Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disease. The World Health Organization estimates that there are currently 18 million people worldwide living with AD and that number is expected to double by early 2025. Currently, there are no therapies to stop or reverse the symptoms of AD. We have developed an antisense oligonucleotide (OL-1) against the amyloid betaprotein precursor (AβPP) that can decrease AβPP expression and amyloid beta protein (Aβ) production. This antisense rapidly crosses the blood-brain barrier, reverses learning and memory impairments, reduces oxidative stress and restores brain-to-blood efflux of Aβ in SAMP8 mice. In the current study, we examined the effects of this AβPP antisense in the Tg2576 mouse model of AD. The Tg2576 overproduces human Aβ, develops age-related learning and memory deficits, and exhibits oxidative damage in the brain. First, we administered the AβPP antisense centrally into the lateral ventricle 3 times at 2 week intervals. Seventy-two hours after the third injection, we tested learning and memory in T-maze foot shock avoidance. In the second study, we injected the mice with AβPP antisense 3 times at two week intervals via the tail vein. Seventy-two hours later, we tested learning and memory T-maze foot shock avoidance, novel object recognition and elevated plus maze. At the end of behavioral testing, mice were sacrificed and brain tissue was collected for evaluation of AβPP, Aβ, and expression of cytokines and chemokines. AβPP antisense administered centrally improved acquisition and retention of T-maze foot shock avoidance. AβPP antisense administered via tail vein improved learning and memory in both T-maze foot shock avoidance and novel object-place recognition. In the elevated plus maze the mice which received OL-1 AβPP antisense spent less time in the open arms and had fewer entries into the open arms indicating reduced disinhibitation. Biochemical analyses reveal significant reduction of AβPP signal and a reduction of measures of neuroinflammation. The current findings support the therapeutic potential of OL-1 AβPP antisense.
Keywords: antisense oligonucleotide, Tg2576, learning, memory, T-maze, object recognition, oxidative stress, blood brain barrier, cytokines
Introduction
Amyloid beta protein (Aβ) is one of the major constituents of plaque [1]. Aβ and Aβ oligomers play a major role in Alzheimer’s disease [2]. To date there are no therapies that can stop or reverse the disease progression. Currently the major drugs affecting Aβ being investigated are antibodies [3]. The most recent report on immunotherapies using passive immunizations indicated that the trials were halted due to lack of an effect (http://www.alzforum.org/new/detail.asp?id=3234).
Antisense oligonucleotides are currently in various stages of development for a number of diseases such as Huntington’s, cancer, spinal muscular atrophy, Duchenne muscular dystrophy, diabetes, Crohns’s disease and Alzheimer’s [4]. We have previously shown that antisense corresponding to the 17–30 region of Aβ (OL-1) produced a significant reversal of the learning and memory deficits in the SAMP8 model of Alzheimer’s disease [5]. The SAMP8 mouse has a natural mutation that induces an overproduction of mouse Aβ [6]. We found that 3 treatments at 2 week intervals before training decreased protein levels of both amyloid beta protein precursor (AβPP) and amyloid beta peptide (Aβ) in whole brain and specific brain regions [5]. OL-1 also reversed oxidative stress, and restored Aβ efflux at the blood-brain-barrier (BBB) [5, 7, 8]. To date, we have found little effect of OL-1 on mRNA levels, suggesting its major effect is at the level of translation.
To be effective in humans, OL-1 would need to be effective at suppressing production of human Aβ. The Tg2576 mouse is another model of AD that overexpresses a mutant form of the human AβPP gene. Like the SAMP8 mouse and human patients with AD, the Tg2576 mouse has an age-related impairment in learning and memory, elevated brain levels of Aβ, decreased efflux of Aβ from the brain, and increased neuroinflammation and oxidative damage in the hippocampus [9–12]. Here, we show in Tg2576 mice that either centrally or intravenously administered OL-1 decreases Aβ levels in brain, reverses cognitive deficits, and reduces neuroinflammation.
Methods
MICE
The subjects for these experiments were 13 month old male Tg2576 (APPswe) mice and Tg2576 wild type mice obtained from Taconic Farms (Germantown, NY). Mice were 2 months old when received from the vendor and housed 2 to 4 per cage until testing. Mice were individually housed for 3 weeks during behavioral testing to ensure each mouse was equally aroused and equally exposed to external stimuli (T-maze buzzer) during testing. Food (Richland 5001) and water were available on an ad lib basis and the rooms had a 12 hour light-dark cycle with lights on at 0600 hours. Experiments were conducted between 0730 and 1100 hours. All studies were conducted with the approval of the Animal Care and Use Committee at the VA Medical Center, St. Louis, MO.
TREATMENT
Antisense oligonucleotide (OL-1) and random antisense (RA) were purchased from Midland Certified Reagent Co. (Midland, TX) and dissolved in saline. The sequence of OL-1 is 5’-(P=S)GGCGCC TTTGTTCGAACCCACATCTTCAGCAAAGAACACCAG-3’; the sequence of RA is 5’-(P=S)GATCACGTACACATCGACACCAGTCGCCATGACTGAGCTT-3’.
Intracerebroventricular (ICV) Treatment
For the initial injection, mice were anesthetized with isoflurane, placed in a stereotaxic instrument, the scalp was deflected and a hole drilled through the skull over the injection site. The injection coordinates for the ICV injection were 0.5 mm posterior to the bregma and 1.0 mm to the right or left of the sagittal suture. A 30 gauge needle was dropped to 2.0 mm and a 2µl injection given over 60 seconds. The needle was removed the scalp was closed and the mice were returned to their cages. For the second and third injections, mice were anesthetized with isoflurane, placed in a stereotaxic instrument and the scalp reopened. A 30 gauge needle was reinserted and 2.0 µl of antisense delivered. The scalp was closed after each injection.
Behavioral Testing
T-Maze training and testing procedures
The T-maze is both a learning task based on working-memory and a reference-memory task. The T-maze consisted of a black plastic alley with a start box at one end and two goal boxes at the other. The start box was separated from the alley by a plastic guillotine door that prevented movement down the alley until raised at the onset of training. An electrifiable floor of stainless steel rods ran throughout the maze to deliver a mild scrambled foot-shock.
Mice were not permitted to explore the maze prior to training. A block of training trials began when a mouse was placed into the start box. The guillotine door was raised and a cue buzzer sounded simultaneously; 5 sec later foot-shock was applied. The arm of the maze entered on the first trial was designated “incorrect” and the mild foot-shock was continued until the mouse entered the other goal box, which in all subsequent trials was designated as “correct” for the particular mouse. At the end of each trial, the mouse was returned to its home cage until the next trial.
