Abstract
Curcumin is derived from the spice tumeric and has anti-inflammatory and antineoplastic effects in vitro and in animal models, including preventing aberrant crypt foci (ACF) and adenomas in murine models of colorectal carcinogenesis. Inhibiting the production of the procarcinogenic eicosanoids prostaglandin E2 (PGE2) and 5-hydroxyeicosatetraenoic acid (5-HETE) can suppress carcinogenesis in rodents. Curcumin reduces mucosal concentrations of PGE2 (via inhibition of cyclooxygenases 1 and 2) and 5-HETE (via inhibition of 5-lipoxygenase) in rats. Although preclinical data support curcumin acitivity in many sites, the reported poor bioavailability of this agent supports its use in the colorectum We assessed the effects of oral curcumin (2 g or 4 g per day for 30 days) on PGE2 within ACF (primary endpoint), 5-HETE, ACF number, and proliferation in a non-randomized, open-label clinical trial in 44 eligible smokers with 8 or more ACF on screening colonoscopy. We assessed pre- and post-treatment concentrations of PGE2 and 5-HETE by liquid chromatography tandem mass spectroscopy in ACF and normal-tissue biopsies, ACF number via rectal endoscopy, proliferation by Ki-67 immunohistochemistry; and curcumin concentrations by high-performance liquid chromatography in serum and rectal mucosal samples. 41 Subjects completed the study. Neither dose of curcumin reduced PGE2 or 5-HETE within ACF or normal mucosa or Ki-67 in normal mucosa. A significant 40% reduction in ACF number occurred with the 4 g dose (P < 0.005); while ACF were not reduced in the 2 g group. This reduction was associated with a significant change in plasma curcumin/conjugate levels pre- and post-treatmeeng (5-fold increase; P = 0.009) in the 4 g group. Curcumin was well tolerated at both 2 g and 4g. Our data suggest that curcumin can decrease ACF number, and this is potentially mediated by curcumin conjugates delivered systemically.
Keywords: ACF, Tobacco, Curcumin, Colon cancer
Introduction
The phytochemical curcumin is derived from the spice tumeric and has substantial, complex anti-inflammatory, antioxidant, and antineoplastic effects in vitro and in animal carcinogenesis models, including preventing colorectal aberrant crypt foci (ACF) and adenomas in mice (1). Long used for treating inflammation, skin wounds, and tumors in India and Southeast Asia (2, 3), curcumin inhibits the production of at least two eicosanoids, prostaglandin E2 (PGE2) and 5-hydroxyeicosatetraenoic acid (5-HETE), via inhibiting cyclooxygenase 1 (Cox-1) and Cox-2 (to reduce PGE2) and 5-lipoxygenase (5-Lox; to reduce 5-HETE; ref. 4). PGE2 and 5-HETE are important procarcinogenic factors, the inhibition of which is associated with reduced carcinogenesis in rodent models of colorectal carcinogenesis (4). Clinical effects of curcumin on potential colorectal cancer biomarkers in humans have not been reported previously.
We and others found ACF in the rectum of current and former smokers. This association was independent of age and increased with years of tobacco use (5,6). These findings led us to speculate that the ability of curcumin to reduce concentrations of PGE2 and 5-HETE in the flat mucosa of the colorectum would reduce colorectal epithelial crypt proliferation and ACF formation in current smokers. ACF reduction has been used in both human (7, 8) and rodent models (9) to assess the potency of cancer-preventive drugs and dietary supplements.
Several phase I studies have shown that curcumin is well tolerated at doses up to 12 g (no maximum tolerated dose has been defined; refs. 10-12) but has very poor bioavailability (12, 13). Current curcumin or curcuminoid formulations may be more beneficial in the gastrointestinal tract, where they come into contact with gastrointestinal mucosa without need of systemic absorption. This suggestion is supported by a pilot study of five patients showing that curcumin plus quercetin reduced adenoma number and recurrence in subjects with familial adenomatous polyposis (14). Single-agent curcumin may also prevent relapse of ulcerative colitis (15), which is a chronic inflammatory process and precursor of colon cancer. All of these preliminary data led us to conduct the phase IIa clinical prevention trial of single-agent curcumin in colorectal neoplasia reported here.
Materials and Methods
Materials
Pure (Good Manufacturing Practice) curcumin powder [98.0% by high performance liquid chromatography (HPLC), Sabinsa Corp., East Windsor, NJ] was micronized and provided by the National Cancer Institute’s Division of Cancer Prevention for human use. Sodium acetate, sodium phosphate, β-17-estradiol acetate, and the enzymes β-glucuronidase (type IX-A from Escherichia coli) and sulfatase (type H-1 from Helix pomatia) were purchased from Sigma Chemical Co. (St. Louis, MO). HPLC-grade ethyl acetate, hexane, methanol, and water were purchased from Burdick and Jackson (Honeywell International Inc., Muskegon, MI).
Participants
Participants were recruited from patients referred to the University of Illinois at Chicago (UIC) for screening colonoscopy or to the Colorectal Screening Clinic at the University of Michigan (UM) for screening flexible sigmoidoscopy. To be eligible, men and women had to be ≥ 50 years of age and a current smoker with a smoking history of > 3 pack-years and had to have ≥ 8 rectal ACF by magnification chromoendoscopy. Written consent was obtained from each subject recruited to the study, which was reviewed and approved by the Institutional Review boards of UIC and UM.
Subjects were excluded for use of nonsteroidal anti-inflammatory drugs including acetylsalicylic acid (ASA, or aspirin) > 10 days per month unless they completed a 30-day washout period, and for a history of chronic inflammatory bowel disease, prior pelvic irradiation, or history of peptic ulcer disease endoscopically confirmed < 5 years from the enrollment date.
Study plan
We conducted a phase IIa cancer prevention trial of oral curcumin given daily for 30 days to reduce the concentrations of PGE2 and 5-HETE within ACF (the primary endpoint) and in associated normal mucosa. Secondary endpoints included total ACF number and an estimate of proliferation in normal mucosa using the proliferation marker Ki-67. The trial was conducted in two stages--curcumin at 2 g (8 capsules) once daily in the first stage and at 4 g (16 capsules) once daily in the second, succeeding stage, with 20 evaluable participants in each stage (total = 40). The protocol mandated a formal toxicity review and finding of acceptable toxicity after completion of stage 1 (2 g) prior to initiating stage 2 (4 g). The criteria for acceptable toxicity in stage 1 were < 20% grade-1 or -2 toxicity requiring drug discontinuation and < 10% grade-3 toxicity attributable to drug; these criteria were established in consultation with the Food and Drug Administration and National Cancer Institute Division of Cancer Prevention prior to opening this study. ACF were counted via magnifying endoscopy before and after treatment. Biopsies of ACF and normal rectal mucosa were taken both at baseline and post-treatment. All participants had venous blood drawn in a fasting state for a complete blood count, liver function tests, blood urea nitrogen, and creatinine levels after the baseline and post-treatment endoscopic exam. The post-treatment endoscopic (flexible sigmoidoscopy) exam was performed between day 30 and day 35. Adherence to treatment was tracked by a follow-up telephone call at day 14. At the post-treatment endoscopy procedure, adherence was assessed by pill counts and a diary in which participants recorded the date and time of each dose. was taken. Adherence to intervention was defined as taking ≥ 80% of the required dose. After completing the intervention with curcumin at 2 g, the study protocol was amended to evaluate plasma for curcumin and its conjugates pre- and post-intervention with curcumin at 4 g. Laboratory analyses of all enpoints were blinded as to subject and timing (pre versus post-treatment) and dose of curcumin. The clinical endpoint ACF reduction could not be blinded as the same investigator performed pre and post intervention examinations.
