Abstract
Background
Minimal Change Disease (MCD) is characterized by increased urinary excretion of CD80, whereas Focal Segmental Glomerulosclerosis (FSGS) is associated with increased serum suPAR. The aim of the study was to assess whether the simultaneous measurement of urinary CD80 and serum suPAR helps differentiate MCD and FSGS.
Methods
Urine and sera were collected from patients with MCD in relapse or in remission, from FSGS patients with nephrotic syndrome, and from normal subjects. CD80 and suPAR were measured by ELISA.
Results
Urinary CD80 was significantly increased in MCD patients in relapse compared with those in remission, as well as with FSGS patients and control subjects. Serum suPAR levels were significantly higher in patients with FSGS when compared to MCD patients in relapse. Urinary suPAR showed a positive correlation with proteinuria in MCD in relapse and FSGS patients, whereas urinary CD80 correlated with proteinuria only in MCD patients in relapse.
Conclusion
Urinary CD80 is elevated in MCD patients in relapse compared to FSGS patients. In contrast, serum suPAR is significantly elevated in FSGS patients. The consistent pattern of these two biomarkers in MCD and FSGS suggests that these two conditions represent different entities rather than a continuum spectrum of one disease.
Keywords: CD80, minimal change disease, focal segmental glomerulosclerosis, suPAR, nephrotic syndrome
Introduction
Idiopathic Nephrotic Syndrome (INS) is defined as the association of nephrotic syndrome with nonspecific glomerular abnormalities including Minimal Change Disease (MCD), Focal Segmental Glomerulosclerosis (FSGS), and mesangial proliferative glomerulonephritis (1). MCD and FSGS are currently thought to be podocytopathies that can share similar renal pathology especially at early stages (2). Proteinuria in these conditions arises from intrinsic or extrinsic “primary” podocyte injury and is represented by foot process effacement.
In MCD, podocytes exhibit diffuse foot process effacement and the cell number is preserved. In FSGS, the number of podocytes is decreased as a consequence of sublethal injury or from apoptotic or necrotic death. Podocytes detach from the GBM leaving denuded GBM areas. This naked GBM contacts the parietal epithelium, forming a synechia (3) leading to focal glomerulosclerosis. Although these glomerulopathies are clearly defined histopathologically, in some cases, because of biopsy sampling, initial findings may be consistent with MCD but repeat renal biopsy would show features typical of FSGS (4). All together, this is raising the issue that MCD and FSGS can be easily misdiagnosed or may in some instances represent a continuum spectrum of the same disease.
Podocyte dysfunction in MCD and FSGS is probably the result of response of local or systemic mediators (vascular permeability factor) (5–8), and in some cases of FSGS, slit diaphragm protein mutations (9). In MCD the circulating factor has not yet been identified. Local mediators may include podocyte-derived Angiopoietin-like-4 (Angptl4) (8) and CD80 (10, 11). We have shown that there is increased podocyte CD80 expression with a concomitant increased in urinary CD80 excretion in MCD (10, 11). No such increase was found in FSGS patients (11).
Recently, suPAR (soluble urokinase type plasminogen activator receptor) has been reported to be elevated in the serum of subjects with primary FSGS (12) and may represent a circulating factor driving proteinuria in this condition. In experimental mouse models (12), circulating suPAR deposits in the glomeruli, binds to and activates podocyte αvβ3 integrin, causing foot process detachment from the glomerular basement membrane and resulting in proteinuria. Removal of suPAR using plasmapheresis in patients with recurrent FSGS to a level below the threshold to activate αvβ3 integrin on human podocytes, was associated with remission (12).
The aim of the study was therefore to begin to assess the diagnostic potential of the simultaneous measurement of urinary CD80 and serum and urinary suPAR to differentiate between patients with MCD and FSGS.
Methods
Patients
Twenty-six children and adolescents with biopsy-proven MCD (aged 2- 21 years, 11 females, 15 males) were included (table 1). Five patients were studied only during relapse and six only during remission. Fifteen were studied both in relapse and remission. Three patients were included in the relapse group although their serum albumin was slightly greater than 3.5 g/dl. They were considered to be in early relapse, according to their urinary protein/creatinine ratio. On follow up, a week later, they presented with edema and low serum albumin. Six patients were included in the remission group despite the serum albumin being less than 3.5 g/dl based on their low protein/creatinine ratio. All MCD patients were followed at the University of Florida.
Table 1.
