Abstract
Modern immune therapies [PD-1/PD-L1 and CTLA-4 checkpoints blockade, and adoptive cell transfer (ACT)] have remarkably improved the response rates of metastatic melanoma. These modalities rely on the killing potential of cytotoxic T lymphocytes (CTL) as proximal mediator of anti-melanoma responses. Mechanisms of tumor resistance to and the predominant cytotoxic pathway(s) employed by melanoma-reactive CTL are important outcome determinants. We hypothesized that down-modulation of death receptors in addition to aberrant apoptotic signaling might confer resistance to death signals delivered by CTL. To test these two hypotheses, we used an in vitro model of MART CTL resistant melanoma sublines. TCR transgenic and patient-derived CTLs employed the TNF-related apoptosis-inducing ligand (TRAIL) cytotoxic pathway, through DR5. Further, rhTRAIL and Drozitumab (anti-DR5 agonistic mAb) were used to explicitly verify the contribution of the DR5/TRAIL pathway in killing melanomas. CTL-resistance was due to DR5 down-regulation and an inverted ratio of pro- to anti-apoptotic molecules, both of which were reversed by the histone deacetylase inhibitor (HDACi) SAHA. Apoptosis negative (c-IAP-2 and Bcl-xL) and positive (DR5) regulators were potential incriminators partly regulating CTL sensitivity. These pre-clinical findings suggest that exposure to this chromatin remodeling drug of immune-resistant melanomas can skew towards an intracellular pro-apoptotic milieu, increase death receptor expression, and overcome acquired immune-resistance.
Keywords: TRAIL/Apo2L, DR5, Apoptosis, Immunotherapy, Signal transduction, Adoptive Cell Transfer, Melanoma, SAHA, Chromatin Remodeling, Drozitumab, Gene Regulation
Introduction
Various modern immune therapies – CTLA-4 and PD-1/PDL-1 blockade, and adoptive cell transfer (ACT) – have increasing effectiveness in producing clinical responses in metastatic melanoma. In all of these modalities, the proximal mediators of anti-tumor response are tumor-reactive cytotoxic T cells (1).
Advanced melanomas are composed of heterogenous cell populations: the variable sensitivity of individual melanoma cells to apoptotic death signals delivered by these modalities may account for the variability of anti-tumor responses. Thus, delineation of the underlying mechanisms of CTL-resistance, and strategies to reverse resistance and enhance sensitivity, could potentially inform clinical trials and improve response rates.
TNF-related apoptosis-inducing ligand, TRAIL (Apo2L), frequently employed by cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, is a potent and selective apoptosis-inducer in tumors while sparing normal cells. TRAIL and agonistic antibodies to its death receptors are attractive therapeutic approaches. Their potential use has been broadly evaluated in various cancers including melanoma (2–5), which are currently being evaluated in clinical trials (6–9). TRAIL engages death-inducing TRAIL-R1 (DR4) and TRAIL-R2 (DR5), and decoy TRAIL-R3 (DcR1) and TRAIL-R4 (DcR2) receptors. Upon ligation of TRAIL with trimeric death receptors, the adaptor protein Fas-associated death domain (FADD), and initiator caspases (2, 8, 10) are recruited to the cytoplasmic death domain (DD) of the receptor forming the multimeric death inducing signaling complex (DISC). This leads to caspase-8 activation, Bid cleavage, and subsequent apoptosis either through direct caspase-3 activation (extrinsic pathway) or mitochondrial destabilization and apoptosome formation (intrinsic pathway) (4, 5, 10). Despite its potent anti-tumor activity, some tumors are inherently resistant or acquire secondary resistance to TRAIL.
Histone deacetylase inhibitors (HDACi) activate transcription of genes responsible for creating an intracellular proapoptotic milieu (11), render tumors more recognizable targets by the immune system, and are considered immune sensitizers (12). We have recently reported significant improvement of anti-tumor activity of ACT by HDACi in an in vivo melanoma model (13), providing the rationale for further examining the sensitizing effects of HDACi on CTL-resistant melanomas. The FDA approved class II HDACi SAHA increases surface death receptors and regulates the expression of apoptosis-associated genes. As single agent or combined with other agents, SAHA has some anti-melanoma activity in vitro and in vivo (12, 13).
Several melanoma lines, which present the melanoma antigenic epitope MART-127–35 in the context of HLA A*0201 and are sensitive to MART-specific CTL (F5 CTL) killing, were serially exposed to F5 CTL yielding completely resistant (R) sublines. These R sublines expressed intact MART-1/A*0201 complex, had reduced DR5 expression and an inversion of apoptotic genes programs favoring resistant phenotype. Pretreatment of R sublines with SAHA increased DR5 expression, restored the gene expression profile to favor an intracellular proapoptotic milieu, and restored CTL sensitivity of R sublines through TRAIL/DR5. This study provides rational molecular basis for combining small molecule sensitizing agents in modern melanoma immune therapy protocols.
Materials and Methods
Cell lines and sublines
Human melanoma lines were established from surgical specimens as described. The generation of F5 CTL R lines has been reported previously (14, 15). Briefly, P cells were grown in the presence of step-wise increasing numbers of F5 CTLs for a total of 8 weeks (2–3 weeks for each E:T). Thirty percent to 50% of melanoma cells survived the first cycle of selection (20:1, 2 weeks), percentage of which drastically reduced during subsequent selection cycles until no further killing was observed. Remaining viable melanoma cells were then subjected to two consecutive rounds of limiting dilution analysis. Single cells were propagated and maintained in RPMI-1640 supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS). After immunoselection, sublines were maintained in medium containing excess (10:1) F5 CTLs, but were grown in F5 CTL-free medium at least 1 week prior to analysis. Cultures were incubated in controlled atmosphere incubator at 37°C with saturated humidity at 0.25 × 106 cells/mL and were used at 50% to 70% confluency for each experiment. Cultures were routinely (once/month) checked for mycoplasma contamination (Lonza).