Mice were trained until they made one avoidance. Training used an intertrial interval of 35 sec, the buzzer was of the door-bell type sounded at 55 dB, and shock was set at 0.35 mA (Coulbourn Instruments scrambled grid floor shocker model E13-08). Retention was tested one week later by continuing training until mice reached the criterion of 5 avoidances in 6 consecutive trials. The results were reported as the number of trials to first avoidance for acquisition and the number of trials to criterion for the retention test. The latency to escape shock in seconds during trial one of acquisition was also reported.
Novel Object -Place Recognition
Novel object-place recognition is a declarative memory task that involves the hippocampus when, as performed here, the retention interval is 24 hours after initial exposure to the objects [13]. Mice were habituated to an empty apparatus for 5 minutes a day for 3 days prior to entry of the objects. During the training session, the mouse was exposed to two like objects (plastic frogs) which it was allowed to examine for 5 minutes. The apparatus and the objects were cleaned between each mouse. Twenty-four hours later, the mouse was exposed to one of the original objects and a new novel object in a new location and the percent of time spent examining the new object was recorded. The novel object was made out of the same material as the original object and of the same size, but a different shape. This eliminated to possibility of smell associated with a particular object being a factor. The underlying concept of the task is based on the tendency of mice to spend more time exploring new, novel objects than familiar objects. Thus, the greater the retention/memory at 24 hours, the more time spent with the new object. The value is presented as the discrimination index which is determined by dividing the time spent exploring the novel object by the total exploration time multiplied by 100.
Elevated Plus Maze
The procedure used was similar to those used by Lister, 1987 [14]. The experimental apparatus is shaped like a “plus” sign and consists of a central platform, two open arms and two equal-sized closed arms opposite to each other. The maze is made of grey Plexiglas, elevated to a height of 50 cm above the flood and illuminated by a dim light. The test consisted of placing a mouse in the central platform facing an enclosed arm and allowing it to freely explore the maze for 5 min. The test arena was wiped with a damp cloth after each trial. The number of entries into the open and closed arms and the time spent in open arms was measure by an observer blind to the drug treatment.. The frequency of arm changes was also calculated.
Tissue processing for analysis of oxidative stress markers, AβPP protein expression, and soluble Aβ levels
Hemibrains were homogenized on ice in a 5× volume of PBS plus 1mM EDTA and protease inhibitor cocktail (Sigma). The homogenate was then portioned, and Triton X-100 added to samples at a final concentration of 0, 0.1, or 1%. Samples containing detergent were further processed by shaking on a vortex shaker adapter for 30 minutes at 4°C. All samples were then centrifuged at 4°C for 10 minutes at 20,000g. The detergent-containing supernatants were collected, aliquotted, and stored at −80°C. The detergent-free supernatants were de-proteinated for subsequent analysis of glutathione according to kit instructions (Cayman Chemicals). Deproteinated supernatants were stored at −20°C until subsequent analysis. Protein concentration in each sample was measured by BCA assay (ThermoScientific).
AβPP Protein Expression
AβPP expression of human and rodent forms was measured by dot blot analysis using Epitomics 1565-1 antibody 1ug/ml. This antibody is directed against the C-terminus of AβPP, has been validated for specificity in brain tissue of APP knockout mice [15], and specifically reacts with full-length AβPP. Negligible immunoreactivity against C-terminal fragments is observed on Western blots. Dot blots were prepared by loading 2 ug of protein from the 1% Triton X-100 extract in a volume of 250 ul PBS in duplicate wells of a Bio-dot microfiltration apparatus (Biorad). A standard curve was also prepared using one of the Tg2576 samples, with protein amounts per well ranging from 0.125 to 4 ug in a volume of 250 ul PBS and loaded in duplicate. Samples and standards were then applied to a nitrocellulose membrane by vacuum. The membrane was washed, blocked in 5% milk, and incubated overnight with AβPP antibody. The membrane was washed in PBS, and anti-rabbit secondary (Santa Cruz) prepared 1:5000 in 5% milk and incubated with the membrane for 1 hour. The membrane was then washed, and dots visualized with West Pico chemiluminescent substrate. Images were captured using an ImageQuant LAS4000, and IQTL software used to quantify spot intensities. A standard curve was generated using spot intensities of the standards using Prism software (GraphPad, Inc, San Diego CA), and spot intensity of the samples were normalized to those of the standard curve prior to statistical analysis.
Soluble A-beta Expression
Samples extracted in 0.1% Trition X-100 were assayed using kits which detect both human and murine Aβ (Wako). Extracts were diluted 1:1 in standard diluent provided in the kit for wild-type samples, and for Tg2576 mice 1:9 or 1:49 for Aβ 42 and 40, respectively. To maintain consistency in the sample matrix, the final concentration of the detergent in all samples was held constant at 0.05%. As a control, brain tissue from APP knockout mice was processed identically, and loaded at equivalent dilutions for both Aβ 40 and 42 assays. The absence of signal for these samples confirmed specificity of the assay.
Total A-Beta Expression
Total Aβ 40 and 42 expression was measured using the same assays described for detection of soluble Aβ. Tg2576 hemibrains were extracted in 5M guanidine and stored at −20°C. On the same day as the assay, extracts were diluted 1/10 in cold casein buffer and centrifuged at 16000g for 20 min at 4°C. The supernatants were then further diluted 1/50 in standard diluent (final dilution 1/500) provided in the kit, and the remainder of the assay conducted according to kit instructions. Only Tg2576 brains were analyzed for total Aβ, as the guanidine extraction method was found to cause false positives in signal from AβPP knockout tissue in the less diluted samples that are necessary to measure Aβ in wild-type mice.
Cytokines and Chemokines
A panel of 23 cytokines and chemokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-17, Eotaxin (CCL11), G-CSF, GM-CSF, IFN-γ, KC (CXCL1), MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5), and TNF-α) were measured in 0.1% detergent extracts using a murine Bioplex assay kit from Biorad. This panel was chosen as it was the most inclusive of the cytokines and chemokines implicated in AD [16] and we have found in a model of systemic inflammation that cytokines on this panel are inhibited by antioxidants [17]. All samples were diluted 1:3 in sample diluent provided in the kit, and processed according to assay directions. Plates were read on a Bioplex 200 (Biorad). Cytokines which were undetected for a sample were assigned a value of zero, and only cytokines which were greater than 50% detectable in any group were considered for analysis.
Statistics
All data were analyzed using GraphPad Prism 5.0 software. The number of mice used in each study is indicated, with error bars representing the standard error of the mean (SEM). Student’s t-test was used for comparison of two groups. More than two groups were compared by analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test.