Characteristics of children with minimal change disease (MCD) in (a) relapse and in (b) remission
| a | ||||||
|---|---|---|---|---|---|---|
| Patient (n=20) |
Age (years) |
Urinary Pr/Cr ratio |
Serum Albumin (g/dl) |
eGFR∫ (ml/min/1.73m2) |
Urinary CD80 (ng/g creat) |
Urinary suPAR (ng/g creat) |
| 1 | 11 | 12.9 | 2.3 | 123 | 494 | 91.9 |
| 2 | 4 | 2.5 | 3 | 186 | 155 | 40 |
| 3 | 3 | >300 mg/dl | 2.4 | 188 | 842 | 99.5 |
| 4 | 6 | 6.4 | 3.2 | 189 | 282 | 32 |
| 5 | 4 | 7.1 | 2.2 | 198 | 36 | 16.1 |
| 6 | 12 | 3.48 | 3.7 | 257 | 332 | 101.6 |
| 7 | 4 | 12.7 | 1.3 | 216 | 398 | 62.2 |
| 8 | 13 | 1.7 | 2.4 | 189 | 98 | 22.6 |
| 9 | 2 | 13.7 | 1.8 | 221 | 578 | 74.7 |
| 10 | 17 | 19.6 | 2.2 | 110 | 186 | 32.5 |
| 11 | 6 | 6.5 | 3.7 | 182 | 328 | 31.9 |
| 12 | 8 | 12 | 1.7 | 202 | 333 | 41 |
| 13 | 6 | 18.8 | 2.1 | 170 | 487 | 59 |
| 14 | 10 | 9.5 | 2.4 | 195 | 2670 | 768.1 |
| 15 | 6 | 1.5 | 4 | 189 | 71 | 45.5 |
| 16 | 2 | 15.3 | 3.1 | 170 | 713 | 40.1 |
| 17 | 7 | 8.5 | 2.7 | 176 | 324 | 24.2 |
| 18 | 5 | 5.9 | 2.7 | 205 | 315 | 33.5 |
| 19 | 7 | 3.8 | 1.4 | 149 | 161 | 49.3 |
| 20 | 6 | 6.2 | 2.4 | 117 | 158.5 | 41 |
| mean ± SD | 6.9±3.9 | 8.8±5.5 | 2.5±0.7 | 181.6±34.7 | 448±564 | 85.3±162.6 |
| b | ||||||
|---|---|---|---|---|---|---|
| Patient (n=21) |
Age (years) |
Urinary Pr/Cr ratio |
Serum Albumin (g/dl) |
eGFR∫ (ml/min/1.73m2) |
Urinary CD80 (ng/g creat) |
Urinary suPAR (ng/g creat) |
| 2 | 4 | 0.16 | 3.9 | 186 | 156 | 30 |
| 3 | 3 | 0.16 | 2.2 | 188 | 92 | 30.2 |
| 4 | 6 | negative | NA* | 153 | 0.4 | 15.7 |
| 6 | 12 | 1.7 | 3.2 | 152 | 1 | 8.9 |
| 7 | 3 | 0.15 | 3.1 | 174 | 0.3 | 8.55 |
| 8 | 12 | 0.6 | 2.2 | 153 | 17 | 17.9 |
| 10 | 17 | 0.03 | 4.8 | 127 | 0.4 | 4.31 |
| 11 | 6 | 0.08 | NA | 182 | 24 | 3.16 |
| 12 | 8 | 0.19 | NA | 202 | 4 | 20.6 |
| 13 | 6 | negative | 4.26 | 158 | 0.4 | 3 |
| 14 | 10 | 0.13 | 3.4 | 156 | 37 | 27.8 |
| 15 | 6 | 0.11 | NA | 198 | 0.5 | 4.3 |
| 18 | 5 | negative | NA | 159 | 0.4 | 1.33 |
| 19 | 7 | negative | NA | 140 | 0.3 | 16.2 |
| 20 | 5 | 0.09 | NA | 184 | 48 | 21.81 |
| 21 | 4 | 0.09 | NA | 163 | 227 | 2.3 |
| 22 | 15 | 0.3 | 4.4 | 161 | 25.4 | 13.4 |
| 23 | 21 | negative | 4.6 | 123 | 27 | 7 |
| 24 | 16 | 0.26 | 4.2 | 312 | 4 | 13.1 |
| 25 | 2 | 2 | 3.4 | 173 | 46 | 19.6 |
| 26 | 11 | 0.08 | 4.9 | 155 | 28 | 40.1 |
| mean ± SD | 8.5±5.2 | 3.7±0.9✧ | 171.3±37.5 | 35.2±58 | 14.7±10.8 | |
| median | 0.11 | |||||
Based on the original Schwartz formula.
NA: not available.
Mean includes only available data.
eGFR – estimated glomerular filtration rate, Pr/Cr – protein/creatinine, SD –standard deviation
Two cohorts of patients with biopsy-proven FSGS were included in this study (table 2). None of the patients presented secondary type of FSGS. One cohort included 15 patients aged 19 – 64 years who were followed at the Hospital Vall d'Hebron- Barcelona, Spain. The second group included 11 patients aged 7 – 19 years seen at the University of Florida. All FSGS patients had active nephrotic syndrome at the time of sample collection.
Table 2.