Magnification endoscopy (ACF quantification)
The Fujinon XL-401 videoscope, (Fujinon Inc, Wayne NJ) at UIC and Olympus GIF 200Z (Olympic Optical, Tokyo Japan) at UM were used to perform the initial standard colonoscopy or sigmoidoscopy. These videoscopes can magnify the mucosa by a factor of 35 and have an autofocusing device. The initial exam was followed by a magnified survey of the rectum for ACF using a modified dye infusion technique that is significantly faster and better tolerated by subjects (16). ACF were counted in a sequential fashion during a single withdrawal of the scope to prevent double counting. The rectum was divided into thirds using the 15 cm mark on the scope and counting proximal (15-10 cm), middle (10-5 cm) and distal (5-out). The ACF structures were identified using the criteria of McLellan and Bird (17).
Biopsy and sample management procedure
During endoscopy, subjects underwent 9 cold forceps biopsies—three from ACF and six from normal mucosa. Normal mucosa was defined as a control area of normal crypts > 5 cm from an ACF. Two ACF biopsies and three normal mucosal biopsies were placed in an indomethacin solution to prevent prostaglandin degradation (18) and then snap frozen in liquid nitrogen and stored at −70°C until assayed. The remaining biopsies were fixed in 10% buffered formalin solution.
Laboratory methods
Eicosanoid assays
Frozen mucosa was pulverized to fine powder under dry ice to extract PGE2 and 5-HETE. The samples were homogenized in ice cold PBS buffer containing 0.1% BHT and 1 mM EDTA using an ultrasonic Processor. Eicosanoids were extracted with hexane: ethyl acetate (1:1, v/v, 2 mL) after adding citric acid (1N, 20μL) and deuterated internal standards. The organic layer from three extractions were dried and reconstituted in HPLC mobile phase (methanol: ammonium acetate buffer (10 mM, pH 8.5, 70:30). Eicosanoids were separated using a ThermoElectron Finnegan triple quadrupole mass spectrometer equipped with a Waters HPLC inlet and a Luna-3 μ, phenyl hexyl, 2 × 150-mm analytical column (Phenomenex, Torrance, CA) with ammonium acetate: methanol gradient. PGE2 and 5-HETE were detected using electrospray negative ionization with multiple-reaction monitoring similar to the methods of Yang P et al (19).
Crypt cell proliferation (Ki-67 immunohistochemistry)
The Ki-67 antibody is a rabbit polyclonal antibody to a nuclear protein expressed in proliferating cells and is a validated marker of proliferation and cancer risk in both malignant (20) and normal colonic mucosa (21). Mucosal proliferation was determined from cold forceps biopsy specimens, oriented flat at the time of biopsy, processed separately in a Sakura VIP-3000 bench top automated tissue processor within 6-8 hrs of fixation in formalin, and paraffin embedded with the crypt axis parallel to block surface prior to shipment. Immunohistochemistry was performed using a standard but automated two-stage modification of an immunoperoxidase technique (22). Briefly, for each biopsy, 4-μm-thick sections were placed on capillary gap slides and deparaffinized with Histoclear (National Diagnostics, Atlanta, GA). Sections were then rehydrated through decreasing concentrations of isopropyl alcohol. Sections for immunoperoxidase staining were steam pretreated for 20 minutes in Antigen Retrieval Citra Buffer (Biogenex, San Ramon, CA). Avidin-biotin complex immunoperoxidase reactions were done using an Immunotech 500 automated immunostainer (Ventana Systems, Inc., Tucson AZ) according to the manufacturer’s instructions. Briefly, the automated steps included blockage of endogenous peroxidase with 3% hydrogen peroxide and reaction with monoclonal antibodies against human Ki-67 (clone MIB-1, Dako) diluted 1:100. The reaction was followed by a biotinylated goat anti-mouse IgG secondary antibody and then an avidin-biotin peroxidase complex. The chromogen was diaminobenzadine for all reactions. Cells were counterstained by 0.4% methyl green in 0.1 mol/L sodium acetate buffer (pH 4.0) followed by three washes each of water, 1-butanol, and Histoclear. Negative controls were done in the same fashion, except that the primary antibody was substituted with mouse immunoglobulin. A section of squamous cell carcinoma provided the positive controls for Ki-67.
Image analysis of sections stained by immunohistochemistry was performed using the Quantitative Proliferation Index Program of the CAS 200, according to the manufacturer’s instructions. The operator chose thresholds at a wavelength of 620 nm for nuclei exhibiting methyl green counterstaining for identification by the machine as a cell nucleus and a separate threshold at 500 nm for the program to identify a nucleus as having a positive immunohistochemical reaction. This was done because the green counterstain is transparent at 500 nm; thus, the instrument will detect only nuclei with a positive immunoperoxidase reaction. The analysis was displayed as the percentage of positively staining cells/total cells.
Detection of curcumin and its conjugates in tissue and in plasma
Curcumin was extracted from biopsy tissue similar to the extraction procedure in a previously published study (23). Frozen normal colon tissue (8-10 mg wet weight) was ground to a fine powder in a precooled Multisample Biopulverizer® (Research Products International Co.) under dry ice. The sample was then transferred into a precooled sealed microcentrifuge tube and treated with ice cold sodium acetate buffer (0.20 mL, pH 5.0). The powdered tissue was then homogenized by an ultrasonic Processor (Misonix, Farmingdale, NY) at 0°C for 3 minutes, with a 20 second homogenization cycle and a 20 second brake cycle to cool the homogenization probe to reduce the degradation of curcumin and its metabolites. An aliquot (0.1 ml) of the colon tissue suspension was then mixed with β-17-estradiol acetate (internal standard, 10 μL), sodium acetate buffer (0.5 M, 0.1 ml, pH 5.0) and vortexed 20 sec. The solution was extracted three times with extracting reagent and vortexed for 3 minutes. The sample was centrifuged at 3500 rpm for 15 minutes at 4°C. The upper organic layer was collected. The organic phases from the three extractions were pooled and evaporated under a stream of argon at room temperature.