Results
Effect on memory of central administration of AβPP antisense in Tg2576 mice
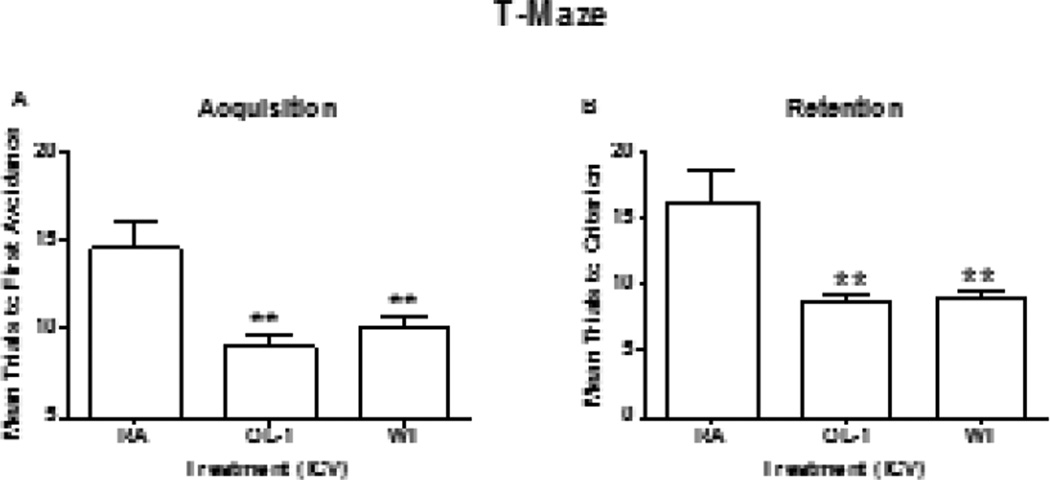
Seventy-two hours after the third injection of antisense treatment regimen, 13 month old Tg2576 mice and wild type mice were tested for hippocampal-dependent learning and memory in T-maze foot shock avoidance [18]. The one-way ANOVA for trials to criterion during acquisition produced a significant effect for treatment F(2,29) = 7.53, P<0.006. Neuman-Keuls post hoc test indicated that the Tg2576 mice which received OL-1 took significantly fewer trials to reach criterion than the Tg2576 mice that received RA. There was no difference between the Tg2576 mice that received OL-1 and the wild type mice (Figure 1a). One week later, retention was tested. The one-way ANOVA for trials to criterion on the retention test produced a significant effect for treatment F(2,29) = 8.78, P<0.001. The Tg2576 mice that received OL-1 took significantly fewer trials to reach criterion than the Tg2576 mice that received RA. There was no difference between the wild type mice and the Tg2576 mice that received OL-1. (Figure 1b). The latency to escape foot shock on the first trial of acquisition was not significant [F(2,29) = 0.53, P NS] (data not shown).
Figure 1.
Tg2576 mice administered OL-1 antisense ICV had improved acquisition and retention compared to the Tg2576 mice that received RA. The Tg2576 mice that received OL-1 were not different from the wild type (WT) controls. The numbers per group were Tg2576 RA = 10, Tg2576 Ol-1 = 11 and WT RA = 11. The ** indicates P<0.001.
Effect of tail vein administration of AβPP antisense in Tg2576 mice on Learning and Memory
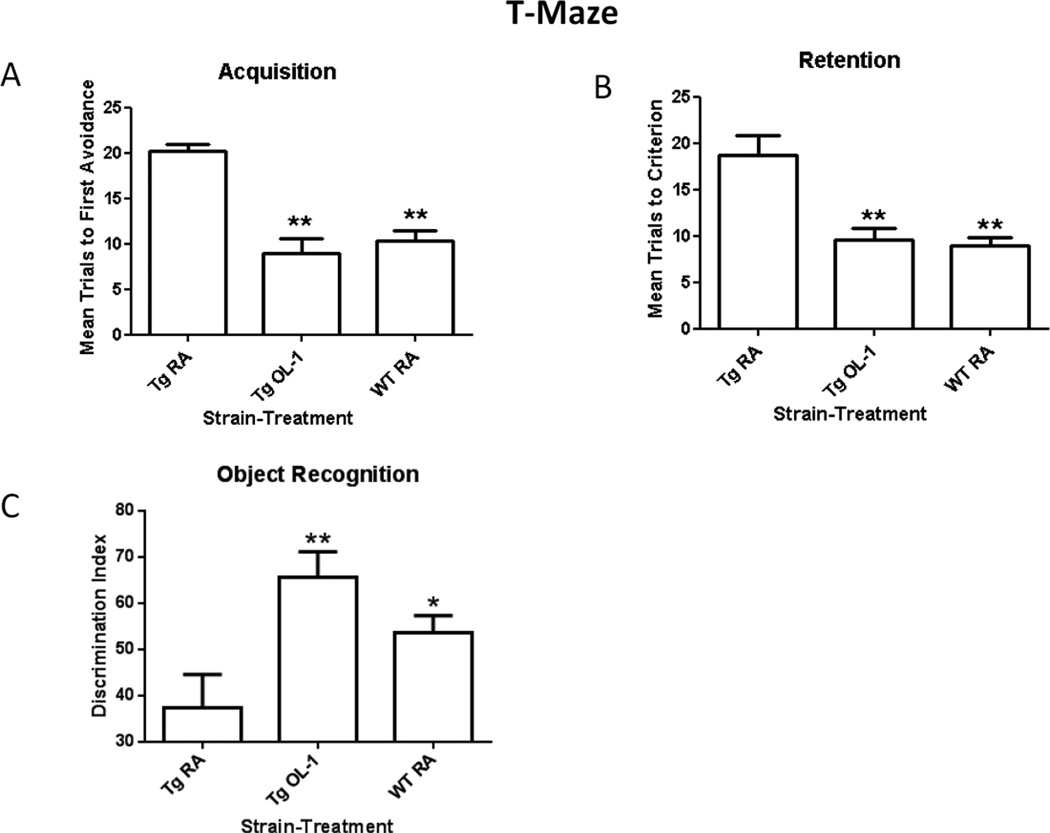
We have previously shown that AβPP antisense crosses the BBB and improves learning and memory in SAMP8 mice [8, 15]. Here, we administered OL-1 or RA via tail vein (6ug) to Tg2576 mice and RA via tail vein (6ug) to the wild type 4 times at 2 week intervals, then 72 h after the third injection of antisense treatment regimen, 13 month old Tg2576 mice and wild type controls were tested for hippocampal-dependent learning and memory in T-maze foot shock avoidance and novel object recognition (NOR), both hippocampal dependent tasks [13, 18]. A one-way ANOVA for trials to first avoidance during acquisition in the T-maze revealed a significant effect [F(2,25) = 21.89; P<0.0001] and Newman Keuls post hoc test indicated that the Tg2576 mice that received OL-1 and the wild type mice that received RA took significantly fewer trials to reach criterion than the Tg2576 mice that received RA (Figure 2a). A one-way for trials to criterion for retention in the T-maze revealed a significant effect [F(2,25) =14.07; P<0.0002]. Newman Keuls post hoc test indicated that the Tg2576 mice that received OL-1 and the wild type mice that received RA took significantly fewer trials to reach criterion than the mice Tg2576 mice that received RA. (See Figure 2b). The latency to escape foot shock on the first trial of acquisition was not significant [F(2,25) = 0.03, P NS].