Reagents
Antibodies specific to MART-1 and anti-cytochrome C and smac/DIABLO were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and DAKO (Carpinteria, CA), respectively. Mouse anti-actin mAb was obtained from Chemicon. rhTRAIL was purchased from Peprotech (NJ). DR5, Bcl-xL and c-IAP-2 siRNA was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). DR5 expression plasmid and Drozitumab were kindly provided by Dr. Avi Ashkenazi (Genentech Inc., San Fransisco, CA) under Material Transfer Agreement (MTA). Blocking Abs and fluorochrome conjugated Abs for FACS analyses were purchased from eBiosciences (San Diego, CA). Suberoylanilide hydroxanic acid (SAHA) procured commercially, was diluted in dimethyl sulfoxide (DMSO). DMSO concentration did not exceed 0.1% in any experiment.
Quantitative real-time PCR (qPCR)
Samples were analyzed with iQ SYBR Green Supermix using iCycler Sequence Detection System (BioRad) using RT2 profiler apoptosis PCR arrays. Total RNA was extracted from 107 cells for each condition with RNeasy mini kit (Qiagen) and quantified by 3.1.2 NanoDrop ND-1000 spectrophotometer. Three micrograms of total RNA was reverse transcribed to first-stranded cDNA for 1 hour at 42°C with 200 units SuperScript II RT and 20µM random hexamer primers. Amplification of 2.5µL of cDNAs was performed using gene-specific primers. Expression of each molecule was calculated with the assumption that control samples were considered as 1.
Transduction of CD8 CTLs with F5 MART-1 TCRα/β retrovirus
Nonadherent population of healthy donor human peripheral blood mononuclear cells (PBMC) were cultured in AIM-V media supplemented with 5% human AB serum, αCD3 antibody (50 ng/mL), and IL-2 (300 IU/ mL) for 48 hours. CD3+CD8+ CTLs were isolated by EasyStep Negative Selection enrichment kits (Stem Cell Technologies, Vancouver, Canada) according to manufacturer’s instructions. CTLs were transduced with MSCV MART-1 TCR as described (14, 15). CD8+ CTLs with more than 95% MART-1 TCRα/β expression were used in all experiments.
Cell-mediated Cytotoxicity assay
Melanoma cultures were trypsinized for 5 minutes, washed once in cold PBS and labeled with 100 µCi of Na251CrO4 for 1 hour (37°C/5%CO2). After 3× washes, 104 cells were added to V-bottom 96-well plates and used immediately as described (14, 15). Percentage of specific 51Cr-release was measured as: % cytotoxicity = (experimental release − spontaneous release)/(total release − spontaneous release) × 100.
Immunoblot analysis
A total of 107 cells were grown in complete medium (±inhibitors), lysed at 4°C in RIPA buffer [50 mmol/L Tris-HCl (pH 7.4), 1% NP-40, 0.25% sodium deoxycholate, 150 µmol/L NaCl] supplemented with protease inhibitor cocktail (Complete Mini; Roche) and subjected to standard immunoblot analysis as described (14–16). The relative intensity of bands, hence, relative alterations in expression was assessed by densitometric analysis of digitized images obtained from multiple independent blots. For confirmation, membranes were stripped and reprobed with anti β-actin mAb.
Evaluation of Active Caspase-3 Levels by Flow Cytometric Analysis
Melanoma cells were treated under the conditions explained for PI staining. At the end of the incubation period, the cells were washed once with ice-cold 1× PBS/0.1% BSA and were resuspended in 100 µl ice-cold 1× PBS/0.1% BSA. Fifty microliters of cell suspension (containing 2×106 cells) were aliquoted to each sample and fixed with the perm/fix solution (PharMingen) for 20 min. Thereafter, the cells were washed twice with 1× perm/wash (PharMingen) solution and stained with the FITC-labeled anti-active caspase-3 antibody (1:1000 predetermined dilution) for 30 min (light protected). Thereafter, the samples were washed once with 1× perm/wash solution followed by flow cytometric analysis (Coulter Electronics, Miami, FL). As negative control, the cells were stained with isotype control (pure IgG1) under the same conditions described above (16).
Gene knock-down by small interfering RNA (siRNA)
Cells were seeded in the wells of 6-well plates in 2 mL antibiotic-free growth medium and incubated until cell confluency reached 50%. A total of 8–10 µL (40 nM) of various siRNA constructs (cIAP-2, Bcl-xL, DR5) or a relevant amount of a control siRNA solution was mixed with 4 µL of Lipofectamin 2000 in OptiMeM solution (Invitrogen). After 6 hours supernatant was aspirated, 2 mL of fresh medium was added and transfection was performed for 80 hours according to the manufacturer's instructions (Santa Cruz Biotechnology, Santa Cruz, CA (14, 15)).
Statistical analysis
Assays were set up in duplicates or triplicates and results were expressed as mean ± standard error of the mean (SEM). Statistical analysis and P values were calculated by two-tailed paired t test with confidence interval (CI) of 95% for determination of significance of differences between treatment groups (P < 0.05: significant). ANOVA was used to test significance among the groups using InStat 2.01 software.
Results
MART TCR-transduced T cells (F5 CTL) induce apoptosis in melanoma targets through TRAIL/DR5 pathway
Human peripheral blood T cells, transduced to high efficiency (>95%) with a retroviral vector encoding MART TCR α/β chains (F5 CTL), efficiently recognize and kill three representative MART-1+/A*0201+ melanoma cell lines with cytotoxic indices of 43.4±3.2% (M202), 68.7±1.8% (M249), and 61.3±1.9% (M329) (Figure 1A). These melanoma lines were predominantly killed in a caspase-dependent manner and through DR5 receptor as blocking antibodies to DR5, but neither to DR4 nor FasR, inhibited killing (Figure 1B). Treatment of these targets with rhTRAIL or Fas agonistic antibody (CH-11) resulted in apoptosis induction by the former, but not the latter, as measured by active caspase-3 levels. In addition, antagonistic TRAIL Ab significantly reduced the cytotoxic potential of F5 CTL in 51Cr-release assays (Figure 1C). Blocking FasL on F5 CTLs did not affect their cytotoxic potential (data not shown). These results indicate that F5 CTL primarily use TRAIL/DR5 pathway in killing TRAIL-sensitive melanomas.
Figure 1. Death receptor-mediated killing of melanomas.
