CI (P = 0.044) and CII&III (P = 0.046) groups had higher total IMTG content compared to L. There was no difference in fiber type specific IMTG content between groups (data not shown). Dihydrosphingosine (P = 0.050) and sphingosine (P = 0.001) were elevated in CII&III compared to L. We also observed higher levels of specific ceramide species containing the following fatty acids, in CII&III: C20:1 (P = 0.021), C22:1 (P = 0.001), C24 (P = 0.015), and C24:1 (P = 0.001). Additionally, ceramide C14 was elevated in the CI group compared to L (P = 0.048). Higher levels of total muscle sphingolipid (including ceramide species) were observed in CII&III compared to L (P = 0.031). Total unsaturated (P < 0.001), but not total saturated (P = 0.115), ceramide species were elevated in CII&III compared to L.
FIGURE 2.
Muscle content of diacylglycerol and long chain acyl CoA species
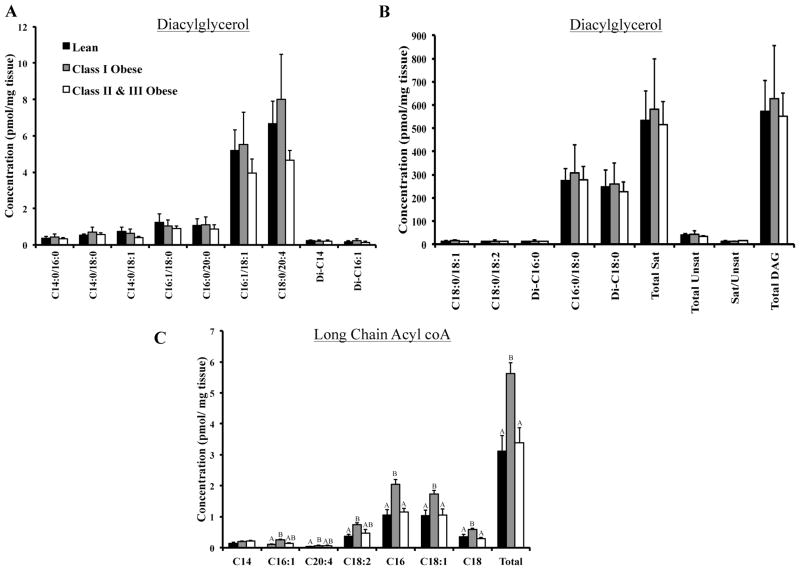
No significant differences in total or individual diacylglycerol species were observed between groups (Figure 3). Total long chain acyl CoA content was elevated in CI, but not in CII&III, compared to L (P = 0.011). This pattern was consistent for C16:1 (P = 0.047), C20:4 (P = 0.027), C18:2 (P = 0.04), C16 (P = 0.009), C18:1 (P = 0.017), and C18 (P = 0.009). When data from all three groups were combined (CII&III, n = 6; CI, n = 6; L, n = 8), total long chain acyl CoA content was correlated with total sphingolipid content (R = 0.601, P = 0.005), the percentage of type I myocytes in muscle (R = 0.464, P = 0.039) and cardiolipin content (R = 0.754, P = 0.002).
FIGURE 3.
Diacylglycerol and long chain acyl CoA content in vastus lateralis of lean, class I and class II&III obese subjects. Panel A: Diacylglycerol species present at low levels in vastus lateralis. Panel B: Diacylglycerol species present at high concentrations in vastus lateralis. Total saturated, total unsaturated and total diacylglycerol content are also included. (CII&III; n = 15, CI; n = 7; L; n = 8). Panel C: Long chain acyl CoA content. (CII&III; n = 6, CI; n = 6; L; n = 8). The letters A, B, and C denote significant differences between groups (P < 0.05, ANOVA).
