Abstract
Neuropathic pain is characterized by persistent, intractable pain following damage or dysfunction of the nervous system. Analgesics that include central, rather than purely peripheral, targets are more effective when treating neuropathic pain, highlighting the spinal and/or supraspinal mechanisms that contribute to this aberrant pain condition. The striatum represents one of the brain regions that have been implicated in pain processing. Release of dopamine in the ventral striatum is normally associated with analgesia. Clinical and human imaging studies suggest dopamine is disrupted in neuropathic pain patients, although the conclusions drawn from these studies are limited by their non-invasive imaging or pharmacological approaches. In this study, we used a C57Bl/6 mouse model of neuropathic pain to describe the changes in neurotransmitter content in the striatum and their relationship to evoked pain thresholds. Striatal dopamine content negatively correlated with mechanical thresholds in sham animals. Neuropathic pain animals had reduced dopamine content that was not correlated with mechanical thresholds. In contrast, norepinephrine content was significantly increased and correlated with mechanical thresholds in neuropathic, but not sham animals. These results describe changes in striatal signaling in neuropathic pain animals, and contribute to the literature defining the role of dopamine and norepinephrine in mediating sensory thresholds in healthy and neuropathic pain states.
Keywords: basal ganglia, catecholamines, chronic pain, neuralgia, pain thresholds
Introduction
Neuropathic pain is a chronic pain condition resulting from damage or dysfunction of the nervous system. The chronic, intractable nature of the pain results in adaptations in the nervous system that contribute to the chronicity of this condition and undermines the efficacy of classical analgesics, such as opioids28. The ventral striatum receives inputs from limbic structures such as the amygdala, hippocampus, and thalamus, as well as from the mesolimbic dopamine system originating in the ventral tegmental area (VTA). Accordingly, the ventral striatum is a critical system in attributing incentive salience and emotional valence to sensory stimuli in the environment, including pain4. However, the ventral striatum is also directly involved in modulating pain itself. Lesions of striatal neurons enhance nociception and interfere with opioid analgesia25. Conversely, activation of the ventral striatum, whether by electrical stimulation or local injection of a dopamine receptor 2 (D2) agonist, produce analgesia30, 31. How signaling within the ventral striatum changes in neuropathic pain, and the influence these changes have on pain is a question that has remained minimally addressed in the literature.
Clinical and human imaging studies suggest that dopaminergic signaling in the striatum is perturbed in neuropathic pain. Human imaging studies using positron emission tomography (PET) found fibromyalgia patients have reduced presynaptic Fluor DOPA reuptake and increased dopamine receptor availability18–20, 35. Patients with burning mouth syndrome and atypical facial pain exhibited higher D2 receptor availability in the dorsal striatum when compared to healthy age and sex matched controls17, 18. These studies suggest neuropathic pain patients exhibit either lower endogenous dopamine or increased dopamine receptor expression in the striatum. Drugs that restore dopaminergic tone, such as the dopamine reuptake inhibitor, bupropion, can be effective treatments for neuropathic pain27. Further, Parkinson’s Disease, a condition characterized by a loss of dopaminergic neurons in the nigrostriatal pathway, is sometimes associated with lower pain thresholds that can be recovered with Levo-Dopa treatment14–16, 33. In fact, pain is the most common sensory disturbance in Parkinson’s Disease, and is often co-morbid with neuropathic pain conditions8, 26, 29.
While the studies presented above clearly point to a disruption in striatal dopamine in neuropathic pain, most rely on imaging techniques with radiolabeled ligands that bind to dopamine receptors. These studies are limited because changes in dopamine receptor availability could reflect a difference in receptor number or a change in endogenous dopamine occupancy. Further, it is difficult to determine whether dopaminergic hypofunction is a result of neuropathic pain or a premorbid risk factor in the development of these painful conditions. Finally, changes in other neurotransmitter systems within the ventral striatum, such as norepinephrine and serotonin, have been even less studied. Unfortunately, animal research addressing these questions is rare, although a decrease in opioid stimulated dopamine release in the ventral striatum has been reported24. In the present study, we investigated more thoroughly changes in the ventral striatum neurotransmitters and their relationship to mechanical thresholds in animals with a neuropathic pain condition imposed by a partial injury of the sciatic nerve.
Materials and Methods
Subjects
Male C57Bl/6J (The Jackson Laboratory, Bar Harbor, ME), 8–9 weeks old at beginning of experimentation were used. Animals were housed in groups of 4 and kept on a 12-hour light/dark cycle with food and water available ad libitum. All behavioral experimentation was performed during the light phase. All procedures were conducted in accordance with the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (IASP) and approved by the UCLA Institutional Animal Care and Use Committee.
Surgery
Mice were randomly assigned to either neuropathic (cuff), sham, or naïve (no surgery) groups. A sample size of 7–8 mice were included in each group. Mice undergoing surgery were anesthetized with gaseous isoflurane (induction at 5% and maintenance at 2.0–2.5% in oxygen). The lateral left thigh was shaved and disinfected with isopropyl alcohol and iodine. A 2cm incision through the skin was made followed by a blunt dissection of the muscle to exposure the sciatic nerve. Peripheral nerve ligation was performed as previously described. Briefly, a 2 mm piece of PE50 tubing was opened and wrapped around the nerve using fine forceps. The skin was closed with absorbable sutures (Vicryl). Sham animals received a similar surgery, but without the isolation or ligation of the nerve. After surgery, the wound was covered in antibiotic ointment and 0.5% Marcaine. After recovery from anesthetic, animals were returned to their home cage with food and water available ad libitum.