A. 51Cr-labeled parental and resistant (R) cells were incubated with F5 CTLs at various E:T ratios in 6-hour standard 51Cr-release assay. B. Caspase and death Receptor blockade. Parental cells were either left untreated or pretreated with zVAD-fmk (1µmol/L – 18 hours) or antagonistic anti–FasR, –DR4, and –DR5 (1–2µmol/L – 6 hours), coincubated with F5 CTLs and subjected to 51Cr-release assay. C. TRAIL killing of melanomas. Parental cells were incubated with (i) rhTRAIL or (ii) agonistic FasL Ab (CH-11) (5–25ng/ml–18hr) and percentage apoptosis was assessed by active caspase-3 levels using FACS analysis. The FasL sensitive DT140 murine lymphoma line was used as control. (iii) F5 CTL either left untreated or pretreated with TRAIL blocking Ab (1µmol/L–6hr) prior to coincubation with melanomas in 51Cr-release assay. Results are presented as mean ± SEM of duplicate samples (n = 3). * P values <0.05 significant.
CTL resistant melanomas have reduced DR5 expression
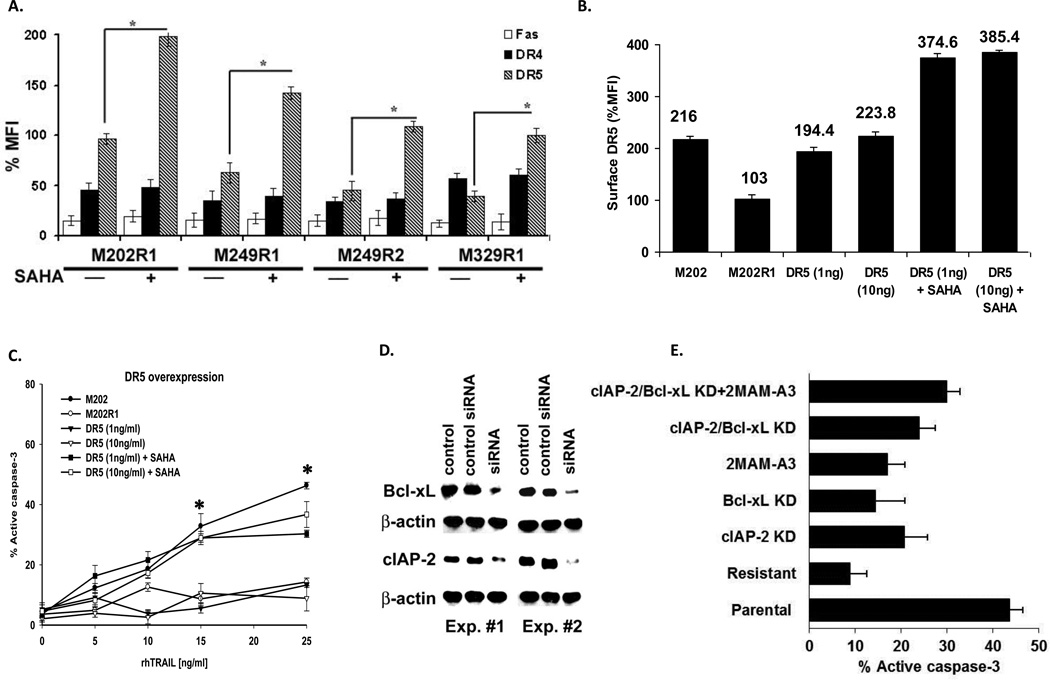
These three F5 CTL-sensitive melanoma cell lines – M202, M249, M329 – were subjected to serial cycles of in vitro exposure to these cytotoxic transgenic T cells to generate sublines resistant to killing (Figure 1A). Some of the characteristics of these cells have been described previously (14, 15) and the details for their generation are described in the Materials and Methods section. The respective resistant sublines (designated M202R1, M249R1, R2 and M329R1) retained surface expression of A*0201 and MART-1 expression, and were comparable to the parental sensitive cells in presentation of MART-1/A*0201 complex as measured by IFN-γ and IL-2 cytokine release recognition assay (Figure 2A). The three resistant sublines could not be killed with rhTRAIL (Figure 2B) and all had >50% reduction in levels of surface DR5 compared with their parental counterparts. Expression levels of surface DR4 and FasR very low and remained unchanged (Figure 2C). These findings raised the question whether CTL resistance in these cells might be due, in part, to DR5 down regulation.
Figure 2. Recognition of R cells by F5 CTLs.
A. Melanomas (106) and F5 CTL were cocultured overnight at 1:1 and 0.5:1 E:T ratios. Amount of cytokine released was measured by ELISA. The MART-1−/A*0201− melanoma line M238 and the myelogenous line K562 were used as control. B. rhTRAIL resistance. CTL R melanomas were treated with rhTRAIL (0–25 ng/ml –18hr) and apoptosis was evaluated by active caspase-3 levels. C. Death receptor surface expression. Melanomas were stained with anti–DR4, –DR5, –FasR fluorochrome-conjugated mAbs (1µmol/L) and subjected to FACS analysis. As negative control cells were stained with isotype control. Results are presented as mean ± SEM of duplicate (quadruplicate for ELISA) samples (n = 3). * P values <0.05 significant.
Adequate levels of DR5 expression are necessary for CTL killing but do not fully account for resistance
We transiently transfected the resistant cell line M202R1 with two concentrations of a DR5 expression plasmid (17), which while restoring DR5 surface expression to the levels seen in the parental M202 (and even above) (Figure 3A), did not render these cells sensitive to rhTRAIL killing (Figure 3B). Recognizing that these melanoma targets were killed through TRAIL/DR5, we knocked down DR5 levels in sensitive M202 melanoma cells using siRNA. As DR5 expression was reduced, somewhere between 70%–40% of normal levels, we observed a precipitous drop in TRAIL-induced apoptosis (Figure 3C). These results, taken together, suggest that resistant cells could not be killed regardless of the levels of DR5 expression, whereas otherwise sensitive cells required adequate levels of DR5 for killing. Thus, DR5 expression in M202R –about 40% of that of the parental M202– clearly contributes to CTL-resistance, but is not the full explanation.
