Abstract
Background
Short telomere lengths are found in a subset of idiopathic pulmonary fibrosis (IPF) patients, but their clinical significance is unknown. The aim of this study was to investigate whether patients with various blood leukocyte telomere lengths had different overall survival.
Methods
Telomere lengths were measured in 370 genomic DNA samples isolated from peripheral blood collected from patients with interstitial lung disease (149 with IPF) at the time of their initial evaluation. Associations of telomere length with transplant-free survival were determined. Findings were validated in two independent IPF cohorts.
Findings
Patients with IPF had shorter telomere lengths than controls, but similar telomere lengths when compared to patients with other interstitial lung disease diagnoses after adjusting for age, male sex and ethnicity. Telomere length was independently associated with transplant-free survival time for patients with IPF (HR 0·22 [0·08–0·63], P-value = 0·0048), but not for patients with interstitial lung disease diagnoses other than IPF (HR 0·73 [0·16–3·41], P-value = 0·69). The association between telomere length and IPF survival was independent of age, male sex, forced vital capacity or diffusing capacity of carbon monoxide (and was replicated in two independent IPF cohorts (HR 0·11 [0·03–0·39], P-value 0·00066; HR 0·25 [0·07–0·87], P-value = 0·029). Addition of telomere length to clinical prediction models improved the integrative discrimination index, especially for IPF cohorts with milder disease.
Interpretation
These findings suggest that shorter leukocyte telomere lengths are associated with worse survival in IPF. Additional studies will be needed to determine clinically relevant thresholds for telomere length and how this biomarker may influence future risk stratification of IPF patients. Furthermore, this study offers mechanistic insight as disease progression in certain IPF patients may be related to aberrant signaling from short telomeres.
Funding
US National Heart, Lung, and Blood Institute; the National Center for Advancing Translational Science, the Harroun Family Foundation and the Nina Ireland Lung Disease Program.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, fibrotic lung disease affecting older individuals. There are currently no approved therapies for IPF in the United States. The natural history of this disease is progressive with a highly variable rate of decline in lung function(1–3). The cause of IPF remains unknown, but recent investigations have implicated telomere shortening in the pathogenesis of IPF(4, 5).
Telomeres are specialized nucleoprotein structures that protect chromosomal ends. Mutations in the genes encoding telomerase, the multi-subunit enzyme that extends telomere lengths, have been found in patients with IPF(6, 7). Heterozygous telomerase mutations, primarily those in the gene encoding the protein component of telomerase (TERT), are found in approximately fifteen percent of kindreds with familial pulmonary fibrosis and three percent of patients with sporadic IPF with no known family history of disease(7, 8). Pulmonary fibrosis associated with TERT mutations is progressive and lethal with a mean survival of three years after diagnosis(8). Compared to the other disorders of telomere dysfunction caused by a single gene defect, such as dyskeratosis congenita, aplastic anemia and liver cirrhosis, IPF is the most common manifestation of telomere-mediated disease(9). Telomere lengths of the offspring of telomerase mutation carriers can be affected by epigenetic inheritance of short telomere lengths independent of mutant alleles(8, 10). In addition, genome-wide association studies of pulmonary fibrosis have identified susceptibility loci near TERT(11, 12), TERC(12) and OBFC1(12), implicating common variants in genes that are associated with telomere length in the general population(13) as contributors to the development of pulmonary fibrosis.
Peripheral blood telomere lengths have been found to be shorter in IPF patients compared to age-matched controls, suggesting that they may be a marker of increased disease susceptibility(4, 5). Non-genetic contributions to telomere shortening relevant to lung disease include exposure to oxidative damage and cigarette smoking(14–16). At this time, the clinical significance of telomere lengths in patients with IPF and other forms of interstitial lung disease (ILD) remains unknown. In this study we tested the hypothesis that telomere length is associated with survival in IPF and non-IPF interstitial lung disease.
Methods
Participants
This study was approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center (Dallas cohort), the University of Chicago (Chicago cohort), and the University of California San Francisco (San Francisco cohort). Written informed consent was obtained from all subjects. Patients were enrolled from 6/17/2003 – 8/25/2011, from 9/1/2005 – 1/31/2012, and from 10/14/2005 – 7/8/2011 for the Dallas, Chicago and San Francisco cohorts, respectively. The diagnosis of IPF (and other ILDs represented in the Dallas cohort) was made in accordance with established criteria(3, 17). Patients with a diagnosis other than IPF (non-IPF ILD) included those with connective tissue disease associated ILD (CTD-ILD), an idiopathic interstitial pneumonia other than UIP (Other IIP), hypersensitivity pneumonitis, an ILD related to an exposure, sarcoidosis or another etiology. Ethnicity was self-reported. Blood samples were collected at the time of enrollment. Genomic DNA samples of normal control subjects (n=195) ranging in age from 19–89 years used in previous studies(4, 8), were obtained from a cohort of unrelated, multiethnic individuals from Dallas, Texas. Spouses of patient participants (n=46) who accompanied the patient to the initial visit were collected as a second independent control group. All subjects performed pulmonary function testing according to ATS guidelines; different reference equations were used at the different sites to convert measurements to percent predicted values. Absolute pulmonary function test values were not available for all subjects. All subjects included in this study were prospectively followed for at least one month before death or lung transplant.