Mechanical withdrawal thresholds
Withdrawal thresholds to a mechanical stimulus applied with calibrated von Frey filaments were measured in naïve, sham and cuff animals, as previously described5. Mice were placed atop a mesh grid floor in a clear plexiglass enclosure. Von Frey filaments were applied to the plantar surface of the ipsilateral hindpaw in an up-down manner, whereby filaments with increasing stiffness were applied until a paw retraction was observed. Following the first positive reaction, the next less stiff filament was applied. If no reaction, the next stiffer filament was applied; if a reaction was observed, the next less stiff filament was applied. This was repeated 5 times per animal and the 50% withdrawal threshold was calculated. Baseline measurements were taken for all animals before surgery and 2 weeks postoperatively.
Neurochemical Analysis
Twenty-four hours after final behavioral testing (2 weeks after nerve injury), animals were sacrificed and brains dissected. Punches (~6mm3) of the ventral striatum (1.0, 0.445 to 1.42, −5.0), habenula (0.5, −2.155 to −1.555, −2.75), anterior cingulate cortex (0.0, 0.44 to 1.42, −1.5), hippocampus (1.5, −2.1555 to −1.555, −1.5), and amygdala (3.5, −2.155 to −1.555, −5.5) were isolated, flash frozen, and stored at −80° until extraction. Punches were homogenized in 300 μl of 0.2M perchloric acid / 0.1mM ethylenediaminetetraacetic acid (EDTA) with 1 uM isoproterenol as an internal standard. An aliquot was removed for protein content analysis using the Bradford assay (BioRad, Hercules, CA) and remaining samples were centrifuged at 15,000 RPM for 10 min at 4°C. Supernatants were collected and filtered through 0.22 μm centrifugal filter units by spinning at 14,000 rpm for 5 min. The filtrate was assayed for neurotransmitter and metabolite content by high-performance liquid chromatography (HPLC) coupled to electrochemical detection. The mobile phase consisted of 0.1 M citrate-acetate buffer, 15% methanol, 110 mg/L sodium 1-octanesulfonate and 5 mg/L EDTA pumped at 0.5 ml/min through a SC-5ODS 3.0×150 mm column (Eicom, San Diego, CA) maintained at 25°C. The glassy carbon working electrode (WE-3G, Antec Leyden, Boston, MA) of the electrochemical detector was set at 0.75 V against an Ag/AgCl reference. HPLC data were collected and analyzed using EZChrom software (Agilent Technologies, Santa Clara, CA). The system was calibrated daily and sample neurochemical content normalized to the isoproterenol internal standard. Neurotransmitter content was normalized to total protein.
Immunocytochemistry
A separate group of sham and cuff animals were sacrificed 2 weeks after nerve injury. Animals were deeply anesthetized with pentobarbital (100mg/kg, i.p.) and transcardially perfused with 4% paraformaldehyde in 0.1M phosphate buffer (PB), pH 7.4 for 5 minutes. The brain was dissected from the skull, post-fixed for 30 minutes in the above-described fixative, and cryoprotected in 30% sucrose in 0.1M PB for 48 hours at 4°C.
Whole brains were flash-frozen in isopentane and embedded in an optimal cutting temperature medium (Tissue Tek OCT; Sakura Finetek Europe, Alphen aan den Rijn, The Netherlands). Forty μm sections were cut at −20°C on a cryostat (Leica, Wetzlar, Germany) and collected in phosphate buffered saline containing 0.2% Triton X-100 (PBS-T). Sections were incubated overnight with an antibody against tyrosine hydroxylase (TH; 1:1500; EMD Millipore, Billerica, MA) at 4°C. Sections were washed and incubated with a highly cross-adsorbed goat anti-rabbit IgG conjugated to Alexa Fluor 488 (1:400; Invitrogen, Grand Island, NY) for 2 hours at room temperature. Sections were washed in PBS, mounted on slides and coverslipped with Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI) stain (Vector Labs, Burlingame, CA).
Images were taken on a Nikon Eclipse 90i fluorescence microscope (Nikon, Tokyo, Japan) with a 20x objective equipped with a high-resolution digital camera. For quantification, images were taken with constant exposure and gain settings. Three sections per animal were measured for each quantification criteria, and values were averaged to give a single number per animal. Cell body size was measured by measuring the smallest possible diameter in TH+ cells in the VTA. Only cells with a visible nucleus and strong TH labeling were measured, and between 8 and 20 cells per section were recorded. Cell counts were done on similar images with standardized area of analysis.
Statistics
Data sets were tested for normality and variances compared using the Brown Forsythe test. When samples were normally distributed with equal variances, group means were compared using a Student’s unpaired t-test or one-way ANOVA with Tukey’s multiple comparison post hoc analysis. When samples showed unequal variance, a Mann-Whitney U test or a Kruskal-Wallis one-way ANOVA with Dunn’s multiple comparison post hoc analysis was used to compare groups. Groups were considered statistically significant when p<0.05. For neurotransmitter analysis, samples were not included in analysis if total protein concentration of the sample was below a predetermined cut off (0.1ug/uL). Outliers, defined as data points that lie outside the range of three times the standard deviation of the group, were also removed from analysis. Normalized neurotransmitter content was correlated with individual evoked mechanical thresholds using a Pearson’s correlation test. Results were considered statistically significant when p<0.05. Data are expressed as mean ± standard error of the mean (S.E.M.) throughout.