Figure 3. Role of DR5 in killing.
A. M202R1 cells were transfected with DR5 expression plasmid (1, 10ng) using standard techniques. Surface DR5 expression was measured by FACS analysis. Cells were then treated with rhTRAIL (5–25ng/ml-18hr) and were subjected to apoptosis assay measured by active caspase-3 levels. M202 cells were used as control. Results are presented as mean ± SEM of duplicate samples (n = 2). C. DR5 knockdown. M202 parental cells were transfected with DR5 or scrambled siRNA, subjected to immunoblot for confirmation of specific gene-silencing, apoptosis (active caspase-3) assay, and DR5 expression by FACS analysis (presented as %MFI). Cells at 50–60% confluency were used for transfection. Results are representative of two independent experiments.
Pretreatment of resistant melanomas with the HDACi SAHA restores their sensitivity to killing
Pretreatment of the resistant melanoma sublines with SAHA (1µmol/L – 48hr) restored their sensitivity to killing by TCR transgenic MART CTL. SAHA also sensitized the resistant cells to killing by two patient-derived MART-specific CD8+ CTL, by rhTRAIL and DR5 agonistic mAb Drozitumab (p < 0.05) (8) (Figure 4A–D, Suppl. Figure 1A–E). Anti-DR5 antagonistic mAb (2µmol/L–6hr) significantly reduced CTL sensitivity of SAHA-pretreated R sublines (Figure 4E, Suppl. Figure 1F).
Figure 4. SAHA-mediated sensitization of CTL R sublines to killing.
Cells (106) were left either untreated or pretreated with SAHA (1µmol/L – 48hr) and used in killing assay using A. TCR transgenic, B. two patient-derived MART-1 specific CTLs as effectors (51Cr-release assay), C. rhTRAIL (0–25ng/ml) or B. cross-linked Drozitumab (0–50ng/ml) for 18 hours; apoptosis was evaluated by active caspase-3 levels. E. DR5 blockade. SAHA-pretreated CTL R cells were washed, incubated with antagonistic DR5 mAb (2µmol/L – 6hr) and used in cytotoxicity assays. Results are presented as mean ± SEM of duplicate samples (n = 3). * P values <0.05 significant.
Kinetics of SAHA-mediated immunosensitization
SAHA-mediated sensitized M202R1 cells became progressively resistant over 72hrs period after drug withdrawal (Figure 5A). DR5 surface expression paralleled CTL sensitivity (Figure 5B), reinforcing a strong correlation between SAHA-mediated DR5 induction and CTL sensitivity. Treatment of both the parental and SAHA-treated CTL-resistant cells with MHC-I blocking mAb (1µmol/L-20min) significantly reduced the recognition (Figure 5C) and killing (Figure 5D) of melanomas by F5 CTL. These results indicate that F5 CTLs recognize melanomas in an MHC-I restricted fashion. Obviously, in the absence of recognition, MART-1/A*0201-specific F5 CTLs fail to induce cytotoxicity in melanoma tumor targets.
Figure 5. Kinetics of SAHA effects on melanomas.
M202R1 cells were grown in media supplemented with SAHA (1µmol/L – 48 hours). The cells were then grown in SAHA-depleted media for various intervals (24–72hr), subjected to A. 51Cr-release assay suing F5 CTL as effectors. B. stained with anti–DR5 mAb and subjected to FACS analysis. Results are presented as mean ± SEM of duplicate samples (n = 3). Melanomas were either left untreated or treated with SAHA (1µM -48hr). The cells were then treated with anti-MHC I blocking Ab (1µM-20 min) and used in C. recognition assay as measured by IFN-γ release of overnight cocultures of melanomas and F5 CTLs (1:1 E:T ratio), or D. incubated with various E:T ratios of F5 CTLs in a 6h 51Cr-release assay. Results are presented as mean ± SEM of duplicate samples (n = 2). * P values <0.05 significant.
Mechanism of SAHA action in restoring CTL sensitivity
SAHA treatment of resistant melanoma cell lines largely restores levels of DR5 expression (Figure 6A), and with kinetics that closely parallels those in killing assays. DR5 overexpression data (Figure 3) argue against this being the only operative mechanism. However, when DR5-overexpressed cells were treated with SAHA, CTL sensitivity was restored (Figure 6B, C). These data suggest that the sensitizing effect of SAHA is mediated via at least two mechanisms: induction of surface DR5 and inhibition of intracellular resistant factors. From our previous work (14, 15), we identified inverted apoptotic signaling pathways as an important mechanism to CTL-induced apoptosis. Focused apoptosis qPCR arrays showed expression patterns of several categories of apoptotic genes were altered in the CTL R cells compared to parental cells, consistent with an anti-apoptotic resistant phenotype (Table 1). Treatment of these CTL-resistant sublines with SAHA increased the expression levels of positive regulators of apoptosis, caspases, TNF/TNFR family and death domain proteins and decreased the expression levels of several negative regulators of apoptosis (Table 1, Supplemental Table 1). These events ultimately force the generation of a proapoptotic intracellular environment.
Figure 6. DR5 induction alone is insufficient for killing.
Resistant melanomas were stained with anti–DR4, –DR5, –FasR fluorochrome-conjugated mAbs (1µmol/L) and subjected to FACS analysis. As negative control, cells were stained with isotype Ig control. DR4 and Fas R expression levels were low and SAHA did not increase their expression. B. DR5 overexpressing M202R1 cells (adopted from figure 3) were grown in complete media (±SAHA) and subjected to C. cytotoxicity assay. M249(CTL R1) cells were left either untreated or treated with cIAP-2 or Bcl-xL siRNA or DR5 overexpression constructs. D. Efficacy of gene knock-down (KD) of two independent experiments is shown by western blot. E. Cells were then incubated with rhTRAIL (15ng/ml-18hr) and cytotoxicity was assessed by measuring levels of active caspase-3. Results are presented as mean ± SEM of duplicate samples (n = 3). Untreated M249(CTL R1) and M249 cells were used as control. * P values <0.05 significant.
Table 1.