Figure 2.
OL-1 administered via tail vein hadimproved acquisition and retention in Tg2576 mice compared to the Tg2576 mice that received RA (A&B). The Tg2576 mice that received OL-1 had improved retention in object recognition compared to the Tg2576 mice that received RA (C). The discrimination index is determined by dividing the time exploring the novel object divided by the total exploration time of both objects multiplied by 100. The numbers per group were Tg2576 RA = 8, Tg2576 OL-1 = 10 and WT RA = 10. The * indicates P<0.05 and the ** indicates P<0.01.
For object recognition, a fourth injection was given two weeks after the third injection and 72 hours before the start of habituation in the object recognition maze. A one-way ANOVA for percent time exploring the novel object revealed a significant effect [F(2,25) = 6.295; P<0.006]. Newman Keuls post hoc test revealed that the Tg2576 mice which received OL-1 and the wild type mice that received RA spent significantly greater amount of time exploring the novel object than the Tg2576 mice that received RA. (See Figure 2c). There was no statistical difference between the Tg2576 mice that received OL-1 and the wild type control mice that received RA.
A one-way ANOVA for entries into the open arms in the elevated plus maze produced a signicant effect [F(2,20)=5.730, P<0.01]. Newman Keuls post hoc test indicated that the Tg2576 mice which received OL-1 made a significantly greater number of entries into the open arms compared the Tg2576 mice which received RA. There was no difference between the Tg2576 mice that received OL-1 and the wild type mice that received RA nor between the Tg2576 mice that received RA and the wild type mice that received RA. A one-way ANOVA for number of open arm entries produced a significant effect [F(2,20) = 7.839, P<0.004]. Newman Keuls post hoc test indicated that the Tg2576 mice which received OL-1 and the wild type mice which received RA made a significantly greater number of arm changes. In the elevated plus maze OL-1 significantly increased entries into the open arms and frequency of arm changes indicating decreased anxiety in the Tg2576 mice that received OL-1 compared to the Tg2576 mice that received RA (See Table 1).
Table 1.
The means and SEMs for the mice in the elevated plus maze. The Tg2576 mice that received OL-1 spent a greater amount of time in the closed arms compared to the Tg2576 mice that received RA. The Tg2576 mice that received OL-1 entered the open arms more frequently and made more arm changes than the mice that received random antisense. The wild type mice that received RA spent a greater amount percentage of time in the closed arms and changed arms more frequently than the Tg2576 mice that received RA. There were no differences on any measure between the wild type mice and the Tg2576 mice that received OL-1, n = 8 to 10 per group.
| Group | % Closed | Entries Open | Time Open (sec) | Change Freq |
|---|---|---|---|---|
