Abstract
Early diagnosis of tuberculosis can dramatically reduce both its transmission and the associated death rate. The extremely slow growth rate of the causative pathogen, Mycobacterium tuberculosis (Mtb), however, makes this challenging at the point of care, particularly in resource-limited settings. Here we report the use of BlaC (an enzyme naturally expressed/secreted by tubercle bacilli) as a marker and the design of BlaC-specific fluorogenic substrates as probes for Mtb detection. These probes showed an enhancement by 100–200 times in fluorescence emission on BlaC activation and a greater than 1,000-fold selectivity for BlaC over TEM-1 β-lactamase, an important factor in reducing false-positive diagnoses. Insight into the BlaC specificity was revealed by successful co-crystallization of the probe/enzyme mutant complex. A refined green fluorescent probe (CDG-OMe) enabled the successful detection of live pathogen in less than ten minutes, even in unprocessed human sputum. This system offers the opportunity for the rapid, accurate detection of very low numbers of Mtb for the clinical diagnosis of tuberculosis in sputum and other specimens.
Tuberculosis is one of the most deadly diseases that kills over one million people each year and infects one-third of the world's population1. The disease is spread by infection with Mycobacterium tuberculosis (Mtb). Owing to its airborne transmission, early diagnosis is critical to the prevention and control of TB. Standard diagnostic methods, acid-fast smear from sputum, often do not become positive until after transmission occurs, which allows the spread of the disease. Culture-based techniques are more sensitive, but take weeks to obtain results because of the extremely slow growth rate of Mtb. Recently, nucleic acid-based diagnostic strategies were shown to have a higher sensitivity than that of sputum smear microscopy2–4. With a semi-automated DNA amplification system (Cepheid GeneXpert), it is thought that TB can be diagnosed with 98% reliability5. However, its high cost6 and requirement for a high technical competence to carry out the test prevent it from being widely available in resource-limited settings. In addition, the sensitivity of this test has not reached that of the gold standard culture, and it cannot evaluate bacterial viability, a critical aspect of evaluating therapeutic outcome. TB eradication efforts would be facilitated greatly by methods that can detect tubercle bacilli in a sensitive, rapid, specific and quantitative manner in vitro at a low cost, particularly in resource-limited settings where TB is the most prevalent.
Tubercle bacilli naturally express BlaC, an enzyme that belongs to the class A β-lactamase family7,8. Extended spectrum class A β-lactamases are capable of hydrolysing all classes of β-lactam substrates, including cephalosporins. The mechanism of cephalosporin hydrolysis by β-lactamases yields hydrolysed β-lactam and, more importantly, may be concomitant with the loss of a 3′ leaving group9–12. Based on this mechanism, a number of fluorogenic and bioluminogenic probes were developed for the detection of β-lactamase activity in vitro, in living cells and even in whole animals13–17. We developed cephalosphorin-based fluorogenic substrates that enable the sensitive detection of Mtb and bacillus Calmette–Guérin (BCG) in vitro and in living mice18. Previous probes lack specificity for BlaC in Mtb; the common TEM-1 β-lactamase (TEM-1 Bla) in gram-negative bacteria can also generate fluorescence, which reduces its utility for TB diagnosis. Earlier probes are generally large and display slow hydrolytic kinetics for BlaC. Here we report a rational design of a series of fluorescent probes based on chemically modified cephalosporins by taking advantage of the unique flexibility of the BlaC substrate-specificity loop. Enzymatic kinetic, structural analyses and whole-cell assays confirmed their high specificity and sensitivity for BlaC over its close class A homologue TEM-1 Bla as well as over β-lactamases produced by Pseudomonas, Staphylococcus and the environmental mycobacterium M. smegmatis. Successful detection and imaging of BCG directly in unprocessed patient sputum at levels of less than 100 bacilli are demonstrated, even with a simple, inexpensive imaging system of a cellular phone. This new BlaC-specific probe has potential for the clinical diagnosis of Mtb in patient sputum and other diagnostic specimens. Structural insights obtained from the BlaC acyl intermediates in this work should facilitate the development of more BlaC-specific probes for Mtb detection and imaging, and of BlaC-specific inhibitors to be used in anti-Mtb therapeutic regimens.