Characteristics of (a) children and (b) adults with focal segmental glomerular sclerosis (FSGS) in full relapse
| a | |||||||
|---|---|---|---|---|---|---|---|
| Patient (n=11) |
Age (years) |
Type of FSGS |
Urinary Pr/Cr ratio |
Serum Albumin (g/dl) |
eGFR∫ (ml/min/1.73m2) |
Urinary CD80 (ng/g creat) |
Urinary suPAR (ng/g creat) |
| 43 | NA* | NA | 15.7 | NA | NA | 38 | 149.5 |
| 44 | 11 | NA | 10.9 | 2.4 | 160 | 224 | 56.8 |
| 45 | 16 | Collapsing | 18.2 | 2 | 34 | 40 | 62 |
| 46 | 14 | NOS | 17.2 | 2.3 | 167 | 0.3 | 164 |
| 47 | 13 | NOS | >300 mg/dl | 2.3 | 137 | 67.7 | 24.1 |
| 48 | 15 | Collapsing | 5.08 | 3.1 | 96 | 1 | 73.2 |
| 49 | 7 | NOS | 7.44 | 2.3 | 225 | 155 | 50.3 |
| 50 | 19 | NOS | 12.7 | 2.4 | 84 | 0.4 | 56 |
| 51 | 11 | NA | 9.4 | NA | 100 | 0.4 | 25 |
| 52 | 12 | Collapsing | 6.51 | 2.6 | 134 | 81 | 31.1 |
| 53 | 17 | NA | 4.4 | 4.1 | 43 | 0.4 | 30.3 |
| mean ± SD | 13.5±3.2✧ | 10.7±4.7 | 2.6±0.5✧ | 118±55.5✧ | 55.2±74 | 65.6±47.9 | |
| b | |||||||
|---|---|---|---|---|---|---|---|
| Patient (n=15) |
Age (years) |
Type of FSGS |
Urinary Pr/Cr ratio |
Serum Albumin (g/dl) |
eGFR (ml/min/1.73m2) |
Urinary CD80 (ng/g creat) |
Urinary suPAR (ng/g creat) |
| 54 | 30 | NOS | 11 | 2.8 | 104 | 184 | 41 |
| 55 | 52 | NOS | 4.6 | 3.4 | 126 | 19 | 43 |
| 56 | 39 | Perihilar | 4.8 | 3.6 | 98 | 26 | 67 |
| 57 | 42 | NOS | 2.9 | 3.2 | 113 | ND** | 18 |
| 58 | 53 | NOS | 3.8 | 2.9 | 110 | ND | 14 |
| 59 | 21 | TIP | 6.1 | 1.9 | 98 | 44 | 17 |
| 60 | 60 | TIP | 3.98 | 3.1 | 106 | 2 | 14 |
| 61 | 47 | NOS | 3.1 | 3.5 | 125 | ND | 23 |
| 62 | 64 | NOS | 7.5 | 2.9 | 132 | 85 | 17 |
| 63 | 35 | NOS | 8 | 2.7 | 121 | 37 | 28 |
| 64 | 43 | NOS | 6.8 | 2.9 | 100 | 26 | 15 |
| 65 | 36 | Collapsing | 6.5 | 2.4 | 96 | 39 | 58 |
| 66 | 19 | Collapsing | 12 | 1.7 | 110 | 250 | 65 |
| 67 | 48 | TIP | 7.9 | 2.2 | 104 | ND | 8 |
| 68 | 52 | NOS | 4 | 3.1 | 118 | 5 | 33 |
| mean ± SD | 42.7±13 | 6.2±2.7 | 2.8±0.5 | 110.7±11 | 65.1±79.6✫ | 30.7±19.6 | |
Based on the original Schwartz formula.
NA: not available.
Mean includes only available data.
ND: not detectable.
Mean includes only detectable values.
eGFR – estimated glomerular filtration rate; Pr/Cr – protein/creatinine; NOS – not otherwise specified; SD – standard deviation
Sixteen subjects, aged 4 to 17 years, served as control (table 3). These individuals were followed at the University of Florida- Pediatric Nephrology Outpatient Clinic (n=5 hypertension, n=4 hematuria, n= 4 urinary tract infection and n=1 lithiasis, primary lymphedema and asymptomatic elevated renin activity). None of these control subjects had any underlying immunological disorder.
Table 3.
Characteristics of control subjects
| Subject (n=16) |
Age (years) |
Urinary protein in dipstick |
Serum Albumin (g/dl) |
Urinary CD80 (ng/g creat) |
Urinary suPAR (ng/g creat) |
|---|---|---|---|---|---|
| 27 | 16 | negative | Not done | 35 | 26.15 |
| 28 | 9 | negative | Not done | 0.35 | 55.78 |
| 29 | 16 | negative | Not done | 0.1 | 14.74 |
| 30 | 16 | negative | Not done | 0.1 | 3.04 |
| 31 | 15 | negative | Not done | 0.1 | 34.8 |
| 32 | 16 | negative | Not done | 0.1 | 9.04 |
| 33 | 9 | negative | Not done | 0.3 | 6.22 |
| 34 | 14 | negative | Not done | 0.1 | 17.67 |
| 35 | 13 | negative | Not done | 0.1 | 17.18 |
| 36 | 17 | negative | Not done | 0.2 | 18.31 |
| 37 | 5 | negative | Not done | NA* | 31.28 |
| 38 | 13 | negative | Not done | 19.39 | 1.21 |
| 39 | 4 | negative | Not done | NA | 55.18 |
| 40 | 11 | negative | Not done | 151 | 81.14 |
| 41 | 15 | negative | Not done | NA | 25.45 |
| 42 | 6 | negative | Not done | 44.6 | 42.47 |
| mean ± SD | 12.1±4.3 | 19.3±42.3✧ | 27.4±22 |
NA: not available.