Differential expression of apoptotic genes in M249 CTLR cells and their regulation by SAHA as measured by real time quantitative PCR-based apoptosis array
| Gene name | CTL R vs. Parental mRNA fold change |
CTL R + SAHA vs. CTL R mRNA fold change |
|
|---|---|---|---|
| Negative apoptosis regulator | ACC-1/ACC2 (A1) | 4.43 | 3.05 |
| Bcl2L1 (Bcl-xL) | 3.31 | −5 | |
| Bcl-B/BOO (Bcl2L10) | 2.68 | 6.6 | |
| Bcl-W (Bcl-2 like) | 4.04 | −6.22 | |
| BAR/RNF47 | −2.5 | 4.5 | |
| AIP/AIP2 (BIRC3) | 13.03 | −18.11 | |
| BNIP-2/NIP2 | −2.8 | 2.8 | |
| AKT | −2 | 5.25 | |
| Positive apoptosis regulator | Apaf-1 | −2.1 | 4 |
| Bcl2L8 (BAD) | −5.1 | 8.15 | |
| BAK | −3 | 5.3 | |
| Bcl2L4 (Bax) | −5.3 | 5 | |
| DFF-45/DFF1 | −2.54 | 4.41 | |
| TNFSR6/Apo-1 (Fas) | −4.42 | 3.5 | |
| CD18/D12S370 (LTβR) | −3.6 | 5 | |
| TNFSF10 (TRAIL) | −23.05 | 7.3 | |
| TRAF4/LART-1 | −3 | 4.5 | |
| DP5/Harakiri (BH3 only) | −6.52 | 6.72 | |
| LTα/TNFβ | 6.1 | −10.1 | |
| DAPK/DKF2P | −3.62 | 4.5 | |
| Caspase activator | CINCIN1/CARD6 | −2.51 | 3.9 |
| ICE/IL1BC (Casp1) | −4.54 | 13.1 | |
| CASP-2/ICH1L | −2 | 7.3 | |
| ICEREL11 (Casp4) | 3.01 | 2.51 | |
| ALPS2B/CAP4 (Casp8) | −2.3 | 4.81 | |
| death domain | GIG3/MORT-1 (FADD) | −2.8 | 6.9 |
| TNFRSF10B (TRAIL DR5) | −4.5 | 5.14 | |
| TRADD | −2.15 | 3.5 | |
| TRAF2/TRAD | −3.02 | 3.81 | |
| DNA damage | TRP53 (p53) | −6.39 | 8.9 |
| p73 | −3.04 | 3.1 |
To further identify apoptotic gene(s) products potentially responsible for enhanced sensitivity observed by SAHA, we performed siRNA gene knock-down (KD) experiments on two anti-apoptotic molecules (Figure 6D). In DR5 overexpressing CTL R cells, c-IAP-2 and Bcl-xL knock-down alone, or Bcl-2 family inhibitor (2MAM-A3) slightly enhanced TRAIL sensitivity. TRAIL sensitivity of c-IAP-2 and Bcl-xL double KD resistant cells was superior to individual KD; an effect that was modestly potentiated by 2MAM-A3 (Figure 6E, Suppl. Figure 2). These results further confirm that simultaneous down-regulation of negative (e.g., Bcl-xL and c-IAP-2) and upregulation of positive (DR5) apoptotic regulators are required to overcome TRAIL-resistance.
We also observed cytosolic accumulation of cytochrome c and Smac/DIABLO (Suppl. Figure 3A lanes 4 and 8), which paralleled their mitochondrial depletion (lanes 12 and 16), as well as mitochondrial depolarization (Suppl. Figure 3B) only by combination of SAHA plus rhTRAIL or Drozitumab, indicating that combination treatment reduces mitochondrial membrane potential (ΔΦm), induces mitochondrial collapse and cytosolic release of apoptotic molecules.
Discussion
Resistance to apoptotic death signals delivered by cytotoxic T cells is likely to become an important limiting factor with increasingly effective immune therapies. We report herein that TCR trangenic CTLs primarily use the TRAIL apoptotic pathway, via DR5 receptor, in killing sensitive melanomas. CTL-resistant melanomas had both DR5 down-regulation and aberrant expression of apoptotic molecules. Clinically achievable concentration of the FDA approved chromatin remodeling drug SAHA increased surface DR5 expression, favorably inverted the expression of apoptotic genes, and restored the sensitivity of R melanomas to MART CTL. TCR transgenic MART CTLs were used as a reliable and reproducible source of highly potent melanoma-reactive CTLs. Acquisition of CTL-resistance and its reversal by SAHA was also confirmed by using more clinically relevant naturally occurring T cells expressing endogenous MART-1 TCR derived from metastatic melanoma patients. Further, rhTRAIL and Drozitumab were used to explicitly verify the contribution of the DR5/TRAIL pathway in killing melanomas. Apoptosis negative (c-IAP-2 and Bcl-xL) and positive (DR5) regulators were potential candidates partly regulating CTL sensitivity. Combination of SAHA and rhTRAIL or Drozitumab destabilized mitochondria and facilitated cytosolic release of apoptotic molecules.
Immune effector cells eradicate tumor cells mainly by apoptosis. Tumors, in turn, have adopted various apoptosis-resistant mechanisms. Alterations in gene expression and cell signaling dynamics account for resistance to specific CTL-killing, where selective targeting of aberrant pathways or apoptosis-related proteins can overcome resistance to immune effector mechanisms (18–23). Expression of theses resistant factors is regulated by the NF-ΦB, MAPK, PI3/AKT, JAK/STAT and other signal transduction pathways that are constitutively activated/deregulated in melanomas (24, 25). Our results indicate that continuous exposure to melanoma-reactive CTLs results in the generation of R cells with diminished DR5 expression and altered apoptotic gene profile (consequence of NF-ΦB and ERK1/2 activation (14, 15)). Loss or down-modulation of death receptors and alterations in the ratio of pro- to anti-apoptotic gene products are the most frequently observed TRAIL-resistance mechanisms (10, 26, 27). In fact, superior contribution of DR5 than DR4 to TRAIL killing is reported (28). Thus, an agent that simultaneously upregulates DR5 and modulates apoptotic gene programs, to favor a sensitive phenotype, can potentially restore CTL sensitivity.