The extraction of plasma was performed by adding 200 μL of plasma to 2 mL microcentrifuge tubes (USA Scientific, Ocala, FL, USA). To each tube, 80 mL de-ionized water was added. The tubes were capped and mixed for 20 seconds at medium speed by vortex (Fisher Scientific). Forty μL internal standard (250 μg/mL β-17-estradiol acetate) was added to each tube. The tubes were capped and mixed by vortexing for 30 seconds. Then, 500 μL of the extraction reagent (95% ethyl acetate/5% methanol) were added to each tube. The tubes were capped and vortexed at high speed for 30 seconds and then centrifuged at 13,500 RPM for 5 minutes in an Eppendorf micro-centrifuge (Brinkmann Instruments, Westbury, NY, USA). Following centrifugation, the supernatant organic layer, approximately 420 μL, was carefully removed into a clean micro-centrifuge tube and dried under a stream of room air using a low heat setting. For assay of conjugates, a second 200 μL plasma sample was mixed with β-glucuronidase (50 μL, 446 units) in 0.1 M phosphate buffer (pH 6.8), and sulfatase (45 μL, 52 units) in 0.1M sodium acetate buffer (pH 5.0), incubated at 37°C for 3.5 hours, and then extracted. All the extraction procedures were performed under dim light to prevent the degradation of curcumin. The extract was then reconstituted in methanol (100 μL) before ultraflow liquid chromatograph (UFLC) analysis.
Curcumin in colon biopsies was quantified by using Prominence® UFLC (Shimadzu Corp.) equipped with EZStart software, Prominence UV-Vis detector. The separation was performed on a Symmetry® C18 column (2.1 × 100 mm i.d.; 3.5 μm; Waters, Millford, MA) with a Waters absorbosphere 30 × 4.6 mm, C18 guard column (Waters, Milford, MA, USA) at room temperature. The mobile phase used under gradient conditions was 25% acetonitrile with 74.9% containing 0.1% acetic acid (25:74.9:0.1; v/v/v; A) and 100% acetonitrile containing 0.1% acetic acid (99.9:0.1; v/v; B). The gradient conditions start from 20% B to 37% B in 8 min and then to 100% B in 4 min and maintained for another 2 min at 100% B before returning to opening conditions over 4 minutes. The flow rate was 0.3 mL/min and injection volume for all samples was 10 μL. The detection of curcumin and β-17-estradiol acetate were performed at 420 and 280 nm, respectively.
The assays were validated by within and between day calibration curves and quality control low, medium, and high accuracy standards. The lower limit of detection for curcumin from human plasma was 5 ng/mL and from human colonic mucosa was 10 μg/g tissue.
Statistical considerations
We calculated that a sample size of 20 patients at each dose level would be sufficient for the two-sided distribution-free Sign test, which is a non-parametric Sign test for detecting any significant reduction or change of value (either positive or negative), such that the change is not due to the null hypothesis of random symmetric variability, in the primary endpoint (level of PGE2 in ACF tissue) with a significance level of 0.05 and power of 80%. The Sign test was chosen because three previous studies, even though demonstrating a significant reduction in prostaglandin concentrations, showed that subjects had a wide variation in prostaglandin concentrations at baseline (24-26). A 30 day intervention period was chosen because two of these studies demonstrated a significant reduction in prostaglandin concentrations in less than than 30 days administration of Cox inhibitors (25, 26). We estimated a dropout rate of 15% and therefore set the accrual goal at 24 to end up with 20 evaluable participants for each dose level. Changes from baseline of tissue PGE2 and 5-HETE concentrations were evaluated by the distribution-free Sign test. To assess proliferation and ACF change, mean change in labeling per crypt compartment and ACF number were examined by paired t-test. Pearson’s correlation coefficient was calculated to assess the correlation between pre- and post intervention concentrations of curcumin.
Results
Participants
We consented 93 eligible patients (current smokers) referred for colorectal screening from October 2006 through October 2008 (Fig. 1). Of these eligible patients, 62 (67%) were enrolled into the trial and 31 failed to meet study entry criteria for the following reasons: 19 for endoscopy failure (< 8 ACF); 6 unable to complete the 30-day aspirinwashout period; 6 for disqualifying illnesses on chart review. Forty-four enrolled patients began treatment after 7 withdrew consent and 1was lost to follow-up prior to starting the curcumin intervention. Three of these 44 patients were not fully evaluable: 1 was lost to follow-up; 1 withdrew; and 1 was non-compliant with treatment and were not further evaluated because the risk of a second endoscopy was thought to be unjustified. Forty-one patients completed the trial and were included in our final toxicity and biomarker analyses, 22 on the 2g and 19 on the 4 g dose (Table 1); 32 of these patients were enrolled at UIC and 9 at UM. We evaluated 17 males and 24 females; overall, 66% of patients were African-American, 27% Caucasian, and 4% Latin American. The two participant groups (2 g and 4 g) were similar with regard to mean age (P = 0.91) and pack-years of smoking (P = 0.74).
Fig. 1.
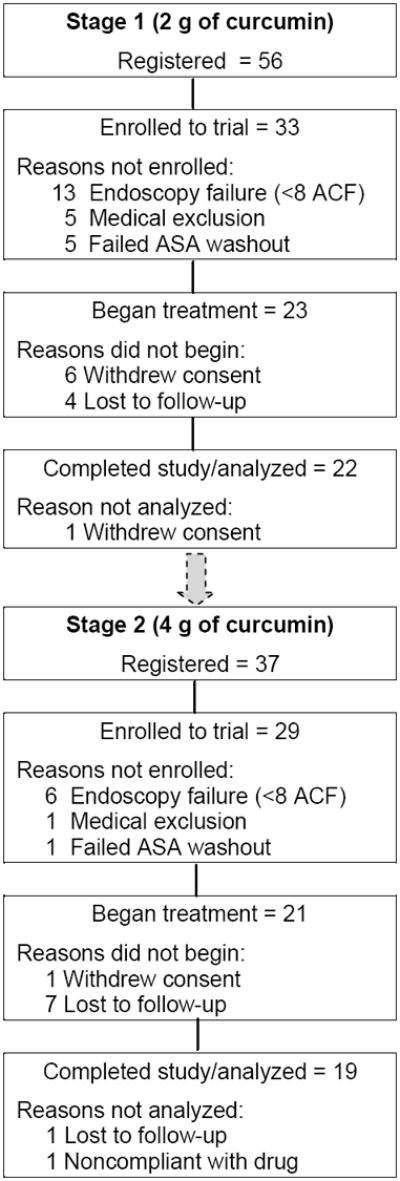
Study flow diagram. The overall 93 patients registered to this study included 56 registered in stage 1 and 37 in stage 2. Stage 2 (4 g) did not begin until a formal toxicity review of stage 1 (2 g) approved continuation of the study. ASA, acetylsalicylic acid (aspirin).
Table 1.