Correlations of intramyocellular lipid with HOMA-IR, FAO and cardiolipin content
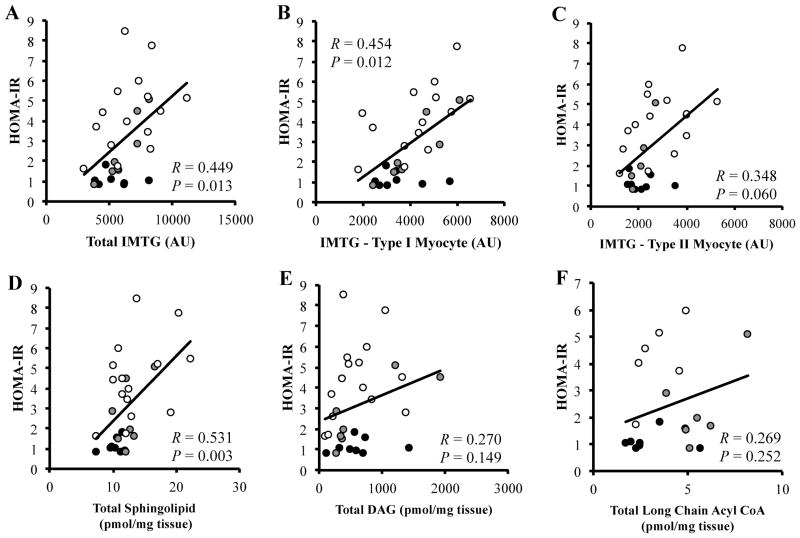
When data from all groups were combined (CII&III, n = 15; CI, n = 7; L, n = 8), IMTG content in type I myocyte (R = 0.454, P = 0.012) and total IMTG content (R = 0.449, P = 0.013) was positively correlated with HOMA-IR (Figure 4A and 4B). The correlation between HOMA-IR and IMTG content in type II myocytes was not as strong (R = 0.348, P = 0.06, Figure 4C). Total sphingolipid content was correlated with HOMA-IR (R = 0.531, P = 0.003, Figure 4D), whereas total diacylglycerol (R = 0.270, P = 0.149, Figure 4E) and in a subgroup (CII&III, n = 6; CI, n = 6; L, n = 8), long chain acyl CoA content were not correlated with HOMA-IR (R = 0.269, P = 0.252, Figure 4F). Complete and incomplete palmitate oxidation was not correlated with content of any lipid species.
FIGURE 4.
Pearson correlations of HOMA-IR with IMTG, sphingolipid, diacylglycerol, and long chain acyl CoA content in lean (black circles), class I obese (gray circles) and class II&III obese (white circles) subjects. Pearson correlation of HOMA-IR with; Total IMTG content (Panel A), IMTG content in type I fibers (Panel B), IMTG content in type II fibers (Panel C), Total sphingolipid content (Panel D), Total diacylglycerol content (CII&III; n = 15, CI; n = 7; L; n = 8) (Panel E), and Total long chain acyl CoA content (CII&III; n = 6 CI; n = 6; L; n = 8) (Panel F).
Lipid droplet protein expression
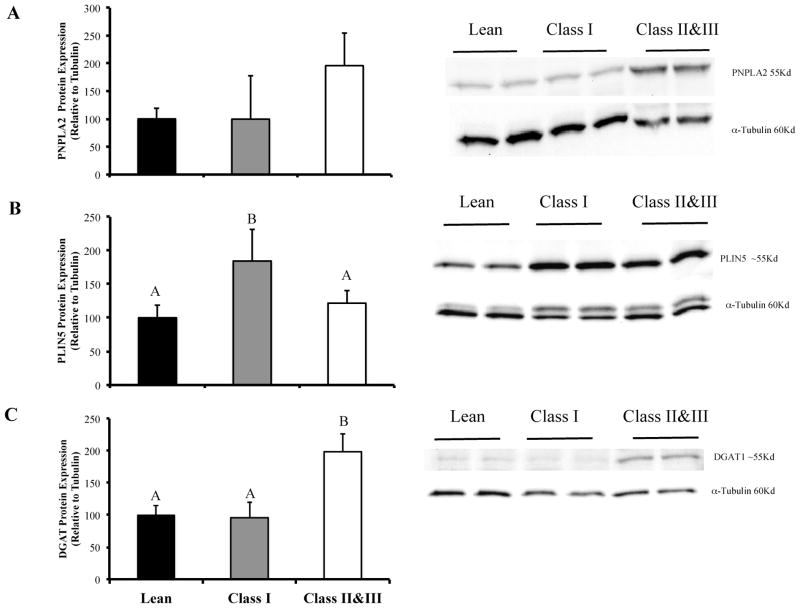
PNPLA2 expression was not different between groups. PLIN5 expression was elevated in CI compared to the L group (P = 0.045). DGAT1 expression was elevated in CII&III compared to L (P = 0.005) and CI (P = 0.007) groups (Figure 5).
FIGURE 5.
Lipid droplet protein content in vastus lateralis of lean, class I, and class II&III obese subjects. Panel A: PNPLA2 expression. Panel B: PLIN5 expression. Panel C: DGAT1 expression. (CII&III; n = 15, CI; n = 7; L; n = 8). The letters A, B and C denote significant differences between groups (P < 0.05, ANOVA).
