Abstract
Cyclooxygenase-2 (COX-2) is an enzyme involved in tumorigenesis, and inhibitors of the enzyme are increasingly used as adjuvant modulators in anticancer therapies due to their synergistic effects. The enzyme is also reported to cause resistance against antitumor agents, such as cisplatin. Here we report the first covalently linked conjugates of cisplatin and COX inhibitors, which allow concerted transport of both drugs into tumor cells and simultaneous action on intracellular cleavage. These platinum(IV) complexes show highly increased cytotoxicity compared to cisplatin and are even able to overcome cisplatin-related resistance of tumor cells. Furthermore, the conjugates provide tools for the elucidation of the influence of COX inhibitors on the efficacy of antitumor agents.
Keywords: antitumor agents, cyclooxygenase inhibitors, drug delivery, drug design, prodrugs
Cisplatin and its derivatives are among the most widely used chemotherapeutic agents for the treatment of multiple types of cancer.[1] However, platinum-based antitumor therapy is complicated by severe side effects as well as intrinsic and acquired resistance of tumor cells. Resistance mechanisms include decreased influx, increased efflux, and detoxification of the drugs, as well as interference with apoptotic pathways that are usually activated by the compounds.[2] Implicated in cisplatin resistance is cyclooxygenase-2 (COX-2), a key enzyme in the biosynthesis of prostaglandins. COX-2 is overexpressed in many tumors, and it plays a role in tumor initiation and progression.[3] It is also associated with poor outcome in several types of cisplatin-treated cancer.[2d,4] Thus, COX inhibitors, including nonsteroidal anti-inflammatory drugs (NSAIDs, e.g., indomethacin) and COX-2-selective inhibitors (e.g., celecoxib) are used as chemopreventive and adjuvant chemotherapeutic agents. Clinical studies have shown synergistic effects when COX inhibitors are administered in combination with various antitumor agents, such as cisplatin, paclitaxel, or doxorubicin.[5] However, the mechanism by which COX-2 is involved in tumorigenesis is still mainly unknown, and also controversial results have been reported. While several studies showed positive effects of COX inhibitors on tumor treatment to be COX-2-independent,[6] others even reported antagonistic effects.[7] Furthermore, preclinical studies also revealed that cisplatin and other antitumor agents even increased COX-2 expression in tumor cells.[8]
Prior studies of the influence of COX inhibitors on the efficacy of antitumor agents have used combinatorial treatments resulting in potential discrepancies between clinical and cell culture studies. Due to differential pharmacokinetics, delivery of the drugs to a tumor in vivo may fail to recapitulate administration of the compounds to cells in culture. To address this issue, we report the first cisplatin–COX inhibitor conjugates. The NSAIDs indomethacin or ibuprofen were coordinated at cisplatin as axial ligands, resulting in platinum(IV) complexes. More stable than cisplatin and its platinum(II) analogues, platinum(IV) compounds can be administered orally,[9] and increased lipophilicity imparted by axial ligands facilitates compound uptake.[10] Reduction of platinum(IV) compounds by redox-active biomolecules such as glutathione and ascorbate cleaves the linkage between the NSAID and cisplatin intracellularly, allowing these conjugates to act as prodrugs.[10b,11] Release of cisplatin together with two equivalents of the NSAID could enable a dual action with the latter preventing COX from tumor promoting activities and interfering with the action of cisplatin. The covalent conjugation ensures concerted transport of both drugs into tumor cells and may promote enrichment of the complexes in COX-2-expressing tumors.[12]
The NSAIDs were coordinated at the metal center of cisplatin via their carboxyl groups (Figure 1). Thus, intracellular reduction of the platinum(IV) complexes directly releases the drugs without any derivatization (Figure 2) and enables direct comparison with studies of combinatorial treatments. The synthesis was achieved by treating oxoplatin (cis,trans,cis-[PtCl2(OH)2(NH3)2]) with the acyl chloride of the respective NSAID in the presence of a base (for methods and characterization data, see Supporting Information).[13] Due to the large aromatic ligands, the conjugates are practically insoluble in water, but can be dissolved in polar aprotic solvents such as DMSO and DMF.