Summary of patient demographics
| Curcumin Dose | 2g N=22 | 4g N=19 |
|---|---|---|
| Age yrs (mean ± SD) | 57±5 | 54±7 |
| Range yrs | 51-70 | 42-69 |
| Personal/Family History, | ||
| Adenoma | 1/1 (9%)1 | 1/2(15.8%)2 |
| Colon Cancer | 0/2 (9%)1 | 0/0(0%)2 |
| Gender | ||
| Female | 13(59%)1 | 11(58%)2 |
| Male | 9(41%)1 | 8 (42%)2 |
| Race/Ethnicity, % | ||
| Caucasian | 3(13.6%)1 | 8(42.1%)2 |
| African-American | 18(81.8%)1 | 9(47.4%)2* |
| Latino-American | 1(4.6%)1 | 1(5.3%)2 |
| Amerindian | 0(0%)1 | 1(5.3%)2 |
| Asian | 0(0%)1 | 1(5.3%)2 |
| Tobacco Pack -years (mean) | 21.75 | 22.8 |
| # 1-15 pack-years | 10(45%)1 | 5(26%)2 |
| # 16-29 pack-years | 5(23%)1 | 8(42%)2 |
| # ≥ 30 pack-years | 7(32%)1 | 6(32%)2 |
| ACF number pre-treatment (mean±SEM) | 15.0±4.8 | 17.8±2.0 |
1 Participant self-identified as Latino and AA
Effects on PGE2 and 5-HETE tissue concentrations
PGE2 concentrations did not change significantly in the 2 g or 4 g cohort between baseline and post-intervention (Table 2). In the 2 g cohort, PGE2 concentrations in ACF tissue were 1.1, ±1.9, picogram (pg)/μg protein (baseline) versus 1.5, ±1.9, pg/μg protein (post-intervention; Mean ±SD P = 0.344) and in normal mucosa were 2.2, ±3.2, pg/μg protein (baseline) versus 2.9, ±3.8, pg/μg protein (post-intervention; P = 0.119). Although higher at baseline (with greater variability/standard deviations) than in the 2 g cohort (Supplemental Fig. 1and 2), PGE2 concentrations also did not change significantly in the 4 g cohort between baseline and post-intervention either in ACF tissue (3.4, ±4.8, pg/μg protein [baseline] versus 3.5, ±3.2, pg/μg protein [post-intervention]; P = 0.388) or in normal mucosa (2.8, ±1.9, pg/μg protein [baseline] versus 2.5 ±3.0, pg/μg protein [post-intervention]; P = 0.092)
Table 2.
Effects of curcumin on PGE2 and 5 HETE
| PGE2 and 5-HETE Concentrations
|
||||
|---|---|---|---|---|
| 2 g pre | 2 g post | 4 g pre | 4 g post | |
|
|
|
|||
| PGE-2 ACF | 1.1±1.8* | 1.6±1.8 | 3.4±4.8* | 3.7±3.2 |
| PGE-2 NL mucosa | 2.1±3.2* | 2.7±3.7 | 2.7±1.9* | 2.6±2.9 |
| 5-HETE ACF | 1.4±0.9* | 1.4±1.0 | 2.3±1.6* | 1.9±0.9 |
| 5-HETE NL mucosa | 2.3±1.3* | 2.4±1.2 | 2.5±1.5* | 2.2±1.6 |
Data are expressed as mean ± standard deviation, μg/g protein.
No significant difference between pre- and post- treatment values by the Sign test, at a significance level of 0.05.
Curcumin also did not reduce 5-HETE concentrations in ACF and normal mucosa of the 2 g or 4 g cohort (Table 2). 5-HETE concentrations in the 2 g cohort were 1.4 ±1.1, pg/μg protein (post-intervention) versus 1.4, ±0.9, pg/μg protein (baseline) in ACF (P = 0.453) and 2.3 ±1.3, pg/μg protein (post-intervention) versus 2.4 ±1.1, pg/μg protein (baseline) in normal mucosa (P = 0.688). Concentrations in the 4 g cohort were 2.4 ±1.6, pg/μg protein (post-intervention) versus 1.8 ±1.0 pg/μg protein (baseline) in ACF (P = 0.727) and 2.5 ±1.5, pg/μg protein (post-intervention) versus 1.6 ±1.6, pg/μg protein (baseline) in normal mucosa (P = 0.344).
Effects on ACF number and mucosal proliferation
The mean number of rectal ACF remained similar between baseline (15.0, ±4.8) and post-intervention (16.3, ±6.6; Mean ±SEM P = 0.51) after curcumin at 2 g (Fig. 2). Curcumin at 4 g significantly decreased the mean number of rectal ACF (17.8, ±2.0, baseline, versus 11.1, ±2.8, post-intervention; Mean ±SEM P <0.005; Fig. 2).
Fig. 2.
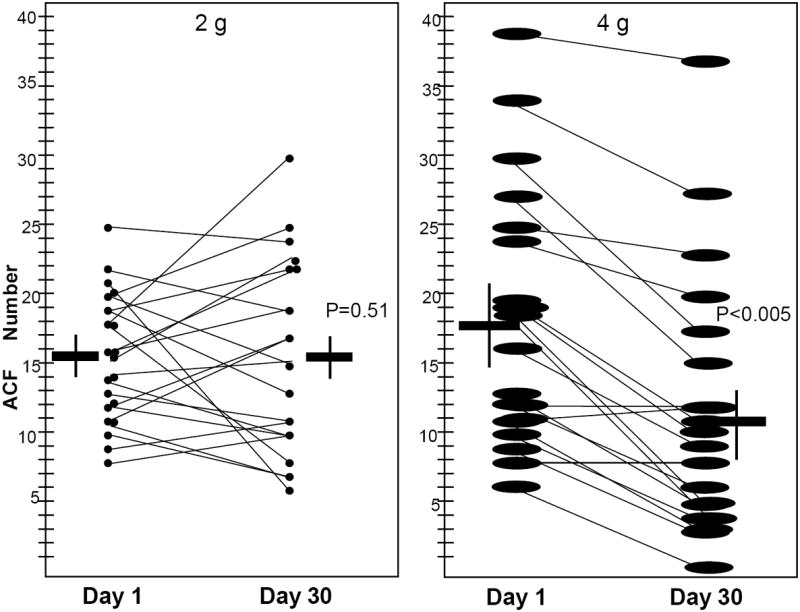
Human rectum with ACF number at initial exam and 30 days post treatment with 2 g or 4 g of curcumin. For each dose group, horizontal lines indicate the mean value for pre- and post-treatment levels in each dose group, with the standard error of the mean indicated by the endpoints of the corresponding vertical lines. Pre- and post-treatment levels of ACF number are plotted for each individual patient. The paired t-test was applied to examine change from pre-treatment levels in ACF number at a significance level 0.05.
No significant differences in Ki-67-detected mucosal proliferation corresponded to the reduced ACF number in the 4-g group; Ki67 was assessed in normal mucosa (Table 3), however, and not in ACF tissue. Proliferation in the proximal, middle, and distal 1/3 of the crypt remained unchanged in response to 2 g and 4 g of curcumin and show that the expected increase in proliferative index from the apex to the base of the crypt was maintained (at either dosing level; Table 3).
Table 3.