Determination of Telomere Length and Genetic Analyses
Genomic DNA was isolated from circulating leukocytes from the Dallas cohort using an Autopure LS (Qiagen, Valencia, CA). For the Chicago and San Francisco cohorts, genomic DNA was isolated using the FlexiGene and Gentra PureGene DNA isolation kits (Qiagen), respectively. Telomere lengths of genomic DNA isolated from blood leukocytes from the Dallas cohort and controls were measured using a quantitative PCR assay as previously described(4, 8) and are reported as logarithm-transformed relative T/S ratios. Telomere lengths were measured for the Chicago and San Francisco cohorts using an identical protocol, except the stock concentrations of DNA was 10 ng/μL (instead of 50 ng/μL) prior to its addition to the PCR reaction. Reference DNA samples from a cell line (MCF7) and from two control individuals were included on each run as internal controls. The observed mean coefficient of variation for the telomere length measurement was 5·2%, 8·2%, and 6·5% for the Dallas, Chicago and San Francisco cohorts, respectively. The telomere length assay was performed blinded to the study endpoint. Sequencing of both the TERT and TERC genes was performed as previously described(7).
Statistical Methods
Primary analyses were performed using the Dallas cohort, with the Chicago and San Francisco cohorts serving as replication cohorts for selected analyses. All analyses were performed using the R software package, v 3.0.1 (http://www.R-project.org/). Clinical characteristics of the study cohorts were compared using the analysis of variance (ANOVA) for continuous variables and Fisher’s exact tests for categorical variables. Relative T/S ratios were logarithm transformed to ensure that the residuals were normally distributed and had constant variance. The relationship between telomere length and age was estimated using linear regression of control subjects as described(4, 8). The estimated regression coefficients were used to calculate the observed minus expected (O-E), or age-adjusted telomere length for each subject. Approximate age-adjusted prediction bands were calculated from the linear regression model. The age range of the Dallas ILD cohort was within the full age range of the controls; the median (25th–75th percentile) age was 58 (45–66), 57 (48–65) and 66 (59–71) for the controls, Dallas non-IPF patient and the Dallas IPF patient cohorts.
The primary outcome was time to death or lung transplantation (i.e. transplant-free survival). The association between telomere length and survival time was tested using Cox proportional hazards regression. Telomere length was modeled as a continuous variable in univariate and multivariable analyses. A multivariable model was created adjusting for age, male sex, forced vital capacity (FVC) % predicted, and diffusing capacity for carbon monoxide (DLCO) % predicted. The proportionality of hazards assumption was checked by plotting scaled Schoenfeld residuals against transformed time for each covariate. No evidence of non-proportional hazards was observed. Patients in each cohort were stratified by quartiles of age-adjusted telomere lengths and the estimated survival functions were plotted for each quartile based on the Cox model with values of individual covariates fixed at the sample means. To assess the sensitivity of the results, we repeated the analysis with time to death as the outcome, right-censoring lung transplantation events. We also performed an additional multivariable analysis, adjusting for the Gender-Age-Physiology (GAP) score, a model-based score generated from individual clinical variables (used in the primary analysis model) that can be used to predict mortality in patients with IPF (18).
To determine the contribution of telomere length to mortality risk prediction in IPF, we calculated the Harrell c-index to assess the discrimination performance of models including and excluding telomere length(19). The optimism-corrected estimates of the c-index were obtained by using twenty repetitions of ten-fold cross-validation. Bootstrap resampling with 1000 repetitions was used to calculate 95% bias corrected confidence intervals of the c-index. The difference between c-indices for models including and excluding telomere length were assessed using a permutation test with 10,000 permutations of telomere length with respect to other covariates and survival times. All tests were two-sided; P-values <0.05 were considered statistically significant.
None of the study sponsors had a role in study design, data collection, data analysis, data interpretation, or writing of the report. B.D.S., J.K. and C.K.G. had access to all the raw data. The corresponding author had full access to the data in the study and had final responsibility for the decision to submit for publication.