Results
Mechanical thresholds
Baseline mechanical 50% thresholds as measured with von Frey filaments averaged 0.9g ± 0.06 (Figure 1). Two weeks after baseline measurements were taken and surgery performed, mechanical thresholds were unaltered in naïve and sham groups (Figure 1A). In the cuff group, mechanical thresholds were significantly lower than both naïve and sham groups (F(2,21)=15.9, p<0.0001).
Figure 1.
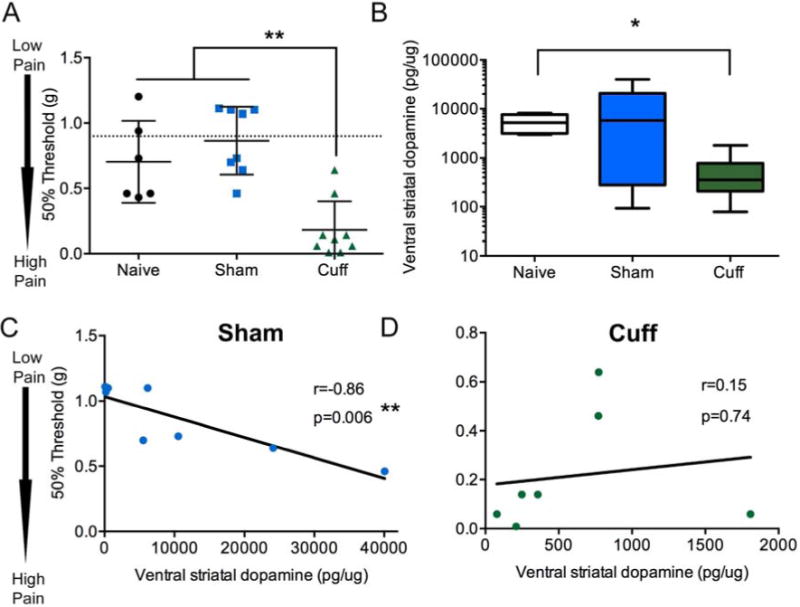
Total dopamine (DA) content in the ventral striatum increase in the cuff group, but lose correlation with mechanical thresholds. A) Two weeks following nerve injury, mechanical thresholds of the hindpaw, as measured with von Frey filaments, were significantly lowered in the cuff group. Naïve and sham groups did not have significantly different thresholds from baseline (pre-surgery, indicated by dotted line). Data represented as scatter plot. Horizontal bar represents mean and error bars represent standard error (S.E.M). Groups were compared using a one-way ANOVA followed by Tukey’s post hoc analysis. **=p<0.01, n=6–8. B) Dopamine content normalized to total protein in ventral striatum was measured by HPLC. Two weeks after nerve injury, cuff animals had significantly lowered dopamine content compared to naïve group. Sham animals were not significantly different, although had a larger variability. Data represented as box plot with horizontal line representing the mean, the outer box limits representing the interquartile span, and the whiskers the furthest data point. Groups were compared with a Kruskal-Wallis ANOVA followed by Dunn’s multiple comparison post hoc analysis. *=p<0.05, n=6–8. C) Dopamine content normalized to total protein in the ventral striatum were correlated with mechanical thresholds 2 weeks after nerve injury. The sham group showed significant negative correlation between dopamine and mechanical thresholds, while the (D) cuff group failed to show this correlation. Correlation was performed with Pearson’s correlation. n=7–8.
Dopamine content and correlation with mechanical thresholds in control and cuff animals
Total dopamine content in the ventral striatum was not significantly different between naïve and sham groups, although the variability was much greater in the sham group (Figure 1B). Two weeks after nerve injury, total ventral striatal dopamine content was significantly reduced in the cuff group compared to the naïve group when compared using a Kruskal-Wallis ANOVA followed by Dunn’s multiple comparison post hoc analysis (K(2,21)=6.75, p=0.03). Dopamine content negatively correlated with mechanical thresholds in the sham group (r=−0.86, p=0.006) and naïve group (r=−0.81, p=0.04), with lower mechanical thresholds (higher pain sensitivity) associated with lower dopamine content in the ventral striatum (Figure 1C). This correlation between dopamine and mechanical thresholds was not evident in the cuff group (Figure 1D; r=0.16, p=0.74). Dopamine content was not significantly altered in extra-striatal brain regions including the habenula, amygdala and anterior cingulate cortex, but was significantly lower in the hippocampus of the cuff group (Table 1; U=4, p=0.04). Dopamine content was not correlated with mechanical thresholds in either sham or cuff groups in any of these areas (Table 1).
Table 1.