Proliferation index (% labeled cells) before and after treatment
| Crypt Segment | 2g pre | 2 g post | 4 g pre | 4 g post |
|---|---|---|---|---|
| Proximal third | 0.5±0.9* | 0.6±1.4 | 0.3±0.6* | 0.3±0.8 |
| Middle third | 11.9±4.2* | 11.8±7.2 | 12.8±6.1* | 16.4±10.3 |
| Distal third | 25.2±5.3 * | 23.5±8.0 | 28.7±9.1* | 30.9±7.7 |
Data are expressed as mean ± standard deviation. Proliferation index is calculated as # labeled cells /# total cells in that portion of the crypt which is arbitrarily divided into three segments.
No significant difference between pre- and post- treatment values by paired t-test, at a significance level of 0.05.
Curcumin and curcumin-conjugate concentrations
Concentrations of curcumin (parent compound) and curcumin conjugates were assayed in rectal mucosal biopsies and venous plasma (Table 4). Rectal mucosal biopsy samples for these assays were obtained from 39 of the 41 participants who completed curcumin treatment (21 at 2 g and 18 at 4 g). Curcumin was detected in baseline biopsy samples from 1 patient in the 2-g cohort and in post-intervention samples from 5 patients in the 2 g and three in the 4 g cohort (Table 4). Curcumin conjugates were detected in baseline biopsy samples from 1 patient in the 4 g cohort and in post-intervention samples from 13 patients in the 2 g and 12 in the 4 g cohort (a total of 25, or 64%, of the 39 patients with assayed biopsies). The mean post-intervention level of curcumin (±standard deviation) was 8.2.5±2.9 μg/g protein (range, 4.7–11.6 μg/g protein) in the 2 g cohort (5 samples) and 3.8±0.6 μg/g protein (range, 3.1–4.3 μg/g protin) in the 4 g cohort (3 samples). The detection of curcumin or its conjugates in rectal mucosal biopsies did not correlate with significant changes in concentrations of either tissue PGE2 or 5-HETE or reductions in ACF formation (data not shown).
Table 4.
Curcumin concentrations in tissue and plasma by dose
| 2g | 4g | |||
|---|---|---|---|---|
|
|
|
|||
| Pre | Post | Pre | Post | |
|
|
|
|||
| Rectal mucosa* | N = 21 | N = 18 | ||
| Curcumin | 4.03 (1) | 8.2±2.9 (5) | ND | 3.8±0.6 (3) |
| Conjugates | ND | 5.9±2.6 (13) | 4.21 (1) | 4.5±1.7 (12) |
| Plasma† | N = 0 | N = 19 | ||
| Curcumin | NA | NA | 7.3±8.1 (4) | 3.8±1.3 (2) |
| Conjugates | NA | NA | 15.8±14.8 (19) | 78.5±84.3‡ (19) |
Measures are mean±SD in μg/g protein (number of samples with detectable levels).
Measures are mean±SD in ng/mL (number of samples with detectable levels).
P = 0.009 for increased post-intervention level (versus pre-intervention level).
Abbreviations: Pre, pre-intervention; Post, post-intervention; ND, not detected; NA, not assayed.
Venous plasma samples for curcumin/curcumin-conjugate assays were obtained from all 19 participants who completed study in the 4 g cohort (Table 4); no venous plasma samples were assayed in the 2 g cohort. The mean concentrations (±SD) at baseline were 7.3±8.1 ng/mL (range, 1.4–19.1) for curcumin (detected in 4 of 19 samples) and 15.8±14.8 ng/mL (range, 4.6–56.5 ng/mL) for curcumin conjugates (detected in all 19 samples). Two post-intervention plasma samples had detectable curcumin (mean 3.8±1.3 ng/mL; range, 2.9–4.7 ng/mL), whereas all 19 samples had detectable curcumin conjugates (mean 78.5±84.3 ng/mL; range 9.2–382.7). Post-intervention curcumin conjugate concentrations rose significantly during treatment in the 4 g group (P = 0.009).
Toxicity
Toxicity was reviewed by both site principal investigators and Data Monitoring boards at the University of California Irvine and UM. Curcumin was well tolerated at either 2 g or 4 g. Stage 2 (curcumin at 4 g) was initiated after a formal review of stage 1 (2 g) found that toxicity in this stage was acceptable (according to criteria described in Methods). In the 22 stage-1 patients, 13 (59%) had grade-1 and -2 toxicity (none requiring drug discontinuation) and none had grade-3 toxicity. In the 19 stage-2 particpants, 12 (63%) had grade-1 and -2 toxicity and 1 (2%) had grade-3 toxicity. Overall, 25/41 participants (61%) had grade-1 and -2 toxicity, primarily gastrointestinal disturbances (most prevalently diarrhea, as well as distension, and gastroesophageal reflux disease which responded to loperamide and metoclopramide, respectively), with none requiring drug discontinuation. The single grade-3 toxicity was atypical chest pain and was unrelated to curcumin. Liver function tests were monitored for subclinical toxicity, and no clinically significant elevations were detected for curcumin at 2 g or 4 g.
Discussion
Curcumin at either 4 g or 2 g per day for 30 days did not reduce concentrations of PGE2 (the primary endpoint) or 5-HETE in ACF or normal flat mucosa, in contrast to previous data in preclinical rodent colorectal cancer models (27). Curcumin at 4 g/day for 30 days, however, significantly reduced ACF formation.
Unlike the elevated concentrations of the eicosanoids PGE2 and 5-HETE measured in the bronchial epithelium of smokers compared with non-smokers; (28), PGE2 and 5-HETE were not significantly altered in flat rectal mucosa of smokers compared with that described in the same mucosa of non-smokers (25). This similarity in concentrations suggests a lack of an inflammatory response to tobacco components in the epithelium of the distal gastrointestinal tract in marked contrast to inflammatory changes which occur in bronchial mucosa of smokers. Neither dose of curcumin reduced or changed the concentrations of these two eicosanoids in the present study. A trial of celecoxib in familial adenomatous polyposis patients similarly found that the intervention failed to reduce PGE2 concentrations in adenomas or normal rectal mucosa despite significantly reducing the number of adenomas (29). Furthermore, a recent study showed no significant change in leukotriene B4 (which is downstream of 5-HETE) during colorectal carcinogenesis (30). Unlike data in rodent models (31), our present human data suggest that Cox-1 or -2 or 5-Lox activity does not correlate with a reduction in ACF. Curcumin may exert its anticarcinogenic effects via alternative signaling pathways or via inhibition of upstream events, for example, its known inhibition of nuclear factor kappa B (NFκB) release (32) upstream of the eicosanoid system.
