Abstract
Fatigue is often described by patients as a lack of energy, mental or physical tiredness, diminished endurance, and prolonged recovery after physical activity. Etiologic mechanisms underlying fatigue are not well understood; however, fatigue is a hallmark symptom of mitochondrial disease, making mitochondrial dysfunction a putative biological mechanism for fatigue. Therefore, this review examined studies that investigated the association of markers of mitochondrial dysfunction with fatigue and proposes possible research directions to enhance understanding of the role of mitochondrial dysfunction in fatigue. A thorough search using PubMed, Scopus, Web of Science, and Embase databases returned 1220 articles. After the application of inclusion and exclusion criteria, a total of 25 articles meeting eligibility criteria were selected for full review. Dysfunctions in the mitochondrial structure, mitochondrial function (mitochondrial enzymes and oxidative/nitrosative stress), mitochondrial energy metabolism (ATP production and fatty acid metabolism), immune response, and genetics were investigated as potential contributors to fatigue. Carnitine was the most investigated mitochondrial function marker. Dysfunctional levels were reported in all the studies investigating carnitine; however, the specific type of carnitine that was dysfunctional varied. Genetic profiles were the second most studied mitochondrial parameter. Six common pathways were proposed: metabolism, energy production, protein transport, mitochondrial morphology, central nervous system dysfunction and post-viral infection. Coenzyme Q10 was the most commonly investigated mitochondrial enzyme. Low levels of Coenzyme Q10 were consistently associated with fatigue. Potential targets for further investigation were identified as well as gaps in the current literature.
Keywords: Fatigue, Mitochondria, Review
Graphical abstract
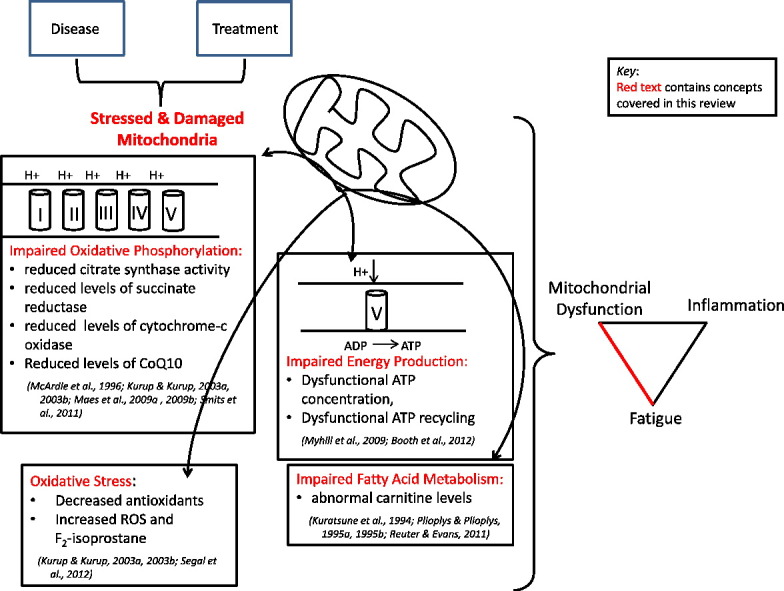
Highlights
-
•
Etiologic mechanisms underlying the symptom of fatigue are not well understood.
-
•
This review investigates the association of mitochondrial dysfunction with fatigue.
-
•
Inconsistent associations observed between mitochondria dysfunction and fatigue.
1. Introduction
Mitochondria are increasingly recognized as major contributors to human health and disease because of their location and influence [7]. Mitochondria have an essential role in energy production through the process of oxidative phosphorylation where nutrients are converted into adenosine triphosphate (ATP), which powers many of the cells' activities. In addition to energy production, mitochondria have been implicated in various physiologic processes including the production of reactive oxygen species (ROS), pyrimidine and lipid biosynthesis, regulation of cellular levels of substrates (amino acids, metabolites, enzyme cofactors), apoptosis, metal (Fe–S cluster and heme) metabolism, calcium homeostasis and flux, neurotransmitter synthesis, heat production, and insulin secretion [8], [26], [27]. Therefore, damage to mitochondria can have widespread consequences [8].
Health conditions such as cancer, diabetes, fibromyalgia, and serious mental disorders such as schizophrenia and bipolar disease are also proposed to result from mitochondrial dysfunction, though the links are less clear [27], [34]. Mechanisms underlying mitochondria-related disease states have predominantly focused on DNA damage and ROS generation [7], [27]. Mitochondrial dysfunction can be of primary (inherent) or secondary (acquired dysfunction) origin [7], [32]. Primary dysfunction results from mitochondrial DNA (mtDNA) mutations inherited from mothers, who are the sole contributors of mitochondria to their offspring [7]. Mitochondrial DNA has a much higher mutation rate than nuclear DNA because it lacks protective histones [32], is readily exposed to damage from ROS production [1], [27], [34], and lacks certain DNA repair mechanisms [7]. Secondary mitochondrial dysfunction results from the influence of external mechanisms such as environmental or pharmacologic toxins that can damage the mtDNA [7]. Mitochondria can protect themselves from the accumulation of damage through various quality control mechanisms (e.g., fission and fusion) [26], [43]; however, if these mechanisms are altered, mitochondrial dysfunction can lead to disease [4], [8]. Past research has predominantly focused on the role of mitochondrial dysfunction on disease pathology. However, some studies have investigated how mitochondrial dysfunction is associated with the development of distressing symptoms such as fatigue, neuropathic pain, weakness, and depression [27]; however, these investigations are still in their infancy. This paper reviewed studies that investigated the association of mitochondrial dysfunction with fatigue.
Fatigue is a hallmark symptom of mitochondrial disease. Fatigue is often described by patients as a lack of energy, mental or physical tiredness, diminished endurance, and the need for a prolonged recovery after physical activity [35]. Fatigue is reported by patients to be unrelieved by rest [16], [35]. As pervasive and debilitating as fatigue is, the etiology of fatigue remains poorly understood. Without a known pathophysiological mechanism for fatigue, there is a minimal consistency in its clinical definition [1], [11], [16], [39]. Furthermore, the lack of a proper clinical definition of fatigue contributes to its underdiagnosis and poor management, which in turn contributes to increased symptom burden and poorer quality of life in patients with fatigue [44]. The purpose of this systematic review was to examine markers of mitochondrial function (in adults) that have evidence of an association with fatigue in order to identify areas needed for further research. In this paper markers of mitochondrial function that associate with fatigue in adult patients were reviewed in order to describe empirical evidence of a relationship between mitochondrial dysfunction and fatigue and to propose possible research directions that would enhance understanding of the role of mitochondrial dysfunction in fatigue.
2. Methods
An initial literature query was conducted in the following four reference databases using search strategies as summarized in Table 1.
Table 1.
Search criteria.
| Database | Search terms | Filters | Yield |
|---|---|---|---|
| PubMed | Mitochondria OR mitochondrial AND fatigue | Humans English |
N = 358 |
| Scopus | (TITLE (mitochondria OR mitochondrial) AND TITLE (fatigue)) (TITLE (mitochondria OR mitochondrial) AND TITLE (fatigue)) |
English | N = 519 |
| Web of Science | Topic = (mitochondria OR mitochondrial) AND Topic = (fatigue) | English | N = 624 |
| Embase | ‘Fatigue’/exp AND (‘mitochondrion’/exp OR ‘mitochondrial respiration’/exp OR ‘mitophagy’/exp OR ‘mitochondrial dna’/exp OR ‘disorders of mitochondrial functions’/exp OR ‘mitochondrial dynamics’/exp OR ‘mitochondrial energy transfer’/exp OR ‘mitochondrial enzyme’/exp OR ‘mitochondrial gene’/exp OR ‘mitochondrial genome’/exp OR ‘mitochondrial membrane potential’/exp OR ‘mitochondrial protein’/exp OR ‘mitochondrion swelling’/exp) | Humans English |
N = 554 |
The initial search resulted in 2055 articles. After removing duplicates, the abstracts of 1220 articles were assessed for relevance to the area of interest. Abstracts that discussed the association of markers of mitochondrial function and fatigue were selected to be included in this review. In addition, studies were excluded if they were more than 20 years old, were not original research, were animal or cell-based studies, investigated the effect of medication or treatment on fatigue, mitochondrial markers or both, or measured induced fatigue through the use of exercise or electric stimulation. Also excluded were letters, literature reviews, notes, conference or meeting abstracts, book chapters, editorials, dissertations, case reports or series, short reports, workshop reports, and practice guidelines. A total of 54 articles were selected for full-text review. Of these, 25 were excluded based upon the aforementioned criteria, 3 were excluded because they focused on neuroimaging, and 1 was excluded because it included children. A total of 25 articles were selected for full review.
