Abstract
Components of the blood have been proposed as potential therapeutic targets for improving cellular regeneration after injury and neurodegenerative disease. In this work, thrombin is shown to increase endogenous neural progenitor proliferation in the intact murine spinal cord. A local injection of heparin before a spinal cord injury reduces cell proliferation and astrogliogenesis associated with scarring. We sought to create depot-formulations of PLGA microsphere and Pluronic F-127 for sustained local delivery of two thrombin inhibitors, heparin and hirudin. Each hydrogel depot-formulation showed delayed drug release compared to microspheres or hydrogel alone. Animals with a lateral demyelination lesion showed a reduction in CD68+ macrophages when treated with hirudin-loaded PLGA/F-127 gels compared to control and heparin-treated animals. Moreover, hirudin-loaded materials showed an accelerated recovery in coordinated stepping and increased oligodendrocyte densities. Together, these data demonstrate that controlled delivery of hirudin accelerates functional recovery from a demyelination lesion in the spinal cord.
Keywords: Drug Release, Hydrogel, Poly(lactic-co-glycolic) acid microspheres, Pluronic, thrombin, oligodendrocyte, spinal cord, gliosis, progenitor cell
1. Introduction
The adult mammalian central nervous system (CNS) does not undergo significant regeneration after injury due to chronic scar formation. Therefore, biomaterials engineered to reduce reactive gliosis and glial scarring is important for the regenerative medicine field. Recent studies have begun to describe changes within the stem cell microenvironment (niche) in response to injury, including inflammatory cues that restrict differentiation [1] and pathophysiology linked to thrombin activation.[2] Yet surprisingly few therapies or biomaterials have been developed to counteract the deleterious effects of blood-borne proteins in the CNS. Targeting of blood components using intraparenchymal delivery of inhibitors is potentially critical to ensure the safety and efficacy of this approach.
In mammalian systems, vascular damage resulting in the breakdown of the blood brain barrier (BBB) and blood extravasation has been postulated to play a role in glial scar formation, edema, and neuronal cell-death [3] while thrombin inhibition has been shown to attenuate tissue damage and neurological deficits imparted by ischemia [4]. Within an intact stem cell niche, vascular elements have been shown to modulate homeostasis by directing self-renewal and differentiation[5] and thrombin has been shown to modulate growth factor production to affect endothelial progenitors and angiogenesis.[6] Yet, the mechanism by which thrombin is able to impart the physiological consequences that occur in the CNS after traumatic injury remains unknown [7], and few studies have investigated whether thrombin inhibitors would be viable therapies to mitigate the effects for vascular damage in the CNS.
Anticoagulants are a pivotal class of agents that serve to inhibit thrombin and treat thrombotic disorders.[8] Heparin and hirudin, a leech-derived peptide, represent two classes of FDA-approved thrombin inhibitors. While heparin inhibits thrombin activity via recruitment and binding of antithrombin[9], hirudin is a bivalent inhibitor that interacts with thrombin’s active site and binds to exosite 1.[10] However, systemic approaches to inhibit thrombin in the CNS have had marginal effects due to dosage limitations, poor delivery past the blood brain barrier, and rapid clearing.[11] However, a self-forming hydrogel implanted in the CNS could serve as an attractive parenteral depot formulation[12] to prolong the release of a thrombin inhibitor and increase the local effective dosage at injury sites while minimizing off-target drug-effects that result from the high-dosing regiments needed for systemic drug delivery.[13]
Biodegradable polymeric microspheres have been extensively investigated for the past two decades as injectable depots for peptide drugs [14]. Poly(D,L-lactic-co-glycolic acid: PLGA) is an FDA-approved, biodegradable copolymer that has been used to encapsulate a variety of proteins [15]. However, a drawback of drug delivery from PLGA microspheres is the potential for burst release of cargo that is often observed.[16] Previous work has shown that burst release can be reduced and prolonged delivery attained when PLGA microspheres are mixed with Pluronic F-127[17], an FDA-approved block copolymer that forms a thermo-reversible gel.[18]
In this work, we hypothesized that thrombin is able to affect proliferation and reactive gliosis after spinal cord injury. We asses cell proliferation and astrogliagenesis in a model of spinal cord contusion-injury. Using Pluronic F-127 hydrogels with embedded PLGA microspheres, we sought to develop an injectable depot hydrogel-formulation to temper burst release and prolong delivery of hirudin (a direct thrombin-inhibitor) at the site of a spinal cord injury. To assess efficacy, we quantify cell proliferation and oligodendrocyte populations within a lateralized demyelination lesion and used a cat-walk analysis to monitor locomotor recovery after injury.