Figure 1.
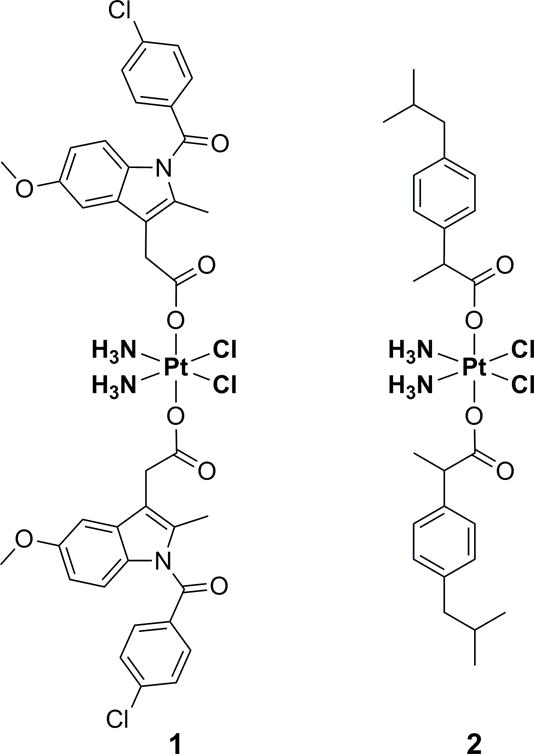
Structures of the conjugates 1 (with indomethacin) and 2 (with ibuprofen).
Figure 2.
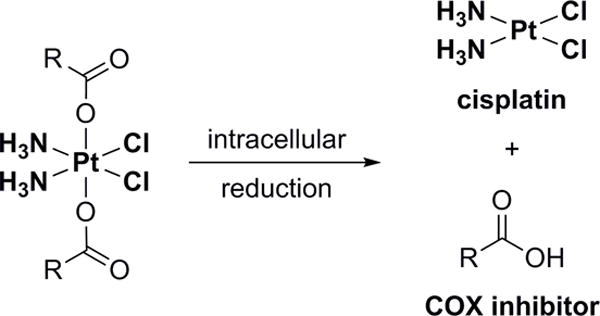
Reduction of the platinum(IV) conjugates releases cisplatin and the respective COX inhibitor.
In solution, 1 and 2 are inert towards ligand exchange but can be irreversibly reduced to more labile platinum(II) compounds (Figure 2). Thus, their pharmacological profile is significantly influenced by their reduction potential, and this in turn depends on the nature of the axial ligands. Reduction occurs most readily with axial chloro ligands and least readily for axial hydroxo ligands, and is intermediate for axial carboxylato ligands.[14] If the potential is too high, reduction occurs in the blood resulting in severe side effects (e.g., tetraplatin, systemic toxicity).[15] In contrast, an excessively low reduction potential results in excretion of the intact compound (e.g., iproplatin, low activity).[16] The cyclic voltammograms of the conjugates showed only one peak, corresponding to the irreversible reduction of platinum(IV) (Figures S1 and S2). 1 (EP = −0.36 V vs. NHE in DMF) showed a higher reduction potential than 2 (EP = −0.68 V vs. NHE in DMF), which could be caused by the more strongly electron-withdrawing indole system and increased steric bulk favoring ligand dissociation. The reduction potentials of the conjugates, which were comparable to those of other reported platinum(IV) carboxylato complexes,[17] should ensure in vivo stability in blood during transport but enable intracellular reduction. Indeed, the conjugates were reduced by ascorbate, an important cytoplasmic reductant.
Reduction rates also increase with increasing electronegativity of the axial ligands and are likely to influence biological properties of platinum(IV) compounds.[14] The reduction of the conjugates by ascorbic acid at 37°C was monitored by 1H NMR spectroscopy (Figure 3, Figures S3–S6). In each case, about 40% of the platinum(IV) complex was reduced over 3 days, a rate that could be compatible with the clearance rate of such drugs from the body. While reduction potential and rate probably influence the pharmacological properties of the platinum(IV) complexes, they do not necessarily correlate with their cytotoxicity.