Chromatin remodeling drugs including SAHA increase acetylation of both histones and cytoplasmic proteins, thus, exerting multiple effects on gene transcription and protein function (29) specifically: transcribing genes responsible for creating an intracellular pro-apoptotic milieu (11), increasing expression of: surface death receptors (30–32), MHC molecules (33), tumor Ags recognized by CTL (34), and NK ligands recognized by NKG2A (35, 36), while having minimal toxicity to normal cells (34). Epigenetic drugs render tumors more recognizable targets by the immune system (37), thus, could serve as immune sensitizers (12). Significant enhancement of anti-tumor activity of ACT by HDACi in an in vivo melanoma model was recently reported by our group (13), therefore, in this study we examined whether SAHA can regulate the TRAIL pathway and sensitizes CTL-resistant melanomas.
While DR5 played an essential role in killing sensitive melanomas, surprisingly, its overexpression alone was insufficient for restoring sensitivity. Sensitization was evident only when DR5 overexpressed R cells were exposed to SAHA. Downregulation of anti-apoptosis genes (e.g., IAP and Bcl-2 family, AKT), concurrent with upregulation of DR5 as well as other positive regulators of apoptosis [e.g., caspases and caspase activators (e.g., CARD6, Casp-1, 2, 4, 8), death domain proteins (FADD, DR5, TRADD, TRAF2), TRAIL, TNF superfamily members (TNF, LTA, TNFRSF)], DNA damage molecules (TRP53, p73), and apoptosis inducers (e.g., Bax, Bad, Bak) suggests that SAHA sensitizes the R melanomas via combinatorial cooperation among several groups of apoptotic genes. However, cell fate is ultimately determined by an imbalance in the ratio of pro- to anti-apoptotic proteins. In fact, knock-down studies revealed that reduction of various anti-apoptotic proteins alone or combined with Bcl-2 family inhibitor fail to significantly enhance TRAIL sensitivity. Exposure of these cells (double KD ± 2MAM-A3) to SAHA overcame the resistance indicating the necessity of additional gene regulatory effects of SAHA. While DR5 overexpression alone was insufficient, knock-down of both anti-apoptotic genes in DR5 overexpressing R cells (±2MAM-A3) enhanced TRAIL sensitivity. Failure of these manipulations to fully restore TRAIL sensitivity of R cells at the levels of parental cells indicates that concurrent enhanced expression of DR5 and reduced expression of Bcl-xL and c-IAP-2 are required, but insufficient, to fully overcome resistance. Yet other unidentified resistant factor(s), whose regulation is modified by SAHA, are involved. Obviously, SAHA favors the generation of a pro-apoptotic milieu, which predestines melanomas to die upon receiving CTL-delivered death signals. Consistent with our observations, death receptor induction, caspase activation, and reduction of anti-apoptotic Bcl-2 members by SAHA sensitizes various human tumors to TRAIL (38–43).
TCR transgenic and patient-derived MART CTL recognition of parental (14, 15) and SAHA-treated R cells was accomplished in an MHC-I restricted manner. Tumor recognition via TCR is a prerequisite for the subsequent delivery of death signals by various means (TRAIL, FasL, perforin/granzyme). In the absence of peptide/MHC recognition and conjugate formation, TRAIL expressing MART-1/A*0201 specific CTLs (patient-derived as well as TCR engineered) fail to engage DR5 and transmit the death signal. Hence, a reduction in killing is observed by MHC-I blockade. The partial involvement of the perforin/granzyme pathway is plausible; blocking the TRAIL pathway by various approaches did not entirely abrogate killing. Consistent with reports by other investigators (44–48), our data, however, indicate TRAIL being the predominant cytotoxic pathway preferentially employed by MART CTLs.
In conclusion, TCR transgenic and patient-derived CTL mainly use the TRAIL pathway in killing melanomas, exclusively via DR5 receptor. CTL-resistance was due to DR5 down-regulation and aberrant expression of apoptotic molecules. Brief exposure to the HDACi SAHA increased surface DR5 expression, regulated the expression of apoptotic molecules, and restored the sensitivity of R melanomas to patient-derived MART-specific and TCR transgenic CTL, rhTRAIL, and Drozitumab. Anti-apoptotic cIAP-2 and Bcl-xL, and proapoptotic DR5 are potential candidates partly regulating CTL sensitivity. Further, combination of SAHA and rhTRAIL or Drozitumab destabilized mitochondria and facilitated apoptosis to proceed. These results are encouraging as one can safely design and implement a clinical trial using a combination of ACT, rhTRAIL (dulanermin), or Drozitumab [both being clinically evaluated (4, 7–9, 49, 50)], in conjunction with SAHA, to reduce the apoptosis threshold of melanomas and to achieve better clinical outcomes.
Supplementary Material
Acknowledgements
The authors acknowledge Dr. Avi Ashkenazi (Genentech Inc, San Francisco, CA) for generously providing Drozitumab and DR5 expression plasmid, Dr. Steven Rosenberg (NCI, Surgery Branch) for the kind gift of MCV-MART-1 F5 TCR vector, Dr. Bijay Mukherji (University of Connecticut, Medical Center) for providing MART-1 specific patient CTLs and Dr. Elizabeth Grimm (University of Texas, MD Anderson Cancer Center) for the review of the manuscript.
Grant Support: This work was supported by the National Center for Research Resources and the National Cancer Institute (NCI) of the National Institutes of Health through Grants Number NIH R21CA 149938 (ARJ), RO1 CA129816 (JSE), PO1 1088934 (JSE), The Stacy and Evelyn Kesselman Research Fund (JSE), The Joy and Jerry Monkarsh Fund (JSE), and a California Institute for Regenerative Medicine (CIRM) grant (SKK).
Footnotes
Conflict of Interest: The authors claim no conflicts of interest.