2. Materials and Methods
2.1 Ethics Statement
These studies have been approved and meet the standards of the Guide for the Care and Use of Laboratory Animals and applicable University of Washington policies and procedures. The University of Washington has an approved Animal Welfare Assurance (#A3464-01) on file with the NIH Office of Laboratory Animal Welfare (OLAW), and the University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
2.2 Animal Surgery, demyelination lesions, and spinal cord tissue processing
All animal procedures were approved and completed under the strict guidelines of the University of Washington IACUC. Adult mice (8 weeks) were anesthetized with avertin (12.6% tribromoethanol, 0.6% tert-amyl alcohol; Sigma, St Louis, MO) and a T9 laminectomy was performed to exposure the dorsal cord. To create a lateral demyelination lesion, a micropipette was used to make three injections (1 μl each) 150–200 microns off mid-line at 100, 200, and 300 μm depths of a 1% lysolecithin solution (Sigma-Aldrich, Inc.). To create a contusion spinal cord injury, a laminectomy at thoracic level 8 was performed and a third generation OSU spinal contusion device was lowered onto the spinal cord to induce a 0.5 mm displacement of the spinal cord (as previously described).[19] Recorded tissue displacement and force data during the injury was used to assure consistency of injury severity between animals. After a successful injury, the muscle and skin werer sutured in layers to close the wound and lactated Ringer’s solution was administered subcutaneously to rehydrate the animals. Analgesics (buprenorphine, 0.05 mg/kg) and antibiotics (4.8 μg/g gentamicin, Abraxis Pharmaceutical Products, Schaumburg, IL) were administered as needed and animals recovered from surgery in warmed cages and manual bladder expression was administered until recovery.
For thrombin injections in to control, uninjured mice, similar pre- and postoperative methods were followed. Thrombin (5000U/ml, Thrombin-JMI, GenTrac, Inc.) or artificial cerebrospinal fluid (aSCF: 119 mM NaCl, 26.2 mM NaHCO3, 2.5 mM KCl, 1 mM NaH2PO4,1.3 mM MgCl2,10 mM glucose) was injected via a micropipette (1 μl at ~100 μm depth into the dorsal columns).
Anesthetized mice were euthanized via intra-cardiac perfusion. Animals exsanguinated with saline were fixed with 4% paraformaldehyde (containing 4% sucrose) and spinal cords were harvested. Perfused tissue was dehydrated in 30% sucrose for cyroprotection in OTC and sectioned coronally (20 μm thickness) with a Leica SM1850 cryostat.
2.3 Preparation of poly(lactic-co-glycolic) acid microspheres and F-127 Hydrogel
Poly(lactic-co-glycolic) acid (PLGA) nanoparticles encapsulating cargo were prepared using a double emulsion solvent evaporation method, as previously described.[20] Briefly, PLGA was dissolved in dichloromethane, forming a 5% w/v solution. Separately, albumin, heparin, or hirudin (residues 54-65)[21] was dissolved in DI water and then transferred to the PLGA solution, and the mixture was emulsified by using homogenizer (T-18 basic, IKA, Wilmington, DE) for 1 minute on high speed. The resultant emulsion was added drop-wise to PVA (2% w/v in DI water). The mixture was then homogenized and stirred overnight. The products were washed and the final pellet was resuspended and lyophilized. The particle size of PLGA nanoparticles was measured using ZetaPlus dynamic light scattering (DLS) instrument (Brookhaven Instrument Co, Holtsville, NY). Loading efficiency for albumin and hirudin was determined by NaOH/HCl extraction followed by and protein quantification by a standard BCA protocol (ThermoPierce). Heparin loading was quantified by Azure A assay.[22]
In order to quantify release from each formulation, PLGA or mixed hydrogel adjuvant formulations were loaded into a trans-well insert and bathed in PBS. At each time-point, a complete media change was performed and the amount of heparin, albumin, or hirudin was quantified accordingly. Each assay was performed in 2 mls of PBS in a 24-well plate at 37 °C.
2.4 Immunofluorescence, Immunohistochemistry, and Immunoblotting
Molecular marker expression was evaluated by immunofluorescence to determine cellular phenotypes. Primary antibodies previously shown to identify astrocytes (glial fibrillary acidic protein, GFAP), immature oligodendrocytes (adenomatous polyposis coli tumor suppresser gene, APC; CC1 clone), and macrophages (CD68). The CC1 clone antibody is directed against the amino terminus of APC and is specific for glial cells (CalBiochem, La Jolla, CA), but CC1 does not cross react with neurons, as reported with antibodies directed against the c-terminus of APC. A cocktail of three compatible primary antibodies, combined in blocking buffer (TBS +0.3%Triton +5% donkey serum) was used to stain neural phenotypes in spinal cord sections. A dilution series determined the optimum antibody concentration: rabbit α-GFAP (1:2500, Millipore), mouse α-APC (1:100, Calbiochem), rat α-BrdU (1:100, Accurate), rabbit anti-CD68 (1:500, Santa Cruz Bio.). Excessive rinses in 0.1 M TBS pH 7.5 and once in blocking buffer (supplemented with α-mouse IgG, where needed) removed unbound primary antibodies, and secondary antibodies were applied in blocking buffer for 2 hours at room temperature, or overnight at 4°C: donkey α-mouse IgG conjugated to CY3 (1:500; Jackson Labs West Grove, PA), α-rabbit IgG conjugated to CY2 (1:500, Jackson Labs), donkey α-guinea pig conjugated to CY5 (1:500, Jackson Labs,), and donkey α-chicken Alexa-488 (Invitrogen). Unbound antibody was removed by extensive washes with blocking buffer.