3. Results
Twelve of the 25 articles (48%) were published within the last five years. Twenty-two (88%) of the studies used a cross-sectional design; three used a repeated-measures design. Eighteen studies investigated only patients with chronic fatigue syndrome (CFS); remaining studies investigated a combination of myalgic encephalomyelitis (ME) and CFS (n = 2), ME (n = 1), multiple sclerosis (n = 1), HIV-related fatigue (HRF) (n = 1), systemic lupus erythematosus (SLE) (n = 1), and cancer-related fatigue (CRF) (n = 1). One study restricted participant gender and included only males [15]. A complete description of study attributes are found in Table 2, Table 3.
Table 2.
Studies investigating mitochondrial dysfunction in CFS and/or ME.
| Authors | Study design | Sample characteristics | Fatigue definition | Fatigue measurement | Mitochondrial marker assessed | Sample source | Association to fatigue |
|---|---|---|---|---|---|---|---|
| Edwards et al. [9]. | Cross-sectional, descriptive | n = 74 CFS patients Controls: a. n = 34 patients with myalgia b. n = 22 asymptomatic controls |
Not specified | CFS diagnosis (criteria/guidelines not specified) | 1. Mitochondrial hyperplasia 2. Cytochrome c oxidase 3. Myoadenylate deaminase |
Muscle biopsy from either: a. Tibialis anterior b. Quadriceps c. Gastrocne-mius, medial head |
No significant differences between CFS patients and controls. |
| Kuratsune et al. [18]. | Cross-sectional, descriptive | n = 38 CFS patients Controls: n = 308 healthy volunteers |
CDC 1988 criteria | CFS diagnosis according to CDC criteria | 1. Free l-carnitine 2. Acylcarnitine |
Serum | Free l-carnitine levels lower in male CFS patients and higher in female CFS compared to controls; however, not statistically significant. Acylcarnitine levels lower (p < .001) in CFS patients compared to controls. In CFS patients, lower acylcarnitine levels associated with worse performance and increased symptom burden at initial exam. Upon symptom improvement, acylcarnitine levels increased (p < .02). |
| Behan et al. [2]. | Cross-sectional, descriptive | n = 31 CFS patients Controls: n = 20 volunteers with no muscle disease |
CDC 1988 criteria | CFS diagnosis according to CDC criteria | 1. Histological 2. Histochemical 3. Ultrastructural |
Vastus lateralis muscle biopsy | Size and morphology of mitochondria showed differences between CFS and controls. No statistical data provided. |
| Plioplys and Plioplys [29]. | Cross-sectional | n = 15 CFS patients Controls: n = 15; age and sex-matched healthy volunteers |
1. CDC 1988 & 1994 criteria 2. British & Australian definitions for CFS |
1. Fatigue Severity Scale (FSS) 2. Beck Depression Inventory (BDI) 3. Symptom Checklist 90-R 4. CFS Impairment Index (CFS-II). 5. Structural Clinical Interview for the DSM III-R-Nonpatient Edition |
Ultrastructural exam of mitochondria | Right vastus lateralis muscle biopsy | No significant differences upon structural exam between CFS patients and controls. |
| Carnitine levels | Serum | Negative association between acylcarnitine levels and CFS-II mental index score (r = − 0.761, p < 0.01) and total score (r = − 0.634, p < 0.05). | |||||
| Plioplys and Plioplys [30]. | Cross-sectional | n = 35 CFS patients Controls: a. Mayo clinic normative data (n = 85) b. Historical study ([18].) |
CDC 1988 criteria | 1. FSS 2. BDI 3. Symptom Checklist 90-R 4. CFS-II. |
Carnitine levels: total, free, aceylcarnitine | Serum | Total carnitine lower in female (p < .001) and male (p < .05) CFS patients compared to Mayo clinic data. Free carnitine lower in female (p < .01) and male (p < .05) CFS patients compared to Mayo clinic data. Acylcarnitine lower in CFS patients compared to historical study controls (p < .00001). Free carnitine lower in CFS patients compared to historical controls (p < .00001). Total carnitine lower in CFS patients compared to historical controls (p < .00001). Free carnitine correlated with CFS-II physical impairment subset (r = .412, p < .05). Negative correlation between FSS and free carnitine (r = − .496, p = .02), and total carnitine and FSS (r = − .473, p = .02). |
| McArdle et al. [24]. | Cross-sectional | n = 54 CFS patients only included n = 34 for viral analysis Controls: a. For enzyme comparison n = 16 from a previous study b. For RNA detection n = 10 patients undergoing orthopedic surgery; no muscle damage or fatigue. |
1. Diagnosis with CFS on the basis of complaints of muscle pain and fatigue 2. Diagnosis conformed to the Oxford Consensus Criteria |
Not mentioned | 1. Mitochondrial enzymes: a. Citrate synthase b. Succinate reductase c. Cytochrome-c oxidase |
Anterior tibialis muscle biopsy | Reduction in all 3 mitochondrial enzyme activities (p < .05) in CFS patients compared to control values from a previous study. |
| 2. Presence of enteroviral RNA | Failed to detect evidence of enteroviral RNA. | ||||||
| Behan et al. [3]. | Cross-sectional | n = 16 CFS patients Controls: n = 10 healthy volunteers |
CDC 1994 criteria. | CFS diagnosis according to CDC criteria | 1. Aerobic capacity a. Pyruvate b. Lactate c. L/P ratio d. Respiratory capacity e. Cytochrome-c oxidase f. LDH 2. DNA analysis a. Total mtDNA volume b. mtDNA rearrangements c. Point mutation at nucleotide pair 3243 d. Two deletions: mtDNA 4977 mtDNA 7436 3. Histological, Histochemical, and Ultrastructural examination |
Right or left vastus lateralis muscle biopsy | Increased pyruvate levels in CFS patients (p = .053). All other biological parameters showed no significant findings between the groups. |
| Soetekouw et al. [38]. | Cross-sectional | n = 25 Caucasian, female CFS patients Controls: n = 25 age- and sex-matched healthy volunteers |
1. CDC 1994 criteria. 2. Fatigue with substantial ADL impairment: a. A score of 35 + on the Checklist Individual Strength subjective fatigue subscale b. a score of 750 + on the weighted total score of the Sickness Impact Profile. |
1. Checklist Individual Strength (CIS) 2. Sickness Impact Profile (SIP) |
1. Carnitine levels: total, free, & acylcarnitine 2. Carnitine ester profiles |
Serum | CFS patients were more fatigued (p < .001) and had more functional impairment (p < .001) as determined by questionnaires. No significant differences with any of the biologic markers between the groups. |
| Kurup and Kurup [20]. | Cross-sectional | n = 15 CFS patients Controls: n = 45 age and sex-matched healthy volunteers |
CDC criteria | Structured clinical interview to assess CFS and comorbid conditions | Mitochondrial markers: 1. Ubiquinone 2. ROS/RNS a. MDA b. Hydroperoxide c. Conjugated dienes d. NO 3. Antioxidants a. Glutathione b. SD c. Catalase d. GSH peroxidase e. GSH reductase |
RBCs and plasma/serum | 1. Ubiquinone lower in CFS patients (F = 259.36, p < .01) 2. ROS markers higher in CFS patients a. MDA (F = 4.56, p < 0.05) b. Hydroperoxide (F = 3.25, p < 0.05) c. Conjugated dienes (F = 16.21, p < 0.01) d. NO (F = 6.54, p < 0.05) 3. Antioxidants lower in ME patients a. Glutathione (F = 8.36, p < 0.05) b. SD (F = 7.56, p < 0.05) c. Catalase (F = 3.98, p < 0.05) d. GSH peroxidase (F = 11.26, p < 0.01) e. GSH reductase (F = 4.26, p < 0.05) |
| Kaushik et al. [17]. | Repeated measures: two time points 6 months apart during which symptoms did not vary significantly | n = 25 CFS patients Controls: 1. Microarray, n = 25 age- and sex-matched normal blood donors 2. Real-time PCR, n = 21 age- and sex-matched normal blood donors |