Our and others’ previous data (10)(11) suggest that the s ystemic bioavailability of curcumin is very poor in humans. Is the present study’s lack of curcumin effect on eicosanoids in flat rectal mucosa due to poor bioavailability of both directly delivered/topical and systemic curcumin? Our present data demonstrate that curcumin is not detectable in rectal mucosa or rectal ACFs at concentrations as low as 50 ng/5-mg biopsy after 30 days of daily administration. We found detectable concentrations of glucuronide and sulfate conjugates of curcumin both prior to and following a month of daily curcumin. As expected, curcumin concentrations in plasma were much higher following the month-long daily dosing. Our previous data did not find circulating conjugate concentrations prior to curcumin administration (10,12). The discrepancy in curcumin conjugate detection prior to curcumin administration in the present study compared to our previous report may be explained by improved analytical sensitivity. Our prior analytical sensitivity ranged from 50 ng/mL to 75 ng/mL (12, 33). Using UFLC and improved UV detection technology, we have reduced our low limit of quantitation of curcumin extracted from plasma to 5 ng/mL, similar to other recently reported UFLC analytical detection limits (34). The concentrations we now report are below our previously reported analytical detection limits (10,12) because of the more-sensitive analytical assay for curcumin.
These data might be interpreted as demonstrating that curcumin is not absorbed in rectal mucosa and that conjugate concentrations detected in the rectal mucosa are delivered systemically from absorption and conjugation higher in the gastrointestinal tract. Our matched plasma concentrations support this hypothesis. This result is surprising because the stools in our participants turned yellow, the color of curcumin. We did not assay stool samples to confirm that the yellow color indeed reflected the presence of curcumin. Alternatively, our data might suggest that curcumin is absorbed and rapidly and fully conjugated in the rectal mucosa. The recently published rodent data of Marczylo et al. suggest much higher curcumin concentrations in intestinal mucosa compared with plasma in rats given 340 mg/kg of Meriva, a phosphatidylcholine formulation of curcumin (35). This formulation may protect curcumin from rapid conjugation in intestinal mucosa and the liver.
Our present data suggest that healthy people eating a Western diet may ingest regular, small quantities of curcumin perhaps in the form of mustard or spice mixtures. The absorbed, dietary curcumin is conjugated, circulates systemically and partitions to tissues as shown by measured curcumin conjugate concentrations in rectal biopsies pre- and post-curcumin treatment in this trial, thus leading to a large volume of distribution and long conjugate half lives. We previously demonstrated the long half-lives (6 to 24 hours) of curcumin glucuronide and sulfate conjugates in human plasma (12).
Curcumin has anticarcinogenesis when delivered systemically in immunodeficent mouse models (36) and in humans with pancreatic adenocarcinoma (23). Yet in humans, both we nor Dhillon et al. infrequently detected curcumin concentrations systemically (plasma) and locally (rectal mucosal). These findings raise the question of whether curcumin conjugates or other breakdown products of curcumin, such as vanillin, ferulic acid, and feruloylmethane may be active metabolites. A mixture of these curcumin breakdown products did not alter the numbers or the mitotic cycle of Ishikawa cells (37), but no in-vitro or in-vivo data assessing the conjugate cellular pharmacology or potential anticarcinogenesis effects are available to date.
Despite the evidence of poor local tissue concentrations and lack of effect upon key rectal mucosal eicosanoids, our data demonstrate an important anticarcinogenic effect of curcumin—significant reduction of ACF at the 4 g dosing level. This finding raises the question of the in-vivo mechanism(s) by which curcumin may have cancer-preventive effects in the setting of early pre-invasive neoplastic lesions. Potential mechanisms may involve down-regulating different signaling cascades such as epidermal growth factor receptor (EGFR) or insulin-like growth factor receptor (IGFR) signaling, either of which has been linked to ACF formation (38, 39) and is known to be inhibited by curcumin in cell-culture models (40). Alternatively, curcumin may act via influencing Notch signaling, as shown in pancreatic cell lines (41), and Wnt signaling, as shown in breast-cancer stem cells (42). These Wnt and Notch signaling pathways have recently been shown to cooperatively influence proliferation and tumor formation in colon crypts (43).
Another potential curcumin mechanism involves tobacco-smoke biology. Tobacco smoke is known to contain the carcinogen benzo-[a]-pyrene, which is activated by cytochrome P450 1B1 (CYP 1B1) expressed in the colon to more-mutagenic and -carcinogenic epoxide intermediates that induce DNA adduct formation (44). Curcumin is capable of inhibiting CYP 1B1 activity in vitro and may inhibit the phase-1 activation of tobacco carcinogens, subsequently reducing DNA damage directly (45).
The reduction of ACF by agents in preclinical animal models of chemically induced carcinogenesis typically is associated with reduced proliferation (46), reduced inflammation (47), and/or increased apoptosis (48). Although we did not see changes in either proliferation or inflammatory markers in the present clinical study, we did not assay apoptotic markers in ACF or normal mucosa. Curcumin enhances apoptosis effects in colon adenocarcinoma cell lines through sphingomyelinase inhibition (49) and p21-independent mechanisms (50). Furthermore, apoptotic markers have recently been shown to be reduced in cancer subjects with ACF (51), suggesting that cancer-reductive agents that upregulate apoptotic pathways could reduce the incidence of ACF.
ACF as a biomarker of colon carcinogenesis in human studies remains controversial. Two clinical studies in Japan showed a significant association between increased ACF number in the distal rectum with colorectal adenomas and/or cancer, compared with normal controls (52, 53). Recent studies from the United States, however, failed to show a significant correlation between ACF number and true adenomas (54, 6). Such differences may be related to racial disparities. In such a context, the positive effect of curcumin at 4 g on ACF in the predominately AfricanAmerican population of our trial may be important. African Americans have increased rates of and mortality from colon cancer (21), in addition to a disproportionate burden of smoking-attributable cancer mortality, (55), compared with Caucasian Americans.
Tobacco smoke contains active carcinogens that concentrate in colonic mucosa (56). For example, benzo-[a]-pyrene is known to produce ACF within the colons of rats and mice (57) and is associated with increased DNA adduct formation. Similar adducts have been identified in the lung tissue of human smokers, increase linearly with tobacco consumption (58), and are strongly linked to colorectal cancer when present in the distal colon (59). In a study of curcumin given for seven days preoperatively to colorectal cancer patients, 3.6 g of curcumin was found to reduce DNA adducts in malignant but not adjacent normal mucosa, an effect not observed at lower doses (0.45 g and 1.8 g) (60), consistent with our findings of ACF reduction at 4g but not at 2 g. These data support the concept that ACF are a neoplastic lesion and might be a useful biomarker of both DNA damage and smoking-associated carcinogen exposure in humans. These earlier and our present data suggest that ACF reduction is a potentially important biomarker for monitoring chemopreventive efficacy in African Americans.
A major direction of future curcumin studies should be the optimization of its bioavailability through improved gastrointestinal absorption and systemic distribution to tissues (61). Pharmaceutical approaches to overcome these limitations include synthesis of curcumin analogues, the use of agents to increase curcumin absorption (e.g., piperine), and the development of modified drug-delivery systems, including liposomal, nanoparticulated, and phospholipid complex formulations of curcumin (62,63).