Dopamine content in various brain regions and correlations with mechanical thresholds of the hind paw
| Dopamine content | Correlation with mechanical threshold | Correlation with mechanical threshold | ||||||
|---|---|---|---|---|---|---|---|---|
| Sham | Cuff | Test | p value | Sham | Cuff | |||
| Mean pg/ug ± SEM (n) | Mean pg/ug ± SEM (n) | r | p value | r | p value | |||
| Habenula | 833.0 ± 317.9 (6) | 387.4 ± 73.9 (7) | M-W | 0.52 | −0.57 | 0.18 | −0.09 | 0.86 |
| Amygdala | 23473 ± 5172 (8) | 16788.2 ± 3396 (7) | t-test | 0.31 | −0.29 | 0.49 | 0.02 | 0.96 |
| ACC | 314.4 ± 96.5 (7) | 156.5 ± 22.3 (7) | M-W | 0.38 | −0.25 | 0.55 | 0.14 | 0.79 |
| Hippocampus | 22105 ± 5880 (8) | 3954 ± 1469 (4) | M-W | *0.04 | −0.23 | 0.59 | −0.29 | 0.57 |
Dopamine levels in extrastriatal brain regions of naïve, sham and cuff groups 2 weeks after nerve lesion. Dopamine levels were measured by HPLC and normalized to total protein of the sample. Values expressed as group means±SEM, followed by sample size in brackets. ACC=anterior cingulate cortex. Groups were compared with an unpaired Student’s t-test or a Mann-Whitney test. Pearson’s correlation was used to compare mechanical thresholds with neurotransmitter levels.
= statistically significant.
Ventral striatal dopamine metabolite content and correlation with mechanical thresholds in control and cuff animals
The two major intermediary dopamine metabolites, 3,4-Dihydroxyphenylacetic acid (DOPAC) and 3-Methoxytyramine (3MT), were measured in the ventral striatum and were normally distributed with equal variances between groups. DOPAC and 3MT content were not significantly altered between groups, although 3MT had a tendency to be lower in the cuff group (Table 2). 3MT and DOPAC contents were not correlated with mechanical thresholds in naïve (DOPAC: r=−0.65, p=0.11; 3MT: r=0.1, p=0.87), sham, or cuff groups (Table 2). The ratio of DOPAC or 3MT to total dopamine is thought reflect stored and released dopamine1. DOPAC/DA and 3MT/DA ratios were not significantly altered in any of the groups, nor were they correlated with mechanical thresholds in naïve (DOPAC/DA: r=0.33, p=0.42; 3MT/DA: r= 0.42, p=0.48), sham or cuff groups (Table 2).
Table 2.
Ventral striatal neurotransmitter content and correlations with mechanical thresholds of the hind paw
| Neurotransmitter content | Correlation with mechanical threshold | Correlation with mechanical threshold | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Naïve | Sham | Cuff | Test | K-W/F Statistic (df) (p value) | Sham | Cuff | |||
| Mean pg/ug±SEM (n) | Mean pg/ug±SEM (n) | Mean pg/ug±SEM (n) | r | p value | r | p value | |||
| DA | 5405.6 ± 998.1 (6) | 10908 ±5029.4 (8) | 606.6 ± 225.0 (7) | K-W | 6.7 (2,20) (0.03)* |
−0.86 | 0.006** | 0.16 | 0.74 |
| 5HT | 253.3 ± 27.3 (6) | 479.3 ± 63.4 (8) | 394.0 ± 49.1 (8) | K-W | 5.6 (2,22) (>0.05) |
0.0004 | 0.99 | 0.14 | 0.72 |
| NE | 527.7 ± 46.2 (7) | 1331.4 ± 310.5 (8) | 1399.2 ± 136.6 (7) | K-W | 8.6 (2,21) (0.01)* |
−0.003 | 0.99 | 0.76 | 0.04* |
| DOPAC | 758.2 ±195.8 (7) | 615.7 ± 190.5 (8) | 551.1 ± 173.0 (8) | ANOVA | 0.31 (2,23) (>0.05) |
−0.68 | 0.58 | −0.06 | 0.89 |
| 3MT | 192.5 ±41.1 (5) | 128.2 ± 53.2 (6) | 59.0 ± 22.1 (6) | ANOVA | 2.56 (2,16) (>0.05) |
−0.08 | 0.85 | −0.38 | 0.46 |
| DOPAC/DA | 0.2 ± 0.1 (6) | 0.7 ± 0.3 (8) | 0.6 ± 0.1 (7) | ANOVA | 0.19 (2,21) (>0.05) |
0.62 | 0.14 | 0.76 | 0.14 |
| 3MT/DA | 0.03 ± 0.002 (5) | 0.03 ± 0.01 (6) | 0.06 ± 0.02 (6) | K-W | 0.85 (2,16) (>0.05) |
−0.16 | 0.76 | −0.23 | 0.66 |
| DA/5HT | 18.5 ± 3.5 (6) | 13.4 ± 5.2 (8) | 2.2 ± 1.0 (7) | K-W | 6.5 (2,20) (0.03)* |
−0.92 | 0.003** | −0.01 | 0.97 |
Neurotransmitter levels in the ventral striatum of naïve, sham and cuff groups 2 weeks after nerve lesion. Protein levels were measured by HPLC and normalized to total protein of the sample. Values expressed as group means±SEM, followed by sample size in brackets. DA=dopamine, 5HT=serotonin, NE=norepinephrine, DOPAC=3,4 Dihydroxyphenylacetic acid, 3MT=3-Methoxytyramine. Groups were compared with a one-way ANOVA followed by a Tukey’s multiple comparison post hoc analysis or a Kruskal-Wallis (K-W) ANOVA with a Dunn’s multiple comparison post hoc analysis. Pearson’s correlation was used to compare mechanical thresholds with neurotransmitter levels.