Mean includes only available data.
SD – standard deviation
The study was approved by the Institutional Review Boards of both Institutions and informed consent and assent when indicated was obtained from participants.
Definitions
MCD was defined according to the established criteria by the International Study for Kidney Diseases in Children (13). Patients with FSGS had renal biopsies demonstrating focal and segmental consolidation of the glomerular tuft with and without synechia and hyalinosis, with either negative immunofluorescence or only segmental IgM or minimal C3 staining, and no electron dense deposits on electron microscopy. The different subtypes of FSGS were classified according to D’Agati et al. (14).
Relapse was defined as the presence of proteinuria (urinary protein creatinine ratio > 3.0 mg/mg or 3 + or greater by using the tetrabromophenol-citrate buffer colorimetric qualitative dipstick test), hypoalbuminemia (< 3.5 g/dl) and edema. Remission was defined as no proteinuria using the colorimetric qualitative test or by urinary protein creatinine ratio < 0.2 mg/mg (or less than 0.5 for children under the age of 5 years).
Urinary CD80 measurements
CD80 was measured using a commercially available ELISA kit (Bender MedSystems, Burlingame, CA, USA) and results were adjusted for urinary creatinine excretion.
suPAR measurements
Serum and urinary suPAR was measured with the Quantikine Human suPAR Immunoassay (R&D Systems) (9).
Urinary protein, creatinine and serum albumin were measured using an Autoanalyzer.
Statistical analyses
Data graphics and statistical analysis were performed using Prism 5 (GraphPad). Kruskal–Wallis tests and Mann–Whitney tests were applied to evaluate differences between the groups and Spearman correlation coefficient was calculated between urinary CD80 and proteinuria and urinary suPAR and proteinuria. P < 0.05 was regarded as statistically significant. Values are presented as means ± s.d. unless otherwise stated.
Results
Demographics and laboratory tests at the time of sample collection are shown on Table 1, 2 and 3.
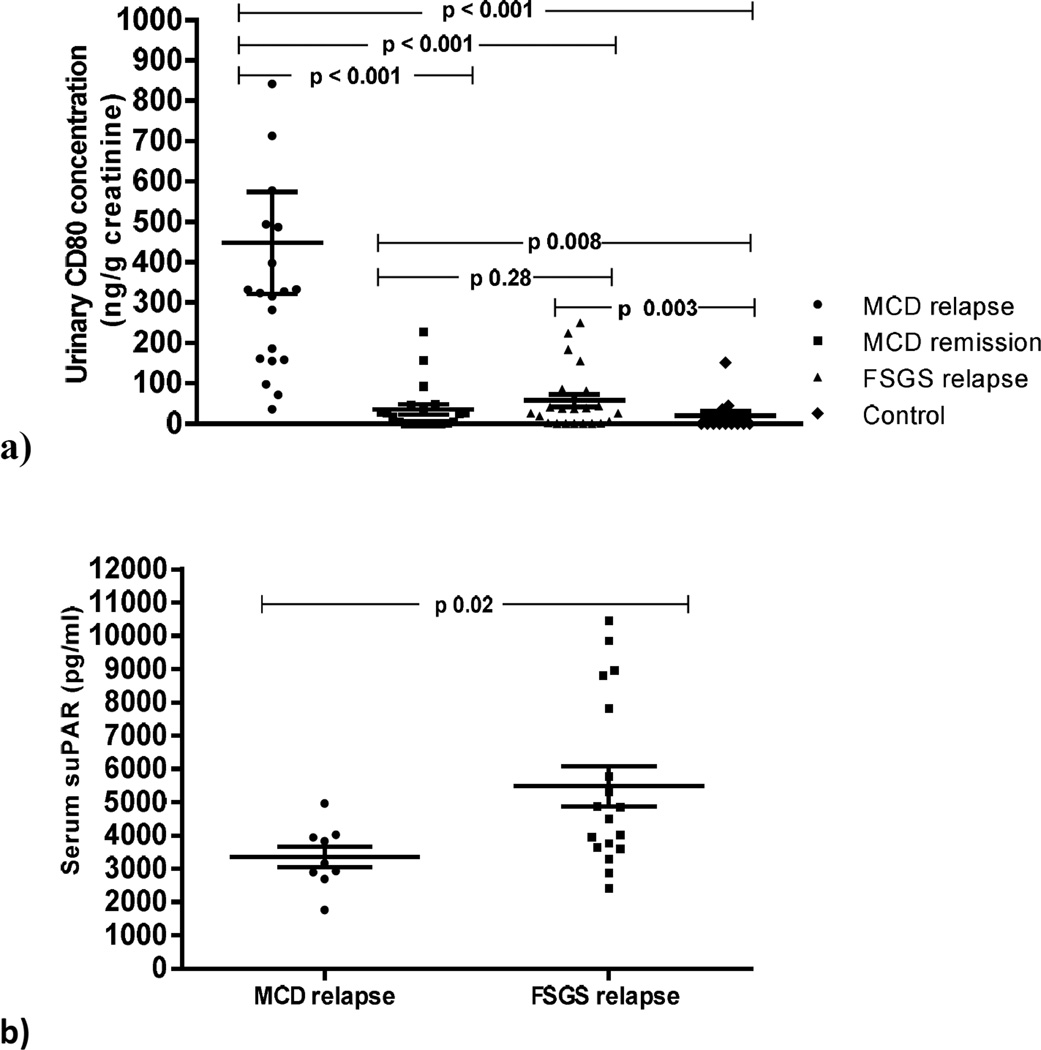
Urinary CD80 excretion in MCD and FSGS (Figure 1a
Figure 1.