Immunostains for BrdU incorporation required tissue to be boiled in 0.01M sodium citrate followed by a 15-minute incubation at 37 °C in 2N HCl. A biotinylated donkey α-Rat (1:500, Jackson) was used with fluorescent or HRP-streptavidin (Vector labs) and 3,3′-Diaminobenzidine (DAB). Nickel chloride (10 mg/ml, Sigma) was used to amplify DAB immunocytochemistry. Similar immunohistochemical procedures were used to stain for macrophages (anti-CD68). For quantitation of astrogliosis, scarring, and tissue sparing tissue was stained to visualize the GFAP of astrocytes, chondroitin sulphate proteoglycan (CSPG) of scar margin and lesion barrier to determine the lesion border and to assess the proportion spared tissue to outline and quantify tissue area (Stereo Investigator, Microbrightfield, Inc.).
2.5 Confocal Microscopy and Stereology
A Nikon TS2000E equipped with a krypton/argon laser, a red diode laser, and an infinity corrected 40X 1.4na lens was used to capture confocal images of stained tissue. Volocity (Improvision Software) managed each digital images and reconstructed z-series stacks. Final image presentation was produced with Photoshop (Adobe Software). Cells populations within the spinal cord and lesion epicenter were etimated using the fractionator method on a serial tissue sections (1 in 6 series). Regional area measurements were taking by creating electronic templates of the lesion, white- and grey-matter borders.
2.6 Statistical analysis
The difference in cell phenotypes of the control, heparin, or hirudin-treated groups was analyzed by non-paired students t-test. Similarly, the significant difference in proliferation was determined by t-tests. For all statistical analyses, significance was accepted at a p value of 0.05 and lower.
3. Results and Discussion
3.1 Proliferation after thrombin injection
Progenitor cells in the adult spinal cord are known to proliferate in response to traumatic injury.[23] Since thrombin extravasates into the parenchyma after BBB disruption, we examined the effects of thrombin on cell proliferation in the intact spinal cord. One day after injection of thrombin (1 unit) into the spinal cord parenchyma, bromodeoxyuridine (BrdU) was administered to label cycling cells (Fig. 1A, n=5).[24] The number of cells that incorporated BrdU was quantified within whole spinal cord sections (20 μm thick) of a known fraction of sections (i.e. optical fractionator method[25]) to count BrdU-labeled cells. In an experiment to assess at acute proliferative effects, mice were given a single BrdU (i.p.) injection immediately after an intraspinal injection of thrombin (1U) or artificial cerebrospinal fluid (aCSF). A 3-fold increase in cell proliferation in thrombin- versus aCSF-injected controls was measured (Fig. 1B). Similarly, animals that received successive BrdU injections (four daily injections) after treatment revealed that a single intraspinal injection of thrombin initiated a sustained increase in cell proliferation instead of a mere transient increase the day of exposure. Remarkably, a single injection of thrombin induces a higher level of cell proliferation than has been reported for a single injection of the potent stem cell mitogen bFGF. [26]
Fig. 1.
Direct injection of thrombin stimulates proliferation within the spinal cord. (A) Adult mice received an intraspinal injection of artificial CSF (aCSF, control) or thrombin (1000U/ml) in aCSF and cell proliferation was marked by BrdU incorporation at 24 hours post-injection (Bar, 250 μm; inset 50 μm). (B) Stereology counts of a one-in-six series of BrdU+ cells/mm2 in the dorsal and ventral aspects of the spinal cord after vehicle (white bars) or thrombin (red bars) injection (1 Unit). The mitotic index (BrdU+/nuclei·mm2) was determined as a function of DAPI-labeled nuclei within adjacent sections (shaded region. n=5 per group). (C) The chronic effect (5 days post) of a single thrombin injection (red bar) versus control (white bar) on proliferation (BrdU+-cells) was determined by stereology. (t-test *, p<0.05; **, p<0.001; ***, p<0.0001).
3.2 Effects of heparin on proliferation and reactive gliosis
Since thrombin stimulated a proliferative effect within the spinal cord, and since previous studies have shown that thrombin inhibition prevents ischemic brain-damage [4], we hypothesized that heparin-mediated inhibition of thrombin would reduce cell proliferation and secondary damage after injury. To test this hypothesis, heparin was injected into the spinal cord 2 minutes prior to a thoracic contusion injury and BrdU was administered 24 hours post-injury (PI) to monitor proliferation(Fig. 2A, n=4–6). In order to examine the initial scarring response, tissues were collected three days post-injury to examine peak proliferation and initial gliogenesis.[24]
Fig. 2.