References
- 1.Mackiewicz J. What is new in the treatment of advanced melanoma? State of the art. Contemp Oncol (Pozn) 2012;16:363–370. doi: 10.5114/wo.2012.31763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollack IF, Erff M, Ashkenazi A. Direct stimulation of apoptotic signaling by soluble Apo2L/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin Cancer Res. 2001;7:1362–1369. [PubMed] [Google Scholar]
- 3.Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- 4.Yang A, Wilson NS, Ashkenazi A. Proapoptotic DR4 and DR5 signaling in cancer cells: toward clinical translation. Curr Opin Cell Biol. 2010;22:837–844. doi: 10.1016/j.ceb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Holland PM. Targeting Apo2L/TRAIL receptors by soluble Apo2L/TRAIL. Cancer Lett. 2013;332:156–162. doi: 10.1016/j.canlet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Walczak H, Miller R, Ariail K, Gliniak B, Griffith T, Kubin M, Chin W, Jones J, Woodward A, Craig A, Smith T, Smolak P, Goodwin R, Rauch C, Schuh J, Lynch D. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nature Medicine. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 7.Lim B, Scicchitano A, Beachler C, Gusani N, Sarwani N, Yang Z, Staveley-O'Carroll K, Ashkenazi A, Portera C, El-Deiry WS. FOLFIRI plus dulanermin (rhApo2L/TRAIL) in a patient with BRAF-mutant metastatic colon cancer. Cancer Biol Ther. 2013;14:711–719. doi: 10.4161/cbt.25310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang Z, Chen JJ, Yu Y, Li B, Sun SY, Zhang B, Cao Y. Drozitumab, a human antibody to death receptor 5, has potent antitumor activity against rhabdomyosarcoma with the expression of caspase-8 predictive of response. Clin Cancer Res. 2011;17:3181–3192. doi: 10.1158/1078-0432.CCR-10-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin. Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hersey P, Zhang XD. How melanoma cells evade trail-induced apoptosis. Nature Rev. Cancer. 2001;1:142–150. doi: 10.1038/35101078. [DOI] [PubMed] [Google Scholar]
- 11.Peart MJ, Smyth GK, van Laar RK, Bowtell DD, Richon VM, Marks PA, Holloway AJ, Johnstone RW. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102:3697–3702. doi: 10.1073/pnas.0500369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jazirehi AR. Regulation of apoptosis-associated genes by histone deacetylase inhibitors: implications in cancer therapy. Anti-Cancer Drugs. 2010;21:805–813. doi: 10.1097/CAD.0b013e32833dad91. [DOI] [PubMed] [Google Scholar]
- 13.Vo DD, Prins RM, Begley JL, Donahue TR, Morris LF, Bruhn KW, de la Rocha P, Yang MY, Mok S, Garban HJ, et al. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009;15:8693–8699. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jazirehi AR, Baritaki S, Koya RC, Bonavida, B B, Economou JS. Molecular mechanism of MART-1+/A*0201+ human melanoma resistance to specific CTL-killing despite functional tumor-CTL interaction. Cancer Res. 2011;71:1406–1417. doi: 10.1158/0008-5472.CAN-10-1296. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Jazirehi AR, Economou JS. Proteasome inhibition blocks NF-κB and ERK1/2 pathways, restores antigen expression, and sensitizes resistant human melanoma to TCR-engineered CTLs. Mol Cancer Ther. 2012;11:1332–1341. doi: 10.1158/1535-7163.MCT-11-0814. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Jazirehi AR, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin's lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2004;3:71–84. [PubMed] [Google Scholar]
- 17.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L, Ashkenazi A. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 18.Hersey P, Zhang XD. Treatment combinations targeting apoptosis to improve immunotherapy of melanoma. Cancer Immunol. Immunother. 2009;58:1749–1759. doi: 10.1007/s00262-009-0732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Kang TH, Noh KH, Bae KC, Kim SH, Yoo TD, et al. Enhancement of dendritic cell-based vaccine potency by antiapoptotic siRNAs targeting key pro-apoptotic proteins in cytotoxic CD8(+) T cell-mediated cell death. Immunol Lett. 2009;122:58–67. doi: 10.1016/j.imlet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Seeger JM, Schmidt P, Brinkmann K, Hombach AA, Coutelle O, Zigrino P, et al. The proteasome inhibitor bortezomib sensitizes melanoma cells toward adoptive CTL attack. Cancer Res. 2010;70:1825–1834. doi: 10.1158/0008-5472.CAN-09-3175. [DOI] [PubMed] [Google Scholar]
- 21.Huber C, Bobek N, Kuball S, Thaler S, Hoffarth S, Huber C, et al. Inhibitors of apoptosis confer resistance to tumour suppression by adoptively transplanted cytotoxic T-lymphocytes in vitro and in vivo. Cell Death Differ. 2005;12:317–325. doi: 10.1038/sj.cdd.4401563. [DOI] [PubMed] [Google Scholar]
- 22.Abouzahr S, Bismuth G, Gaudin C, Caroll O, Van Endert P, Jalil A, et al. Identification of target actin content and polymerization status as a mechanism of tumor resistance after cytotoxic T lymphocyte pressure. Proc Natl Acad Sci USA. 2006;103:1428–1433. doi: 10.1073/pnas.0510454103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chouaib S, Meslin F, Thiery J, Mami-Chouaib F. Tumor resistance to specific lysis: a major hurdle for successful immunotherapy of cancer. Clin. Immunol. 2009;130:34–40. doi: 10.1016/j.clim.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Huang S, DeGuzman A, Bucana CD, Fidler IJ. Nuclear factor-kappaB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin. Cancer Res. 2006;6:2573–2581. [PubMed] [Google Scholar]
- 25.McNulty SE, del Rosario R, Cen D, Meyskens FL, Jr, Yang S. Comparative expression of NfkappaB proteins in melanocytes of normal skin vs. benign intradermal naveus and human metastatic melanoma biopsies. Pigment Cell Res. 2004;17:173–180. doi: 10.1111/j.1600-0749.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 26.Jazirehi AR, Arle D. Epigenetic Regulation of the TRAIL/Apo2L Apoptotic Pathway by Histone Deacetylase Inhibitors: An Attractive Approach to Bypass Melanoma Immunotherapy Resistance. American J. of Clinical and Experimental Research. 2013;2:55–74. [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Z, McDonald ER, Dicker DT, III, El-Deiry WS. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J. Biol. Chem. 2004;279:35929–35839. doi: 10.1074/jbc.M405538200. [DOI] [PubMed] [Google Scholar]