CDC 1994 criteria. | 1. Diagnosis of CFS according to CDC criteria 2. Chalder Fatigue Scale |
Real-time PCR | Peripheral blood mononuclear cells (PBMCs) | 16 Genes differentially expressed in CFS patients (15 genes up-regulated, 1 down-regulated). 3 Up-regulated genes are located in the mitochondria: a. EIF2B4 (p = 1.8 × 10− 5) b. EIF4G1 (p = 7.63 × 10− 13) c. MRPL23 (p = 1.25 × 10− 6) 2 Up-regulated genes for peroxisomal function, ABCD4 (p = .00190) and PEX16 (p = .0126), suggesting enhanced defense to oxidative damage. |
| Vernon et al. [41]. | Repeated measures: baseline, 2–3 weeks, 4–6 weeks, 3 months, 6 months (in those with symptoms), & 12 months. | n = 5 with symptoms suggestive of infectious mononucleosis with provisional lab confirmation Controls: n = 5 controls that recovered promptly from infectious mononucleosis; HLA-A and -B, sex, and age-matched. |
CDC 1994 criteria | 1. Diagnosis of CFS according to CDC criteria 2. Self-report and interview assessment of psychological and physical health. |
Gene transcription patterns | PBMCs | Due to small n in each group, data was categorized by time periods: early (baseline-3 months) and late (> 6 months following disease onset). Early Phase: 23 genes differentially expressed between cases and controls; 8 expressed in cases and involved binding and metabolism ontologies. Early & Late Phase: 24 genes significantly different between cases and controls; 12 genes are associated with mitochondrial function. |
| Hokama et al. [13]. | Cross-sectional | n = 328 CFS patients n = 18 CCFP patients n = 8 GWVs n = 15 PC patients n = 49 normal, healthy controls |
CDC 1994 criteria | CFS diagnosis according to CDC criteria | 1. Phospholipids 2. Anti-cardiolipin (aCL) antibodies |
Serum | CFS, CCCP, GWV, and PC patients have cardiolipin associated with mitochondrial membrane. The presence of aCL was also detected. |
| Hokama et al. [12]. | Cross-sectional | n = 40 CFS patients | CDC 1994 criteria | CFS diagnosis according to CDC criteria | Anti-cardiolipin antibodies | Serum | IgM isotope present in 95% of CFS patients. IgG isotype present in 10% and the IgA isotype present in 5% of CFS patients. |
| Maes et al. [23]. | Cross-sectional | n = 35 major depressed patients; n = 17 patients had a diagnosis with CFS Controls: n = 22 healthy volunteers |
1. 1994 CDC criteria 2. Criteria: a. Severe chronic fatigue for at least 6 months b. At least 3 Additional symptoms from a checklist |
1. CFS diagnosis according to CDC criteria 2. Fibromyalgia and CFS Rating Scale (FF scale) |
CoQ10 levels | Plasma | Depressed patients with CFS had lower plasma CoQ10 than depressed patients without CFS (F = 8.7, df = 1/33, p = .006). CFS independently predicted low CoQ10 values (F = 4.3, df = 1/31, p = .04). |
| Myhill et al. [25] | Cross-sectional | n = 71 CFS patients Controls: n = 53 normal, healthy volunteers |
CDC 1994 criteria | 1. CFS diagnosis according to CDC criteria 2. CFS Ability Scale |
1. “ATP Profile” test: a. ATP concentration and ATP ratio b. The efficiency of ox-phos process c. Translocator (TL) protein function (TL-OUT and TL-IN) 2. Mitochondrial Energy Score (MES) |
Neutrophils | Patients grouped into 3 groups based on CFS Ability Scale scores: very severe (VS), severe (S), and moderate (M). For most of the 5 factors of the “ATP Profile Test” the percentage of patients who are in the normal region increases from VS, S, to M. The MES is highly correlated with the CFS Ability scale (R2 = .645, p < .001). |
| Pietrangelo et al. [28]. | Cross-sectional | n = 4 CFS patients n = 2 female patients, meeting CDC criteria n = 2 male patients meeting CDC criteria for CFS Controls: n = 9 healthy volunteers |
CDC 1994 criteria | 1. CFS diagnosis according to CDC criteria 2. Skeletal muscle membrane testing |
Global transcriptome analysis | aaRNA obtained from vastus lateralis muscle biopsy | 47 Genes significantly altered in CFS patients: 2 up-regulated, 38 down-regulated and 7 up-regulated in females, but down-regulated in males. Gene Pathways: Control of Ox-Phos; 3 mitochondrial genes were down-regulated: SOD2, FDX1, and NQO1. Energy Balance; depressed transcription of several genes implicated in the energy metabolism: PFKFB3, PDK4, GOT1, AMPD3, and ATP-binding cassette member 5. One gene, VLDLR was up-regulated Apoptosis; FOS, MYC, SOX17, AATF, and CEBPD were all down-regulated. |
| Reuter and Evans [33]. | Cross-sectional | n = 44 CFS patients Controls: n = 49 age and gender-matched healthy subjects |
Royal Australasian College of Physicians CFS clinical practice guidelines | 1. Medical diagnosis of CFS using the Royal Australasian College of Physicians CFS clinical practice guidelines 2. FSS |
1. Endogenous carnitine: total, l-, and acylcarnitine 2. 35 individual carnitines |
Plasma | CFS patients had lower individual carnitines: C8:1 (p = .0201), C14 (p = .0023), C16:1 (p = .0383), C18 (p = .0104), C18:1 (p < .0001), and C18:2 (p < .0001). CFS patients had higher C12DC (p < .0001) and C18:1-OH (p = .0191). Negative correlation between FSS scores and C8:1, C14, C16:1, specifically with C18:1 (R = − .3547, p = .0009) and C18:2 (R = -.4191, p < .0001). Significant positive correlations between fatigue severity and C12DC, C16, and C18:1-OH. |
| Smits et al. [37]. | Cross-sectional | n = 16 CFS patients Controls: n = 11 male healthy volunteers |
1. CDC 1994 criteria 2. Severe fatigue determined as > 35 on the fatigue subscale of the CIS. 3. Fatigue longer than 6 months 4. Fatigue not explained by somatic or psychiatric illness or ongoing exertion and is not relieved by rest. 5. Myalgia and/or exercise intolerance. 6. Substantial functional impairment determined by a score of 700 + on the SIP-8. |
1. Diagnosis of CFS according to CDC criteria 2. CIS 3. SIP-8. |
1. ATP production rate 2. Respiratory chain complexes activity (Complex I, II + III, II, and IV) 3. Citrate Synthase levels |
Right quadriceps muscle biopsies | No significant differences in ATP production or respiratory chain complex activity in CFS patients. Citrate synthase levels were lower in CFS patients (p < .001). |
| Maes et al. [22]. | Cross-sectional | n = 58 ME/CFS patients Controls: n = 22 healthy volunteers |
1994 CDC criteria | 1. CFS diagnosis 2. FF scale |
CoQ10 levels | Plasma | Plasma CoQ10 lower in ME/CFS patients (F = 31.0, df = 1/78, p = .00001) Negative association between CoQ10 and total FF scale score (r = .28, p = .03). Negative correlation between CoQ10 and fatigue (r = − .86, p < 10− 5). |
| Booth et al. [5]. | Cross-sectional | n = 61 ME/CFS patients (Cohort 1; still ill after interventions; from the previous study) n = 138 ME/CFS patients (Cohort 2; no prior interventions) Controls: n = 53 normal, healthy volunteers [25] |
1. CDC 1994 criteria 2. ICCME (most, if not all were met) |
1. CFS diagnosis according to CDC criteria 2. The Bell CFS Ability Scale |
1. 5 Parameters of the ATP Profile test: a. ATPmg b. ATPend c. Ox-Phos d. TL OUT e. TL IN 2. MES 3. Nfn 4. MESinh |
Neutrophils | ME/CFS patients had reduced ATP production Mitochondrial dysfunction, mainly through partial blockage of the TL was demonstrated in both cohorts. It was observed that neutrophils used at least two different pathways to compensate for mitochondrial dysfunction. |
Table 3.