Urinary CD80 concentration in minimal change disease (MCD) patients in relapse, MCD patients in remission, FSGS patients and control subjects (a). Serum suPAR concentration in MCD patients in relapse and FSGS patients (b).
Urinary CD80 excretion was significantly increased in MCD patients in relapse (448±564 ng/g creat.) when compared to patients with MCD in remission (35.2±58 ng/g creat.) (p < 0.001), patients with FSGS (60.2±73.4 ng/g creat.) (p < 0.001) and control subjects (19.3±42.3 ng/g creat.) (p < 0.001). No differences were observed in CD80 urinary excretion between MCD patients in remission and those with FSGS. Urinary CD80 levels were significantly higher in MCD patients in remission and in FSGS patients when compared to control subjects. Nine out of 13 available control subjects had a urinary CD80 below 0.5 ng/g creat.
Serum suPAR (Table 4
Table 4.
Serum suPAR level in different FSGS subtypes
| Patient (n=17) |
Type of FSGS |
suPAR (pg/ml) |
|---|---|---|
| 46 | NOS | 8964 |
| 54 | NOS | 3289 |
| 55 | NOS | 4504 |
| 57 | NOS | 3645 |
| 58 | NOS | 3602 |
| 61 | NOS | 4017 |
| 62 | NOS | 7813 |
| 63 | NOS | 8794 |
| 64 | NOS | 5302 |
| 68 | NOS | 5769 |
| mean ± SD 5569.9±2195.6 | ||
| 59 | TIP | 2418 |
| 60 | TIP | 2866 |
| 67 | TIP | 10452 |
| mean ± SD 5245.3±4514.6 | ||
| 52 | Collapsing | 4847 |
| 65 | Collapsing | 9857 |
| 66 | Collapsing | 3763 |
| mean ± SD 6155.6±3250.9 | ||
| 56 | Perihilar | 4868 |
FSGS – focal segmental glomerular Sclerosis; NOS – not otherwise specified;
Serum suPAR levels were significantly higher in patients with FSGS when compared to MCD patients in relapse (p=0.02) (Figure 1b) though there was considerable overlap. Serum suPAR levels did not show significant difference among FSGS individual histological FSGS subtypes.
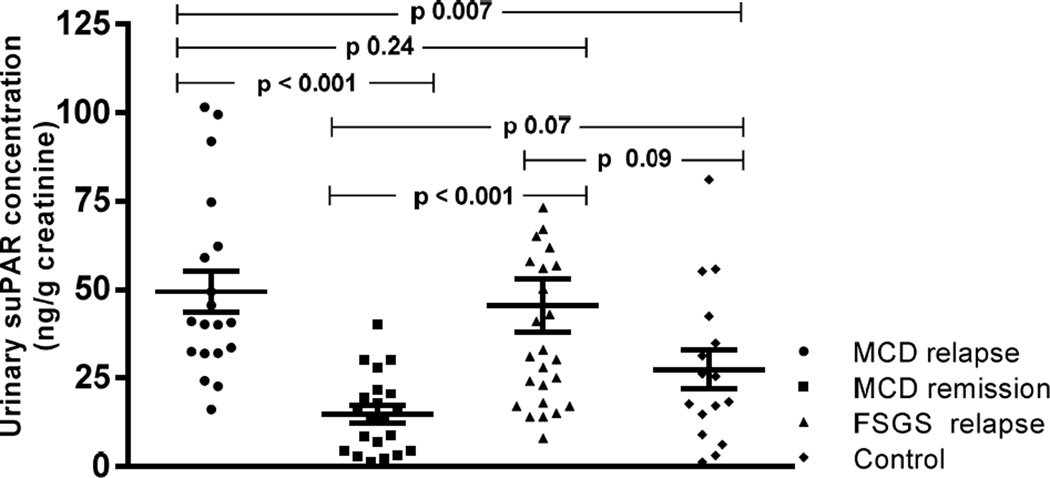
Urinary suPAR excretion in MCD and FSGS (Figure 2
Figure 2.
Urinary suPAR concentration in minimal change disease (MCD) patients in relapse, MCD patients in remission, FSGS patients and control subjects.
Urinary suPAR levels were increased in both MCD patients in relapse and those with FSGS when compared to MCD patients in remission (p < 0.001). MCD patients in relapse showed greater concentration of urinary suPAR than control subjects (p=0.007). There was no significant difference in urinary suPAR levels between MCD patients in relapse and those with FSGS. No statistical significance differences were observed in urinary suPAR between FSGS patients and control subjects.