Results
Cohort characteristics, telomere lengths, and telomerase mutations
The primary (Dallas) cohort consisted of 370 patients (149 with IPF); the replication cohorts consisted of 139 IPF patients from Chicago and 54 IPF patients from San Francisco. Baseline characteristics of the cohorts are listed in Table 1. The Dallas IPF patients were older and had a higher proportion of males, smokers and persons of European American descent than the Dallas non-IPF patients. In addition, Dallas IPF patients had reduced baseline measures of pulmonary function (FVC and DLCO) compared to non-IPF ILD patients.
Table 1.
Comparison of Clinical Characteristics of ILD patients
| Dallas Cohorts: | P-value (Dallas non-IPF vs. IPF) | Chicago IPF cohort: | San Francisco IPF cohort: | P-value (Three IPF cohorts3) | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| All Non-IPF ILD (n=221) | IPF cohort (n=149) | IPF cohort (n=139) | IPF cohort (n=54) | |||
| Age at enrollment, mean (SD), years | 56 (13) | 65 (10) | <0·0001 | 68 (9) | 69 (9) | 0·0047 |
| Male, n (%) | 75 (34) | 104 (70) | <0·0001 | 100 (72) | 43 (80) | 0·39 |
| Ethnicity, n (%) | ||||||
| European American | 147 (67) | 126 (85) | 0·00010 | 122 (88) | 40 (75) | 0·11 |
| African American | 38 (17) | 10 (7) | 0·0041 | 10 (7) | 2 (4) | 0·80 |
| Hispanic | 25 (11) | 11 (7) | 0·28 | 5 (4) | 3 (6) | 0·39 |
| Other | 11 (5) | 2 (1) | 0·084 | 2 (1) | 8 (15) | 0·00011 |
| Ever smokers, n (%) | 83 (38) | 94 (63) | 0·00010 | 88 (70) | 39 (72) | 0·35 |
| Baseline Spirometry, mean (SD) | ||||||
| FVC, % predicted | 67 (23) | 62 (21) (n=148) | 0·023 (0·00026) | 64 (18) | 69 (15) | 0·026 (0·12) |
| FEV1, % predicted | 67 (23) | 66 (20) (n=147) | 0·74 (0·067) | 77 (20) (n=137) | NA | <0·0001 (<0·0001) |
| Ratio of FEV1/FVC | 81 (12) (n=211) | 82 (9) (n=148) | 0·49 (0·20) | 84 (8) (n=122) | NA | 0·066 (0·046) |
| DLCO, % predicted | 41 (18) (n=187) | 35 (16) (n=136) | 0·0035 (0·0063) | 48 (18) (n=123) | 46 (16) (n=49) | <0·0001 (<0·0001) |
| Unable to perform DLCO, n (%) | 15 (6·8) | 8 (5·6) | 0·83 | 1 (0·8) | 1 (2·0) | 0·057 |
| Follow up time, median (IQR), months | 27 (13–35) | 15 (9–27) | 0·00013 | 26 (15–49) | 56 (17–72) | <0·0001 |
| Time to Death or Transplant, median (95% CI), months1 | 69 (69-)1 | 21.5 (16–28) | 0·00010 | 67 (46- )1 | 64 (32- )1 | <0·0001 |
| Number of patients undergoing transplant (%) | 13 (6) | 38 (26) | <0·0001 | 11 (8) | 7 (13) | 0·00023 |
| Telomere Length, mean (SD) | 1·46 (0·24) | 1·33 (0·25) | <0·0001 (adjusted 0·47)2 | 1·34 (0·26) | 1·67 (0·37) | <0·0001 |
Abbreviations: SD, standard deviation; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; DLCO, diffusion capacity for carbon monoxide; IQR, interquartile range; 95% CI, 95 percent confidence interval; NA, not available.
The median survival time was estimated using Kaplan-Meier analysis. More than 50% of non-IPF ILD subjects were still alive at the end of the observation period, therefore the median cannot be estimated. The upper 95% confidence limits of median survival time for the San Francisco cohort and Chicago cohorts could not be determined because of the large proportion of transplant-free surviving subjects at the end of the observation period.
The P-values for comparison of Dallas IPF vs. non-IPF patients adjusted for age, male sex, and ethnicity.
The P-values for comparison of Dallas IPF, Chicago IPF and San Francisco IPF cohorts.