Studies investigating mitochondrial dysfunction in other fatigued populations.
| Authors | Study design | Sample characteristics | Fatigue definition | Fatigue measurement technique | Mitochondrial dynamic assessed | Sample source | Findings |
|---|---|---|---|---|---|---|---|
| Fukazawa et al. [10]. | Cross-sectional | n = 25 MS patients; n = 11 with disabling fatigue and n = 14 without fatigue Controls: n = 25 age- and sex-matched healthy volunteers |
1. Debilitating, persistent, or relapsing fatigue noted after the onset of MS 2. Lack of other causes of fatigue based on a history, physical examination, and laboratory tests. |
Medical diagnosis of fatigue | Carnitine levels: total, free, and acylcarnitine | Serum | No significant differences in carnitine levels between the groups. |
| Kurup and Kurup [19]. | Cross-sectional | n = 15 ME patients Controls: n = 45 age and sex-matched healthy volunteers |
CDC criteria | Structured clinical interview to assess CFS and comorbid conditions | Mitochondrial markers: 1. Ubiquinone 2. ROS/RNS a. MDA b. Hydroperoxide c. Conjugated dienes d. NO 3. Antioxidants a. Glutathione b. SD c. Catalase d. GSH peroxidase e. GSH reductase |
RBCs and plasma/serum | 1. Ubiquinone lower in ME patients (F = 259.36, p < .01) 2. ROS markers higher in ME patients a. MDA (F = 4.56, p < 0.05) b. Hydroperoxide (F = 3.25, p < 0.05) c. Conjugated dienes (F = 16.21, p < 0.01) d. NO (F = 6.54, p < 0.05) 3. Antioxidants lower in ME patients a. Glutathione (F = 8.36, p < 0.05) b. SD (F = 7.56, p < 0.05) c. catalase (F = 3.98, p < 0.05) d. GSH peroxidase (F = 11.26, p < 0.01) e. GSH reductase (F = 4.26, p < 0.05) |
| Segal et al. [36]. | Cross-sectional | n = 71 SLE patients Controls: n = 51 healthy volunteers |
Not specified | 1. Visual Analogue Scale-fatigue 2. FSS 3. Profile of Fatigue (ProF) |
F2-isoprostane | Plasma | SLE patients with fatigue had higher levels of F2-isoprostane than non-fatigued SLE patients (p = .0076). Positive correlation between F2-isoprostane and fatigue in SLE patients. F2-isoprostane predicts higher FSS scores in SLE patients (p = .0002). |
| Hsiao et al. [15]. | Repeated measures; Baseline Day 1, Day 7, Day 14, Day 19/21, Day 38–42 of EBRT, and Day 68–72 after EBRT | n = 15 men with non-metastatic prostate cancer receiving ADT and scheduled to receive EBRT. Controls: n = 15 age, gender, and race matched controls. |
Not specified | Revised Piper Fatigue Sale | Radiation-induced changes in mitochondria-related gene expression | WBCs-RNA | Eleven genes related to mitochondrial function were differentially expressed over time during EBRT (p < .05). After Bonferroni, only SLC25A23 was significantly down-regulated post-EBRT (p = .008 - .02). Eight of the 11 differentially expressed genes were significantly associated with fatigue scores (p = .012 - .0003). |
| Voss et al. [42]. | Cross-sectional | n = 5 HIV patients with high fatigue n = 5 HIV patients with low fatigue Controls: n = 5 healthy controls |
HIV-related fatigue | Revised 26-Item Piper Fatigue Scale | Genomic (mitochondrial and nuclear) expression markers of mitochondrial dysfunction | CD14 + cells | Genes pertaining to mitochondrial function include: CHD1L (τ = − .49) and ALDOB (τ = − .62), TIMM17B (τ = .62), GSR (τ = .62), IMMT (τ = .57), and SLC25A26 (τ = .62). 2 HIV-associated genes related to mitochondrial function, fatty acid metabolism: ACAD9 (τ = .20) and PPAR-alpha (τ = − .44). |
CFS = chronic fatigue syndrome; ME = myalgic encephalomyelitis; CDC = Centers for Disease Control and Prevention; DSM III-R = Diagnostic & Statistical Manual of Mental Disorders 3rd Edition Revised RNA = ribonucleic acid; L/P ratio = lactate/pyruvate ratio; DNA = deoxyribonucleic acid; mtDNA = mitochondrial DNA; ADLs = activities of daily living; ROS = reactive oxygen species; RNS = reactive nitrogen species; MDA = malondialdehyde; NO = nitric oxide; SD = superoxide dismutase; GSH = glutathione; RBCs = red blood cells; PCR = polymerase chain reaction; PBMCs = peripheral blood mononuclear cells; EIF2B4 = eukaryotic translation initiation factor 2B, subunit 4δ, tv-1; EIF4G1 = eukaryotic translation initiation factor 4γ, 1, tv-5; MRPL23 = mitochondrial ribosomal protein L23; ABCD4 = ATP binding cassette, subfamily D (ALD), member 4, tv-4; PEX16 = peroxisomal biogenesis factor 16, tv-1; HLA = human leukocyte antigen; CCFP = chronic ciguatera fish poisoning; GWVs = Gulf War Veterans; PC = prostate cancer; IgM = immunoglobulin subtype M; IgG = immunoglobulin subtype G; CoQ10 = Coenzyme Q10; ATP = adenosine triphosphate; TL-IN = transports ATP to cytosol; TL-OUT = transports ADP from cytosol to mitochondria; aaRNA = amino allyl RNA; Ox-Phos = oxidative phosphorylation; SOD2 = superoxide dismutase 2, mitochondrial; FDX1 = ferredoxin 1; NQO1 = nicotinamide adenine dinucleotide phosphate dehydrogenase quinone 1; PFKFB3 = 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3(allosteric enzyme); PDK4 = pyruvate dehydrogenase kinase, isoenzyme 4; GOT1 = glutamic-oxaloacetic transaminase 1; AMPD3 = adenosine monophosphate deaminase (isoform E); VLDLR = very low density lipoprotein receptor; FOS = V-fos FBJ murine osteosarcoma viral oncogene homolog; MYC = v-myc myelocytomatosis viral oncogene homolog; SOX17 = SRY-related HMG-box transcription factor; AATF = apoptosis antagonizing transcription factor; CEBPD = nuclear factor-IL6-beta; ICCME = International Consensus Criteria Myalgic Encephalomyelitis; ATPmg = whole cell ATP measured by adding excess Mg; ATPend = ATP measured with endogenous Mg only; Nfn = number of factors with normal; values; MESinh = revised MES using the % ATP inhibited instead of TL IN; MS = multiple sclerosis; EBRT = external beam radiation therapy; WBCs = white blood cells; SLC25A23 = solute carrier family 25, member 23; HIV = human immunodeficiency virus; CD14 = monocyte; LDH = lactate dehydrogenase.
CFS patients were the most studied (72% of all articles reviewed). CFS subjects were phenotyped by clinical diagnosis using the 1988 or 1994 Center for Disease Control (CDC) diagnostic criteria alone (n = 17) or in combination with other developed criteria (n = 3), such as the Australian definition for CFS (n = 1), the British definition for CFS (n = 1), and the International Consensus Criteria for Myalgic Encephalomyelitis (ICCME) (n = 1). Two studies used other diagnostic guidelines; one used the Oxford Consensus criteria and one group used the diagnostic criteria established by the Royal Australasian College of Physicians Working Group.
Fatigue was defined through either clinical diagnosis alone or in combination with self-report questionnaires. Most articles (n = 16) quantified fatigue using a variety of questionnaires such as the Fatigue Severity Scale (n = 4), CFS Impairment Index (CFS-II) (n = 2), Checklist Individual Strength (CIS) fatigue subscale (n = 2), Sickness Impact Profile (n = 2), Chalder Fatigue Scale (n = 1), the Fibromyalgia and the CFS Rating Scale (FF Scale) (n = 2), Symptom Checklist 90-R (n = 2), CFS Ability Scale (n = 2), the revised Piper Fatigue Scale (n = 2), Visual Analogue Scale-fatigue (VAS) (n = 1), the Profile of Fatigue (ProF) (n = 1), and structured interviews (n = 3).
A number of mitochondrial parameters were investigated as potential markers for the fatigue conditions. In about 70% (n = 18) of the studies, mitochondrial biomarkers were obtained from peripheral blood samples; in the remaining 7 studies, lower extremity skeletal muscle biopsy specimens were used. Dysfunctions in the mitochondrial structure, mitochondrial function, mitochondrial energy metabolism, immune/inflammatory response, and genetics were investigated as potential contributors to fatigue. Studies are grouped into these four areas for review.