Results
Design of BlaC-specific fluorogenic probes
The substrate specificity of class A β-lactamases is conferred by Ambler residues (163–178) that comprise the omega loop, or substrate-specificity loop, and is partially dependent on the ability of the catalytic base, E166, to assume the optimal conformation for hydrolytic water coordination and deacylation19,20. To design a cephalosporin substrate specific for BlaC to achieve the specific detection of Mtb, we exploited the unique flexibility of the BlaC substrate-specificity loop by introducing bulky substitutions on the lactam. Such a cephalosporin may be accommodated by the BlaC substrate-specificity loop with the coordination of E166 still optimal for efficient deacylation.
We designed a series of fluorescent probes with substitutions on the side chain (R1) of the 7-amino group or the 7-position of the lactam ring (R2) (Fig. 1). Each probe contains the alkylated umbelliferone at the 3′-position and initially fluoresces little when excited at 400 nm. We hypothesized that these substituted substrates could be hydrolysed readily by BlaC and release free fluorophore to turn on the fluorescence, but their hydrolysis by other β-lactamases, such as TEM-1 Bla, would proceed with much slower kinetics. These blue fluorescent probes were synthesized21,22 as outlined in Supplementary Fig. S1 for CDC-1 and CDC-OMe, and unambiguously characterized (see the Supplementary Information).
Figure 1.
General structures of blue fluorescent probes and their hydrolysis by β-lactamase (Bla), which triggers the release of umbelliferone and turns on fluorescence.
Enzymatic kinetics for BlaC and TEM-1 Bla
We first recorded the fluorescent spectra of CDC-1, CDC-3, CDC-OMe and CDC-OEt before and after BlaC treatment to examine their responses to BlaC. With excitation at 400 nm, all the probes exhibited over a 100-fold increase in the fluorescence intensity at 455 nm after incubation with BlaC (Fig. 2a), but their reaction rates varied significantly (Fig. 2b and Supplementary Fig. S2). Under the same conditions, the hydrolytic rate by BlaC decreased in the order CDC-1 > CDC-OMe > CDC-OEt, which appears to correlate with the size of the R2 group. CDC-OMe showed more than a 30-fold enhancement of fluorescence intensity in less than 30 minutes. The larger substitution (ethoxy versus methoxy) provided CDC-OEt with only a slight fluorescence enhancement within 30 minutes. In comparison, the same concentration of TEM-1 Bla (50 nM) gave no change in the fluorescence intensity within 30 minutes with CDC-OMe, but produced rapid fluorescence with CDC-1 and CDC-3. These results demonstrate that CDC-OMe can preferentially detect BlaC over TEM-1 Bla.
Figure 2. Kinetic comparison of CDC probes with β-lactamases.
a, Fluorescent emission spectrum of CDC-OMe (1 μM in PBS) before and after treatment with BlaC (1 μM) (excitation, 400 nm). b, Time courses of fluorescent activation of CDC-1 and CDC-OMe with BlaC (filled circles) or TEM-1 Bla (open squares). c,d, Enhanced fluorescent intensity of CDC-OMe (c) and CDC-1 (d) (5 μM in PBS) by BlaC or TEM-1 Bla at the indicated concentrations for one hour. F/F0 represents the turn-on ratio of fluorescence intensity by β-lactamase. Error bars are±standard deviation (s.d.). a.u. = arbitrary units.
We next examined the response of CDC-OMe to varying concentrations of BlaC and TEM-1 Bla (in the range 1 pM to 100 nM) to investigate its specificity (Fig. 2c). BlaC (1 nM, 100 μl) induced a 130% increase in the fluorescence intensity within one hour (F/F0 = 2.3), but a 100-fold higher TEM-1 Bla concentration (100 nM) produced only a 30% fluorescence enhancement (F/F0 = 1.3). In contrast, TEM-1 Bla generated a stronger signal with CDC-1 than with BlaC (Fig. 2d).
The kinetic parameters of fluorescent probes for both BlaC and TEM-1 Bla, including the catalytic constant kcat and the Michaelis constant Km, were obtained from Lineweaver–Burk plots (Supplementary Fig. S3) and are summarized in Table 1. Consistent with the above observations, the kinetic efficiency (kcat/Km) of CDC-OMe for BlaC is 2.1 × 104 s−1 M−1, over 1,000 times higher than that for TEM-1 Bla (16 s−1 M−1). When an ethoxy group is introduced at the 7-position (CDC-OEt), no hydrolysis by TEM-1 Bla was detected, and the value of kcat/Km by BlaC decreased substantially to 1.9 × 102 s−1 M−1.