Figure 3.
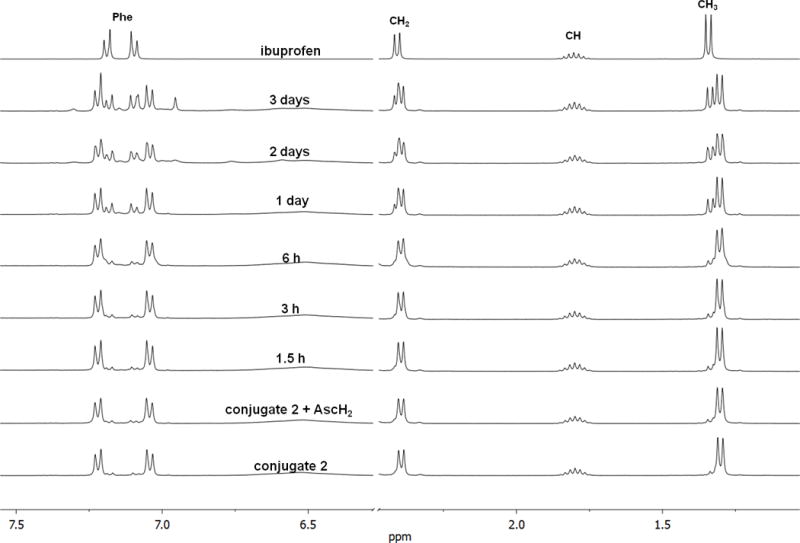
Reduction of conjugate 2 with ascorbic acid (AscH2) at 37°C; time-resolved 1H NMR spectra (400 MHz, [D6]DMSO); sections of the aromatic and aliphatic region.
The COX inhibitory potency of each conjugate was determined using purified COX-2 and the COX-1 isoform. 1 was a potent COX inhibitor with a 100-fold selectivity for COX-2 (IC50(COX-1): 4.1 μM, IC50(COX-2): 0.045 μM) and is the first highly selective COX-2 inhibitor containing a metal center. This complex is much more potent than other reported metal complexes of NSAIDs, which were less potent or COX-1-selective.[18] The structure of 1 resembles that of indomethacin esters, which were shown to be COX-2-selective inhibitors.[19] The inhibition potency of 1 suggests a strong interaction with the COX-2 enzyme that could contribute to targeted delivery into COX-2-overexpressing tumor cells.[12] In contrast, 2 showed no COX inhibition (Table S3). Ibuprofen itself is a weak, rapidly reversible inhibitor of COX,[20] probably resulting in a weak binding of 2.
On entering tumor cells, the conjugates could act as platinum(IV) compounds or be reduced with release of cisplatin and the NSAID.[21] To study the cytotoxicity of the conjugates and the influence of the coordinated NSAIDs, proliferation assays were carried out using a panel of four tumor cell lines with different sensitivities towards cisplatin and different levels of COX-2 expression (Table 1, Table S2). Both conjugates showed a markedly increased cytotoxicity compared to cisplatin, with submicromolar IC50 values. The conjugates are some of the most active in the class of platinum(IV) carboxylato complexes, most of which exhibit potencies similar to that of cisplatin.[17a,22] 1 and 2 were even able to completely overcome cisplatin resistance in MDA-MB-231 breast cancer cells, with an up to 400-fold lower IC50 value than that of cisplatin.
Table 1.