Heparin reduces proliferation and reactive gliosis in contused spinal cords. (A) Heparin (5 mg/ml; 1 μl) was injected into the dorsal columns immediately prior to a contusion injury and tissue was harvested 3 days post-injury (PI) (n=5). (B) Immunofluorescence against CSPG, GFAP, and serum albumin show the lesion after SCI (Insets, Bar=100μm). (C) Stereology was used to quantify proliferation (BrdU+-cells) and a Scar Index (GFAP+-cells/DAPI+-nuclei) in the lesion epicenter (green outline), white- and grey-matter (red and cyan outline, respectively). (D) The Mitotic Index (BrdU+/nuclei·mm2) quantified within the lesion epicenter, white- and grey-matter demonstrates a 3-fold decrease in proliferation in heparin (blue bars) versus aCSF (white bars). (E) Similarly, the Scar Index revealed reactive gliosis decreased in heparin injected animals. (F) Tissue sparing was evaluated by the average tissue-area (mm2) in white, grey, lesion, and combined (gray region. t-test *, p<0.05; **, p<0.001; #, p=0.08). (G) Cell density was quantified by DAPI-labeled nuclei per micron.
To assess whether thrombin inhibition affects glial scarring, injured tissues were stained by immunofluorescence against GFAP and CSPG, and counterstained for serum albumin (Fig. 2B) to identify the lesion border/margin.[27] Heparin-injected animals had reduced proteoglycan staining, a component of the glial scar (Fig. 2B, CSPG insets), which was used to identify the lesion boundary. Furthermore, heparin-treated animals showed a ~50% reduction in cell proliferation compared to mice treated aCSF (mitotic index; Fig. 2D). The proportion of GFAP+-cells as a function of total nuclei within sub-regions of the spinal cord was quantified (Fig. 2E, combined regions highlighted within grayed area) to calculate a Scar Index. In heparin treated animals, a significant reduction in the number of astrocytes was noted in the white- and grey-matter, as well as the lesion (Fig. 2E, combined regions highlighted within grayed area). Combined, the areas showed a 35% reduction in reactive gliosis compared to control animals (n=6, p=0.0029). These data suggest that blocking thrombin activity at the time of injury inhibits astrogliosis, which would mitigate the pathophysiology after injury that has been linked to thrombin.[28]
To control for effects of tissue edema or atrophy in our results, we quantified spared tissue areas in regions designated lesion, white-, and gray-matter. Notably, heparin-injected animals trended toward an increase in tissue sparing, with a significant increase in white matter tissue (Fig. 2F) while the overall cell density remained unchanged (Fig. 2G), which suggests cell loss and/or apoptosis remained consistent within control and heparin injected animals. These results indicate that thrombin inhibition by heparin reduces proliferation and gliosis and enhanced tissue sparing after a contusion injury.
3.3 PLGA/Pluronic F-127 hydrogels with heparin and hirudin
Encouraged by the observed effect on reactive gliosis after SCI, we sought to develop a hydrogel formulation to prolong the delivery of a thrombin-specific inhibitor. Unlike heparin, hirudin has been shown to bind and inhibit thrombin directly [21]; yet, both heparin and hirudin have been shown to mitigate the deleterious effects of thrombin after brain injury.[4] Consequently, heparin and hirudin were encapsulated in PLGA. Pluronic F-127 is a FDA-approved triblock copolymer of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) that undergoes a temperature-dependent transition to a gel state at body temperature.[29] It has therefore been used as an injected carrier with microspheres for better retention at the site of injection.[30] Based on this previous work, we hypothesized that cargo release from PLGA microspheres may be further sustained by co-injection with Pluronic F-127. Due to the cost of recombinant hirudin, initial release studies were conducted using model protein albumin and heparin.
PLGA microspheres containing albumin (~500–600 nm in diameter) or heparin (~2500 nm in diameter) were prepared by double emulsion solvent evaporation (Fig. 3A). For these studies, formulations were bathed in PBS buffer and buffer was removed at various time points to quantify cargo release. Albumin release from the PLGA microspheres was accelerated compared with any of the Pluronic F-127 formulations. Pluronic F-127 and PLGA/F-127 hydrogels showed a significantly slowed rate of albumin release at 0.5, 1, 2, and 4 hours (†, p<0.01), and the trend persisted at 6 hours for the PLGA/F-127 formulation (*, p<0.05). Initial release rates of albumin and heparin from PLGA microspheres in Pluronic F-127 was slower from either PLGA microspheres or Pluronic F-127 alone (Fig. 3B). While >50% of albumin was released from PLGA microspheres or Pluronic F-127 within 6 hours, only 36% was released from the PLGA/F-127 formulation in the same time frame. Similar trends were observed with heparin-loaded formulations: combining microspheres encapsulating inhibitors with Pluronic F-127 hydrogels resulted in delayed heparin release (Fig. 3C). While the initial heparin release at 30 minutes was reduced significantly for F-127 and PLGA/F-127 formulations compared to microspheres only (Fig. 3C, inset; ¥, p<0.05), the PLGA/F-127 formulation slowed the lowest cumulative release at 1, 2, 4, and 6 hours among the three tested formulations (*, p<0.01. #, p=0.05). Based on these results, hirudin-loaded PLGA microspheres were prepared and release rate of hirudin from microspheres in Pluronic F-127 hydrogel was determined. An initial fast release of hirudin (50% within the first 8 hours) was observed with the remaining peptide released by 48 hours (Fig. 3D). The faster release characteristics of hirudin compared to heparin and albumin is expected due to its low molecular weight. Still, the PLGA/F-127 formulation provides controlled hirudin release over 2 days from the injection site. The surrounding tissue may also act to further limit release kinetics in the spinal cord environment.