= statistically significant.
= statistically significant.
Tyrosine Hydroxylase (TH)-positive cell labeling in the ventral tegmental area (VTA)
Dopaminergic cell bodies were labeled with an antibody against TH in the VTA. The number and size of TH+ cell bodies were quantified. No difference in cell body size or number was found between the sham and cuff groups (Figure 2). No obvious morphological differences in cell body shape were observed between sham and cuff groups.
Figure 2.
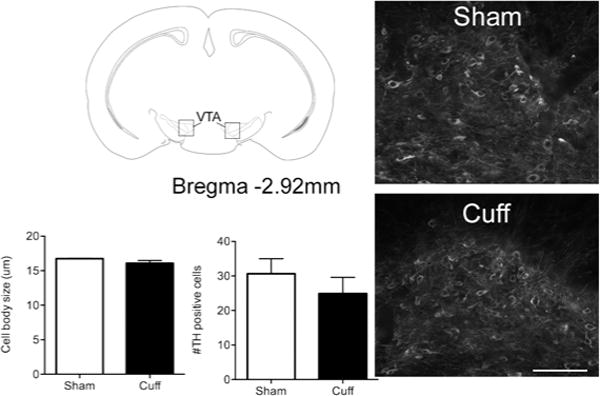
No changes in number or size of dopaminergic cells in the mesolimbic dopamine system in neuropathic pain. B) Tissue slices from the ventral striatum of sham and cuff groups taken two weeks following nerve injury were processed for immunofluorescence with an antibody against tyrosine hydroxylase (TH) to label dopaminergic cell bodies in this region. Representative micrographs showing TH cell bodies from sham and cuff groups. Scale bar = 100 μm. Neither cell body size nor cell number were significantly different between sham and cuff animals. Groups were compared with an unpaired Student’s t test. p>0.05, n=3–4.
Ventral striatal norepinephrine content and correlation with mechanical thresholds in control and cuff animals
Total ventral striatal norepinephrine content was significantly increased in the cuff group compared to naïve when compared with a Kruskal-Wallis ANOVA followed by a Dunn’s multiple comparison post hoc analysis (Figure 3A; K(2,16)= 8.56, p=0.01). In contrast to dopamine content, norepinephrine content did not correlate with mechanical thresholds in the naïve (r=−0.14, p=0.73) or sham (r=−0.003, p=0.99) group (Figure 1C), but correlated with mechanical thresholds in the cuff group (Figure 1D; r=0.76, p=0.04).
Figure 3.
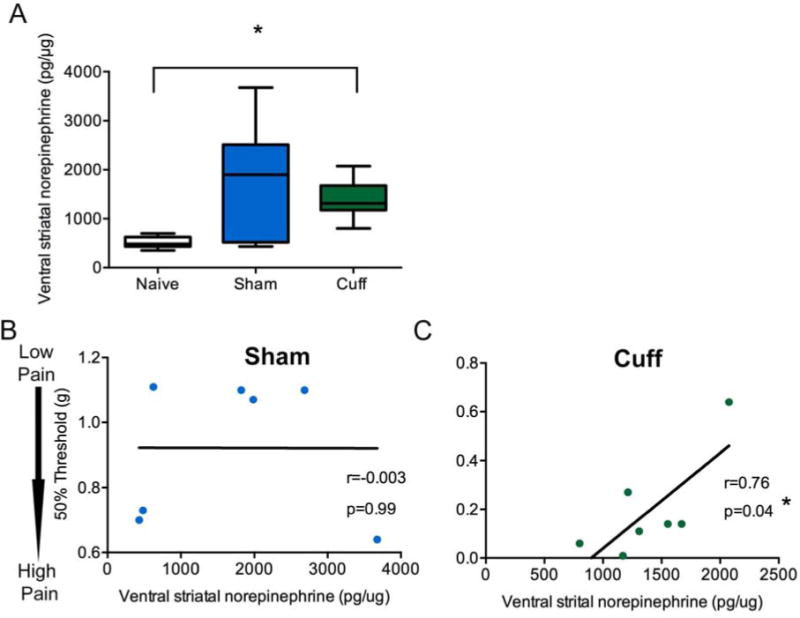
Total norepinephrine (NE) content in the ventral striatum are significantly increased in the cuff group and highly correlated with mechanical thresholds. A) NE content normalized to total protein in the ventral striatum was measured with HPLC. Two weeks after nerve injury, the cuff group had significantly elevated NE, compared to the naïve group. The sham group was not significantly different, although the variability was greater in this group. Data represented as box plot with horizontal line representing the mean, the outer box limits representing the interquartile span, and the whiskers the furthest data point. Groups were compared with a Kruskal-Wallis ANOVA followed by Dunns’ multiple comparison post hoc analysis. *=p<0.05, n=7–8. B) NE content normalized to total protein in the ventral striatum were not correlated with mechanical thresholds in sham animals, but were highly correlated in cuff animals two weeks after nerve injury. Correlation was performed with Pearson’s correlation. n=7.