3.1. Mitochondrial structure
Four studies investigated the association of fatigue with mitochondrial number, size, and/or shape. All four studies were cross-sectional in design, included patients with CFS, and used muscle biopsy specimens to determine the potential mitochondrial markers. One study quantified fatigue through the use of a self-report questionnaire; whereas, three studies assumed fatigue through medical diagnosis. In one study about 7% of tibialis anterior (n = 69), quadriceps (n = 4), or medial head gastrocnemius (n = 1) muscle biopsies from CFS subjects had mitochondrial hyperplasia; however, because there were no structural abnormalities noted, the authors concluded that mitochondrial structural abnormalities were not a feature of CFS [9]. Findings were similar in another study in which no significant mitochondrial structural differences were found in the right leg vastus lateralis muscle biopsies of CFS patients versus controls [29].
One study investigating the vastus lateralis muscle biopsies of CFS subjects compared to normal controls found a significantly larger (hypertrophic) mitochondrial size and shape (3–8 times larger), often with noticeable branching of the cristae, termed compartmentalization, [2]. In another study of CFS patients with decreased energy metabolism, the vastus lateralis muscle biopsy specimens, as categorized by observed lactate to pyruvate production in the biopsy samples, were found to have minor, nonspecific changes to mitochondrial shape and structure (paracrystalline inclusions and increased numbers of pleomorphic mitochondria with proliferation of cristae) [3]. However, a vastus lateralis muscle specimen from a CFS patient with normal energy metabolism showed abundant pleomorphic mitochondria, but these structural changes were not observed in the vastus lateralis muscle specimens from CFS subjects with increased energy metabolism [3].
In summary, three of these four cross-sectional studies investigated the relationship between fatigue and mitochondrial number, shape, and/or size and found no significant differences with mitochondrial shape and structure among CFS patients and controls. Only one study observed significant mitochondrial hypertrophy in the CFS patients compared to healthy controls using skeletal muscle specimens.
3.2. Mitochondrial function
Eleven studies investigated the aspects of mitochondrial function; all were cross-sectional in design. Most studies (n = 8) focused on mitochondrial enzymes, while the rest (n = 3) focused on oxidative and/or nitrosative stress. Most studies looked at CFS or ME/CFS patients, while one study investigated fatigue in SLE patients.
3.2.1. Mitochondrial enzymes
Four studies measured mitochondrial enzymes using muscle biopsy specimens and the remaining ones used peripheral blood specimens. Six studies only enrolled CFS patients, one included ME/CFS patients, and the remaining study enrolled only ME patients. Almost all participants were enrolled based on their medical diagnoses, except for one study which used a self-report questionnaire to measure fatigue. In four studies fatigue was further assessed by structured clinical interviews (n = 2) and self-report questionnaires (n = 2).
A significant reduction in citrate synthase, succinate reductase, and cytochrome-c oxidase was observed in the anterior tibialis muscle biopsy samples of CFS patients compared to healthy controls, which were attributed to the reduction in physical activity commonly present in CFS subjects [24]. A significant reduction in citrate synthase in the right quadriceps muscle biopsy samples from CFS subjects was also confirmed recently [37]. Citrate synthase is located in the mitochondrial matrix and is an essential enzyme in the citric acid cycle [40]. Succinate reductase (Complex II) and cytochrome-c oxidase (Complex IV) are two of the four mitochondrial transmembrane enzyme complexes of the electron transport chain [40].
In contrast, one study observed no significant difference with the skeletal muscle biopsies of partial cytochrome-c oxidase and myoadenylate deaminase (MAD) between CFS patients and healthy controls; MAD was more associated with the symptom of myalgia than fatigue [9]. MAD is the muscle-specific subtype of adenosine monophosphate (AMP) deaminase and is involved in nucleotide metabolism [40], [41], [42], [43], [44], [45]. Another study observed no difference in the vastus lateralis muscle biopsy levels of lactate dehydrogenase and cytochrome-c oxidase activity in CFS patients and controls [3]. Lactate dehydrogenase is an enzyme located in the cytoplasm of cells and contributes to the formation of lactate from pyruvate [40].
Four articles examined the levels of Coenzyme Q10 (CoQ10) in fatigued patients with either ME or CFS compared to healthy controls [19], [20], [22], [23]. ME and CFS patients had significantly lower plasma levels of CoQ10 compared to healthy controls [19], [20]. Plasma CoQ10 was also observed to be significantly lower in ME/CFS and depressed patients with CFS compared to controls [22], [23]. Significant, inverse relationships were observed with plasma CoQ10 levels and severity of illness scores of ME/CFS patients, specifically, greater fatigue and autonomic symptoms were associated with lower levels of CoQ10 [22]. However, in patients with depression there was no correlation observed with severity of illness total or individual scores and plasma CoQ10 levels [23]. The presence of CFS independently predicted low plasma CoQ10 levels [19], [20], [23].
No longitudinal studies investigated the association between mitochondrial enzymes and fatigue. The cross-sectional studies reviewed reported conflicting associations between mitochondrial enzymes and fatigue. CoQ10 was the most studied mitochondrial enzyme, where reduced plasma levels of CoQ10 were found in the fatigued populations compared to healthy controls.
3.2.2. Oxidative/nitrosative stress
Three cross-sectional studies, two from the same research group, investigated oxidative and nitrosative stress in CFS and ME patients compared to healthy controls. All three used peripheral blood specimens for biologic analyses, one study investigated patients with CFS, one study investigated ME, and one study investigated fatigue in SLE. Two of the studies complemented medical diagnosis with a structured clinical interview while one study defined fatigue through the use of self-report questionnaires.
Antioxidants (Glutathione, superoxide dismutase, catalase, GSH peroxidase, and GSH reductase) were significantly decreased, while ROS and reactive nitrogen species (RNS) (malondialdehyde [MDA], conjugated dienes, hydroperoxides, and nitric oxide) were significantly increased in the plasma/serum samples of CFS patients compared to healthy controls [19]. Similar results were observed in subjects with ME [20]. It is hypothesized that the aforementioned disruption in the oxidative stress pathway is the downstream result of an imbalance in intracellular calcium and magnesium, which results in high intracellular calcium and low intracellular magnesium [19], [20].
Another study investigated the association between F2-isoprostance levels and fatigue in patients with SLE. They observed that fatigued SLE patients had higher levels of F2-isoprostane than non-fatigued SLE patients [36]. A positive correlation between F2-isoprostane and fatigue was observed in SLE patients and it was observed that F2-isoprostane predicts higher FSS scores in SLE patients. F2-isoprostane is currently acknowledged as the most reliable measure of in vivo oxidative stress [36]. The results from this study provide further evidence of an association between oxidative stress and the development of fatigue.
3.3. Mitochondrial energy metabolism
Ten studies investigated aspects of mitochondrial energy metabolism; all were cross-sectional in design. Most studies (n = 6) focused on fatty acid metabolism, while the rest (n = 4) focused on ATP production. Most studies looked at CFS or ME/CFS patients, while one study investigated fatigue in MS patients.
3.3.1. ATP production
Four cross-sectional studies investigated mitochondrial energy metabolism as a potential marker in fatiguing conditions. Mitochondrial energy metabolism was assessed from muscle biopsies in two of the studies and from peripheral blood in the remaining studies. Two studies only enrolled CFS patients, while the remaining two studies included both ME and CFS patients.
The mitochondrial energy metabolism was measured using the ATP profile test in two studies [5], [25]. The ATP profile tests measures three parameters of mitochondrial function in neutrophils extracted from peripheral blood, (1) ATP concentration (how much ATP is present) and ATP ratio (what fraction of ATP is available for energy supply), (2) the efficiency of oxidative phosphorylation (ADP to ATP recycling efficiency), and (3) TL OUT (ADP out of cytosol into mitochondria) and TL IN (ATP from mitochondria into the cytosol). A Mitochondrial Energy Score (MES) was calculated by multiplying all five factors (ATP, ATP ratio, Ox Phos, TL OUT, TL IN, TL OUT × TL IN). One of the two studies that used this ATP Profile test observed that the percentage of participants with normal values of mitochondrial function increased as fatigue symptoms decreased. [25]. In addition, the MES was positively correlated with scores on the CFS Ability scale [25] indicating that greater mitochondrial efficiency was associated with higher levels of activity and function in those with CFS/ME.
A second study by the same group confirmed the presence of mitochondrial dysfunction in patients with ME/CFS by observing partial blockage of the ADP–ATP translocator protein, adenosine nucleotide translocase (TL) [5]. The TL protein functions to transfer ATP out of the mitochondria into the cell cytoplasm as well as transferring ADP from the cell cytoplasm into the mitochondria to generate more ATP [5]. Cells can compensate for some of the dysfunction in ATP production through two alternative pathways: by increased glyocolysis and the use of adenlyate kinase pathway of ATP formation [5]. Therefore, partial blockage of the TL protein can lead to impaired energy production.