| Tg2576 RA | 31 ± 19 + | 4.0 ± 0.85 | 196.33 + 46.60 | 7.5 ± 2.13 + |
| Tg2576 OL-1 | 52 ± 12 | 14.00 ± 3.04 ** | 116.29 + 25.27 | 29.71 ± 5.29 ** |
| Wild Type RA | 79 ± 5 | 9.63 ± 1.79 | 41.38 + 9.77 | 23.25 ± 4.14 |
Indicates P<0.05 of the same treatment in the opposite strain.
Indicates P<0.01 compared to the RA given to the same strain.
Biochemical Assays
Mice given the peripheral injections were used to assay for AβPP, Aβ and neuroinflammatory cytokines. One-week after the fourth injection and 24 hours after the final behavrioral test mice were sacrifice and the tissue was flash frozen and stored at −80° C until they were assayed.
Effect of OL-1 on AβPP and Aβ Expression
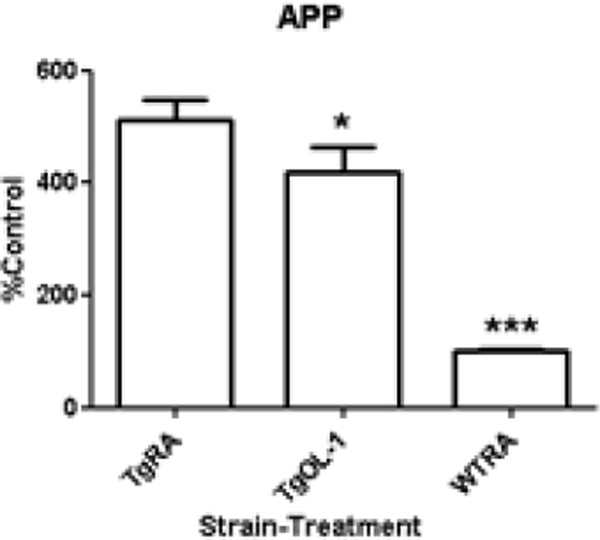
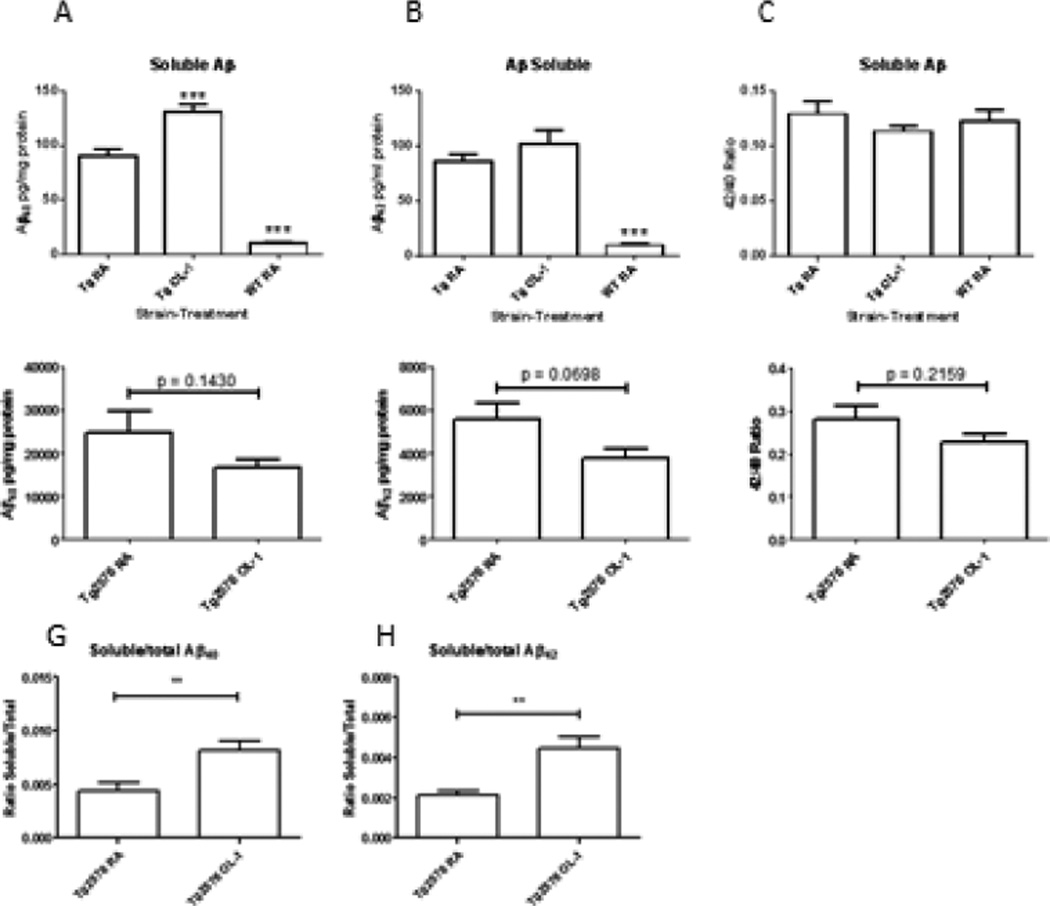
AβPP protein expression as measured by dot blot indicated that Tg2576 mice had an approximately 5-fold increase in AβPP protein levels compared to wild-type controls (Figure 3) which is consistent with initial reports of AβPP overexpression in this strain [11]. OL-1 significantly decreased the expression of AβPP in Tg2576 mice (Figure 3). Surprisingly, OL-1 treatment increased the levels of soluble Aβ40 and 42 in the Tg2576 strain without significantly changing the Aβ42/Aβ40 ratio. The guanidine extraction method routinely used for quantification of total human Aβ was found not to be compatible for measurement of Aβ in wild-type mouse tissue. Therefore, total Aβ could only be measured in the Tg2576 mice. Slight decreases in the mean level of total Aβ40 and 42 or the ratio of total Aβ42/40 were observed in Tg2576 mice, however the differences were not significant. The ratio of soluble to total Aβ40 and 42 approximately doubled in the OL-1 treated Tg2576 group (See Figure 4).
Figure 3.
OL-1 administered via tail vein reduced APP protein levels in Tg2576 mice. Protein expression of APP was measured by dot blot, and analyzed by one-way ANOVA and Newman-Keulsmultiple-comparisons test: *P<0.05, ***P<0.001 vs. RA Tg2576, ###P<0.001 vs. WT RA, n=6–8 per group.
Figure 4.
Effects of OL-1 administered via tail vein 3 times at 2 week intervals on soluble (A–C), total (D–F), and the ratio of soluble/total (G–H) Aβ. Aβ levels were measured by ELISA, and analyzed by one-way ANOVA and Newman-Keuls multiple-comparisons test: **P<0.01, ***P<0.001 vs. RA Tg2576; ###P<0.001 vs. WT RA, n=6–8 per group.
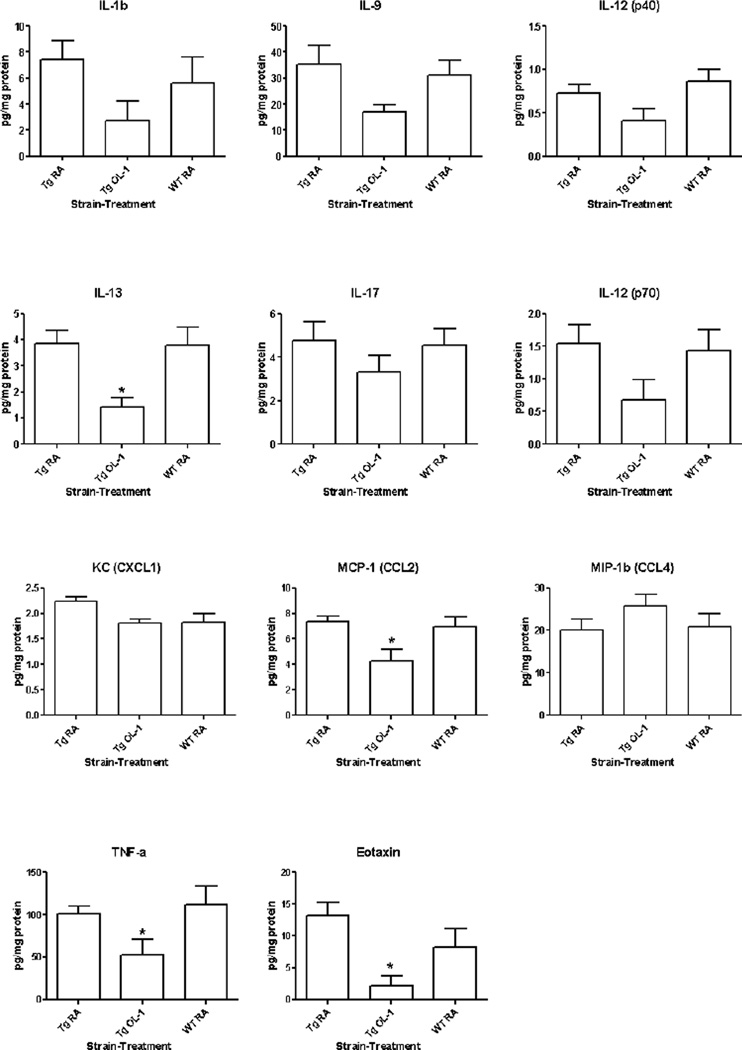
To assess whether OL-1 treatment affects the neuroinflammatory profile, we measured 23 cytokines and chemokines in brain homogenate using a bead-based multiplex ELISA. Of the 23 cytokines on the panel, we detected 11 in the brains of both WT and Tg2576 strains: IL-1β IL-9, IL-12 (p40 and p70), IL-13, IL-17, KC (CXCL1), MCP-1 (CCL2), MIP-1β (CCL4), eotaxin (CCL11), and TNF-α. No significant differences in cytokine levels were detected between the WT and Tg2576 RA groups. However, OL-1 significantly decreased the expression of IL-13, CCL2, CCL11, and TNF-α (Figure 5).