Ventral striatal serotonin content and correlation with mechanical thresholds in control and cuff animals
Total ventral striatal serotonin content was not significantly different between naïve, sham or cuff groups, although the variability was significantly greater in the sham and cuff groups (Table 2). Serotonin content did not correlate with mechanical thresholds in either the naïve (r=0.55, p=0.15), sham or cuff groups (Table 2). However, the dopamine/serotonin ratio in the ventral striatum was significantly reduced in the cuff group (F(2,21)=1.821, p=0.03), reflecting the drop in ventral striatal dopamine in the cuff group, described in Figure 1. Further, the dopamine/serotonin ratio was negatively correlated with mechanical thresholds in the sham group (r=−0.92, p=0.003), but not the cuff group (r=−0.32, p=0.40).
Discussion
The present study describes significant changes in neurotransmitter contents in the ventral striatum of neuropathic pain (cuff) animals. A decrease in ventral striatal dopamine with concomitant rise in norepinephrine content was associated with a decrease in mechanical withdrawal thresholds indicative of neuropathic pain. Mechanical thresholds shifted from being correlated with dopamine to norepinephrine in neuropathic pain, which suggests adaptations in ventral striatal neurochemistry that may underlie the pathological pain associated with nerve injury.
A relationship between striatal dopamine and pain thresholds in healthy and neuropathic pain states has been established. Human imaging PET studies found increased dopamine receptor availability in the striatum that was positively correlated to pain thresholds34. In other words, increased binding of the labeled dopamine agonist was associated with less subjective pain. The findings in this study support these studies, where low ventral striatum dopamine content was associated with high mechanical thresholds in naïve and sham groups. This suggests that occupied dopamine receptors, and, therefore, endogenous dopamine that would occupy those receptors, is related to the pain threshold of the healthy individual. Importantly, much like the results we observed in mice, the relationship between striatal dopamine and pain thresholds was lost in fibromyalgia patients35. However, the conclusions from these PET imaging studies have been limited because they cannot address whether changes in dopamine receptor occupancy are due to changes in receptor number, PET ligand affinity/accessibility, or occupation by endogenous dopamine. Our study supports the human imaging finding that striatal dopamine signaling is disrupted in neuropathic pain, and extends these results by showing that changes in dopamine receptor occupancy in the striatum may be due, at least in part, to decreased endogenous dopamine content. An important limitation to this study is the inability to distinguish between intracellular and released dopamine. While overall reductions in total dopamine content within the striatum suggest lowered dopamine function, further studies are required to explore changes in in vivo dopamine release. Encouragingly, an initial study in this area suggests dopamine release is, in fact, decreased in chronic pain animals24.
The high variability in dopamine content in the striatum of sham animals is surprising given naïve (non-surgery) animals do not show this same variability. This suggests the sham surgery alone is able to induce some neurochemical changes in the brain, and may represent an important endogenous analgesic response of the animal. This is supported by our observation that striatal dopamine content was significantly correlated with mechanical pain thresholds in sham animals. There is considerable animal research to support the idea that striatal dopamine contributes to analgesia. Electrical stimulation of the VTA produced analgesia and lesions of the striatum and VTA increased pain sensitivity25, 30. It should be noted that our sample size is relatively small in this study, and some of the variability could be due to sampling error. While these results are supported by previous literature, further studies replicating these findings are needed to further validate these results.
While the sham group had variable dopamine content, they were not significantly different from the naïve group. This is in contrast to the cuff group, which showed significantly reduced ventral striatal dopamine content (Figure 1B). Depletion of monoamines in the mesolimbic dopamine pathways can lead to a hypersensitive pain state23. Our results support these studies by showing that the depletion of dopamine in neuropathic pain is related to lowered mechanical thresholds, albeit not correlated with mechanical thresholds (Figure 1D). The lack of correlation with mechanical thresholds could be due to the fact that the spread of mechanical thresholds is restricted in cuff animals, given they fall close to the lower limit of the testing threshold. We also measured changes in dopamine content in other brain regions that receive strong or moderate input from the VTA, including the habenula, anterior cingulate cortex, hippocampus, and amygdala (Table 1). Dopamine content did not differ significantly between naïve, sham and cuff groups in the habenula, amygdala, or anterior cingulate cortex. While dopamine content was significantly lower in the hippocampus of the cuff group, dopamine content was never correlated with mechanical thresholds in either sham or cuff groups in any of these brain regions. Therefore, the decreased dopamine content observed in neuropathic pain was not reflective of a global loss of dopamine content throughout the brain, but rather highlight ventral striatum dopamine as being particularly affected by this chronic pain condition. The decreased dopamine in the hippocampus of the cuff group is striking, and may contribute to other elements of the chronic pain state beyond evoked pain thresholds.
In addition to dopamine, we analyzed changes in dopamine metabolites in the ventral striatum (Table 2). The two major intermediary metabolites of dopamine, DOPAC and 3MT are formed from separate metabolic pathways of separate pools of dopamine, with DOPAC formed intracellular and 3MT formed in the extracellular space. Therefore, DOPAC and 3MT content can reflect stored versus released dopamine content, particularly when expressed as an index of total dopamine content1. Neither absolute DOPAC content nor DOPAC/dopamine ratios were significantly different between naïve, sham and cuff groups, suggesting there was not a change in stored dopamine at the terminals. While neither 3MT content nor 3MT/dopamine ratios were significantly different, 3MT content tended to be lower in the cuff group. This suggests a decrease in released dopamine, which is supported by previous research that found decreased morphine-stimulated dopamine release in neuropathic animals24. However, neither DOPAC nor 3MT content (nor DOPAC/dopamine or 3MT/dopamine ratios) correlated with mechanical thresholds in naïve, sham or neuropathic groups, suggesting dopamine metabolites contribute less to mechanical thresholds in healthy or neuropathic pain states than total dopamine content.