Another study measured two aspects of energy metabolism in the vastus lateralis muscle biopsy samples: aerobic respiration and respiratory chain function, which showed no difference between CFS patients and healthy controls for either parameter [3]. In addition, there were no differences in ATP production rate or respiratory chain complex activity found in the right quadriceps muscle biopsy samples of CFS patients compared to healthy controls [37]. Although ME/CFS patients had impaired energy production as determined by the ATP profile test, no differences in either aerobic respiration or respiratory chain complex activity were found in CFS patients compared to healthy controls.
3.3.2. Fatty acid metabolism
Six cross-sectional articles investigated carnitine levels in fatiguing conditions. Five studies included CFS patients and one study included MS patients. Four studies used peripheral blood specimens for biologic analyses and two studies used muscle biopsies. Four studies complimented medical diagnosis with self-report questionnaires, while two studies assumed fatigue through medical diagnosis.
Acylcarnitine serum levels were significantly lower in CFS patients compared to healthy controls; however, free l-carnitine serum levels were not significantly different between the two groups [18]. Another study observed significantly lower serum levels of total and free carnitine in CFS patients of both genders when compared to healthy controls, as well as lower serum levels of acylcarnitine in CFS patients compared to controls, using historical data [30]. However, no difference between total, free, and acylcarnitine serum levels were found in a more recent study of CFS patients versus healthy controls [38], as well as in patients with MS (with and without fatigue) compared to healthy controls [10]. Another study also showed no significant difference in l-carnitine, total carnitine, or total acylcarnitine plasma levels between CFS patients and healthy controls; however, when individual acylcarnitine subtypes were investigated in the CFS sample, 6 acylcarnitine subtypes (C8:1, C14, C16:1, C18, C18:1 and C18:2) were significantly lower, while 2 acylcarnitine subtypes (C12DC and C18:1-OH) were significantly higher in plasma of CFS participants compared to the matched controls [33].
Higher acylcarnitine serum levels were inversely correlated with the Chronic Fatigue Syndrome Impairment Index (CFS-II) mental index score and CFS-II total score [29]. Lower serum acylcarnitine was associated with worse activity levels and symptom presentation, but these relationships were not observed with free l-carnitine serum levels [18]. A later study, however, showed that higher free carnitine serum levels were significantly associated with better physical abilities, and higher free and total carnitine serum levels were significantly associated with lower fatigue severity [30].
4. Immune response
Four studies investigated dysfunctional immune responses to mitochondria in various fatiguing conditions. Three of these studies were cross-sectional and one study used repeated measures. Three studies only enrolled CFS patients and one study included fatigued patients with various diagnoses. Three studies used peripheral blood specimens for biologic analyses, while one study used muscle biopsies. In one study clinical diagnosis was complemented with self-report questionnaires and interview assessments, while three studies assumed fatigue through medical diagnosis.
Two studies from the same research group investigated autoimmune responses to acute phase phospholipids in patients with fatiguing illnesses [12], [13]. CFS subjects had serum lipid fractions that resembled those commonly found in patients poisoned with ciguatoxin, a marine toxin [13]. Sera from patients with CFS, chronic ciguartera fish poisoning (CCFP), gulf war veterans (GWV), and prostate cancer patients contained antibodies to cardiolipin (aCL), a phospholipid of the mitochondrial membrane [13]. Further study found that 95% of CFS patients had anticardiolipin antibody (ACA) of the IgM subtype, 10% showed an IgG response, 2.5% had an IgA response; 4 patients were positive for IgG and IgM, and one patient was positive for all three antibody subtypes [12].
Two studies investigated the role of enteroviral infection with the onset of CFS [24], [41]. One study examined anterior tibialis muscle biopsy specimens for the presence of enteroviral RNA. However, they failed to detect the presence of a persistent enteroviral infection in patients with CFS [24]. Another study observed 23 differentially expressed genes from peripheral blood samples of patients with persistent post-Epstein–Barr (EBV) fatigue compared to controls (those who recovered without persistent fatigue) in the early phase (0–3 months) of infection. Of the 23 differentially expressed genes, 8 were found in subjects with persistent fatigue post-EBV and were involved in binding and metabolism ontologies [41]. When exploring both early and late (> 6 months) phases of infection, 24 genes were differentially expressed between cases and controls. Half of the 24 differentially expressed genes were associated with mitochondrial functions such as fatty acid oxidation (CRAT, carnitine acetyltransferase; APOA2, apolipoprotein A-II), apoptosis (BTG1, B-cell translocation gene 1; FOLR1, folate receptor 1; CTRL, chymotrypsin-like), DNA repair, and mitochondrial membrane (COX8A, cytochrome c oxidase subunit VIII; COX11, cytochrome c oxidase assembly protein; KCNA10, potassium voltage-gated channel; MGP, matrix G1a protein; ATP5L, ATP synthase) [41].
In the four studies reviewed an autoimmune response was found as evidenced by the presence of mitochondrial phospholipids in the sera of CFS patients. Although there was no evidence of persistent enteroviral infection found in muscle biopsies of CFS patients, differential expression of genes associated with mitochondrial function was noted in patients' post EBV infection. Comparing the results among these studies is challenging. Only one research group investigated autoimmune responses to acute phase phospholipids, publishing two different studies. Both of the studies investigating post-infective fatigue investigated different viral infections (enterovirus vs. EBV) and the two studies employed different study designs, cross-sectional [24] and repeated measures [41].
5. Genetics
Five articles explored the association between gene expression profiles in fatigue conditions versus controls. Three studies were cross-sectional and two studies used repeated measures designs. Three studies only included CFS patients, one study included men with prostate cancer, and one study included patients with Human Immunodeficiency Virus (HIV). Three studies used peripheral blood specimens for genomic analyses, while two studies used muscle biopsies. One study complemented clinical diagnosis with self-report questionnaires, two studies used self-report questionnaires alone to measure fatigue, and two assumed fatigue through medical diagnosis.
The first genomic study found no significant differences between CFS patients and healthy controls in the total volume of mitochondrial DNA present, two mtDNA rearrangements, and the presence of one point mutation [3]. A real-time PCR (qPCR) study found 11 mitochondrial function-related genes to be differentially expressed during radiation therapy for prostate cancer and 8 of the 11 genes were significantly associated with fatigue intensification during radiation therapy [15]. These 8 genes are involved in mitochondrial apoptosis and signaling, mitochondrial membrane polarity and potential, mitochondrial morphology and fission/fusion, and mitochondrial and small molecule transport [15].
One microarray analysis found 35 differentially expressed genes in the peripheral blood mononuclear cells (PBMCs) from CFS patients, where 3 up-regulated genes had activities specific to mitochondrial function: EIF2B4 (eukaryotic translation initiation factor 2B, subunit 4δ, tv-1), EIF4G1 (eukaryotic translation initiation factor, 4γ, 1, tv-5), and MRPL23 (mitochondrial ribosomal protein L23) [17]. Another microarray study found 47 differentially expressed genes (2 up-regulated and 38 down-regulated in both genders; 7 up-regulated in females, yet down-regulated in males) from vastus lateralis muscle biopsies of CSF patients compared to healthy controls. The down-regulated genes were associated with an impairment of antioxidant mechanisms, aerobic energy production, and metabolism [28]. Another study investigated gene networks in CD14 + cells from HIV-infected patients who reported high fatigue, versus those who reported low fatigue or healthy controls, where 6 mitochondrial-related genes (CHD1L and ALDOB genes were negatively associated with fatigue; TIMM17B, GSR, IMMT, and SLC25A26 were positively associated with fatigue) are implicated in protein translocation into the mitochondrial matrix, cristae morphology, ATP-binding and protein binding, metabolism, oxidation and reduction processes, and energy production were identified [42].
Five articles explored the association between gene expression profiles in fatiguing conditions versus controls. Common mitochondria-specific functional pathways were reported from the results of the gene expression studies included in review, to include pathways related to metabolism, energy production, protein transport, mitochondrial morphology, central nervous system dysfunction and post-viral infection. The pathways identified in these studies were similar across three different patient populations and supported areas of dysfunction identified in the previous sections.