Discussion
Intramyocellular lipid accumulation, impaired fatty acid oxidation and decreased mitochondrial content have been separately identified as factors in the etiology of insulin resistance in obesity. Until now, these potentially important components of insulin resistance have not been examined together in severely obese humans in whom stronger associations would expected to be found. A primary finding Q-Q plot) and equality of variance (Levene test). Non-normal data were transformed. Correlations between variables were determined by Person’s correlation coefficient. All data are means ± SEM unless otherwise stated. Statistical significance was set at P < 0.05.
Results
Subject characteristics, aerobic capacity, and body composition
Thirty women were recruited to lean (n = 8), class I obese (n = 7), and class II&III obese (n = 15) groups (Table 1). There was a greater proportion of African American subjects in the CII&III group (9AA, 6C). However, there were no statistically significant differences between Caucasian and African American CII&III obese subjects for any of the primary variables. The average age was slightly greater for CI and CII&III groups compared to the lean group but was not anticipated to be a confounding influence based on previously reported IMTG differences between young and old (21). As per study design, BMI, weight, fat mass and fat free mass were greater in the obese groups compared to the lean group. CII&III had elevated fasting insulin, HOMA-IR, and tended to have elevated glucose (P = 0.073) compared to L, indicative of insulin resistance in that group. There was a trend toward elevated insulin (P = 0.060), and HOMA-IR (P = 0.087) in CI compared to L. Aerobic fitness (VO2 peak) was lower in the CII&III group (P < 0.001) compared to L.
Muscle fiber distribution and cross sectional area
There was a higher percentage of type IIx myocytes in CII&III compared to L (Table 2, P = 0.035), with no difference in type I and type IIa myocyte proportion between groups. CII&III had greater type I (P = 0.018), type IIa (P = 0.023), and type IIx (P = 0.009) myocyte cross sectional area compared to L.
Muscle fatty acid oxidation and content of mitochondria
Complete palmitate oxidation (14CO2 production) was significantly lower in CII&III (~58%, P = 0.007) compared to L (Figure 1A). While there was no difference in complete palmitate oxidation between CI and the other groups, this may have been due to the low number in this group (n = 5) and the inherent variability of the fatty acid oxidation assay. The ASM/14CO2 ratio (Incomplete/Complete palmitate This is the first study to compare a comprehensive profile of skeletal muscle sphingolipid, DAG and long chain acyl CoA species in lean, obese and severely obese humans. The sphingolipid ceramide is a putative intermediate linking both excess nutrients and inflammatory cytokines (e.g., tumor necrosis factor-α, TNFα) to the induction of insulin resistance. Muscle sphingolipids have been touted as key mediators of insulin resistance through inhibition of AKT signaling and mediation of inflammation (TNFα) (4). However, a firm consensus has not been reached as to which sphingolipid species are associated with severe obesity, again possibility due to a range of metabolic health within moderate obesity. Previous reports indicate an association between muscle ceramide content and obesity (6,23). However, not all studies have observed an association between muscle ceramide content and obesity (7). Furthermore, many previous studies only measure total ceramide (23) or a small number of species (6). In this study, we have furthered the knowledge base by using state-of-the-art lipidomics to quantify individual molecular species of sphingolipid (16 total, including ceramide) in muscle of subjects with a wide range of BMI, and provide strong evidence that ceramide is elevated in severe obesity and for the first time, we show that this occurs concomitantly with reduced mitochondrial content and oxidative capacity.
Animal models of insulin resistance, including lipid infusion, muscle denervation, and the Zucker diabetic rat model, support a role for DAG in mediating muscle insulin resistance (5,24). Despite this body of research, studies of human muscle have not been conclusive. We did not observe a significant difference in DAG content among lean, obese and severely obese groups, a result in line with our previous findings (11,12). However, we observed relatively higher inter-individual variation with DAG quantification, which combined with the relatively low n per group, makes interpretation of this negative result a little more tenuous. Interestingly, Jocken et al. paradoxically reported lower levels of DAG in obese subjects compared to lean (25), and recent studies using rodent models suggest that muscle insulin resistance can occur independently of DAG accumulation (26). While the consensus in human studies thus far suggests that whole muscle levels of DAG are not associated with insulin resistance, this does not preclude the possibility that organelle/lipid membrane specific accumulation of DAG species plays an important role in mediating insulin resistance in human obesity. Plasma membrane accumulation of the 1-,2- DAG stereoisoform alone leads to activation of PKCθ which, in turn decreases insulin-stimulated IRS-1 tyrosine phosphorylation and downstream insulin signaling (27). Bergman et al. recently demonstrated that membrane accumulation of saturated DAG was associated with insulin resistance in humans (28). Further studies are needed to examine the relationship between subcellular-specific DAG depots, in a stereoiso-form specific manner, in human muscle insulin resistance.