Fig. 3.
Pluronic F-127 slows thrombin inhibitor release from PLGA microspheres. (A) PLGA microspheres (0.5 μm) loaded with albumin, heparin, or hirudin were mixed (25% w/v) at room 25°C and gelled at 37°C. (B) Albumin release over 3 days showed a slower release within the first 10 hours (inset graph) in PLGA/F-127 (triangle) versus PLGA (square) or F-127 (circle) alone. (C) Heparin release showed a longer and slower release from PLGA/F-127 loaded gels despite an overall faster release profile within the first 6 hours (inset graph). (D) PLGA microsphere-encapsulating hirudin in F-127 gels release hirudin over 48 hours.
3.4 Hirudin and heparin PLGA/Pluronic F-127 hydrogels post-injury
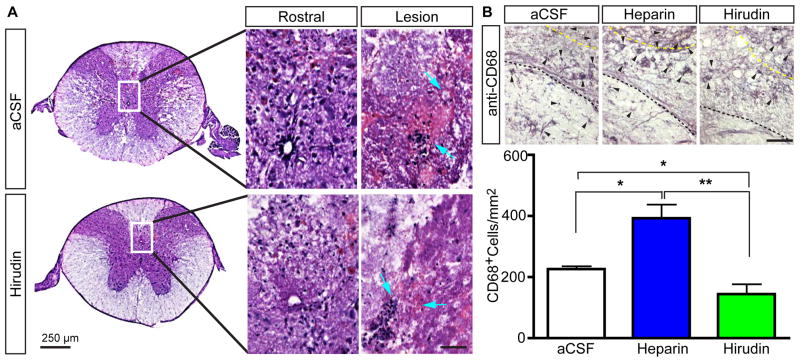
Next, the PLGA/Pluronic F-127 hydrogels formulated with heparin and hirudin were injected into a mouse spinal cord demyelination lesion. Prior to gel implantation, a demyelination lesion was induced in mice by an injection of lysophosphatidylcholine (lysolecithin) at 3 sites ventral-, medial-, and dorsal-lateral to midline. This lateralized demyelination lesion manifests in a severe, incompletely repaired demyelination zone accompanied by locomotor dysfunction.[31] Next, gel formulations were injected into the lysolecithin lesion. One month post-injection, spinal cord sections stained via hematoxylin revealed a significant amount of PLGA microspheres and polymer persisted within the spinal cord (Fig. 4A, cyan arrows), which has been noted in other studies as well.[32] Due to the persistence of the injected material, we quantified the number of macrophages within the spinal cord lesion one month post-injury. Spinal cord tissue was stained with antibodies against CD68 and the number of macrophages was quantified by stereology (Fig. 4B, arrows). In all animals, CD68+ macrophages persisted at the site of hydrogel implantation. However, the hirudin-containing hydrogel had less macrophage infiltration versus control and heparin gel-formulations (Fig. 4C). The observed increase in CD68+ cells within heparin treated animals was unexpected. While CD68+-macrophage populations are sparse in an intact spinal cord,[33] macrophage recruitment as been well documented in the injured spinal cord and has been shown to peak within an injured spinal cord at 1–3 days post-injury (PI), but those macrophage populations diminish greatly by 1 month PI.[34] Similarly, the strong anticoagulant effects of activated protein C have been shown to reduce monocyte recruitment after ischemic stroke.[35] Consequently, the anticoagulant properties of hirudin have the potential to reduce macrophage recruitment and counter the recruitment-effects of the materials that persist at the site of implantation, which could delay remyelination and affect M2 macrophage polarization.[36]
Fig. 4.
Peripheral macrophage populations are reduced by hirudin delivery from PLGA/F-127. (A) Tissue stained by hematoxylin revealed PLGA/F-127 hydrogels persisted up to 1 month and showed pockets of clumped cells (arrows) in the spinal cords of control or hirudin treated animals that received a lateral demyelination lesion. (B) Immunohistochemical stains (DAB) against CD68 was used to quantify macrophages (arrow heads) in control, heparin, and hirudin treated animals. Yellow dashed-line delineates the tissue-hydrogel interface. Hirudin-loaded PLGA/F-127 injected animals showed a lower persistence of macrophages within the demyelination lesion at 1 month PI. t-test; *, p<0.5; **, p<0.01.