IC50 values [μM] of cisplatin and conjugates 1 and 2, as determined in proliferation assays for different tumor cell lines with incubation times of 24 h and 72 h.
| HCT 116[a] | OVCAR3[b] | MDA-MB-231[c] | 1483 HNSCC[d] | |||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 72 h | 24 h | 72 h | 24 h | 72 h | 24 h | 72 h | |
| cisplatin | 22.3 | 12.0 | 10.7 | 2.07 | 159.8 | 20.0 | 8.6 | 2.0 |
| 1 | 2.5 | 1.1 | 7.7 | 2.2 | 20.1 | 1.65 | 3.5 | 0.69 |
| 2 | 0.22 | 0.065 | 1.5 | 0.13 | 1.05 | 0.05 | 0.54 | 0.045 |
Colorectal carcinoma, no COX-2 expression.
Ovarian adenocarcinoma, COX-1 expression.
Breast adenocarcinoma, COX-2 expression.
Head and neck squamous cell carcinoma, high COX-2 expression.
The cytotoxic potency of the conjugates in the cell lines was unrelated to COX-2 expression (Table 1). Furthermore, 2 was more active than 1 despite its complete absence of COX-2 inhibitory activity and the lower potency of ibuprofen as compared to indomethacin. These data suggest that the cytotoxicity of the complexes is unrelated to the COX-2 inhibitory activity of the conjugates or the parent NSAIDs. Consistently, combinatorial treatment of the cancer cells with cisplatin and the respective NSAID (ratio 1:2) did not reveal any increase in cytotoxicity over that of cisplatin alone (Table S2).
The mechanism by which NSAID conjugation increases the cytotoxic potency of cisplatin is unclear. Hydrophobic ligands increase the lipophilicity of the platinum complex thereby facilitating transport through the cell membrane.[10,22a,b] Thus, it is possible that the coordinated NSAIDs promote the transport and accumulation of cisplatin in tumor cells. Oxidized cisplatin derivatives are also kinetically inert, which prevents detoxication of the drugs in the extracellular matrix and intracellularly, for example, by coordination of sulfur-containing proteins.[10b,23] Cisplatin irreversibly binds to a large extent to plasma proteins leading to substantially reduced uptake into cells.[24] Thus, increasing inertness of the metal complex towards ligand exchange should enhance the efficacy of the antitumor drug by increasing the probability that the complex reaches the cellular target intact and thereby also reduce side effects.
In the case of 1 and 2, only two molecules of the NSAID per molecule of cisplatin are transported into the tumor cells. In contrast, the administered concentrations of COX inhibitors in combinatorial treatments are usually much higher than that of the antitumor agent. Used in such an excess, the COX inhibitors may have an influence on cytotoxicity different to that shown in this investigation. However, the ratio of the drugs which finally reaches the tumor cells remains unknown in combinatorial treatments, especially for in vivo studies, in which the COX inhibitors are differently distributed in the tissues than cisplatin. To efficiently and rationally use the inhibitors as chemosensitizing agents, the involvement of COX in tumorigenesis and the action of its inhibitors in tumor cells must be better understood.
In conclusion, the first covalently linked conjugates of cisplatin with NSAIDs are presented. The platinum(IV) complexes show strongly increased cytotoxicity compared to cisplatin and even overcome cisplatin-related resistance in tumor cells. Although the indomethacin conjugate is a potent COX-2-selective inhibitor, these complexes seem to execute their cytotoxic action via COX-2-independent mechanisms. These conjugates provide tools for the elucidation of the influence of COX inhibitors on platinum-based antitumor agents.
Supplementary Material
Acknowledgments
This work was supported by the Fonds der Chemischen Industrie (doctoral grant for W.N.), the Graduate School “Building with Molecules and Nano-objects (BuildMoNa)” funded by the Deutsche Forschungsgemeinschaft, the US National Institutes of Health (CA89450), and the German Academic Exchange Service (PPP USA). The authors thank Umicore AG & Co. KG for the generous donation of chemicals and are grateful to Carol Rouzer for editorial assistance.