- 28.Kelley RF, Totpal K, Lindstrom SH, Mathieu M, Billeci K, Deforge L, Pai R, Hymowitz SG, Ashkenazi A. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR)5 than DR4 to apoptosis signaling. J. Biol. Chem. 2005;280:2205–2212. doi: 10.1074/jbc.M410660200. [DOI] [PubMed] [Google Scholar]
- 29.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat. Rev. Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 30.Insinga A, Monestiroli S, Ronzoni S, Gelmetti V, Marchesi F, Viale A, Altucci L, Nervi C, Minucci S, Pelicci PG. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat. Med. 2005;11:71–76. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- 31.Nebbioso A, Clarke N, Voltz E, Germain E, Ambrosino C, Bontempo P, Alvarez R, Schiavone EM, Ferrara F, Bresciani F, et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat. Med. 2005;11:77–84. doi: 10.1038/nm1161. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XD, Gillespie SK, Borrow JM, Hersey P. The histone deacetylase inhibitor suberic bishydroxamate regulates the expression of multiple apoptotic mediators and induces mitochondria-dependent apoptosis of melanoma cells. Mol. Cancer Ther. 2004;3:425–435. [PubMed] [Google Scholar]
- 33.Magner WJ, Kazim AL, Stewart C, Romano MA, Catalano G, Grande C, Keiser N, Santaniello F, Tomasi TB. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J. Immunol. 2000;165:7017–7024. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 34.Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, Garrido F. Rexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2'-deoxycytidine treatment. Int. J. Cancer. 2001;94:243–251. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 35.Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Odum N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 2005;65:11136–11145. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- 36.Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, Kaiser S, Jobst J, Smirnow I, Wagner A, et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321–6329. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- 37.Frost P, Liteplo RG, Donaghue TP, Kerbel RS. Selection of strongly immunogeneic “TUM” variants from tumors at high frequency using 5-azacytidine. J. Exp. Med. 1984;159:1491–1501. doi: 10.1084/jem.159.5.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanoosten RL, Earel JK, Griffith TS. Enhancement of Ad5-TRAIL cytotoxicity against renal cell carcinoma with histone deacetylase inhibitors. Cancer Gene Ther. 2006:1–5. doi: 10.1038/sj.cgt.7700939. [DOI] [PubMed] [Google Scholar]
- 39.Lakshmikanthan V, Kaddour-Djebbar I, Lewis RW, Kumar MV. SAHA-sensitized prostate cancer cells to TNFalpha-related apoptosis-inducing ligand (TRAIL): mechanisms leading to synergistic apoptosis. Int. J. Cancer. 2006;119:221–228. doi: 10.1002/ijc.21824. [DOI] [PubMed] [Google Scholar]
- 40.Lillehammer T, Engesaeter B, Prasmickaite P, Maelandsmo G, Fodstad O, Engebraaten O. Combined treatment with Ad-hTRAIL and DTIC or SAHA is associated with increased mitochondrial-mediated apoptosis in human melanoma cell lines. J. Gene Med. 2007;9:440–451. doi: 10.1002/jgm.1036. [DOI] [PubMed] [Google Scholar]
- 41.Carlisi D, Lauricella M, D'Anneo A, Emanuele S, Angileri L, Di Fazio P, Santulli A, Vento R, Tesoriere G. The histone deacetylase inhibitor suberoylanilide hydroxamic acid sensitises human hepatocellular carcinoma cells to TRAIL-induced apoptosis by TRAIL-DISC activation. Euro. J. Cancer. 2009;45:2425–2438. doi: 10.1016/j.ejca.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 42.Lauricella M, Ciraolo A, Carlisi D, Vento R, Tesoriere G. SAHA/TRAIL combination induces detachment and anoikis of MDA-MB231 and MCF-7 breast cancer cells. Biochimie. 2012;94:287–299. doi: 10.1016/j.biochi.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 43.Nakata S, Yoshida T, Horinaka M, Shiraishi T, Wakada M, Sakai T. Histone deacetylase inhibitors upregulate death receptor 5/TRAIL-R2 and sensitize apoptosis induced by TRAIL/APO2-L in human malignant tumor cells. Oncogene. 2004;23:6261–6271. doi: 10.1038/sj.onc.1207830. [DOI] [PubMed] [Google Scholar]
- 44.Wennerberg E, Sarhan D, Carlsten M, Kaminskyy VO, D'Arcy P, Zhivotovsky B, Childs R, Lundqvist A. Doxorubicin sensitizes human tumor cells to NK cell- and T-cell-mediated killing by augmented TRAIL receptor signaling. Int. J. Cancer. 2013;133:1643–1652. doi: 10.1002/ijc.28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorothée G, Vergnon I, Menez J, Echchakir H, Grunenwald D, Kubin M, Chouaib S, Mami-Chouaib F. Tumor-infiltrating CD4+ T lymphocytes express APO2 ligand (APO2L)/TRAIL upon specific stimulation with autologous lung carcinoma cells: role of IFN-alpha on APO2L/TRAIL expression and -mediated cytotoxicity. J. Immunol. 2002;169:809–817. doi: 10.4049/jimmunol.169.2.809. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen N, Ødum N, Ursø B, Lanier LL, Spee P. Cytotoxicity of CD56(bright) NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS One. 2012;7:e31959. doi: 10.1371/journal.pone.0031959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirandola P, Ponti C, Gobbi G, Sponzilli I, Vaccarezza M, Cocco L, Zauli G, Secchiero P, Manzoli FA, Vitale M. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood. 2004;104:2418–2424. doi: 10.1182/blood-2004-04-1294. [DOI] [PubMed] [Google Scholar]
- 48.Sato K, Niessner A, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J. Exp. Med. 2006;203:239–2350. doi: 10.1084/jem.20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams C, Totpal K, Lawrence D, Marsters S, Pitti R, Yee S, Ross S, Deforge L, Koeppen H, Sagolla M, Compaan D, Lowman H, Hymowitz S, Ashkenazi A. Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ. 2008;15:751–761. doi: 10.1038/sj.cdd.4402306. [DOI] [PubMed] [Google Scholar]
- 50.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001–1012. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.