Table 1. Kinetic parameters of fluorescent probes for BlaC and TEM-1 Bla.
| Probe | BlaC | TEM-1 Bla | Spontaneous hydrolysis rate (×10−7 s−1) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Km (μM) | kcat (s−1) | kcat/Km (s−1 M−1) | Km (μM) | kcat (s−1) | kcat/Km (s−1 M−1) | |||
| 1 | CDC-1 | 63±6 | 13±0.5 | 2.1 × 105 | 135±16 | 48±3.8 | 3.6 × 105 | 2.4±0.3 |
| 2 | CDC-2 | 136±44 | 0.5±0.1 | 3.7 × 103 | 454±37 | 7±0.7 | 1.5 × 104 | 1.1±0.1 |
| 3 | CDC-3 | 69±7 | 6±0.2 | 8.7 × 104 | 59±10 | 77±6.6 | 1.3 × 106 | 1.8±0.2 |
| 4 | CDC-OMe | 47±9.4 | 1±0.1 | 2.1 × 104 | 50±2 | 8 ± 0.3 × 10−4 | 16 | 2.8±0.1 |
| 5 | CDC-OEt | 131±40 | 0.026±0.005 | 1.9 × 102 | ND | ND | ND | 2.3±0.2 |
| 6 | CDC-4 | 148±50 | 10±1.9 | 6.8 × 104 | 133±18 | 10±1 | 7.5 × 104 | 1.9±0.1 |
| 7 | CDC-5 | 139±22 | 0.52±0.05 | 3.6 × 103 | ND | ND | ND | 2.6±0.3 |
| 8 | CDG-1* | 2.1±0.3 (2±0.4) | 4±0.2 (1.0±0.1) | 1.9 × 106 (5 × 105) | 1.0±0.1 (2±0.2) | 9±0.4 (5±0.1) | 9 × 106 (2.5 × 106) | 3.3±0.1 (6.0±0.1) |
| 9 | CDG-OMe* | 3±0.3 (5±0.3) | 0.7±0.04 (0.8±0.01) | 2.3 × 105 (1.6 × 105) | 30±8 (40±4) | 1±0.2× 1023 (7±0.6 × 1024) | 33 (18) | 2.5±0.1 (1.9±0.1) |
Kinetic data were measured in PBS buffer (1×, pH = 7.4) at room temperature (22 °C) unless otherwise noted. All data indicate averages of three replicate experiments.
Data in parentheses were measured in MES buffer (0.1 M, pH 6.6) at 22 °C. ND = not determined due to extremely slow kinetics.
Additional structural modifications were explored for their effects on the enzymatic kinetics of BlaC and TEM-1 Bla. Probes with a large substitution group on the 7-amine position (R1), such as CDC-3, displayed less catalytic efficiency for BlaC (8.7 × 104 s−1 M−1) than for TEM-1 Bla (1.3 × 106 s−1 M−1). However, substitution by phenylacetyl or acetyl groups (for example, CDC-1 and CDC-4) at this position resulted in only a slight difference in the catalytic efficiency between BlaC and TEM-1 Bla (CDC-1, 2.1 × 105 versus 3.6 × 105 s−1 M−1; CDC-4, 6.8 × 104 versus 7.5 × 104 s−1 M−1, respectively). Oxidation of the sulfur into sulf-oxide (CDC-2) caused a decrease in the kinetic efficiency for both BlaC and TEM-1 Bla.
These kinetic analyses confirm CDC-OMe as a BlaC-specific fluorogenic probe with more than a 1,000-fold higher catalytic efficiency for BlaC than for TEM-1 Bla.