Footnotes
Supporting information for this article (preparation and characterization of conjugates, cyclic voltammetry, reduction with ascorbic acid, cell lines and tissue culture conditions, cell proliferation assay, COX inhibition assay, spectra of conjugates) is available on the WWW under http://www.chemmedchem.org at doi:…
References
- 1.Wheate NJ, Walker S, Craig GE, Oun R. Dalton Trans. 2010;39:8113. doi: 10.1039/c0dt00292e. [DOI] [PubMed] [Google Scholar]
- 2.a) Kartalou M, Essigmann JM. Mutat Res. 2001;478:23. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]; b) Ohmichi M, Hayakawa J, Tasaka K, Kurachi H, Murata Y. Trends Pharmacol Sci. 2005;26:113. doi: 10.1016/j.tips.2005.01.002. [DOI] [PubMed] [Google Scholar]; c) Rabik CA, Dolan ME. Cancer Treat Rev. 2007;33:9. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Stewart DJ. Crit Rev Oncol Hematol. 2007;63:12. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]; e) Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Oncogene. 2012;31:1869. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]; f) Shen DW, Pouliot LM, Hall MD, Gottesman MM. Pharmacol Rev. 2012;64:706. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Simmons DL, Botting RM, Hla T. Pharmacol Rev. 2004;56:387. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]; b) Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Int J Biochem Cell Biol. 2006;38:1654. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]; c) Sarkar FH, Adsule S, Li Y, Padhye S. Mini-Rev Med Chem. 2007;7:599. doi: 10.2174/138955707780859431. [DOI] [PubMed] [Google Scholar]; d) Marnett LJ. Annu Rev Pharmacol Toxicol. 2009;49:265. doi: 10.1146/annurev.pharmtox.011008.145638. [DOI] [PubMed] [Google Scholar]; e) Greenhough A, Smartt HJM, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. Carcinogenesis. 2009;30:377. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]; f) Ghosh N, Chaki R, Mandal V, Mandal SC. Pharmacol Rep. 2010;62:233. doi: 10.1016/s1734-1140(10)70262-0. [DOI] [PubMed] [Google Scholar]
- 4.a) Ferrandina G, Lauriola L, Distefano MG, Zannoni GF, Gessi M, Legge F, Maggiano N, Mancuso S, Capelli A, Scambia G, Ranelletti FO. J Clin Oncol. 2002;20:973. doi: 10.1200/JCO.2002.20.4.973. [DOI] [PubMed] [Google Scholar]; b) Ferrandina G, Lauriola L, Zannoni GF, Fagotti A, Fanfani F, Legge F, Maggiano N, Gessi M, Mancuso S, Ranelletti FO, Scambia G. Annals of Oncology. 2002;13:1205. doi: 10.1093/annonc/mdf207. [DOI] [PubMed] [Google Scholar]; c) Ferrandina G, Ranelletti FO, Legge F, Gessi M, Salutari V, Distefano MG, Lauriola L, Zannoni GF, Martinelli E, Scambia G. Clin Cancer Res. 2004;10:3117. doi: 10.1158/1078-0432.ccr-1090-3. [DOI] [PubMed] [Google Scholar]