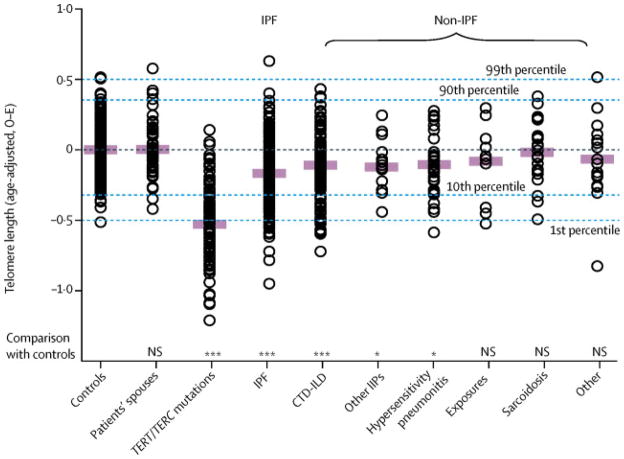
Each of the ILD cohorts demonstrated a continuous range of leukocyte telomere lengths (Figure 1). The mean age-adjusted telomere length of the Dallas sporadic IPF cohort was shorter than two control cohorts, but similar to the overall non-IPF ILD cohort after adjustment for age, male sex and ethnicity (Table 1). We found that 25% of the Dallas IPF cohort had a mean telomere length ≤ 10th predicted percentile of normal controls, consistent with an earlier study(4). Within the non-IPF ILD group, patients with connective tissue disease associated ILD (CTD-ILD), idiopathic interstitial pneumonia (IIP) other than usual interstitial pneumonia (Other IIPs), and hypersensitivity pneumonitis had shorter mean age-adjusted telomere lengths than normal controls (Figure 1). The telomere length comparison between the controls and some of the non-IPF ILD subgroups were not significant, perhaps due to the small sample sizes (which are specified in the figure legend).
Figure 1. Age-adjusted telomere lengths of some interstitial lung disease (ILD) patients are shorter than controls.
Mean observed minus expected (age-adjusted) telomere length for controls (n=195), patient spouses (n=46) and individuals from familial pulmonary fibrosis kindreds who carry an inherited telomerase (TERT or TERC) mutation (n=106). The ILD patients were subdivided by etiology: idiopathic pulmonary fibrosis (IPF, n=149) and non-IPF patients which include those with connective-tissue disease associated ILD (CT-ILD, n=126), idiopathic interstitial pneumonias other than usual interstitial pneumonia (Other IIPs, n=17), hypersensitivity pneumonitis (n=30), drug-related or radiation-associated ILD (Exposures, n=10), sarcoidosis (n=22) and ILD from other causes (Other, n=16). Approximate age-adjusted prediction bands (percentiles) were calculated from the linear regression model.
The coding regions of the telomerase genes (TERT and TERC) were sequenced for the Dallas IPF patients with telomere lengths ≤ 10th predicted percentile of normal. We found four individuals with rare variants in TERT (no TERC variants were found) of the 40 IPF patients that were sequenced (Supplemental Table 1). This number represents 2·6% of the total Dallas IPF patients.
Association between telomere length and survival time in ILD
In unadjusted analysis of the Dallas cohorts, shorter telomere length was associated with decreased survival time for IPF patients (HR 0·32 for each 1-unit difference in the log T/S ratio, 95% CI 0·14–0·72, P-value = 0·0058, Supplemental Table 2), but not for non-IPF ILD patients (HR 0·72 [0·23 – 2·23], P-value = 0·57, Supplemental Table 3). After adjustment for relevant individual covariates (age, male sex, FVC % predicted and DLCO % predicted), telomere length remained an independent predictor of IPF transplant-free survival time (HR 0·22, 95% CI 0·08–0·63, P-value = 0·0048, Table 2). For each quartile decrease in telomere length, there was a step-wise decrease in IPF transplant-free survival (Figure 2). Telomere length was not significantly associated with transplant-free survival of non-IPF ILD patients in multivariable analysis (HR 0·73 [0·16 – 3·41], P-value = 0·69, Table 3).
Table 2.