3.5 Cell proliferation after PLGA/F-127 hydrogels injection post-injury
In order to evaluate whether controlled thrombin inhibitor delivery would produce the the anti-proliferation effect seen by a direct injection of heparin prior to SCI (Fig. 2), animals were given BrdU injections to label dividing cells 24 hours after hydrogel delivery. Delivery of both hirudin and heparin from injected hydrogels reduced proliferation by ~50% after a demyelination lesion (Fig. 5). Surprisingly, unlike the direct injection with heparin (Fig. 2) the population of reactive astrocytes (GFAP-cells) was similar to control animals (data not shown) and is likely attributed to the persistence of the PLGA/F-127 hydrogel, which showed intense immunoreactivity around the remnant gel.
Fig. 5.
Hirudin reduces proliferation after SCI. (A) Immunofluorescence was used to stain BrdU+-cells in the spinal cords of lesioned animals. (B) Stereology of the tissue from animals showed decreased proliferation (1mm caudal) 24 hours PI in heparin- and hirudin-loaded PLGA/F-127. Only hirudin-loaded PLGA/F-127 show reduced proliferation versus controls within the lesion site. Heparin-loaded PLGA/F-127 showed a similar trend within the lesion site, but the difference versus controls was not significant. t-test, p<0.5.
3.6 Locomotor function after hirudin-loaded PLGA/F-127 injection post-injury
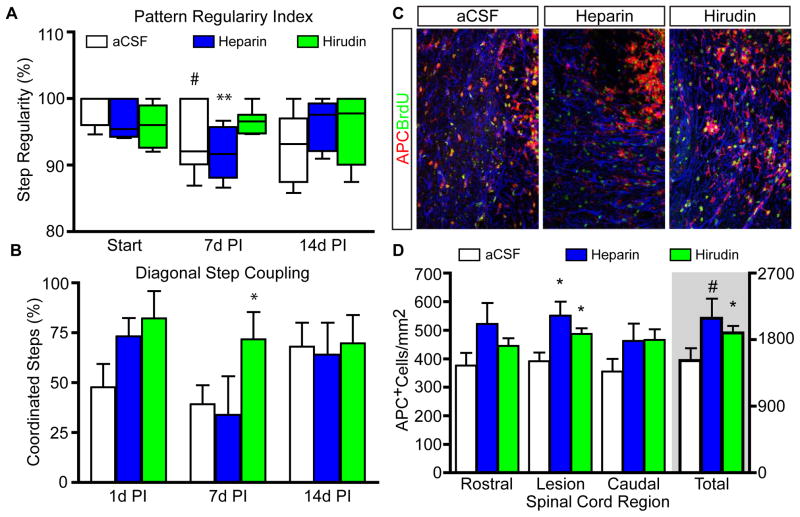
Since heparin was able to reduce reactive gliosis after a spinal cord contusion injury, we evaluated the behavior of animals that were injected with thrombin-inhibiting hydrogels after demyelination was induced. A lateral demyelination lesion (through the dorsal-ventral axis) has been shown to produce subtle but detectable locomotor deficits [31]. Therefore, animals were pre-trained on a catwalk device in order to perform a complex and objective analysis of paw placements amongst lesioned animals. Accordingly, inter-paw coordination as a base-line regularity index score was determined prior to injury (100% equals fully functional) followed by catwalk analysis at 1, 7, and 14 days PI. As expected, all animals displayed a reduction in coordinated stepping patterns, according to a regularity index,[37] but nearly full recovery to pre-surgical functionality is observed by 14 days PI (Fig. 6A). Interestingly, animals injected with hirudin-containing PLGA/F-127 hydrogels showed a marked trend toward improved coordinated stepping at 7 days PI compare to aCSF or Heparin treated animals (**, p<0.01. #, p=0.058). Furthermore, when stepping patterns were analyzed for coordinated stepping by front and hind-paws (Diagonal Step Coupling), animals injected with heparin- and hirudin-loaded gels, tended to have higher scores after injury. Animals treated with hirudin showed a significant recovery in locomotor function at 7 days PI (*, p≤0.05; Fig. 6B) compared to animals injected with aCSF and Heparin-loaded hydrogels although all animals showed similar scores at 14 days PI. Furthermore, neither the hirudin-treated nor heparin-treated animals showed a decline in locomotor function at 14 days, suggesting that the formulations did not delay injury nor prolong the expected recovery.
Fig. 6.
Hirudin-loaded PLGA/F-127 promotes oligodendrocyte formation and improved behavioral recovery after SCI. (A) Catwalk analysis of the regularity index for inter-paw coordination showed Hirudin treated animals recovered faster by 7 days PI. (B) Coordinated stepping between front and diagonal hind-paws in Hirudin treated animals showed a more rapid recovery in coordinated diagonal step coupling on a catwalk by 7 d.p.i., which lagged in both heparin and control groups. *, p<0.5. (C) Immature oligodendrocytes stained by immunofluorescence against APC (red) and counterstained with BrdU (green) within lesioned spinal cords of animals treated with heparin, Hirudin, or PLGA/F-127 alone. (D) Stereology of oligodendrocyte populations within the lesion and 1mm rostral or caudal. t-test; *, p<0.5. #, p=0.7.