A significant correlation was observed between urinary CD80 and urinary suPAR levels in MCD patients in relapse (r=0.63; p=0.002). No correlation was seen between urinary CD80 and urinary suPAR in FSGS patients (r=0.02; p=0.8).
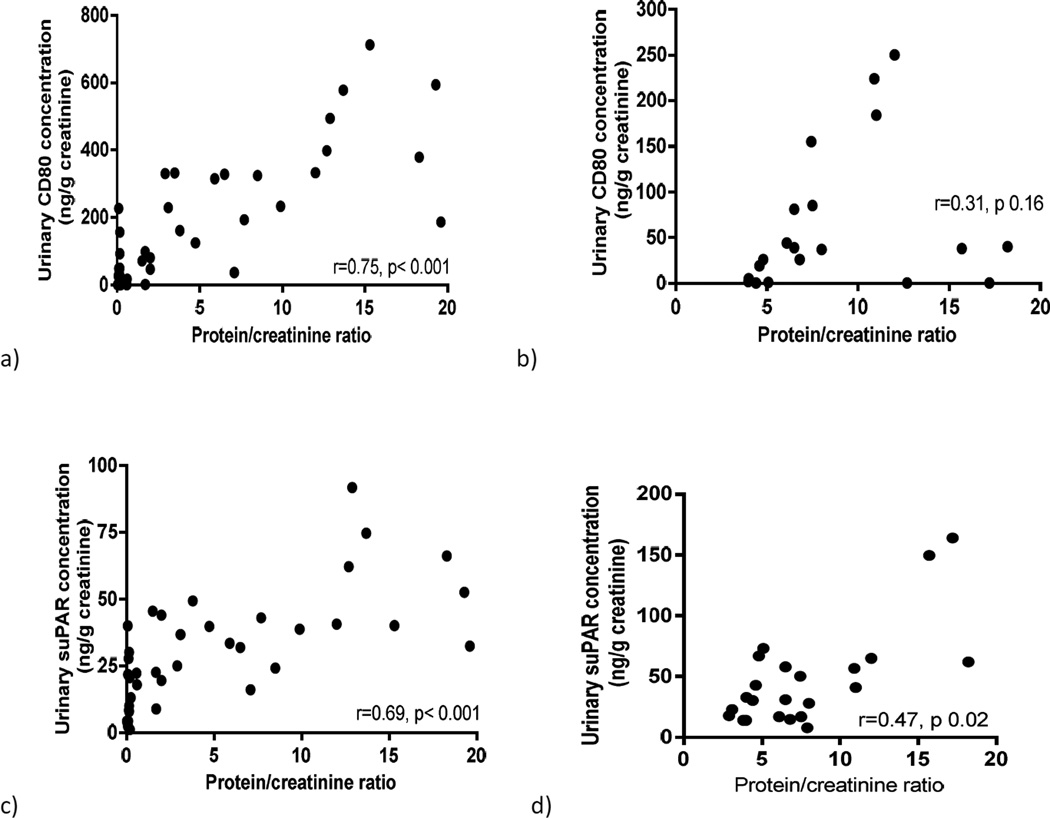
Proteinuria, urinary CD80 and urinary suPAR
Urinary CD80 showed a strong positive correlation with the degree of proteinuria in MCD patients (r=0.75, p < 0.001) (Figure 3a) whereas no such correlation was found in FSGS patients (r=0.31, p=0.16) (Figure 3b). Urinary suPAR was found to have positive correlation with proteinuria in both MCD patients (r=0.69, p < 0.001) (Figure 3c) and FSGS patients (r=0.47, p=0.02) (Figure 3d).
Figure 3.
Correlation between urinary CD80 and proteinuria in minimal change disease (MCD) patients (a) and FSGS patients (b) and between urinary suPAR and proteinuria in MCD patients (c) and FSGS patients (d).
Discussion
This study suggests that MCD and FSGS have different pathogenetic mechanisms relative to the development of proteinuria. CD80 appears to play a major role in MCD while suPAR is implicated in FSGS. These data indicate that MCD and FSGS represent two different entities rather than a continuum spectrum of one disease.
The role of CD80 in proteinuria has been studied in experimental models of proteinuria (15). Reiser et al found that mice injected with lipopolysaccharide (LPS) developed proteinuria with glomerular podocyte CD80 expression likely through podocyte Toll-like receptor 4 expression. Our group has previously reported data supporting the role of CD80 in MCD (10, 11). Indeed, patients with MCD in relapse showed increased urinary CD80 compared to patients with MCD in remission, control subjects or other glomerular diseases including FSGS.
uPAR and suPAR have been identified to play a critical role in human FSGS and animal models of proteinuria resembling FSGS (12, 16). uPAR is a glycosylphosphatidylinositol (GPI)-anchored three-domain protein which not only serves as receptor for urokinase but also interacts with other transmembrane protein such as integrins. Mice administered with LPS developed foot process effacement and proteinuria. In contrast, when LPS was administered to uPAR knock-out mice (Plaur−/−), there was no development proteinuria. Exogenous suPAR administration to uPAR knock-out mice (Plaur−/−) led to albuminuria and suPAR deposition along the podocytes as well as activation of αvβ3 integrin (12).