Long chain acyl CoA accumulation is associated with muscle insulin resistance; however this class of lipid has received considerably less attention than DAG and ceramide. We observed elevated acyl CoA species in class I obesity compared to lean, but perhaps paradoxically, a lower acyl CoA content in Class II&III obesity. Hulver et al., also reported elevated muscle acyl CoA content in overweight/obese and severely obese women compared to lean controls (1). Interestingly in this study there was a tendency, although it was not statistically significant, for long chain CoA content to be lower in the severely obese group (1). Ellis et al., demonstrated a negative relationship between muscle long chain acyl CoA content and insulin sensitivity in a group of men with a wide range of BMI (24–38 kg/m2) (29). Moreover, Houmard et al., showed that weight loss can reduce muscle long chain CoA content in obesity, concomitant with improvements in insulin sensitivity (30). Hence, our observation of lower acyl CoA content in severe obesity compared to less severe obesity may seem counterintuitive. However, our observation that acyl CoA content is associated with higher type I fiber percentage and cardiolipin content suggests that acyl CoA species track with muscle mitochondrial content/oxidative capacity. Our data are in accord with studies in animal models demonstrating that long chain acyl CoA content is higher in oxidative and mitochondrial rich red gastrocnemius and soleus muscle groups, compared to low oxidative white gastrocnemius (29). Others have reported that the majority of acyl CoA content (~95%) reside within the mitochondria (31,32). When considered together, these reports support our novel finding that muscle long chain acyl CoA content is related to mitochondrial content, and that both are lower in severe obesity. We acknowledge the descriptive nature of these observations and that our interpretation maybe be hypothetical and suggest that further confirmatory studies are needed.
In addition to the comprehensive lipidomic profiles (37 individual lipid species) in human muscle in severe obesity, we examined the expression of proteins involved in muscle lipid droplet metabolism. We observed that PLIN5 expression was elevated in class I obesity and attenuated in severe obesity, compared to lean. PLIN5 is highly expressed in oxidative tissues, on the surface of lipid droplets and in mitochondria, and is involved with TAG accumulation and fatty acid oxidation by mitochondria (33). PLIN5 RNA/protein expression is induced in skeletal muscle by prolonged fasting (34) and high fat diet (35), interventions that also increase long chain acyl CoA content in muscle (29). Our acyl CoA and PLIN5 data is congruent with recent reports that high fat feeding and obesity are characterized by elevated lipid import to mitochondria and subsequent elevation of incomplete lipid oxidation (36,37). However, we found that the class II&III obese phenotype is one with reduced muscle mitochondria content (38), PLIN5 expression, and long chain acyl CoA species, and therefore capacity to oxidize lipid. These data tentatively suggest that the nature of mitochondrial dysfunction may change over the spectrum of obesity, from lean to severely obese.
We previously observed a positive correlation between PLIN5 and insulin sensitivity (r = 0.52, P < 0.01) (12), however there was no correlation between PLIN5 and HOMA-IR in this study. In the Amati et al. paper, insulin sensitivity was determined using glucose clamp with stable isotope tracer dilution, providing a more targeted assessment of skeletal muscle insulin sensitivity. Insulin sensitivity as determined by HOMA–IR might also encompass hepatic insulin sensitivity as well as peripheral tissue insulin sensitivity, thus confounding the relationship between PLIN5 and insulin sensitivity in this study.
We also report the observation of increased DGAT1 protein expression in class II&III obesity, but not in CI obesity, which is in line with our previous report (12). Rodent studies of muscle specific DGAT1 over expression suggest a role in augmenting triglyceride synthesis and, paradoxically, fatty acid oxidation (39). PNPLA2 (ATGL) expression was not altered with obesity in this study, a finding that is congruent with the findings of others (12,40). Our observations, while descriptive in nature, suggest an adaptive response in class II&III obesity and reduced mitochondrial oxidative capacity.