Analysis of the crystal structure
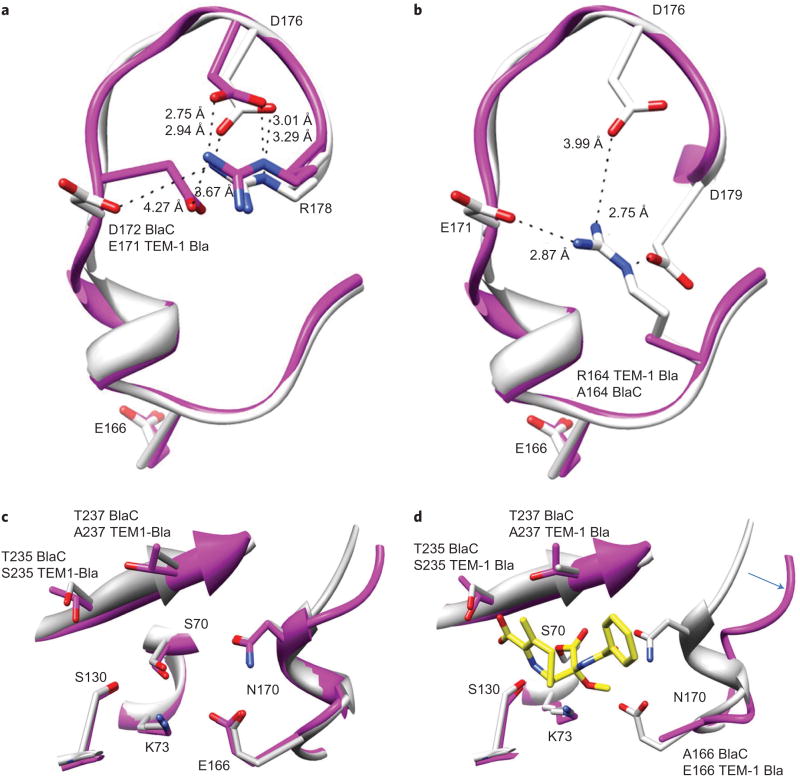
X-ray crystallographic structures of the acyl intermediate complex structures of CDC-OMe and CDC-1 with BlaC were obtained to probe the structural origin of the observed specificity of CDC-OMe for BlaC (Table 1). The substrate-specificity loops of TEM-1 Bla and BlaC share a 67% sequence identity over 20 residues (160–180). Conserved salt bridges in both enzymes formed by D172–R178 (E171–R178 in TEM-1 Bla) and D176–R178 stabilize the loop conformers of the unoccupied active sites (Fig. 3a)23,24.
Figure 3. Comparison of BlaC and TEM-1 Bla active sites and substrate-specificity loops.
a, Conserved salt bridges in both enzymes formed by D172–R178 and D176–R178 stabilize the loop conformers of the unoccupied active sites. b, TEM-1 Bla (1M40, white) R164 forms salt bridges with E171 (2.87 Å), D176 (3.99 Å) and D179 (2.75 Å). BlaC (2GDN, purple) A164 prevents these stabilizing interactions, which results in an increased flexibility of the loop and catalytic base E166. c, Superimposition of unoccupied TEM-1 Bla and BlaC active sites. d, Superimposition of unoccupied TEM-1 Bla (white) and the BlaC–CDC-OMe acyl intermediate complex (purple). The arrow indicates the movement of the substrate loop in BlaC on binding CDC-OMe.
The largest phenotypic difference between the two enzymes results from residue 164, which is an arginine in TEM-1 Bla and an alanine in BlaC. R164 plays a central role in the molecular dynamics of the substrate-specificity loop in TEM-1 Bla. The R164 guanidinium side-chain nitrogens (Nε, NH and NH2) stabilize a triad of carboxylates from the D176, D179 and E171 side chains with electrostatic interaction distances of 3.99 Å, 2.75 Å and 2.87 Å, respectively24, by forming three additional salt bridges (Fig. 3b)23,24.
A164 mutation results in the loss of these stabilizing salt bridges in BlaC, which ultimately increases the flexibility of the BlaC substrate-specificity loop relative to that of TEM-1 Bla. The increased flexibility is underscored by B-factors for residues that surround E166 (164–168), which are 108% of the mean B-factor in BlaC and 87% of the mean B-factor in TEM-1 Bla (refs 23,24). The structural plasticity of these residues allows the catalytic base E166 to sample multiple conformations and increases the capability of BlaC to hydrolyse substrates in the acyl intermediate state.
The acyl intermediate complex structures of BlaC–CDC-1 and BlaC–CDC-OMe exist in similar orientations, and share conserved electrostatic interactions with substrate-recognition residues T235, T237, S130 and S70 (Fig. 4). The phenyl groups in both CDC-1 and CDC-OMe are disordered as a result of the lack of active-site contacts and facing open to the solvent. In contrast, CDC-1 makes a unique hydrogen bond (2.76 Å) with the backbone carbonyl oxygen of T237. The methoxy group of CDC-OMe forms an electrostatic interaction with the side-chain amino group of K73 (3.29 Å). The orientation of the CDC-OMe 7′-phenylacetylamino substituent is rotated approximately 90° relative to the CDC-1 acyl intermediate (Fig. 4c). The CDC-OMe–K73 interaction positions the methoxy group directly in the path between the acyl bond and catalytic residue E166, which may interfere with the hydrolytic water coordination for deacylation and lead to a decreased turnover number for CDC-OMe (Table 1). Replacement of the methoxy group with a bulkier ethoxy group (CDC-OEt) causes a more serious occlusion, which leads to a further 40-fold reduction in turnover number (Table 1). However, these unique interactions in the CDC-OMe acyl intermediate accommodated by the BlaC flexible substrate-specificity loop confer the specificity of CDC-OMe for BlaC (Fig. 3c,d).