Multivariable Survival Analysis of IPF Patients Across All Three Cohorts
| Dallas IPF cohort | Chicago IPF cohort | San Francisco IPF cohort | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | |
| Multivariable analysis (individual covariates) | (n=135) | (n=123) | (n=49) | |||
|
| ||||||
| Age | 1·02 (0·99 – 1·04) | 0·26 | 0·99 (0·96 – 1·03) | 0·66 | 1·02 (0·98 – 1·07) | 0·36 |
| Male Sex | 1·3 (0·78 – 2·14) | 0·31 | 2·24 (0·89 – 5·68) | 0·088 | 1·33 (0·38 – 4·62) | 0·65 |
| FVC, % predicted | 0·81 (0·67 – 0·97) | 0·023 | 0·91 (0·70 – 1·17) | 0·45 | 0·85 (0·64 – 1·15) | 0·30 |
| DLCO, % predicted | 0·72 (0·58 – 0·89) | 0·0021 | 0·60 (0·47 – 0·78) | 0·00013 | 0·77 (0·57 – 1·05) | 0·097 |
| Telomere Length | 0·22 (0·08 – 0·63) | 0·0048 | 0·11 (0·03 – 0·39) | 0·00066 | 0·25 (0·07 – 0·87) | 0·029 |
|
| ||||||
| Multivariable analysis (GAP score) | (n=143) | (n=124) | (n=50) | |||
|
| ||||||
| GAP score | 2·62 (1·79 – 3·84) | <0·0001 | 3·67 (1·94 – 6·95) | <0·0001 | 2·81 (1·35 – 5·87) | 0·0058 |
| Telomere length | 0·5 (0·20 – 1·26) | 0·14 | 0·21 (0·06 – 0·76) | 0·018 | 0·25 (0·08 – 0·84) | 0·024 |
The reported hazard ratios are per a 10% difference in FVC and DLCO measurements and per a 1-unit difference in log T/S ratios. Patients who were unable to perform the DLCO test (n=9 in the Dallas cohort, n=1 in the Chicago cohort, and n=1 in the San Francisco cohort) were excluded from analysis when using individual covariates, but included when using GAP score.
Figure 2. Shorter telomere lengths predict worse survival for IPF patients in three independent cohorts.
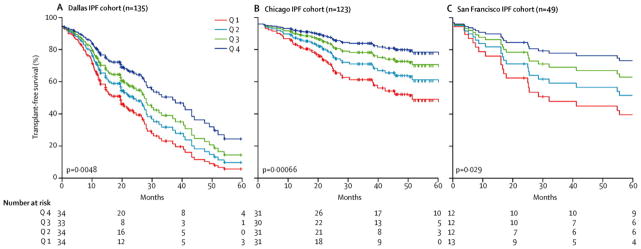
Estimated survival functions for IPF patients from Dallas, Chicago and San Francisco stratified by telomere length quartiles for transplant-free survival.
Table 3.
Multivariable Analysis of Transplant-free Survival of Non-IPF ILD Patients in the Dallas Cohort.
| Dallas non-IPF cohort (n=187)* | ||
|---|---|---|
|
| ||
| Hazard Ratio (95% CI) | P-value | |
| Multivariable analysis | ||
|
| ||
| Age | 1·03 (1·00 – 1·06) | 0·065 |
| Male Sex | 3·07 (1·55 – 6·07) | 0·0013 |
| FVC, % predicted | 1·06 (0·86 – 1·31) | 0·59 |
| DLCO, % predicted | 0·44 (0·31 – 0·62) | <0·0001 |
| Telomere Length | 0·73 (0·16 – 3·41) | 0·69 |
The analysis includes patients with all covariates available.
Replication Cohorts
The clinical features of the independent IPF replication cohorts are shown in Table 1. The three IPF cohorts were significantly different with regard to age, baseline spirometry, and survival time. Telomere length remained a significant independent predictor of transplant-free survival time after adjustment for individual covariates (age, male sex, FVC % predicted and DLCO % predicted) for both the Chicago IPF cohort (HR 0·11 [0·03–0·39], P-value 0.00066) and the San Francisco IPF cohort (HR 0·25 [0·07–0·87], P-value = 0·029, Table 2). IPF patients from the Chicago and San Francisco cohorts stratified by telomere length quartiles demonstrated a similar step-wise decrease in survival (Figure 2).
Sensitivity Analyses
To avoid bias from the consideration of lung transplantation as an event equivalent to death, a sensitivity analysis was performed with censoring of transplantation events. Using this model, telomere length lost statistical significance as an independent predictor of survival in the Dallas cohort, but the estimated hazard ratio was similar to that in the primary analysis (HR 0·35 [0·11 – 1·15], P-value = 0·085). There was a high incidence of lung transplant in the Dallas cohort (26%). Telomere length remained a significant predictor of survival for the Chicago and San Francisco IPF cohorts (HR 0·11 [0·03 – 0.41], P-values = 0·0011 and HR 0·13 [0·03 – 0.52], P-value = 0·0040, respectively; Supplemental Table 4). The transplant incidence was eight percent in the Chicago cohort and thirteen percent in the San Francisco cohort. We analyzed survival of the three IPF cohorts with a second model that utilized the GAP score, a model-based score generated from individual clinical variables (gender, age, and physiologic measurements of FVC and DLCO)(18). Telomere length was not an independent predictor of survival for the Dallas cohort using the GAP model (HR 0·50 [0·20 – 1·26], P-value = 0·14), but was for both the Chicago and San Francisco IPF cohorts (HR 0·21 [0·06 – 0·76], P-value = 0·018 and HR 0·25 [0·08 – 0.84], P-value = 0·024, respectively, Table 2).