Since the group of animals injected with the hirudin-gel showed a significant reduction in proliferation and improved behavioral recovery, next we wanted to evaluate how each hydrogel affected oligodendrocyte populations. Spontaneous remyelination is known to occur amongst a balance of astrogliogenesis and myelin regeneration via oligodendrocyte progenitors cells.[38] Therefore, spinal tissue was stained by immunfluorescence to quantify immature oligodendrocyte populations (APC+-cells. Fig. 6C). As quantified by fractionator methodologies, animals injected with heparin- and hirudin-loaded PLGA/F-127 hydrogels showed a greater number of APC+-oligodendrocytes within the demyelination lesion (*, p<0.05. Fig. 6D). Moreover, the total population (all regions combined) of oligodendroctyes was higher in animals treated with hirudin-hydrogel versus aCSF-hydrogel controls. Animals treated with the heparin PLGA/F-127 hydrogel showed a similar increase in APC+-oligodendrocytes, but the trend did not reach significance (#, p=0.06) These data demonstrate that hirudin was able to accelerate functional recovery after a demyelination lesion and increase oligodendrocyte populations in the spinal cord, likely to result in increased myelin regeneration instead of the gliosis induced by thrombin receptor activation.[28] Given that hirudin is a direct thrombin inhibitor, versus heparin,[8] these data suggest that anticoagulants per se do not prevent astrogliogenesis but rather the inhibition of thrombin is able to promote regenerative pathways.
4. Conclusion
Traumatic injury to the central nervous system initiates a cascade of events that stimulate progenitor proliferation and reactive gliosis to restore tissue integrity, restrict inflammation and prevent neuronal death. Our studies demonstrate that a hirudin-loaded PLGA/F-127 depot hydrogel accelerated recovery of motor function and increased oligodendrocyte populations in animals treated post-injury. Our data suggest that a biomaterial designed to prolong the release of thrombin inhibitor holds promise as a therapeutic to mitigate scarring and to promote regenerative therapies in the central nervous system.
Acknowledgments
This work was funded through grants from the National Institute of Health (NSO46724), a CDMRP SCRIP Investigator Initiated Award (SC130249), the Paralysis Project of America, and the Craig H. Neilsen Foundation (SAN 124679), and the Washington Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–88. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishino A, Suzuki M, Ohtani H, Motohashi O, Umezawa K, Nagura H, Yoshimoto T. Thrombin may contribute to the pathophysiology of central nervous system injury. J Neurotrauma. 1993;10:167–79. doi: 10.1089/neu.1993.10.167. [DOI] [PubMed] [Google Scholar]
- 3.Gingrich MB, Traynelis SF. Serine proteases and brain damage - is there a link? Trends Neurosci. 2000;23:399–407. doi: 10.1016/s0166-2236(00)01617-9. [DOI] [PubMed] [Google Scholar]
- 4.Karabiyikoglu M, Hua Y, Keep RF, Ennis SR, Xi G. Intracerebral hirudin injection attenuates ischemic damage and neurologic deficits without altering local cerebral blood flow. J Cereb Blood Flow Metab. 2004;24:159–66. doi: 10.1097/01.WCB.0000100062.36077.84. [DOI] [PubMed] [Google Scholar]
- 5.Andreu-Agulló C, Morante-Redolat JM, Delgado AC, Fariñas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12:1514–23. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 6.Tarzami ST, Wang G, Li W, Green L, Singh JP. Thrombin and PAR-1 stimulate differentiation of bone marrow-derived endothelial progenitor cells. J Thromb Haemost. 2006;4:656–63. doi: 10.1111/j.1538-7836.2006.01788.x. [DOI] [PubMed] [Google Scholar]
- 7.Maggio N, Shavit E, Chapman J, Segal M. Thrombin induces long-term potentiation of reactivity to afferent stimulation and facilitates epileptic seizures in rat hippocampal slices: toward understanding the functional consequences of cerebrovascular insults. J Neurosci. 2008;28:732–6. doi: 10.1523/JNEUROSCI.3665-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsh J, O’donnell M, Weitz JI. New anticoagulants. Blood. 2005;105:453–63. doi: 10.1182/blood-2003-12-4195. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg R, Damus P. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973;248:6490. [PubMed] [Google Scholar]
- 10.Wallis RB. Hirudins: from leeches to man. Semin Thromb Hemost. 1996;22:185–96. doi: 10.1055/s-2007-999007. [DOI] [PubMed] [Google Scholar]
- 11.Festoff BW, Ameenuddin S, Santacruz K, Morser J, Suo Z, Arnold PM, Stricker KE, Citron BA. Neuroprotective effects of recombinant thrombomodulin in controlled contusion spinal cord injury implicates thrombin signaling. J Neurotrauma. 2004;21:907–22. doi: 10.1089/0897715041526168. [DOI] [PubMed] [Google Scholar]