- 5.a) Knapp DW, Glickman NW, Widmer WR, DeNicola DB, Adams LG, Kuczek T, Bonney PL, Amalia AE, Han C, Glickman LT. Cancer Chemother Pharmacol. 2000;46:221. doi: 10.1007/s002800000147. [DOI] [PubMed] [Google Scholar]; b) Hattori K, Matsushita R, Kimura K, Abe Y, Nakashima E. Biol Pharm Bull. 2001;24:1214. doi: 10.1248/bpb.24.1214. [DOI] [PubMed] [Google Scholar]; c) Ogino M, Minoura S. Int J Clin Oncol. 2001;6:84. doi: 10.1007/pl00012088. [DOI] [PubMed] [Google Scholar]; d) Barnes AP, Miller BE, Kucera GL. Gynecol Oncol. 2007;104:443. doi: 10.1016/j.ygyno.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 6.a) Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. FASEB J. 2001;15:2742. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]; b) Minter HA, Eveson JW, Huntley S, Elder DJ, Hague A. Clin Cancer Res. 2003;9:1885. [PubMed] [Google Scholar]; c) Jendrossek V, Handrick R, Belka C. FASEB J. 2003;17:1547. doi: 10.1096/fj.02-0947fje. [DOI] [PubMed] [Google Scholar]; d) Byun SS, Kim SW, Choi H, Lee C, Lee E. BJU Int. 2005;95:1086. doi: 10.1111/j.1464-410X.2005.05472.x. [DOI] [PubMed] [Google Scholar]
- 7.a) Czembirek C, Eder-Czembirek C, Erovic BM, Turhani D, Selzer E, Turnher D. Oncol Rep. 2005;14:1523. [PubMed] [Google Scholar]; b) Bijman MN, Hermelink CA, van Berkel MP, Laan AC, Janmaat ML, Peters GJ, Boven E. Biochem Pharmacol. 2008;75:427. doi: 10.1016/j.bcp.2007.09.005. [DOI] [PubMed] [Google Scholar]; c) Yu L, Chen M, Li Z, Wen J, Fu J, Guo D, Jiang Y, Wu S, Cho C-H, Liu S. Mol Pharmacol. 2011;79:608. doi: 10.1124/mol.110.069393. [DOI] [PubMed] [Google Scholar]
- 8.Mercer SJ, Di Nicolantonio F, Knight LA, Gabriel FG, Whitehouse PA, Sharma S, Fernando A, Bhandari P, Somers SS, Toh SK, Cree IA. Anti-cancer Drugs. 2005;16:495. doi: 10.1097/00001813-200506000-00004. [DOI] [PubMed] [Google Scholar]
- 9.a) Kelland LR, Abel G, McKeage MJ, Jones M, Goddard PM, Valenti M, Murrer BA, Harrap KR. Cancer Res. 1993;53:2581. [PubMed] [Google Scholar]; b) Lemma K, Sargeson AM, Elding LI. J Chem Soc Dalton Trans. 2000:1167. [Google Scholar]
- 10.a) Mistry P, Kelland LR, Loh SY, Abel G, Murrer BA, Harrap KR. Cancer Res. 1992;52:6188. [PubMed] [Google Scholar]; b) Hall MD, Hambley TW. Coord Chem Rev. 2002;232:49. [Google Scholar]
- 11.a) Graf N, Lippard SJ. Adv Drug Delivery Rev. 2012;64:993. doi: 10.1016/j.addr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wexselblatt E, Gibson D. J Inorg Biochem. 2012;117:220. doi: 10.1016/j.jinorgbio.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Uddin MJ, Crews BC, Blobaum AL, Kingsley PJ, Gorden DL, McIntyre JO, Matrisian LM, Subbaramaiah K, Dannenberg AJ, Piston DW, Marnett LJ. Cancer Res. 2010;70:3618. doi: 10.1158/0008-5472.CAN-09-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galanski M, Keppler BK. Inorg Chem. 1996;35:1709. doi: 10.1021/ic9509490. [DOI] [PubMed] [Google Scholar]
- 14.a) Choi S, Filotto C, Biszano M, Delaney S, Lagasee D, Whitworth JL, Jusko A, Li C, Wood NA, Willingham J, Schwenker A, Spaulding K. Inorg Chem. 1998;37:2500. [Google Scholar]; b) Ellis LT, Er HM, Hambley TW. Aust J Chem. 1995;48:793. [Google Scholar]
- 15.Schilder RJ, LaCreta FP, Perez RP, Johnson SW, Brennan JM, Rogatko A, Nash S, McAleer C, Hamilton TC, Roby D, Young RC, Ozols RF, O’Dwyer PJ. Cancer Res. 1994;54:709. [PubMed] [Google Scholar]