The loss of dopamine in the cuff group is significant, which may result from a loss of dopaminergic cells within the mesolimbic dopamine system. To test this hypothesis, we performed immunocytochemical staining against TH, a marker of dopaminergic neurons. No significant difference in cell body size or number between sham and cuff groups was detected. Therefore, loss of dopamine in the ventral striatum must be due to overall dopamine content in the terminals, rather than dopaminergic cell death.
In contrast to dopamine, norepinephrine content was significantly elevated in the ventral striatum of the cuff group compared to naïve controls (Figure 3A). While norepinephrine content did not correlate with mechanical thresholds in the sham group, they showed a strong positive correlation with mechanical thresholds in the cuff group. While the contribution of ventral striatal norepinephrine to pain states has not been well described, in general, norepinephrine is involved in attention, general arousal and stress reactions in challenging environments2, 3. Increased norepinephrine in the striatum of cuff animals could lead to increased arousal following a painful stimulus that could translate into a lowered mechanical threshold. Further research in this area is warranted to describe the contribution of norepinephrine in the ventral striatum to pain sensitivity.
Serotonin content was also measured in the ventral striatum of naïve, sham, and neuropathic groups and did not differ significantly, although tended to be higher in the cuff group. However, serotonin content in the ventral striatum did not correlate with mechanical thresholds in either sham or cuff groups (Table 2). This was surprising given that the ventral striatum receives strong input from the dorsal raphe and encodes mainly aversive information, which would be particularly relevant in painful conditions9, 10. However, the ratio of dopamine to serotonin was significantly reduced in the cuff group, reflecting the above described drop in dopamine content and rise in serotonin, and this ratio was correlated with mechanical thresholds in the cuff, but not the sham, group. Previous studies have found that dopamine and serotonin interact in an opposing manner in the striatum, with dopamine mediating reward and serotonin mediating aversion12, 13, 21. It has been proposed that it is the balance of serotonin and dopamine that refines the behavioral response to motivationally relevant stimuli by balancing approach and withdrawal11. Our results support this proposition given the tight correlation of the dopamine/serotonin ratio with mechanical thresholds in sham animals. Previous studies have also found the dopamine/serotonin ratio to be correlated with the degree of naloxone place aversion32. The implication of the dopamine/serotonin ratio in mediating evoked pain thresholds remains to be described, although our results suggest it may play a particularly important role.
Overall these results show a striking change in the ventral striatal catecholamine content in neuropathic pain states, with a shift in pain thresholds that are correlated to dopamine to norepinephrine systems. Further research is warranted to further explore the contribution of norepinephrine in the striatum to mediate neuropathic pain.
Perspective.
Results show significant loss of ventral striatal dopamine in neuropathic pain conditions, and the relationship of ventral striatal catecholamines to pain thresholds is changed in neuropathic pain. These results complement human imaging studies, and provide evidence that chronic pain alters the function of reward systems.
Acknowledgments
This study was supported by National Institutes of Health (DA005010) (CJE), Canadian Institutes of Health Research (MOP 123298) (CMC and AMWT) and The Shirley and Stefan Hatos Foundation (CJE, NPM and AMWT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflict of interest.
Table of references
- 1.Arbuthnott GW, Fairbrother IS, Butcher SP. Dopamine release and metabolism in the rat striatum: An analysis by ‘in vivo’ brain microdialysis. Pharmacology and Therapeutics. 1990;48:281–293. doi: 10.1016/0163-7258(90)90050-c. [DOI] [PubMed] [Google Scholar]
- 2.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual review of neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 3.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain research. Brain research reviews. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 4.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain research. Brain research reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 5.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 6.Chudler EH. Response properties of neurons in the caudate-putamen and globus pallidus to noxious and non-noxious thermal stimulation in anesthetized rats. Brain research. 1998;812:283–288. doi: 10.1016/s0006-8993(98)00971-8. [DOI] [PubMed] [Google Scholar]
- 7.Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain. 1995;60:3–38. doi: 10.1016/0304-3959(94)00172-B. [DOI] [PubMed] [Google Scholar]
- 8.Clifford TJ, Warsi MJ, Burnett CA, Lamey PJ. Burning mouth in Parkinson’s disease sufferers. Gerodontology. 1998;15:73–78. doi: 10.1111/j.1741-2358.1998.00073.x. [DOI] [PubMed] [Google Scholar]