A recent study showed that 84.3% and 55.3% of FSGS patients with normal eGFR from two different populations had elevated serum suPAR (17). In addition, Wei et al studied the level of serum suPAR in patients with different glomerulopathies (including 63 patients with FSGS) and in a normal population and found that most of the patients with a serum suPAR above 3000 pg/ml had FSGS as the underlying disease. However, in some FSGS patients, serum suPAR levels were not different from normal controls or patients with MCD. It is not clear yet whether the low suPAR levels in these FSGS patients is due to genetic mutations where the defect is at the level of other podocyte genes and not associated with a circulating factor, or to suPAR with different biochemical properties that are not readily detected by commercial ELISA tests. It is also possible pathologically processed suPAR is podocyte pathogenic even though the total measured suPAR level are low or normal in these FSGS patients.
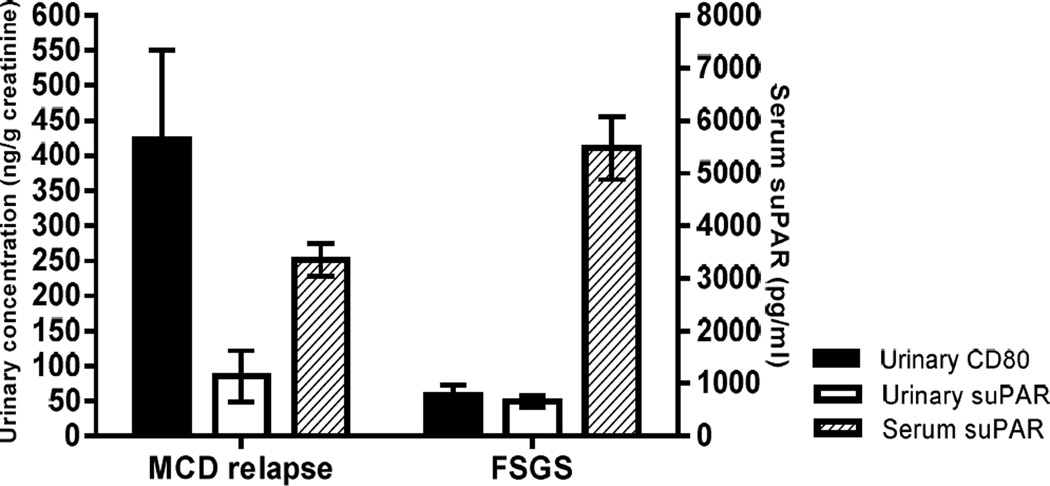
This study suggests that serum suPAR and urinary CD80 are potential biomarkers that can be used in concert and that might allow us to differentiate between FSGS and MCD. Such a biomarker driven additional characterization helps in the correct diagnosis of the proteinuric disorder. A pattern of elevated serum suPAR and normal urinary CD80 is consistent with the diagnosis of FSGS. In contrast, a normal or mildly elevated serum suPAR with an elevated urinary CD80 rather suggests MCD as the underlying pathology (Figure 4) (11). Of interest is a patient with FSGS secondary to podocin mutation in which we found a distinctive mixed pattern consistent of elevated serum suPAR and elevated urinary CD80. Therefore, the combination of these two biomarkers may help differentiate between primary and the genetic forms of FSGS (18). Clearly more work is needed to describe the latter type of patients in greater detail in the future.
Figure 4.
Relationship between urinary CD80, urinary suPAR and serum suPAR for each studied group [minimal change disease (MCD) in relapse and focal segmental glomerular sclerosis (FSGS)].
Urinary suPAR is elevated in both FSGS and MCD patients in relapse when compared to control subjects. This finding supports the notion that suPAR is excreted at larger amounts if the filtration barrier is leaky and in MCD might rather be the consequence than the cause of proteinuria. In fact, this study shows a significant correlation between urinary suPAR and proteinuria in both FSGS patients and MCD patients in relapse.
These findings may have clinical and pathogenic implications. A renal biopsy to define the underlying pathology would be indicated if the suPAR-CD80 pattern suggests FSGS to guide therapy as well as prognosis. Testing for suPAR might currently be best justified in FSGS pretransplant evaluation wherein high levels might call for pre-emptive plasmapheresis, a treatment known to lower suPAR levels and to reduce the number of post-transplant FSGS recurrences. suPAR testing which includes podocyte integrin activation assays (11) might also be of value in the management of post-transplant FSGS recurrence to assess the continued need for plasmapheresis (12).
This study further suggests that proteinuria in MCD and FSGS have different pathogenic mechanisms. We have postulated that increased podocyte CD80 expression in MCD is a result of a circulating factor that stimulates TLR 3 or TLR4 (19). We have demonstrated in cell culture studies that TL3 stimulation is followed by the translocation of the p65 subunit from the cytoplasm to the nucleus resulting in the activation of NFκB and subsequent CD80 upregulation. CD80 upregulation is followed by changes in the actin cellular cytoskeleton resulting in opening of the slit diaphragms and proteinuria.