In conclusion, we have demonstrated that a short duration of curcumin treatment reduces ACF number. Our finding that oral, 98%-pure curcumin significantly reduced ACF number in humans confirms preclinical observations regarding changes in ACF in response to curcuminoid mixtures (consisting of curcumin, bisdemethoxy, and demethoxycurcumin) but leaves the mechanism(s) by which this occurs unanswered. The treatment-related increase in conjugate concentrations in plasma suggests that the ACF reduction resulted from systemic rather than local delivery of conjugates. These data verify the data of others that despite lack of systemically bioavailable concentrations of curcumin, anticarcinogenesis activity occurs at tissue targets. If these results can be confirmed in further trials in high-risk adenoma populations and if the mechanisms by which ACF reduction occurs after the administration of curcumin can be identified, they would further support the use of curcumin as a cancer prevention agent while strengthening the utility of ACF as a biomarker for clinical colon-cancer studies.
Supplementary Material
Acknowledgments
The authors wish to thank Dr Scott Lippman and Mr Kendall Morse for their advice and assistance in the preparation of this manuscript
Grant Support
This work was supported by National Institutes of Health, National Cancer Institute contract NCI-N01-CN-35160 (toREC), NCI-K07-CA12884 (to MK), and grants M01-RR000042 and UL1RR024986 to the University of Michigan, and the Kutche Family Professorship (to DEB). P30-CA62203 and contract NO-1 CN-25000(39) (toFLM)
References
- 1.Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–66. [PubMed] [Google Scholar]
- 2.Mukhopadhyay A, Basu N, Ghatak N, Gujral PK. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982;12:508–15. doi: 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- 3.Srimal RC, Dhawan BN. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol. 1973;25:447–52. doi: 10.1111/j.2042-7158.1973.tb09131.x. [DOI] [PubMed] [Google Scholar]
- 4.Huang M, Lysz T, Ferraro T, Abidi T, Laskin J, Conney A. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activity. Cancer Res. 1991;51:813–9. [PubMed] [Google Scholar]
- 5.Moxon D, Raza M, Kenney R, et al. Relationship of aging and tobacco use with the development of aberrant crypt foci in a predominantly African-American population. Clin Gastroenterol Hepatol. 2005;3:271–8. doi: 10.1016/s1542-3565(04)00623-8. [DOI] [PubMed] [Google Scholar]
- 6.Mutch MG, Schoen RE, Fleshman JW, et al. A multicenter study of prevalence and risk factors for aberrant crypt foci. Clin Gastroenterol Hepatol. 2009;7:568–74. doi: 10.1016/j.cgh.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Bruce WR, Archer MC, Corpet DE, et al. Diet, aberrant crypt foci and colorectal cancer. Mutat Res. 1993;290:111–8. doi: 10.1016/0027-5107(93)90038-h. [DOI] [PubMed] [Google Scholar]
- 8.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila) 2010;3(9):1077–83. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- 9.Corpet DE, Tache S. Most effective colon cancer chemopreventive agents in rats: a systematic review of aberrant crypt foci and tumor data, ranked by potency. Nutr Canncer. 2002;43:1–21. doi: 10.1207/S15327914NC431_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lao CD, Ruffin MTt, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:6–10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 12.Vareed SK, Kakarala M, Ruffin MT, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1411–7. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Correa M, Shoskes DA, Sanchez P, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4(8):1035–8. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502–6. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Carroll RE. Colon preparation for magnification endoscopy: a rapid novel approach. Endoscopy. 2004;36:1–4. doi: 10.1055/s-2004-814516. [DOI] [PubMed] [Google Scholar]
- 17.McLellan EA, Bird RP. Aberrant Crypts: Potential Preneoplastic Lesions in the Murine Colon. Cancer Research. 1988;48:6187–92. [PubMed] [Google Scholar]
- 18.Finley PR, Bogert CL, Alberts DS, et al. Measurement of prostaglandin E2 in rectal mucosa in human subjects: a method study. Cancer Epidemiol Biomarkers Prev. 1995;4:239–44. [PubMed] [Google Scholar]
- 19.Yang P, Chan D, Felix E, et al. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins Leukot Essent Fatty Acids. 2006;75:385–95. doi: 10.1016/j.plefa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Rego RL, Foster NR, Smyrk TC, et al. Prognostic effect of activated EGFR expression in human colon carcinomas: comparison with EGFR status. Br J Cancer. 2010;102:165–72. doi: 10.1038/sj.bjc.6605473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Keefe SJ, Chung D, Mahmoud N, et al. Why do African Americans get more colon cancer than Native Africans? J Nutr. 2007;137:175S–82S. doi: 10.1093/jn/137.1.175S. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter PM, Linden KG, McLaren CE, et al. Nuclear morphometry and molecular biomarkers of actinic keratosis, sun-damaged, and nonexposed skin. Cancer Epidemiol Biomarkers Prev. 2004;13:1996–2002. [PubMed] [Google Scholar]
- 23.Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 24.Giardello FM, Casero Rj, Hamilton S, et al. Prostanoids, ornithine decarboxylase, and polyamines in primary chemoprevention of familial adenomatous polyposis. Gastroenterology. 2004;126:425–31. doi: 10.1053/j.gastro.2003.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnan K, Ruffin MT, Normolle D, et al. Colonic mucosal prostaglandin-E2 and cyclooxygenase expression before and after low aspirin doses in subjects at high risk or at normal risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:447–53. [PubMed] [Google Scholar]
- 26.Ruffin MT, Krishnan K, Rock CL, et al. Suppression of human colorectal mucosal prostaglandins: determining the lowest effective aspirin dose. J Natl Cancer Inst. 1997;89:1152–60. doi: 10.1093/jnci/89.15.1152. [DOI] [PubMed] [Google Scholar]
- 27.Huang M, Lou Y, Ma W, Newmark H, Reuhl K, Conney A. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:584–5817. [PubMed] [Google Scholar]
- 28.Profita M, Sala A, Bonanno A, et al. Chronic obstructive pulmonary disease and neutrophil infiltration: role of cigarette smoke and cyclooxygenase products. Am J Physiol Lung Cell Mol Physiol. 2009;298:L261–9. doi: 10.1152/ajplung.90593.2008. [DOI] [PubMed] [Google Scholar]
- 29.Sinicrope FA, Half E, Morris JS, et al. Cell proliferation and apoptotic indices predict adenoma regression in a placebo-controlled trial of celecoxib in familial adenomatous polyposis patients. Cancer Epidemiol Biomarkers Prev. 2004;13:920–7. [PubMed] [Google Scholar]