Risk Prediction Model
To determine the contribution of telomere length to mortality risk prediction in IPF, we calculated the c-index(19) for prediction models including and excluding telomere length. For both clinical risk prediction models, the c-index was significantly increased with the inclusion of telomere length for the Chicago and San Francisco IPF cohorts (Table 4). The c-index was significantly increased with the inclusion of telomere length for the combined IPF cohort (c-index 0.74 (0.70 – 0.78), P-value 0·00030 for the individual covariate model and c-index 0.73 (0.69 – 0.77), P-value = 0·0020 for the GAP score model).
Discussion
While short telomere lengths have been found in pulmonary fibrosis patients with rare loss-of-function telomerase mutations(6, 7) and have been implicated in disease pathogenesis, to our knowledge this is the first report to demonstrate an independent association between telomere length and IPF survival (See Research in Context panel). We found that the mean telomere length of IPF and non-IPF patients is significantly shorter than controls, although not as short as the mean telomere length of individuals from familial pulmonary fibrosis kindreds in which a telomerase mutation was discovered to segregate with lung disease(4, 7, 8, 20). Intriguingly, telomere length is a predictor of survival only for patients with IPF; it is not associated with survival for patients with non-IPF interstitial lung disease. The association between telomere length and IPF survival is robust, being observed across three independent IPF cohorts with differing baseline demographic, physiologic and survival characteristics.
Prior studies have reported the incidence of telomerase mutations and the degree of telomere shortening in patients with sporadic (non-familial) IPF or other idiopathic interstitial pneumonias(4, 5). In this study rare variants in TERT were found in only four of the forty IPF patients whose telomere lengths were less than the 10th percentile. This number represents only 2·6% of the total Dallas IPF cohort and is consistent with the 1–2% incidence of telomerase mutations found in sporadic IPF patients analyzed in other studies(5, 7). Since the number of telomerase mutation carriers is small, it is unlikely that the effect of telomere length on survival in these sporadic IPF cohorts reflects unrecognized deleterious telomerase mutations. Data collected from patients with the familial form of pulmonary fibrosis due to telomerase mutations suggests that short telomere lengths precede the development of lung disease. Patients with inherited, or germline, TERT mutations have evidence of telomere shortening several decades before the onset of IPF, which generally affects those >50 years of age(8). Telomere length represents not only the effects of inherited genetic variants in the telomerase genes, but also reflects the starting telomere set point, the rate of telomere erosion with age, the history of cellular replication and various environmental effects. As such, it represents the integration of multiple risk factors, both inherited and acquired. Since telomeres progressively shorten with age and cellular division, their measure of “molecular” age may complement chronologic age as a predictor of survival.
The degree of telomere shortening is similar for the Dallas IPF and non-IPF groups after correction for age, male sex, and ethnicity. However, the lack of association of telomere lengths with survival in the non-IPF ILD patients suggests that factors other than telomere length, such as the duration of an environmental fibrogenic exposure or the severity of an underlying connective tissue disease, may be more relevant to disease progression and survival in the non-IPF ILD patient. Mutations in the telomerase genes linked to familial pulmonary fibrosis all cause a loss-of-function in telomerase enzymatic activity(4, 6–8). Peripheral blood telomere lengths correlate with telomere lengths measured from cells isolated from different somatic tissues(21), so the short telomere lengths seen in telomerase mutation carriers likely reflect reduced global telomerase activity. The mechanism for developing lung disease for telomerase mutation carriers is likely related to this decrease in telomerase activity and the resulting short telomeres. Similarly, some sporadic IPF patients may have an intrinsic reduction in total telomerase activity or may have experienced epigenetic or environmental influences that alter telomere integrity. While these effects in sporadic IPF patients may not be to the same degree as seen in mutation carriers, telomere lengths may fall below a critical threshold needed for flawless lung repair. Since the cause of death of IPF patients is usually related to their underlying lung disease and progressive respiratory failure(22), it is conceivable that the worse survival of IPF patients with short telomere lengths is due to relentless fibrosis caused by an intrinsic low level of cellular telomerase activity or the activation of aberrant signal transduction pathways from critically short telomeres. These studies offer mechanistic insights into IPF, as telomere shortening is associated with IPF disease pathogenesis, progression and reduced survival.