Figure 5.
Effects of OL-1 administered via tail vein 3 times at 2 week intervals on cytokine and chemokine levels in the CNS. Cytokine and chemokine levels were quantified in brain homogenates using a bead-based multiplex ELISA. All detectable analytes are shown. Data were analyzed by one-way ANOVA and Bonferroni multiple-comparisons test: *P<0.05 vs. RA Tg2576; #P<0.05, ##P<0.01 vs. WT RA, n=6–8 per group.
Discussion
The current set of studies shows that antisense oligonucleotide improves learning and memory, reduces AβPP and cytokine levels in Tg2576 mice. The Tg2576 mice are genetically manipulated to overproduce human AβPP and, as a result, have increased brain levels of beta amyloid. The overproduction of beta amyloid in this strain is associated with impairment in learning and memory, oxidative stress, and neuroinflammation [19]. Our studies indicate that antisense is able to reverse the beta amyloid-induced learning and memory deficits. Tg2576 mice have neuroinflammation that coincides with the impairment in learning and memory and precedes Aβ deposits [20] [21].
The current results with OL-1 antisense are similar to our studies using SAMP8 mice. SAMP8 mice have an age-related overproduction of Aβ which coincides with impairment in learning and memory, oxidative stress and impaired efflux of Aβ at the blood-brain-barrier [5, 7, 8]. OL-1 improves learning and memory impairment, decreases AβPP and Aβ, reduces oxidative stress, and improves Aβ efflux. Here, we find that the same OL-1 antisense that improved these measures in the SAMP8, improves learning and memory, reduces AβPP protein adducts, and reduces neuroinflammation in the Tg2576 mouse model of AD.
The Tg2576 mice have impaired spatial learning and memory [22]. A systemic review of the literature found that T-maze alternation in the Tg2576 was one of the most sensitive tests in the Tg2576 mice [22]. Here, we used T-maze foot shock avoidance and found it to be very sensitive for detecting deficits and pharmacological improvements. This has also proven to be true in the SAMP8 model of AD [23]. Our current finding along with previous findings in Tg2576 and SAMP8 mice suggest that various forms of the T-maze are useful paradigms in these two models of AD.
Object recognition is a nonspatial recognition memory test [13]. Tg2576 mice have been reported to have deficits in object recognition [24, 25]. We have shown that OL-1 reverses deficits in object recognition in SAMP8 mice [15]. Here, we found that the OL-1 improved recognition memory in the Tg2576 mice.
Behavioral disinhibition is a common characteristic of AD [26, 27]. Tg2576 mice have been reported to have behavioral disinhibition that increases with age compared to the wild type controls [24, 28]. At 9 months of age Tg2576 mice spent significantly greater amount of time in the open arms of an elevated plus maze compared to wild type controls [28] and would freeze less when presented with a loud tone compared to wild type controls [28]. We found that OL-1 increased the amount of time the mice spent in the closed arm and decreased the amount of time spent in the open arms like the wild type controls. These findings suggest an additional benefical effect of OL-1 is the reversal of behavioral disinhibition.
Similar to previous findings in aged SAMP8 mice [15], IV OL-1 is effective at lowering AβPP expression in the brains of Tg2576 strain. This reduction in AβPP is associated with improvement in learning and memory. An unexpected finding of this study was that soluble levels of Aβ in the Tg2576 strain increased with OL-1 treatment. Furthermore, OL-1 treatment increased the proportion of soluble Aβ in the total Aβ pool. Although no significant changes in total Aβ were observed with OL-1 treatment, the trend was decreased for all parameters. Together, these results suggest that OL-1 causes a shift in the Aβ pool, favoring increased soluble and decreased insoluble Aβ. The increase in soluble Aβ would promote its clearance, as aggregated insoluble Aβ is a poor substrate for many Aβ-degrading enzymes [29] or for transport across the blood-brain barrier [30, 31]. Although these findings do raise the concern that soluble Aβ increases could reflect increased levels of toxic oligomeric species, the association of Aβ changes with improved cognitive performance suggests that this is not likely. Improved cognitive performance and reduced neuroinflammation in the absence of Aβ reduction have been reported in the Tg2576 model following administration of an antibody which inhibits β-secretase [32]. This suggests that either secondary or off-target effects are important considerations for Aβ-targeted therapies for AD. Furthermore, the association of increased soluble Aβ with improved cognitive function is consistent with results showing an inert, or possibly even beneficial, effect of certain Aβ species [33].
Neuroinflammation is a feature of AD, and in humans is supported to be an early event in the disease process [34–36]. Cytokines are signaling molecules which are produced in response to inflammatory stimuli, and have important roles in propagating or modulating the inflammatory response. Aβ induces the expression of pro-inflammatory cytokines in cultured glia and brain endothelial cells, and the effects of Aβ on cytokine expression can be modulated when cells are pre-incubated with anti-inflammatory cytokines. Cytokine profiles in the brain are altered in AD. A recent study demonstrated that IL-1β, IL-10, IL-13, IL-18, IL-33, TGF-β1, and TNF-α converting enzyme are elevated in AD [37]. In the Tg2576 model, TNF-α, IL-1β, and MCP-1 are increased with age and plaque deposition [38]. In the panel of 23 cytokines measured, we detected significant levels of 11 cytokines and chemokines. Of these, OL-1 significantly reduced levels of IL-13, MCP-1, eotaxin, and TNF-α in the Tg2576 strain. Because TNF-α and MCP-1 are produced by glia in response to Aβ exposure in vitro [39], and increase with age in Tg2576 mice [38], reductions in MCP-1 and TNF-α by OL-1 are likely caused by the decreased Aβ deposition. IL-13 is considered an anti-inflammatory cytokine, and can facilitate the clearance of Aβ by microglia in a murine model of AD [40]. Eotaxin is a chemokine which has recently been implicated to have negative effects on learning and memory due to its ability to impair hippocampal neurogenesis [41]. Furthermore, eotaxin levels increase with age in human and mouse serum, and in human CSF [41]. Based on this newly discovered role of eotaxin, an interesting possibility is that eotaxin reduction by OL-1 is a beneficial downstream effect of decreased APP expression and Aβ deposition in the Tg2576 strain.