- 9.Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural networks : the official journal of the International Neural Network Society. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- 10.Dayan P, Huys QJ. Serotonin, inhibition, and negative mood. PLoS computational biology. 2008;4:e4. doi: 10.1371/journal.pcbi.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deakin JF, Graeff FG. 5-HT and mechanisms of defence. Journal of psychopharmacology (Oxford, England) 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher PJ. Dopamine receptor blockade in nucleus accumbens or caudate nucleus differentially affects feeding induced by 8-OH-DPAT injected into dorsal or median raphe. Brain research. 1991;552:181–189. doi: 10.1016/0006-8993(91)90082-7. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher PJ. Injection of 5-HT into the nucleus accumbens reduces the effects of d-amphetamine on responding for conditioned reward. Psychopharmacology. 1996;126:62–69. doi: 10.1007/BF02246412. [DOI] [PubMed] [Google Scholar]
- 14.Ford B. Pain in Parkinson’s disease. Clinical neuroscience (New York, N.Y.) 1998;5:63–72. [PubMed] [Google Scholar]
- 15.Gerdelat-Mas A, Simonetta-Moreau M, Thalamas C, Ory-Magne F, Slaoui T, Rascol O, Brefel-Courbon C. Levodopa raises objective pain threshold in Parkinson’s disease: a RIII reflex study. Journal of neurology, neurosurgery, and psychiatry. 2007;78:1140–1142. doi: 10.1136/jnnp.2007.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goetz CG, Tanner CM, Levy M, Wilson RS, Garron DC. Pain in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 1986;1:45–49. doi: 10.1002/mds.870010106. [DOI] [PubMed] [Google Scholar]
- 17.Hagelberg N, Forssell H, Aalto S, Rinne JO, Scheinin H, Taiminen T, Nagren K, Eskola O, Jaaskelainen SK. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106:43–48. doi: 10.1016/s0304-3959(03)00275-6. [DOI] [PubMed] [Google Scholar]
- 18.Hagelberg N, Forssell H, Rinne JO, Scheinin H, Taiminen T, Aalto S, Luutonen S, Nagren K, Jaaskelainen S. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101:149–154. doi: 10.1016/s0304-3959(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 19.Hagelberg N, Jaaskelainen SK, Martikainen IK, Mansikka H, Forssell H, Scheinin H, Hietala J, Pertovaara A. Striatal dopamine D2 receptors in modulation of pain in humans: a review. Eur J Pharmacol. 2004;500:187–192. doi: 10.1016/j.ejphar.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Jaaskelainen SK, Rinne JO, Forssell H, Tenovuo O, Kaasinen V, Sonninen P, Bergman J. Role of the dopaminergic system in chronic pain – a fluorodopa-PET study. Pain. 2001;90:257–260. doi: 10.1016/S0304-3959(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 21.Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. The American journal of psychiatry. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- 22.Mosconi T, Kruger L. Fixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: Ultrastructural morphometric analysis of axonal alterations. Pain. 1996;64:37–57. doi: 10.1016/0304-3959(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 23.Oe T, Tsukamoto M, Nagakura Y. Reserpine causes biphasic nociceptive sensitivity alteration in conjunction with brain biogenic amine tones in rats. Neuroscience. 2010;169:1860–1871. doi: 10.1016/j.neuroscience.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 24.Ozaki S, Narita M, Narita M, Iino M, Sugita J, Matsumura Y, Suzuki T. Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: implication of the reduction in mu-opioid receptor functions in the ventral tegmental area. Journal of neurochemistry. 2002;82:1192–1198. doi: 10.1046/j.1471-4159.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 25.Saade NE, Atweh SF, Bahuth NB, Jabbur SJ. Augmentation of nociceptive reflexes and chronic deafferentation pain by chemical lesions of either dopaminergic terminals or midbrain dopaminergic neurons. Brain research. 1997;751:1–12. doi: 10.1016/s0006-8993(96)01164-x. [DOI] [PubMed] [Google Scholar]
- 26.Sandyk R, Bamford CR, Iacono RP. Pain and sensory symptoms in Parkinson’s disease. The International journal of neuroscience. 1988;39:15–25. doi: 10.3109/00207458808985688. [DOI] [PubMed] [Google Scholar]
- 27.Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology. 2001;57:1583–1588. doi: 10.1212/wnl.57.9.1583. [DOI] [PubMed] [Google Scholar]
- 28.Smith HS. Opioids and neuropathic pain. Pain physician. 2012;15:Es93–110. [PubMed] [Google Scholar]
- 29.Snider SR, Fahn S, Isgreen WP, Cote LJ. Primary sensory symptoms in parkinsonism. Neurology. 1976;26:423–429. doi: 10.1212/wnl.26.5.423. [DOI] [PubMed] [Google Scholar]
- 30.Sotres-Bayon F, Torres-Lopez E, Lopez-Avila A, del Angel R, Pellicer F. Lesion and electrical stimulation of the ventral tegmental area modify persistent nociceptive behavior in the rat. Brain research. 2001;898:342–349. doi: 10.1016/s0006-8993(01)02213-2. [DOI] [PubMed] [Google Scholar]
- 31.Taylor BK, Joshi C, Uppal H. Stimulation of dopamine D2 receptors in the nucleus accumbens inhibits inflammatory pain. Brain research. 2003;987:135–143. doi: 10.1016/s0006-8993(03)03318-3. [DOI] [PubMed] [Google Scholar]
- 32.Weitemier AZ, Murphy NP. Accumbal dopamine and serotonin activity throughout acquisition and expression of place conditioning: correlative relationships with preference and aversion. The European journal of neuroscience. 2009;29:1015–1026. doi: 10.1111/j.1460-9568.2009.06652.x. [DOI] [PubMed] [Google Scholar]
- 33.Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, Poncet M, Cherif AA. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology. 2002;59:408–413. doi: 10.1212/wnl.59.3.408. [DOI] [PubMed] [Google Scholar]
- 34.Wood PB, Patterson JC, 2nd, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J Pain. 2007;8:51–58. doi: 10.1016/j.jpain.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA. Fibromyalgia patients show an abnormal dopamine response to pain. The European journal of neuroscience. 2007;25:3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]