The mechanism by which increased serum suPAR levels results in proteinuria in FSGS is not likely due to podocyte CD80 stimulation. Urinary suPAR did not show a correlation with urinary CD80 in FSGS patients. In addition, urinary suPAR was elevated in FSGS and MCD patients in relapse but no statistical differences were found among the groups tested despite the fact that FSGS patients had a greater serum suPAR concentration compared to MCD patients suggesting production of suPAR in FSGS. Finally, we have not observed CD80 podocyte expression in FSGS patients.
In summary, urinary CD80 is elevated in MCD patients in relapse compared to FSGS patients, who in contrast, have elevated concentration of serum suPAR. The consistent pattern of these two biomarkers in MCD and FSGS would suggest that they represent different entities rather than a continuum spectrum of one disease. Moreover, the combination of these biomarkers may help differentiating between genetic and primary FSGS as well. These findings, therefore, could have important clinical significance regarding diagnosis of these diseases.
Acknowledgments
This study was supported by NIH R01DK080764 to E.G. and in part by grants from the National Institutes of Health DK073495 and DK089394 to J.R.
Footnotes
Disclosures
JR and CW are inventors of pending (JR, CW) and issued (JR) patents on novel technologies aruound proteinuric kidney diseases and stand to gain royalties from their commercialization.
The other authors declare no conflict of interest.
References
- 1.Habib R, Kleinknecht C. The primary nephrotic syndrome in childhood: Classification and clinicopathologic study of 406 cases. Pathol Annu. 1971;6:417–474. [PubMed] [Google Scholar]
- 2.Barisoni L, Schnaper HW, Kopp JB. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol. 2007;2:529–542. doi: 10.2215/CJN.04121206. [DOI] [PubMed] [Google Scholar]
- 3.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 4.Ponticelli C, Glassock RJ. Treatment of Primary Glomerulonephritis. 2nd edn. New York: Oxford University Press; 2010. p. 181. [Google Scholar]
- 5.Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 6.Sharma M, Sharma R, McCarthy ET, Savin VJ. "The FSGS factor:" enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma. J Am Soc Nephrol. 1999;10:552–561. doi: 10.1681/ASN.V103552. [DOI] [PubMed] [Google Scholar]
- 7.Reiser J, Mundel P. Danger signaling by glomerular podocytes defines a novel function of inducible B7-1 in the pathogenesis of nephrotic syndrome. J Am Soc Nephrol. 2004;15:2246–2248. doi: 10.1097/01.ASN.0000136312.46464.33. [DOI] [PubMed] [Google Scholar]
- 8.Franceschini N, North KE, Kopp JB, McKenzie L, Winkler C. NPHS2 gene, nephrotic syndrome and focal segmental glomerulosclerosis: a HuGE review. Genet Med. 2006;8:63–75. doi: 10.1097/01.gim.0000200947.09626.1c. [DOI] [PubMed] [Google Scholar]
- 9.Clement LC, Avila-Casado C, Macé C, Soria E, Bakker WW, Kersten S, Chugh SS. Podocyte-secreted Angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–122. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garin EH, Diaz LN, Mu W, Wasserfall C, Araya C, Segal M, Johnson RJ. Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol. 2009;20:260–266. doi: 10.1681/ASN.2007080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garin EH, Mu W, Arthur JM, Rivard CJ, Araya CE, Shimada M, Johnson RJ. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010;78:296–302. doi: 10.1038/ki.2010.143. [DOI] [PubMed] [Google Scholar]
- 12.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20:765–771. doi: 10.1038/ki.1981.209. [DOI] [PubMed] [Google Scholar]
- 14.D'Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Reiser J, von Gersdorff G, Loos M, Oh L, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 17.Wei C, Trachtman H, Li J, Dong C, Friedman AL, Gassman JJ, McMahan JL, Radeva M, Heil KM, Trautmann A, Anarat A, Emre S, Ghiggeri GM, Ozaltin F, Haffner D, Gipson DS, Kaskel F, Fischer DC, Schaefer F, Reiser J PodoNet and FSGS CT Study Consortia. Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol. 2012;23:2051–2059. doi: 10.1681/ASN.2012030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cara-Fuentes G, Araya C, Wei C, Rivard C, Ishimoto T, Reiser J, Johnson R, Garin EH. CD80, suPAR and Nephrotic Syndrome in a case of NPHS2 mutation. Nefrologia. 2013;33:727–731. doi: 10.3265/Nefrologia.pre2013.Jun.12085. [DOI] [PubMed] [Google Scholar]
- 19.Shimada M, Ishimoto T, Lee PY, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Wymer DT, Yamabe H, Mathieson PW, Saleem MA, Garin EH, Johnson RJ. Toll-like receptor 3 ligands induce CD80 expression in human podocytes via an NF-kB-dependent pathway. Nephrol Dial Transplant. 2012;27:81–89. doi: 10.1093/ndt/gfr271. [DOI] [PubMed] [Google Scholar]