- 16.Trask C, Silverstone A, Ash CM, Earl H, Irwin C, Bakker A, Tobias JS, Souhami RL. J Clin Oncol. 1991;9:1131. doi: 10.1200/JCO.1991.9.7.1131. [DOI] [PubMed] [Google Scholar]
- 17.a) Wilson JJ, Lippard S. Inorg Chem. 2011;50:3103. doi: 10.1021/ic2000816. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Reithofer MR, Bytzek AK, Valiahdi SM, Kowol CR, Groessl M, Hartinger CG, Jakupec MA, Galanski M, Keppler BK. J Inorg Biochem. 2011;105:46. doi: 10.1016/j.jinorgbio.2010.09.006. [DOI] [PubMed] [Google Scholar]; c) Varbanov HP, Valiahdi SM, Kowol CR, Jakupec MA, Galanski M, Keppler BK. Dalton Trans. 2012;41:14404. doi: 10.1039/c2dt31366a. [DOI] [PubMed] [Google Scholar]
- 18.a) Yun Y, Chen P, Zheng CL, Ang Y, Duan WG, Wang L, He B, Ma JQ, Wang DH, Shen ZQ. Yakugaku Zasshi. 2007;127:1869. doi: 10.1248/yakushi.127.1869. [DOI] [PubMed] [Google Scholar]; b) Rubner G, Bensdorf K, Wellner A, Kircher B, Bergemann S, Ott I, Gust R. J Med Chem. 2010;53:6889. doi: 10.1021/jm101019j. [DOI] [PubMed] [Google Scholar]
- 19.Kalgutkar AS, Marnett AB, Crews BC, Remmel RP, Marnett LJ. J Med Chem. 2000;43:2860. doi: 10.1021/jm000004e. [DOI] [PubMed] [Google Scholar]
- 20.Prusakiewicz JJ, Duggan KC, Rouzer CA, Marnett LJ. Biochemistry. 2009;48:7353. doi: 10.1021/bi900999z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talman EG, Kidani Y, Mohrmann L, Reedijk J. Inorg Chim Acta. 1998;283:251. [Google Scholar]
- 22.a) Reithofer MR, Schwarzinger A, Valiahdi SM, Galanski M, Jakupec MA, Keppler BK. J Inorg Biochem. 2008;102:2072. doi: 10.1016/j.jinorgbio.2008.07.006. [DOI] [PubMed] [Google Scholar]; b) Ang WH, Pilet S, Scopelliti R, Bussy F, Juillerat-Jeanneret L, Dyson PJ. J Med Chem. 2005;48:8060. doi: 10.1021/jm0506468. [DOI] [PubMed] [Google Scholar]; c) Ang WH, Khalaila I, Allardyce CS, Juillerat-Jeanneret L, Dyson PJ. J Am Chem Soc. 2005;127:1382. doi: 10.1021/ja0432618. [DOI] [PubMed] [Google Scholar]; d) Reithofer MR, Valiahdi SM, Jakupec MA, Arion VB, Egger A, Galanski M, Keppler BK. J Med Chem. 2007;50:6692. doi: 10.1021/jm070897b. [DOI] [PubMed] [Google Scholar]; e) Chin CF, Tian Q, Setyawati MI, Fang W, Tan ESQ, Leong DT, Ang WH. J Med Chem. 2012;55:7571. doi: 10.1021/jm300580y. [DOI] [PubMed] [Google Scholar]; f) Song Y, Suntharalingam K, Yeung JS, Royzen M, Lippard SJ. Bioconjugate Chem. 2013;24:1733. doi: 10.1021/bc400281a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.a) Mason WR. Coord Chem Rev. 1972;7:241. [Google Scholar]; b) Raynaud FI, Boxall FE, Goddard P, Barnard CF, Murrer BA, Kelland LR. Anticancer Res. 1996;16:1857. [PubMed] [Google Scholar]
- 24.a) DeConti RC, Toftness BR, Lange RC, Creasey WA. Cancer Res. 1973;33:1310. [PubMed] [Google Scholar]; b) Gullo JJ, Litterst CL, Maguire PJ, Sikic BI, Hoth DF, Woolley PV. Cancer Chemother Pharmacol. 1980;5:21. doi: 10.1007/BF00578558. [DOI] [PubMed] [Google Scholar]; c) Melvik JE, Dornish JA, Pettersen EO. Br J Cancer. 1992;66:260. doi: 10.1038/bjc.1992.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