Telomere lengths are not often analyzed by adult pulmonologists, but are more frequently used in clinical practice by geneticists and hematologists in the work up of patients with suspected dyskeratosis congenita. In this study, telomere lengths were measured from genomic DNA samples isolated from blood using a quantitative PCR assay to accommodate the limited amounts of available DNA. This method is less precise and has a higher inter-assay coefficient of variation than other methods(23). Several factors can affect the measurement of telomere length, including DNA integrity and the specific population of leukocytes from which DNA is extracted(24). The telomere lengths of the three IPF cohorts differ significantly. We do not know if these differences are biologically significant or are due to technical variables, such as the method of DNA isolation, the DNA storage concentration, or interference of a DNA storage buffer component(s) with the telomere length PCR assay. Because of these differences, survival analyses were performed separately for each cohort, independent of the telomere length measurements of the other cohorts or the normal control population. Telomere lengths of the controls were used only to calculate the age-adjusted telomere lengths for the Dallas ILD cohorts. The younger median age of the controls as compared to the Dallas IPF cohort may affect the number of patients classified below the 10th predicted percentile, but not the survival analyses. It would be useful in the future to determine patient survival with reference to absolute telomere length cut points. However, in order to test arbitrary cut points of predicted telomere length percentiles, a large validated reference control population is required and the method of blood collection and telomere length measurement would need to be uniform across all controls and patients.
The three IPF cohorts were significantly different from each other with regard to age, ethnicity, baseline spirometry and survival characteristics. In contrast with the Dallas and Chicago IPF cohorts, telomere length was not a significant covariate for survival in univariate analysis of the San Francisco cohort (HR 0.38 (0.14 – 1.06), p-value = 0.065, Supplemental Table 2), although it was significant in the multivariable analysis (HR 0.25 (0.07 – 0.87), p-value = 0.029, Table 2). For this cohort, the effect of telomere length was only significant in the context of the other covariates. The Dallas cohort includes patients referred not only to the ILD clinic, but also those referred directly to the Lung Transplant clinic and the Pulmonary Hypertension clinic. Compared with the other cohorts, the Dallas IPF cohort had worse baseline pulmonary function tests, shorter mean survival and a higher percentage of subjects who underwent lung transplantation, reflecting more advanced disease. For this cohort, telomere length did not add predictive power to the GAP score for survival in IPF. However, telomere length significantly improved the clinical risk prediction models for the Chicago and San Francisco cohorts, which both had longer overall transplant-free survival times and lower incidence of transplantation. These results are suggestive that telomere length may offer more predictive power for IPF patients that are less severely affected at baseline.
Weaknesses of the study include the lack of absolute lung function values and the use of different reference equations to convert measurements to percent predicted values by the different centers. In addition, the lack of serum or RNA samples limited our ability to compare telomere lengths with other biomarkers, such as MMP7(25, 26) or mononuclear cell gene expression profiles(27, 28).
Given the wide range of variability in survival of IPF patients, predicting clinical course is a real challenge. Improved prognostic information may help guide the timing of lung transplantation referrals or allow for appropriate life planning. In this study we demonstrate that telomere length of genomic DNA obtained from blood collected at the time of initial evaluation has value in predicting survival for patients with IPF, but not those with non-IPF interstitial lung disease. Existing clinical predictors and the GAP score, which is calculated from baseline clinical and physiologic parameters, are imperfect in predicting survival for IPF patients, especially for those with early disease. The addition of biomarkers may allow for better risk stratification. If short telomere length represents an individual’s susceptibility to develop progressive lung scarring, then its use as a predictive biomarker of IPF survival may complement other biomarkers that indirectly measure epithelial cell injury or activation of fibrotic remodeling. It is currently unknown if absolute telomere length cut-offs can be used across different patient cohorts in predicting survival. In addition, future studies will be needed to determine if the subdivision of IPF patients by underlying mechanism (telomere shortening vs. non-telomere shortening) predicts responsiveness to any of the novel therapeutics currently under investigation in clinical trials or leads to better outcome predictions.
Supplementary Material
Research in Context.
Systematic Review
We searched PubMed for reports published before April 1, 2014 using the search terms “telomere length,” “IPF” and “survival.” At the time of our search, one study had evaluated individuals from familial pulmonary fibrosis kindreds with inherited telomerase (TERT) mutations and found that they had reduced life expectancy(8). No study had evaluated telomere length and the survival of sporadic IPF patients.
Interpretation
To our knowledge, this is the first study that has evaluated telomere length as a predictor of IPF patient survival. Shorter telomere lengths were associated with worse transplant-free survival for IPF patients independent of age, male gender or physiologic measurements (FVC, DLCO) from the discovery cohort and two independent replication cohorts. The addition of telomere length improved clinical prediction models, especially for IPF cohorts with more mild disease. Future research will be needed to determine if an absolute telomere length cut point can be used as a predictor of survival across cohorts and how this biomarker may influence risk stratification of IPF patients. Since rare mutations in the telomerase genes have been linked to an increased risk of developing IPF, the development of lung fibrosis in some patients may be related mechanistically to low levels of cellular telomerase activity or aberrant signaling from critically short telomeres.