Finally, there were no correlations between palmitate oxidation and any of the quantified lipid species, suggesting a more complex interaction between oxidative capacity and lipid partitioning in obesity. It should be noted that the palmitate oxidation assay does not account for lipid availability, uptake, esterification, all of which may impact the relationship between lipid oxidation and partitioning. There were, however, positive correlations between insulin resistance and both ceramide content and IMTG in type I fibers, underlining the contribution of intramyocellular lipid in whole body insulin resistance.
In summary, many individual molecular species of ceramide, but not diacylglycerol, are significantly elevated within skeletal muscle of severely obese women, concomitant with lower mitochondrial content and capacity to oxidize palmitate. The phenotype of insulin resistance, lower mitochondrial fatty acid oxidation and lipid accumulation in severe obesity is also more clearly distinguished from less severe obesity. This suggests that perhaps there are unique derangements in severe obesity or that studying these subjects simply permitted us to detect a broader magnitude of important obesity-related parameters, including muscle fiber type and protein content related to lipid partitioning. However, given that this study was conducted in women only caution should be taken when generalizing this data to both men and women. Taken together, this data suggest that distinctions should be made with regard to the metabolic deregulation of lipid metabolism across the spectrum of obesity. Future studies should examine whether the results herein are applicable to both men and women, and whether or not interventions such as exercise or weight loss affect fatty acid oxidation, long chain acyl CoA, DAG and ceramide levels and expression of proteins involved in lipid metabolism in the same way across the spectrum of obesity, to better understand the role that dysregulated fatty acid metabolism has in the etiology of insulin resistance of obesity.
Acknowledgments
The authors gratefully appreciate the efforts of our study participants and acknowledge the excellent technical assistance of Krista Clark, Angela Laslavic, Jennifer Gabany, Kristin Valchar, Steve Anthony, and the nursing staff at the University of Pittsburgh CTRC.
Funding agencies: This study was supported by the University of Pittsburgh CTRC (M01RR00056), the Obesity and Nutrition Research Center (1P30DK46204), and a grant from the Commonwealth of Pennsylvania Department of Health.
Footnotes
Disclosure: No potential conflicts of interest relevant to this article were reported. A subset of this data was presented at the Keystone Symposia on Type 2 Diabetes, Insulin Resistance and Metabolic Dysfunction (J1), Keystone Resort, Keystone, Colorado, 2011 (Poster # 134).
References
- 1.Hulver MW, Berggren JR, Cortright RN, et al. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab. 2003;284:E741–E747. doi: 10.1152/ajpendo.00514.2002. [DOI] [PubMed] [Google Scholar]
- 2.Berggren JR, Boyle KE, Chapman WH, et al. Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am J Physiol Endocrinol Metab. 2008;294:E726–E732. doi: 10.1152/ajpendo.00354.2007. [DOI] [PubMed] [Google Scholar]
- 3.Hoeks J, Schrauwen P. Muscle mitochondria and insulin resistance: a human perspective. Trends Endocrinol Metabol. 2012;23:444–450. doi: 10.1016/j.tem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 6.Adams JMII, Pratipanawatr T, Berria R, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 7.Skovbro M, Baranowski M, Skov-Jensen C, et al. Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia. 2008;51:1253–1260. doi: 10.1007/s00125-008-1014-z. [DOI] [PubMed] [Google Scholar]
- 8.Itani SI, Ruderman NB, Schmieder F, et al. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 9.Moro C, et al. Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J Clin Endocrinol Metab. 2009;94:3440–3447. doi: 10.1210/jc.2009-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anastasiou CA, et al. Diabetes mellitus is associated with increased intramyocellular triglyceride, but not diglyceride, content in obese humans. Metab Clin Exp. 2009;58:1636–1642. doi: 10.1016/j.metabol.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Coen PM, Dube JJ, Amati F, et al. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes. 2010;59:80–88. doi: 10.2337/db09-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amati F, Dube JJ, Alvarez-Carnero E, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60:2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vistisen B, Hellgren LI, Vadset T, et al. Effect of gender on lipid-induced insulin resistance in obese subjects. Eur J Endocrinol. 2008;158:61–68. doi: 10.1530/EJE-07-0493. [DOI] [PubMed] [Google Scholar]