Figure 4. Active-site details of the BlaC-CDC-OMe (top) and BlaC–CDC-1 (bottom) acyl intermediate complexes.
a,b, Electrostatic surface potential (a) and cartoon diagrams (b) of acyl intermediates with FO − FC electron-density maps displayed in grey for CDC-OMe (2.0σ) and CDC-1 (1.5σ) prior to fitting each ligand for refinement protocols. Active-site residues are displayed as sticks. FO = observed amplitude; FC = calculated amplitude. c, Ligplot analysis of active-site interactions.
Detection and imaging of live mycobacteria
We first sought to evaluate the feasibility of CDC-OMe for the detection of BlaC expressed by Escherichia coli. CDC-OMe was incubated with the same number of E. coli that expressed no β-lactamase, TEM-1 Bla or BlaC (Supplementary Fig. S5) for two hours and a strong fluorescence was observed only with E. coli that expressed BlaC. As a control, the fluorescence for CDC-1 was present in E. coli that expressed either TEM-1 Bla or BlaC. This result demonstrates that CDC-OMe can detect BlaC specifically in intact E. coli. However, the sensitivity for detecting BlaC expressed by the Mtb var. bovis strain BCG is low because of the high background of BCG at the excitation and emission wavelengths of umbelliferone. Therefore, we replaced umbelliferone with the green fluorescent dye Tokyo Green (Fig. 5a). Tokyo Green allows a stable single-site attachment at its phenolic position25, but the direct coupling product with cephalosporin at the 3′-position, similar to that in CDC probes, displayed a 21-fold less stability (with a spontaneous hydrolysis rate of 7 × 10−6 s−1 in PBS) than that of CDC-OMe. A benzyl ether linker was thus introduced between the 3′-position of the lactam and Tokyo Green to increase its stability (Fig. 5a). The syntheses of green fluorogenic substrates CDG-1 and CDG-OMe are outlined in Supplementary Fig. S6.
Figure 5. β-Lactamase selectivity of green fluorescent probes CDG-1 and CDG-OMe.
a, BlaC hydrolyses green probes and turns on the fluorescence signal. b, Fluorescent emission spectrum of CDG-OMe (20 nM in MES) before and after treatment of BlaC (0.2 μM) (excitation, 490 nm). c, Time courses of fluorescence enhancement with CDG-OMe (8 μM in MES buffer) and various concentrations of β-lactamases. d, Enhanced fluorescence intensity of CDG-OMe (8 μM in MES, front) and CDG-1 (8 μM in MES, back) by serially diluted solutions of BlaC or TEM-1 Bla for eight hours. Data were collected in 384-well plates with a total volume of 25 μl in each well. e, Magnified view of the rectangular region in (d). ΔF represents the difference in fluorescence intensity with and without β-lactamase incubation. Data in (d) and (e) are the average of three replicate experiments. Error bars are±s.d.
On treatment with BlaC, the fluorescence emission of CDG-OMe at 520 nm increased by up to 218-fold (Fig. 5b). In addition to the gain in the stability of the probe obtained from the additional linker, the specificity of CDG-OMe for BlaC improved, with an 11-fold increase in kcat/Km to 2.3 × 105 s−1 M−1 in PBS (Table 1). Even at a more than 1,000-fold higher concentration, the fluorescence signal of CDG-OMe generated by TEM-1 Bla (400 nM) was just 30% of that generated by BlaC (400 pM) in 300 minutes, which confirms its high selectivity for BlaC (Fig. 5c). Less than 1 fmol of BlaC was readily detectable with CDG-OMe after eight hours of incubation (Fig. 5d). When TEM-1 Bla- and BlaC-expressing E. coli were incubated with CDG-1 and CDG-OMe, CDG-1 displayed no selectivity, but CDG-OMe showed excellent selectivity for BlaC over TEM-1 Bla (Supplementary Fig. S7).