- 30.Shureiqi I, Chen D, Day RS, et al. Profiling lipoxygenase metabolism in specific steps of colorectal tumorigenesis. Cancer Prev Res (Phila) 2010;3(7):829–38. doi: 10.1158/1940-6207.CAPR-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe K, Kawamori T, Nakatsugi S, et al. Role of the prostaglandin E receptor subtype EP-1 in colon carcinogenesis. Cancer Research. 1999;59:5093–6. [PubMed] [Google Scholar]
- 32.Sandur SK, Deorukhkar A, Pandey MK, et al. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int J Radiat Oncol Biol Phys. 2009;75:534–42. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heath DD, Pruitt MA, Brenner DE, R CL. Curcumin in plasma and urine: quantitation by high-performance liquid chromatography. J Chromatogr B Analyt technol biomed Life Sci. 2003;783:287–95. doi: 10.1016/s1570-0232(02)00714-6. [DOI] [PubMed] [Google Scholar]
- 34.Marczylo TH, Steward WP, Gescher AJ. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method. J Agric Food Chem. 2009;57:797–803. doi: 10.1021/jf803038f. [DOI] [PubMed] [Google Scholar]
- 35.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–7. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 36.Bachmeier B, Nerlich AG, Iancu CM, et al. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol Biochem. 2007;19:137–52. doi: 10.1159/000099202. [DOI] [PubMed] [Google Scholar]
- 37.Dempe JS, Pfeiffer E, Grimm AS, Metzler M. Metabolism of curcumin and induction of mitotic catastrophe in human cancer cells. Mol Nutr Food Res. 2008;52:1074–81. doi: 10.1002/mnfr.200800029. [DOI] [PubMed] [Google Scholar]
- 38.Carroll RE, Goodlad RA, Poole AJ, et al. Reduced susceptibility to azoxymethane-induced aberrant crypt foci formation and colon cancer in growth hormone deficient rats. Growth Horm IGF Res. 2009;19:447–56. doi: 10.1016/j.ghir.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dougherty U, Sehdev A, Cerda S, et al. Epidermal growth factor receptor controls flat dysplastic aberrant crypt foci development and colon cancer progression in the rat azoxymethane model. Clin Cancer Res. 2008;14:2253–62. doi: 10.1158/1078-0432.CCR-07-4926. [DOI] [PubMed] [Google Scholar]
- 40.Patel BB, Sengupta R, Qazi S, et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267–73. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106:2503–13. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 42.Kakarala M, Brenner DE, Korkaya H, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–85. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fre S, Pallavi SK, Huyghe M, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci U S A. 2009;106:6309–14. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Port JL, Yamaguchi K, Du B, et al. Tobacco smoke induces CYP1B1 in the aerodigestive tract. Carcinogenesis. 2004;25(11):2275–81. doi: 10.1093/carcin/bgh243. [DOI] [PubMed] [Google Scholar]
- 45.Walle T, Walle UK. Novel methoxylated flavone inhibitors of cytochrome P450 1B1 in SCC-9 human oral cancer cells. J Pharm Pharmacol. 2007;59(6):857–62. doi: 10.1211/jpp.59.6.0012. [DOI] [PubMed] [Google Scholar]
- 46.Carroll RE, Goodlad RA, Poole AJ, et al. Reduced susceptibility to azoxymethane-induced aberrant crypt foci formation and colon cancer in growth hormone deficient rats. Growth Horm IGF Res. 2009;19:447–56. doi: 10.1016/j.ghir.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagos GK, Carroll RE, Kouznetsova T, et al. Colon cancer chemoprevention by a novel NO chimera that shows anti-inflammatory and antiproliferative activity in vitro and in vivo. Mol Cancer Ther. 2007;6:2230–9. doi: 10.1158/1535-7163.MCT-07-0069. [DOI] [PubMed] [Google Scholar]
- 48.Poole A, Heap D, Carroll RE, Tyner A. Tumor suppressor functions for the Cdk inhibitor p21 in the mouse colon. Oncogene. 2004;23:8128–34. doi: 10.1038/sj.onc.1207994. [DOI] [PubMed] [Google Scholar]
- 49.Cheng Y, Kozubek A, Ohlsson L, Sternby B, Duan RD. Curcumin decreases acid sphingomyelinase activity in colon cancer Caco-2 cells. Planta Med. 2007;73:725–30. doi: 10.1055/s-2007-981540. [DOI] [PubMed] [Google Scholar]
- 50.Watson JL, Hill R, Lee PW, Giacomantonio CA, Hoskin DW. Curcumin induces apoptosis in HCT-116 human colon cancer cells in a p21-independent manner. Exp Mol Pathol. 2008;8:230–3. doi: 10.1016/j.yexmp.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Figueiredo P, Donato M, Urbano M, et al. Aberrant crypt foci: endoscopic assessment and cell kinetics characterization. Int J Colorectal Dis. 2009;24:441–50. doi: 10.1007/s00384-008-0576-z. [DOI] [PubMed] [Google Scholar]
- 52.Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998;339:1277–84. doi: 10.1056/NEJM199810293391803. [DOI] [PubMed] [Google Scholar]
- 53.Seike K, Koda K, Oda K, et al. Assessment of rectal aberrant crypt foci by standard chromoscopy and its predictive value for colonic advanced neoplasms. Am J Gastroenterol. 2006;101:1362–9. doi: 10.1111/j.1572-0241.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- 54.Adler D, Gostout C, Sorbi D, Burgart L, Wang L, Harmsen W. Endoscopic identification and quantification of aberrant crypt foci in the human colon. Gastrointest Endosc. 2002;56:657–62. doi: 10.1067/mge.2002.128540. [DOI] [PubMed] [Google Scholar]
- 55.Rivo ML, Kofie V, Schwartz E, Levy ME, T RV. Comparisons of black and white smoking attributable mortality, morbidity, and economic costs in the District of Columbia. J Natl Med Assoc. 1989;81:1125–30. [PMC free article] [PubMed] [Google Scholar]
- 56.Boysen G, Hecht SS. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutat Res. 2003;543:17–30. doi: 10.1016/s1383-5742(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 57.Tudek B, Bird RP, Bruce WR. Foci of aberrant crypts in the colons of mice and rats exposed to carcinogens associated with foods. Cancer Research. 1989;49:1236–40. [PubMed] [Google Scholar]
- 58.Phillips DH, Hewer A, Martin CN, Garner RC, King MM. Correlation of DNA adduct levels in human lung with cigarette smoking. Nature. 1988;336:790–2. doi: 10.1038/336790a0. [DOI] [PubMed] [Google Scholar]
- 59.Pfohl-Leszkowicz A, Grosse Y, Carriere V, et al. High levels of DNA adducts in human colon are associated with colorectal cancer. Cancer Res. 1995;55:5611–16. [PubMed] [Google Scholar]
- 60.Garcea G, Berry DP, Jones DJ, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14(1):120–5. [PubMed] [Google Scholar]
- 61.Cui J, Yu B, Zhao Y, et al. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009;371:148–55. doi: 10.1016/j.ijpharm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Bisht S, Maitra A. Systemic delivery of curcumin: 21st century solutions for an ancient conundrum. Curr Drug Discov Technol. 2009;6:192–9. doi: 10.2174/157016309789054933. [DOI] [PubMed] [Google Scholar]
- 63.Shahani K, Swaminathan SK, Freeman D, Blum A, Ma L, Panyam J. Injectable sustained release microparticles of curcumin: a new concept for cancer chemoprevention. Cancer Res. 2010;70(11):4443–52. doi: 10.1158/0008-5472.CAN-09-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