- 14.Whaley MH, Brubaker PH, Otto RM, Armstrong LE. American college of sports medicine: ACSM’s guidelines for exercise testing and prescription. 7. Lippin-cott Williams & Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- 15.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14:101–102. [PubMed] [Google Scholar]
- 16.Pruchnic R, Katsiaras A, He J, et al. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab. 2004;287:E857–E862. doi: 10.1152/ajpendo.00459.2003. [DOI] [PubMed] [Google Scholar]
- 17.Bielawski J, Szulc ZM, Hannun YA, et al. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Sun D, Cree MG, Wolfe RR. Quantification of the concentration and 13C tracer enrichment of long-chain fatty acyl-coenzyme A in muscle by liquid chromatography/mass spectrometry. Anal Biochem. 2006;349:87–95. doi: 10.1016/j.ab.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Cortright RN, Sandhoff KM, Basilio JL, et al. Skeletal muscle fat oxidation is increased in African-American and white women after 10 days of endurance exercise training. Obesity. 2006;14:1201–1210. doi: 10.1038/oby.2006.137. [DOI] [PubMed] [Google Scholar]
- 20.Ritov VB, Menshikova EV, Kelley DE. Analysis of cardiolipin in human muscle biopsy. J Chromatogr B Anal Technol Biomed Life Sci. 2006;831:63–71. doi: 10.1016/j.jchromb.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 21.Johannsen DL, Conley KE, Bajpeyi S, et al. Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J Clin Endocrinol Metab. 2012;97:242–250. doi: 10.1210/jc.2011-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JY, Hickner RC, Cortright RL, et al. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–E1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 23.Straczkowski M, Kowalska I, Baranowski M, et al. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366–2373. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- 24.Turinsky J, O’Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265:16880–16885. [PubMed] [Google Scholar]
- 25.Jocken JW, Moro C, Goossens GH, et al. Skeletal muscle lipase content and activity in obesity and type 2 diabetes. J Clin Endocrinol Metab. 2010;95:5449–5453. doi: 10.1210/jc.2010-0776. [DOI] [PubMed] [Google Scholar]
- 26.De Vogel-van den Bosch J, Hoeks J, Timmers S, et al. The effects of long- or medium-chain fat diets on glucose tolerance and myocellular content of lipid intermediates in rats. Obesity. 2011;19:792–799. doi: 10.1038/oby.2010.152. [DOI] [PubMed] [Google Scholar]
- 27.Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16:400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergman BC, Hunerdosse DM, Kerege A, et al. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia. 2012;55:1140–1150. doi: 10.1007/s00125-011-2419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis BA, Poynten A, Lowy AJ, et al. Long-chain acyl-CoA esters as indicators of lipid metabolism and insulin sensitivity in rat and human muscle. Am J Physiol Endocrinol Metab. 2000;279:E554–E560. doi: 10.1152/ajpendo.2000.279.3.E554. [DOI] [PubMed] [Google Scholar]
- 30.Houmard JA, Tanner CJ, Yu C, et al. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes. 2002;51:2959–2963. doi: 10.2337/diabetes.51.10.2959. [DOI] [PubMed] [Google Scholar]
- 31.Idell-Wenger JA, Grotyohann LW, Neely JR. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem. 1978;253:4310–4318. [PubMed] [Google Scholar]
- 32.Robishaw JD, Neely JR. Coenzyme A metabolism. Am J Physiol. 1985;248:E1–E9. doi: 10.1152/ajpendo.1985.248.1.E1. [DOI] [PubMed] [Google Scholar]
- 33.Bosma M, Minnaard R, Sparks LM, et al. The lipid droplet coat protein perilipin 5 also localizes to muscle mitochondria. Histochem Cell Biol. 2012;137:205–216. doi: 10.1007/s00418-011-0888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalen KT, Dahl T, Holter E, et al. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta. 2007;1771:210–227. doi: 10.1016/j.bbalip.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Wolins NE, Quaynor BK, Skinner JR, et al. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 36.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Turner N, Bruce CR, Beale SM, et al. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56:2085–2092. doi: 10.2337/db07-0093. [DOI] [PubMed] [Google Scholar]
- 38.Holloway GP, Thrush AB, Heigenhauser GJ, et al. Skeletal muscle mitochondrial FAT/CD36 content and palmitate oxidation are not decreased in obese women. Am J Physiol Endocrinol Metab. 2007;292:E1782–E1789. doi: 10.1152/ajpendo.00639.2006. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Shi X, Choi CS, et al. Paradoxical coupling of triglyceride synthesis and fatty acid oxidation in skeletal muscle overexpressing DGAT1. Diabetes. 2009;58:2516–2524. doi: 10.2337/db08-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badin PM, Louche K, Mairal A, et al. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes. 2011;60:1734–1742. doi: 10.2337/db10-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]