6. Discussion
The purpose of this systematic review was to examine markers of mitochondrial function that have evidence of an association with fatigue in order to identify areas needed for further research. This review included studies focusing on the markers of mitochondrial function in relation to fatigue. Dysfunctions in the mitochondrial structure, mitochondrial function (mitochondrial enzymes and oxidative/nitrosative stress), mitochondrial energy metabolism (ATP production and fatty acid metabolism), immune response, and genetics were investigated as potential contributors to fatigue.
Carnitine was the most investigated mitochondrial function marker reported in this review (n = 6). Dysfunctional carnitine levels were reported in all six studies that investigated the biomarker; however, the specifics of the dysfunction varied among the studies. Genetic profiles were the second most studied mitochondrial parameter. Even though different genes were reported across the studies, common pathways (metabolism, energy production, protein transport, mitochondrial morphology, central nervous system dysfunction, and post-viral infection) were identified among the articles. The most commonly investigated mitochondrial enzyme was CoQ10. It was the only mitochondrial biomarker found to have a consistent association with fatigue identified in this review [19], [20], [22], [23]. CoQ10 is an essential enzyme in the electron transport chain, responsible for shuttling electrons and protons [40]. Further investigation is needed to understand the role of CoQ10 in fatigue.
CoQ10 deficiency can be either primary or secondary in nature. Primary deficiency stems from dysfunction with the genes coding for the synthesis of CoQ10, whereas secondary deficiency results from anything that is not primary in nature [14], [21], [31]. CoQ10 is endogenously produced and therefore, dietary intake has minimal influence on the CoQ10 concentration in the body. However, if CoQ10 is found to be depleted, especially with primary deficiency, supplementation is the remaining recommended therapeutic option [31]. In clinical practice, vitamins such as riboflavin B2, niacin B3, vitamin E and other mitochondrial cofactors including levo-carnitine, lipoic acid, and acetyl-l-carnitine are used as supplemental treatment for mitochondrial disorders, in order to enhance ETC enzyme activity as an antioxidant defense [6]. The efficacy of these vitamins and mitochondrial cofactors as treatments for mitochondrial disorders remains controversial [6].
CoQ10 has been shown to have clinical benefits attributed to its antioxidant properties and its role in cellular bioenergetics [21]; hence, it is being used as a therapeutic option for a number of mitochondria-related clinical conditions including those with cardiovascular disease, reproductive issues, and neurodegenerative diseases [21]. In this review, there are only two research teams that investigated the relationship between CoQ10 and fatigue. More clinical studies are needed to confirm the role of vitamins and mitochondrial cofactors in alleviating fatigue symptoms [14], [31].
Based on the findings of this review, alterations in energy metabolism may contribute to fatigue. Support for this conclusion was evident from dysfunctions reported with oxidative phosphorylation, electron transport chain activity, and ATP production and recycling. Impaired oxidative phosphorylation was noted through reduced citrate synthase activity [24], [37]. Electron transport chain activity was disrupted as evidenced by reduced levels of succinate reductase (Complex I), cytochrome-c oxidase (Complex IV), and CoQ10 (electron shuttle from Complex I and II to Complex III), as well as evidenced through disrupted oxidative stress (decreased levels of antioxidants and increased levels of ROS and F2-isoprostance) [19], [20], [22], [23], [24], [36]. ATP concentration, recycling, and the efficiency of oxidative phosphorylation were found to be disrupted through the ATP Profile Test [5], [25]. Fatty acid metabolism was also impaired as evidenced by abnormal carnitine levels [18], [29], [30], [33]. Genetic investigations found abnormal gene transcription pathways related to metabolism, energy production, and protein transport [15], [17], [28], [42]. These genetic pathways support the areas of dysfunction that were found in the aforementioned studies.
The limitations identified in this review are: 83% of the studies were cross-sectional, 79% of the studies enrolled only CFS patients, and there were inconsistent associations found between mitochondrial biomarkers and fatigue. Future studies utilizing longitudinal designs need to be conducted to establish associations between mitochondrial dysfunction and fatigue development. Additionally, if mitochondrial dysfunction and fatigue are observed, longitudinal studies can provide more evidence about the characteristics of the association.
The inclusion of diverse patient populations in future studies would provide evidence regarding common mitochondrial mechanisms as an etiology for fatigue. The predominant patient population included in the reviewed studies was CFS. Knowledge gained from studies of the CFS population needs to be translated to other fatigued populations, such as those with cancer or other chronic diseases. Understanding the biological mechanisms underlying fatigue development is important to enhance clinical evaluation and treatment.
Furthermore, fatigue was defined differently among the reviewed studies and different diagnostic criteria and self-report instruments were used. This variability limited the ability to compare findings across studies. Furthermore, the self-report questionnaires included in the reviewed studies are valid measures of fatigue, but not necessarily for the populations included in the studies, namely CFS. Future research needs to work towards establishing a global agreement on the clinical definition of fatigue. Once a clinical definition of fatigue is established, research will need to focus on developing a valid and reliable tool for measurement of fatigue in the clinical setting. Until a global definition is developed, researchers need to ensure that the existing fatigue tools are validated in their clinical populations of interest.
The literature review identified some potential relationships between mitochondrial dysfunction and fatigue; however, the findings were limited to predominantly one patient population, were from mostly cross-sectional studies, and results were confounded by the use of multiple definitions of fatigue. These limitations culminated in inconsistent findings across studies. Therefore, the results from the review suggest further investigation to address the gaps in the current literature. Once the underlying mechanisms of fatigue are better understood, individualized and tailored therapies can be developed to improve quality of life of patients.
Contributor Information
Kristin Filler, Email: kristin.filler@nih.gov.
Debra Lyon, Email: delyon@ufl.edu.
James Bennett, Email: jpbennett@vcu.edu.
Nancy McCain, Email: nlmccain@vcu.edu.
Ronald Elswick, Email: rkelswic@vcu.edu.
Nada Lukkahatai, Email: nada.lukkahatai@nih.gov.
Leorey N. Saligan, Email: saliganl@mail.nih.gov.