- 12.Kempe S, Mäder K. In situ forming implants — an attractive formulation principle for parenteral depot formulations. J Control Release. 2012;161:668–79. doi: 10.1016/j.jconrel.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Alexander A, Ajazuddin, Khan J, Saraf S, Saraf S. Poly(ethylene glycol)-poly(lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications. J Control Release. 2013;172:715–29. doi: 10.1016/j.jconrel.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Langer R. Biomaterials in drug delivery and tissue engineering: one laboratory’s experience. Acc Chem Res. 2000;33:94–101. doi: 10.1021/ar9800993. [DOI] [PubMed] [Google Scholar]
- 15.Kim HK, Chung HJ, Park TG. Biodegradable polymeric microspheres with “open/closed” pores for sustained release of human growth hormone. J Control Release. 2006;112:167–74. doi: 10.1016/j.jconrel.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima Y, Yamamoto H, Takeuchi H, Hino T, Niwa T. Properties of a peptide containing DL-lactide/glycolide copolymer nanospheres prepared by novel emulsion solvent diffusion methods. Eur J Pharm Biopharm. 1998;45:41–8. doi: 10.1016/S0939-6411(97)00121-5. [DOI] [PubMed] [Google Scholar]
- 17.Barichello JM, Morishita M, Takayama K, Nagai T. Absorption of insulin from pluronic F-127 gels following subcutaneous administration in rats. Int J Pharm. 1999;184:189–98. doi: 10.1016/s0378-5173(99)00119-2. [DOI] [PubMed] [Google Scholar]
- 18.Escobar-Chávez JJ, López-Cervantes M, Naik A, Kalia Y, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharm Sci. 2006;9:339–58. [PubMed] [Google Scholar]
- 19.Sellers DL, Maris DO, Horner PJ. Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J Neurosci. 2009;29:6722–33. doi: 10.1523/JNEUROSCI.4538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng CP, Goodman TT, Park I-K, Pun SH. Bio-mimetic surface engineering of plasmid-loaded nanoparticles for active intracellular trafficking by actin comet-tail motility. Biomaterials. 2009;30:951–8. doi: 10.1016/j.biomaterials.2008.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jandrot-Perrus M, MGH, JLK, AB, MCG Effect of the hirudin carboxy-terminal peptide 54-65 on the interaction of thrombin with platelets. Thromb Haemost. 1991;66:300–5. [PubMed] [Google Scholar]
- 22.Yildiz A, Okyar A, Baktir G, Araman A, Ozsoy Y. Nasal administration of heparin-loaded microspheres based on poly(lactic acid) Farmaco. 2005;60:919–24. doi: 10.1016/j.farmac.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol. 2001;172:115–27. doi: 10.1006/exnr.2001.7798. [DOI] [PubMed] [Google Scholar]
- 24.Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006;498:525–38. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 26.Hagood SK, McGinn MJ, Sun D, Colello RJ. Characterizing the mitogenic effect of basic fibroblast growth factor in the adult rat striatum. J Neurotrauma. 2006;23:205–15. doi: 10.1089/neu.2006.23.205. [DOI] [PubMed] [Google Scholar]
- 27.Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci. 1999;19:8182–98. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, Lyuboslavsky P, et al. Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. J Neurosci. 2005;25:4319–29. doi: 10.1523/JNEUROSCI.5200-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jørgensen EB, Hvidt S, Brown W, Schillén K. Effects of salts on the micellization and gelation of a triblock copolymer studied by rheology and light scattering. Macromolecules. 1997;30:2355–64. [Google Scholar]
- 30.Kwon SK, Kim H-B, Song J-J, Cho CG, Park S-W, Choi J-S, et al. Vocal fold augmentation with injectable polycaprolactone microspheres/pluronic f127 hydrogel: long-term in vivo study for the treatment of glottal insufficiency. PLoS ONE. 2014;9:e85512. doi: 10.1371/journal.pone.0085512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, et al. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol. 2002;177:575–80. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- 32.Cantín M, Miranda P, Galadames IS, Zavando D, Arenas P, Velásquez L, Vilos C. In vivo biocompatibility of the PLGA microparticles in parotid gland. Int J Clin Exp Pathol. 2013;6:2412. [PMC free article] [PubMed] [Google Scholar]
- 33.Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun. 2007;21:599–616. doi: 10.1016/j.bbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–69. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 35.Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, et al. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- 36.Miron VE, Boyd A, Zhao J-W, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–8. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamers FP, Lankhorst AJ, van Laar TJ, Veldhuis WB, Gispen WH. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J Neurotrauma. 2001;18:187–201. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- 38.Blakemore WF, Eames RA, Smith KJ, McDonald WI. Remyelination in the spinal cord of the cat following intraspinal injections of lysolecithin. J Neurol Sci. 1977;33:31–43. doi: 10.1016/0022-510x(77)90179-4. [DOI] [PubMed] [Google Scholar]