Acknowledgments
We are grateful to all participating patients for their contributions to this research, to Hannah Bereuter for technical excellence in measuring telomere lengths, to Martha Kingman for help in patient recruitment and to Helen H. Hobbs for the control DNA samples. The authors are grateful for funding provided by NIH K12 HD-068369 (B.D.S.), NIH, UCSF-CTI KL2TR000143 (J.S.L.), P01 HL108794 (H.R.C., P.J.W.), the Harroun Family Foundation and the Nina Ireland Lung Disease Program (H.R.C., P.J.W.), NIH RC1 HL099619 (I.N.), NIH R01 HL093096 and the UL1TR001105 (C.K.G). None of the funding organizations or sponsors had any role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Author Contributions
B.D.S., J.S.L., I.N., H.R.C., P.J.W. and C.K.G. designed the study. All authors acquired data and undertook quality control. J.K., B.D.S., J.S.L., I.N., H.R.C., P.J.W. and C.K.G. analyzed the data. All authors contributed to the revision of the report.
Declaration of Interests
J.S.L. reports grant funds from the NIH. I.N. reports grants from the NIH and Intermune; consulting fees from Intermune, Gilead and Boehringer Ingelheim; clinical study contracts from Boehringer Ingelheim, Stromedix, Sanofi and Hoffman LaRoche; and pending patents for use of TOLLIP and a PBMC gene expression signature in IPF. C.S.G. reports speaker fees from Intermune. C.E.G. reports a clinical study contract from Intermune. P.J.W. reports a grant from Genentech and consulting fees from Veracyte. C.K.G. reports grants from the NIH and the Doris Duke Charitable Foundation. The other authors declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157(1):199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 2.Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):285–92. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(7):729–37. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13051–6. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317–26. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 7.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104(18):7552–7. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz de Leon A, Cronkhite JT, Katzenstein AL, Godwin JD, Raghu G, Glazer CS, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS ONE. 2010;5(5):e10680. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutation research. 2012;730(1–2):52–8. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004;36(5):447–9. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- 11.Mushiroda T, Wattanapokayakit S, Takahashi A, Nukiwa T, Kudoh S, Ogura T, et al. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Med Genet. 2008;45(10):654–6. doi: 10.1136/jmg.2008.057356. [DOI] [PubMed] [Google Scholar]
- 12.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–20. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy D, Neuhausen SL, Hunt SC, Kimura M, Hwang SJ, Chen W, et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A. 2010;107(20):9293–8. doi: 10.1073/pnas.0911494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Experimental cell research. 1995;220(1):186–93. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 15.Morla M, Busquets X, Pons J, Sauleda J, MacNee W, Agusti AG. Telomere shortening in smokers with and without COPD. Eur Respir J. 2006;27(3):525–8. doi: 10.1183/09031936.06.00087005. [DOI] [PubMed] [Google Scholar]
- 16.Kozlitina J, Garcia CK. Red blood cell size is inversely associated with leukocyte telomere length in a large multi-ethnic population. PLoS ONE. 2012;7(12):e51046. doi: 10.1371/journal.pone.0051046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 18.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–91. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Diaz de Leon A, Cronkhite JT, Yilmaz C, Brewington C, Wang R, Xing C, et al. Subclinical lung disease, macrocytosis, and premature graying in kindreds with telomerase (TERT) mutations. Chest. 2011;140(3):753–63. doi: 10.1378/chest.10-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez Perez ER, Daniels CE, Schroeder DR, St Sauver J, Hartman TE, Bartholmai BJ, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137(1):129–37. doi: 10.1378/chest.09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aubert G, Hills M, Lansdorp PM. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutation research. 2012;730(1–2):59–67. doi: 10.1016/j.mrfmmm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp PM. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol. 1998;16(8):743–7. doi: 10.1038/nbt0898-743. [DOI] [PubMed] [Google Scholar]
- 25.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5(4):e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Kaminski N. Biomarkers in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2012;18(5):441–6. doi: 10.1097/MCP.0b013e328356d03c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herazo-Maya JD, Noth I, Duncan SR, Kim S, Ma SF, Tseng GC, et al. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med. 2013;5(205):205ra136. doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selman M, Carrillo G, Estrada A, Mejia M, Becerril C, Cisneros J, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS ONE. 2007;2(5):e482. doi: 10.1371/journal.pone.0000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.