Antisense treatment with phosphorothioate oligonucleotides (PONs) in general and OL-1 in particular has several advantages. PONs are enzymatically resistant. For OL-1, we have found no degradation products in blood, brain, or peripheral tissues for up to 16 h after IV administration. OL-1 depots in peripheral tissues and is slowly released back into blood. Thus, OL-1 maintains a steady state in blood for hours even after a single injection. Enzymatic resistance and favorable pharmacokinetics explain in part why OL-1 only has to be dosed about every 2 weeks in mice. OL-1 rapidly crosses the BBB by a saturable transport system [42]. The BBB has since been shown to transport several other PONs [43–45]. The transport system is robust and OL-1 reaches a steady state of about 0.25% of the IV injected dose per g of brain. This is over 10 times the concentration of morphine and twice the concentration of leptin, interleukin-1 alpha, or acetaminophen --all substances that cross the BBB to exert clinically significant effects within the CNS [46–48]. OL-1 and other PONs tend to suppress their protein targets only about 40–60% so that it is difficult to overdose with them [4]. This makes PONs ideal for situations such as AD where the target protein is only increased about 50% and has important functions at its physiologic concentration. The mechanisms of antisense molecules are well understood and are increasingly thought to act as pharmacologic mimics of miRNAs.
In conclusion, the current study found that in the Tg2576 mouse which over produces AβPP and subsequently Aβ, the antisense targeting AβPP, OL-1 reverses learning and memory impairment, reverses behavioral disinhibition, reduces AβPP, and neuroinflammatory markers. The current findings suggest that OL-1 is a potential treatment for AD.
Acknowledgements
This work was supported by VA Merit Review.
REFERENCES
- 1.Goate A, Hardy J. Twenty years of Alzheimer's disease-causing mutations. J Neurochem. 2012;120(Suppl 1):3–8. doi: 10.1111/j.1471-4159.2011.07575.x. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira ST, Klein WL. The AB oligomer hypothesis for synapse falure and memory loss in Alzheimer's disease. Neurobiol Learn Mem. 2011;96:529–543. doi: 10.1016/j.nlm.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panza F, Solfrizzi V, Frisardi V, Imbimbo BP, Capurso C, D'Introno A, Calacicco AM, Seripa D, Vendemiale G, Capurso A, Pilotto A. Beyond the neurotransmitter-focused approach in treating Alzheimer's disease: drugs targeting beta-amyloid and tau protein. Aging Clin Exp Res. 2009;216:386–406. doi: 10.1007/BF03327445. [DOI] [PubMed] [Google Scholar]
- 4.Malik R, Roy I. Making sense of therapeutics using antisense techonology. Expert Opin Drug Discov. 2011;6:507–526. doi: 10.1517/17460441.2011.565744. [DOI] [PubMed] [Google Scholar]
- 5.Kumar VB, Farr SA, Flood JF, Kamlesh V, Franko M, Banks WA, Morley JE. Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice. Peptides. 2000;21:1769–1775. doi: 10.1016/s0196-9781(00)00339-9. [DOI] [PubMed] [Google Scholar]
- 6.Morley JE, Kumar VB, Bernardo AI, Farr SA, Uezu K, Tumosa N, Flood JF. Beta-amyloid precursor polypeptide in SAMP8 mice affects learning and memory. Peptides. 2000;21:1761–1767. doi: 10.1016/s0196-9781(00)00342-9. [DOI] [PubMed] [Google Scholar]
- 7.Poon HF, Joshi G, Sultana R, Farr SA, Banks WA, Morley JE, Calabrese V, Butterfield DA. Antisense directed at the Abeta region of the APP decreases brain oxidative maarkers in aged senescence accelerated mice. Brain Res. 2004;1018:86–96. doi: 10.1016/j.brainres.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 8.Banks WA, Kumar VB, Farr SA, Nakaoke R, Robinson SM, Morley JE. Impairments in brain-to-blood transport of amyloid-B protein precursor. J Alzheimers Dis. 2011;23:599–605. doi: 10.3233/JAD-2010-100021. [DOI] [PubMed] [Google Scholar]
- 9.Apelt J, Bigl M, Wunderlich P, Schliefs R. Aging-related increase in oxidative stress correlates with developmental pattern of beta-secretase activyt and beta-amyloid plaque formation in transgenic Tg2576 mice with Alzheimer-like pathology. Int J Dev Neurosci. 2004;22:475–484. doi: 10.1016/j.ijdevneu.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Dickstein DL, Biron KE, Ujie M, Pfeifer CG, Jeffries AR, Jefferies WA. Abeta peptide immunization restores blood-brain barrier integrity in Alzheimer's disease. FASEB Journal. 2006;20:426–433. doi: 10.1096/fj.05-3956com. [DOI] [PubMed] [Google Scholar]
- 11.Hsaio K, Chapman P, Nilsen S, Erkman C, Harigaya Y, Yang F, Cole G. Correlative memory deficits, AB elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 12.Good MA, Hale G, Staal V. Impaired "Episodic-Like" object memory in adult APPswe transgenic mice. Behav Neurosci. 2007;121:443–448. doi: 10.1037/0735-7044.121.2.443. [DOI] [PubMed] [Google Scholar]
- 13.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 15.Erickson MA, Niehoff ML, Farr SA, Morley JE, Dillman LA, Lynch KM, Banks WA. Peripheral admininstration of antisense oligonucleotides targeting the amyloid-B protein precursor reverses ABPP and LRP-1 overexpression in the aged SAMP8 mouse brain. J Alzheimers Dis. 2012;28:951–960. doi: 10.3233/JAD-2011-111517. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson MA, Hansen K, Banks WA. Inflammation-induced dysfunction of the low-density lipoprotein receptor-related protein-1 at the blood-brain barrier: protection by the antioxidant N-acetylcysteine. Brain Behav Immun. 2012;26:1085–1094. doi: 10.1016/j.bbi.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farr SA, Banks WA, La Scola ME, Flood JF, Morley JE. Permanent and temporary inactivation of the hippocampus impairs T-maze footshock avoidance acquisition and retention. Brain Res. 2000;872:242–249. doi: 10.1016/s0006-8993(00)02495-1. [DOI] [PubMed] [Google Scholar]
- 19.Quinn JF, Bussiere JR, Hammond RS, Montine TJ, Henson E, Jones RE, Stackman RWJ. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007;28:213–225. doi: 10.1016/j.neurobiolaging.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Yao Y, Chinnici C, Tang H, Trojanowski JQ, Lee VM, Pratico D. Brain inflammation and oxidative stress in a transgenic mouse model of Alzheimer-like brain amyloidosis. J Neuroinflammation. 2004;1:21. doi: 10.1186/1742-2094-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Practico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart S, Cacucci F, Lever C. Which memory task for my mouse? A systematic review of spatial memory performance in the Tg2576 Alzheimer's mouse model. J Alzheimers Dis. 2011;26:105–126. doi: 10.3233/JAD-2011-101827. [DOI] [PubMed] [Google Scholar]