References
- 1.Alexander N.B., Taffet G.E., Horne F.M., Eldadah B.A., Ferrucci L., Nayfield S., Studenski S. Bedside-to-Bench conference: research agenda for idiopathic fatigue and aging. J. Am. Geriatr. Soc. 2010;58(5):967–975. doi: 10.1111/j.1532-5415.2010.02811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behan W.M.H., More I.A.R., Downie I., Gow J.W. Mitochondrial studies in the chronic fatigue syndrome. EOS Riv. Immunol. Immunofarmacol. 1995;15(1–2):36–39. [Google Scholar]
- 3.Behan W.M.H., Holt I.J., Kay D.H., Moonie P. In vitro study of muscle aerobic metabolism in chronic fatigue syndrome. J. Chron. Fatigue Syndr. 1999;5(1):3–16. [Google Scholar]
- 4.Blackstone C., Chang C.R. Mitochondria unite to survive. Nat. Cell Biol. 2011;13(5):521–522. doi: 10.1038/ncb0511-521. [DOI] [PubMed] [Google Scholar]
- 5.Booth N.E., Myhill S., McLaren-Howard J. Mitochondrial dysfunction and the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Int. J. Clin. Exp. Med. 2012;5(3):208–220. [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen B.H. Mitochondrial cytopathies: a primer. 2000. http://www.umdf.org/atf/cf/%7B858ACD34-ECC3-472A-8794-39B92E103561%7D/mitochondrial_cytopathies_APrimer.pdf Retrieved from:
- 7.Cohen B.H., Gold D.R. Mitochondrial cytopathy in adults: what we know so far. Cleve. Clin. J. Med. 2001;68(7):625–626. doi: 10.3949/ccjm.68.7.625. (629–642) [DOI] [PubMed] [Google Scholar]
- 8.Duchen M.R. Roles of mitochondria in health and disease. Diabetes. 2004;53(Suppl. 1):S96–S102. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- 9.Edwards R.H., Gibson H., Clague J.E., Helliwell T. Muscle histopathology and physiology in chronic fatigue syndrome. CIBA Found. Symp. 1993;173:102–117. doi: 10.1002/9780470514382.ch7. (discussion 117–131) [DOI] [PubMed] [Google Scholar]
- 10.Fukazawa T., Sasaki H., Kikuchi S., Hamada T., Tashiro K. Serum carnitine and disabling fatigue in multiple sclerosis. Psychiatry Clin. Neurosci. 1996;50(6):323–325. doi: 10.1111/j.1440-1819.1996.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 11.Hardy S.E., Studenski S.A. Qualities of fatigue and associated chronic conditions among older adults. J. Pain Symptom Manag. 2010;39(6):1033–1042. doi: 10.1016/j.jpainsymman.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hokama Y., Campora C.E., Hara C., Kuribayashi T., Le Huynh D., Yabusaki K. Anticardiolipin antibodies in the sera of patients with diagnosed chronic fatigue syndrome. J. Clin. Lab. Anal. 2009;23(4):210–212. doi: 10.1002/jcla.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hokama Y., Empey-Campora C., Hara C., Higa N., Siu N., Lau R., Yabusaki K. Acute phase phospholipids related to the cardiolipin of mitochondria in the sera of patients with chronic fatigue syndrome (CFS), chronic Ciguatera fish poisoning (CCFP), and other diseases attributed to chemicals, Gulf War, and marine toxins. J. Clin. Lab. Anal. 2008;22(2):99–105. doi: 10.1002/jcla.20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvath R. Update on clinical aspects and treatment of selected vitamin-responsive disorders II (riboflavin and CoQ10) J. Inherit. Metab. Dis. 2012;35:679–687. doi: 10.1007/s10545-011-9434-1. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao C.P., Wang D., Kaushal A., Saligan L. Mitochondria-related gene expression changes are associated with fatigue in patients with nonmetastatic prostate cancer receiving external beam radiation therapy. Cancer Nurs. 2013;36(3):189–197. doi: 10.1097/NCC.0b013e318263f514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jason L.A., Evans M., Brown M., Porter N. What is fatigue? Pathological and nonpathological fatigue. PM R. 2010;2(5):327–331. doi: 10.1016/j.pmrj.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Kaushik N., Fear D., Richards S.C., McDermott C.R., Nuwaysir E.F., Kellam P., Kerr J.R. Gene expression in peripheral blood mononuclear cells from patients with chronic fatigue syndrome. J. Clin. Pathol. 2005;58(8):826–832. doi: 10.1136/jcp.2005.025718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuratsune H., Yamaguti K., Takahashi M., Misaki H., Tagawa S., Kitani T. Acylcarnitine deficiency in chronic fatigue syndrome. Clin. Infect. Dis. 1994;18(Suppl. 1):S62–S67. doi: 10.1093/clinids/18.supplement_1.s62. [DOI] [PubMed] [Google Scholar]
- 19.Kurup R.K., Kurup P.A. Hypothalamic digoxin, cerebral chemical dominance and myalgic encephalomyelitis. Int. J. Neurosci. 2003;113(5):683–701. doi: 10.1080/00207450390200026. [DOI] [PubMed] [Google Scholar]
- 20.Kurup R.K., Kurup P.A. Isoprenoid pathway dysfunction in chronic fatigue syndrome. Acta Neuropsychiatr. 2003;15(5):266–273. doi: 10.1034/j.1601-5215.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 21.Littarru G.P., Tiano L. Clinical aspects of coenzyme Q10: an update. Nutrition. 2010;26:250–254. doi: 10.1016/j.nut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol. Lett. 2009;30(4):470–476. [PubMed] [Google Scholar]
- 23.Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Lower plasma Coenzyme Q10 in depression: a marker for treatment resistance and chronic fatigue in depression and a risk factor to cardiovascular disorder in that illness. Neuroendocrinol. Lett. 2009;30(4):462–469. [PubMed] [Google Scholar]
- 24.McArdle A., McArdle F., Jackson M.J., Page S.F., Fahal I., Edwards R.H. Investigation by polymerase chain reaction of enteroviral infection in patients with chronic fatigue syndrome. Clin. Sci. (Lond.) 1996;90(4):295–300. doi: 10.1042/cs0900295. [DOI] [PubMed] [Google Scholar]
- 25.Myhill S., Booth N.E., McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int. J. Clin. Exp. Med. 2009;2(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 26.Nunnari J., Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pieczenik S.R., Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007;83(1):84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Pietrangelo T., Mancinelli R., Toniolo L., Montanari G., Vecchiet J., Fano G., Fulle S. Transcription profile analysis of vastus lateralis muscle from patients with chronic fatigue syndrome. Int. J. Immunopathol. Pharmacol. 2009;22(3):795–807. doi: 10.1177/039463200902200326. [DOI] [PubMed] [Google Scholar]
- 29.Plioplys A.V., Plioplys S. Electron-microscopic investigation of muscle mitochondria in chronic fatigue syndrome. Neuropsychobiology. 1995;32(4):175–181. doi: 10.1159/000119233. [DOI] [PubMed] [Google Scholar]
- 30.Plioplys A.V., Plioplys S. Serum levels of carnitine in chronic fatigue syndrome: clinical correlates. Neuropsychobiology. 1995;32(3):132–138. doi: 10.1159/000119226. [DOI] [PubMed] [Google Scholar]
- 31.Potgieter M., Pretorius E., Pepper M. Primary and secondary coenzyme Q10 deficiency: the role of therapeutic supplementation. Nutr. Rev. 2013;71:180–188. doi: 10.1111/nure.12011. [DOI] [PubMed] [Google Scholar]
- 32.Read C.Y., Calnan R.J. Mitochondrial disease: beyond etiology unknown. J. Pediatr. Nurs. 2000;15(4):232–241. doi: 10.1053/jpdn.2000.8042. [DOI] [PubMed] [Google Scholar]
- 33.Reuter S.E., Evans A.M. Long-chain acylcarnitine deficiency in patients with chronic fatigue syndrome. Potential involvement of altered carnitine palmitoyltransferase-I activity. J. Intern. Med. 2011;270(1):76–84. doi: 10.1111/j.1365-2796.2010.02341.x. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds T. From small things. BMJ. 2007;335(7623):747–748. doi: 10.1136/bmj.39328.503785.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal T.C., Majeroni B.A., Pretorius R., Malik K. Fatigue: an overview. Am. Fam. Physician. 2008;78(10):1173–1179. [PubMed] [Google Scholar]
- 36.Segal B.M., Thomas W., Zhu X., Diebes A., McElvain G., Baechler E., Gross M. Oxidative stress and fatigue in systemic lupus erythematosus. Lupus. 2012;21:984–992. doi: 10.1177/0961203312444772. [DOI] [PubMed] [Google Scholar]
- 37.Smits B., van den Heuvel L., Knoop H., Kusters B., Janssen A., Borm G., van Engelen B. Mitochondrial enzymes discriminate between mitochondrial disorders and chronic fatigue syndrome. Mitochondrion. 2011;11(5):735–738. doi: 10.1016/j.mito.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Soetekouw P., Wevers R.A., Vreken P., Elving L.D., Janssen A.J.M., van der Veen Y., van der Meer J.W.M. Normal carnitine levels in patients with chronic fatigue syndrome. [Article] Neth. J. Med. 2000;57(1):20–24. doi: 10.1016/s0300-2977(00)00030-9. [DOI] [PubMed] [Google Scholar]
- 39.Swain M.G. Fatigue in chronic disease. Clin. Sci. (Lond.) 2000;99(1):1–8. [PubMed] [Google Scholar]
- 40.Tymoczko J., Berg J., Stryer L. W. H. Freeman and Company; New York, NY: 2010. Biochemistry: A Short Course. [Google Scholar]
- 41.Vernon S.D., Whistler T., Cameron B., Hickie I.B., Reeves W.C., Lloyd A. Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr virus. BMC Infect. Dis. 2006;6:15. doi: 10.1186/1471-2334-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voss J.G., Dobra A., Morse C., Kovacs J.A., Danner R.L., Munson P.J., Dalakas M.C. Fatigue-related gene networks identified in CD14(+) cells isolated from HIV-infected patients-part I: research findings. Biol. Res. Nurs. 2013;15(2):137–151. doi: 10.1177/1099800411421957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youle R.J., van der Bliek A.M. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norheim K., Jonsson G., Omdal R. Biological mechanisms of chronic fatigue. Rheumatology. 2011;50:1009–1118. doi: 10.1093/rheumatology/keq454. [DOI] [PubMed] [Google Scholar]
- 45.Verzijl H.T., van Engelen B.G., Luyten J.A., Steenbergen G.C., van den Heuvel L.P., ter Laak H.J., Padberg G.W., Wevers R.A. Annals of Neurology. 1998;44(1):140–143. doi: 10.1002/ana.410440124. [DOI] [PubMed] [Google Scholar]