- 23.Farr SA, Uezu K, Creonte TA, Flood JF, Morley JE. Modulation of memory processing in the cingulate cortex. Pharmacology, Biochemistry and Behavior. 2000;65:363–368. doi: 10.1016/s0091-3057(99)00226-9. [DOI] [PubMed] [Google Scholar]
- 24.Dobarro M, Gerenu G, Ramirez MJ. Propranolol reduces cognitive deficits, amyloid and tau pathology in Alzheimer's transgenic mice. Int J Neuropsychopharmacol. 2013;16:2245–2257. doi: 10.1017/S1461145713000631. [DOI] [PubMed] [Google Scholar]
- 25.Sivilia S, Lorenzini L, Giuliani A, Gusciglio M, Fernandez M, Baldassarro VA, Mangano C, Ferraro L, Pietrini V, Baroc MF, Viscomi AR, Ottonello S, Villetti G, Imbimbo BP, Calza L, Giardino L. Multi-target action of the novel anti-Alzheimer compound CHF5074: in vivo study of long term treatment in Tg2576 mice. BMC Neurosci. 2013;14:44. doi: 10.1186/1471-2202-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry DC, Miller BL. Frontotemporal dementia. Semin Neurol. 2013;33:336–341. doi: 10.1055/s-0033-1359316. [DOI] [PubMed] [Google Scholar]
- 27.Manoochehri M, Huey ED. Diagnosis and management of behavioral issues in frontotemporal dementia. Curr Neurol Neurosci Rep. 2012;12:528–536. doi: 10.1007/s11910-012-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ognibene E, Middei S, Daniele S, Adriani W, Ghirardi O, Caprioli A, Laviola G. Aspects of spatial memory and behavioral disinhibition in Tg2576 transgenic mice as a model of Alzheimer's disease. Behav Brain Res. 2005;156:225–232. doi: 10.1016/j.bbr.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer's disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Norstrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Ito S, Ohtsuki S, Kamiie J, Nezu Y, Terasaki T. Cerebral clearance of human amyloid-beta peptide (1–40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J Neurochem. 2007;103:2482–2490. doi: 10.1111/j.1471-4159.2007.04938.x. [DOI] [PubMed] [Google Scholar]
- 32.Rakover I, Arbel M, Solomon B. Immunotherapy against APP beta-secretase cleavage site improves cognitive function and reduces neuroinflammation in Tg2576 mice without a significant effect on brain abeta level. Neurodegener Dis. 2007;4:392–402. doi: 10.1159/000103250. [DOI] [PubMed] [Google Scholar]
- 33.Morley JE, Farr SA, Banks WA, Johnson SN, Yamada KA, Xu L. A physiological role for amyloid-beta protein: enhancement of learning and memory. J Alzheimers Dis. 2010;19:441–449. doi: 10.3233/JAD-2009-1230. [DOI] [PubMed] [Google Scholar]
- 34.Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 35.Eidson P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, Hammers A, Tai YF, Fox N, Kennedy A, Rossor M, Brooks DJ. Microglia, amyloid, and cognition in Alzheimer's disease: An [11C](R) PK1195-PET study. Neurobiol. Dis. 2008;32:412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboon P. Neuroinflammation and regeneration in the early stages of Alzheimer's disease pathology. Int J Dev Neurosci. 2006;24:157–165. doi: 10.1016/j.ijdevneu.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Morimoto K, Horio J, Satoh H, Sue L, Beach T, Arita S, Tooyama I, Konishi Y. Expression profiles of cytokines in the brain of Alzheimer's disease (AD) patients compared to the brain of non-demented patients with and without increasing AD pathology. J Alzheimers Dis. 2011;25:59–76. doi: 10.3233/JAD-2011-101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sly LM, Krzesicki RF, Brashler JR, Buhl AE, McKinley DD, Carter DB, Chin JE. Endogenous brain cytokine mRNA and inflammatory responses to lipolysaccharide are elevated in the Tg2576 mouse model of Alzheimer's disease. Brain Res. Bull. 2001;56:581–588. doi: 10.1016/s0361-9230(01)00730-4. [DOI] [PubMed] [Google Scholar]
- 39.Lue LF, Rydel R, Brigham EF, Yang LB, Hampel H, Murphy GMJ, Brachova L, Yan SDW, D G, Shen Y, Rogers J. Inflammatory repertiore of Alzheimer's disease and nondemented elderly microglia in virto. Glia. 2001;35:72–79. doi: 10.1002/glia.1072. [DOI] [PubMed] [Google Scholar]
- 40.Kawahara K, Suenobu M, Yoshida A, Koga K, Hyodo A, Ohtsuka H, Kuniyasu A, Tamamaki N, Sugimoto Y, Nakayama H. Intracerebral microinjection of interleukin-4/interleukin-13 reduces B–amyloid accumulation in the ipsilateral side and improves cognitive deficits in young amyloid precursor protein 23 mice. Neuroscience. 2012;207:243–260. doi: 10.1016/j.neuroscience.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 41.Villeda SA, Luo J, Mosher KO, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The aging systemic millieu negatively regulates neurogenesis and cognitive funciton. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banks WA, Farr SA, Butt W, Kumar VB, Franko MW, Morley JE. Delivery across the blood-brain barrier of antisense directed against amyloid beta: reversal of learning and memory deficits in mice overexpressing amyloid precursor protein. J Pharmacol Exp Ther. 2001;297:1113–1121. [PubMed] [Google Scholar]
- 43.Banks WA, Jaeger LB, Urayama A, Kumar VB, Hileman SM, Gaskin FS, Llanza NV, Farr SA, Morley JE. Preproenkephalin targeted antisenses cross the blood-brain barrier to reduced brain methionine enkephalin levels and increase voluntary ethanol drinking. Peptides. 2006;27:784–796. doi: 10.1016/j.peptides.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Schneider T, Becker A, Ringe K, Reinhold A, Firsching R, Sabel BA. Brain tumor therapy by combined vaccination and antisense oligonucleotide delivery with nanoparticles. J Neuroimmunol. 2008;195:21–27. doi: 10.1016/j.jneuroim.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Hemmrich K, Kroncke KD, Suschek CV, Bolb-Bachofen V. What sense lies in antisense inhibition of inducible nitric oxide synthase expression? Nitric Oxide. 2005;12:183–199. doi: 10.1016/j.niox.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Banks WA. Leptin transport across the blood-brain barrier: implications for the cause and treatment of obesity. Curr Pharm Des. 2001;7:125–133. doi: 10.2174/1381612013398310. [DOI] [PubMed] [Google Scholar]
- 47.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 48.Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: effects of cognitive processes. Neuroimmunomodulation. 2002–2003;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]