We further tested the sensitivity and specificity of CDG-OMe for detecting bacteria present in raw unprocessed sputum samples obtained from cystic fibrosis patients. BCG or other bacteria that expressed β-lactamase, including E. coli, methicillin-resistant S. aureus (MRSA), P. aeruginosa strain PA01 and M. smegmatis, were incubated with CDG-OMe in human sputum. We could detect Mtb readily in sputum down to ten colony-forming units (c.f.u.) (P<0.05), which demonstrates the high sensitivity of this system for evaluating clinical samples (Fig. 6a). Fluorescence emission generated by 10 c.f.u. of BCG in sputum is significantly (P< 0.05) higher than fluorescence from the negative control and 105 c.f.u. of E. coli, MRSA, PA01 and M. smegmatis (Fig. 6b).
Figure 6. Sensitivity and specificity of CDG-OMe in raw, unprocessed human sputum.
a, Detection of BCG in sputum with CDG-OMe. b, Specificity of CDG-OMe for detecting BCG in sputum. 10 c.f.u. of BCG or 105 c.f.u. of the indicated β-lactamase-expressing bacteria were added to human sputum samples obtained from cystic fibrosis patients and incubated with CDG-OMe (2.5 μM) at room temperature for 160 minutes. Fluorescence was measured with excitation at 490 nm and emission at 535 nm. *P, 0.05 as compared to 10 c.f.u. of BCG. c, Imaging of BCG in sputum with CDG-OMe using a cellular phone. BCG-spiked sputum samples were incubated with CDG-OMe (5 μM) at room temperature for ten minutes before they were imaged in a simple handmade light box: light from an off-the-shelf LED assembly (>US$10.00) passed through a 490 nm excitation filter (bandpass 20 nm) was reflected through a 530 nm emission filter (bandpass 43 nm) and photographed with an iPhone 4S. The photograph was analysed with iPhotoLux v. 1.0. Error bars are ±s.d.
To investigate the applicability of this system in low-resource settings, we built a handmade box equipped with a simple LED light source, excitation filter and emission filter, and took a picture with a cell phone through a hole in the box (Supplementary Fig. S8). As shown in Fig. 6c, 10 c.f.u. of BCG in 200 μl of human sputum was detected readily within ten minutes of incubation with the probe, and the use of luminance mapping allowed facile optimization of image visualization. In summary, CDG-OMe demonstrates excellent sensitivity and specificity for detecting Mtb var. bovis strain BCG in clinical specimens, even using low-cost detection systems.
Discussion
One of the critical needs for improving the diagnosis of TB is to identify accurate biomarkers for active Mtb (ref. 26). Recently, the trehalose mycolyltransferase enzymes were utilized to incorporate unnatural trehalose analogues into Mtb for TB detection27. As it requires cell culturing for the probe incorporation, the process can take a long time because of the slow growth rate of Mtb. BlaC has the potential to serve here and has many advantages as a diagnostic marker. All TB complex bacteria examined displayed a strong signal dependent on β-lactamase, including BCG, TB laboratory strains and TB clinical isolates, with a total of more than 20 different strains. In addition, complete genome sequencing of more than 54 Mtb strains from numerous geographical regions, multidrug resistant and extensively drug-resistant strains demonstrates that BlaC is highly conserved in all Mtb clinical isolates, with only two non-synonymous single-nucleotide changes, but they still produce an active enzyme28. These observations are consistent with reports in the literature that indicate 100% (18 examined for Mtb, nine for M. bovis, five for M. africanum) of the strains within the TB complex and M. kansasii (17 examined) produce β-lactamase and are positive for enzymatic activity29. As BlaC is an enzyme, its activity promises great sensitivity for the detection of tubercle bacilli, as we have shown with fluorogenic substrates. However, previously available probes lack specificity for BlaC (ref. 18), which allows activation by other β-lactamases, such as TEM-1 Bla, commonly found in gram-negative bacteria30,31, which reduces their accuracy for TB diagnosis.
Extensive research is devoted to β-lactamases because of their role in rendering bacteria resistant to lactam antibiotics. However, most previous studies focused on designing molecules that are poor β-lactamase substrates32,33, in contrast to the goal of this study, to design substrate probes that have rapid kinetics and are selective for BlaC. We took advantage of the unique substrate-specificity loop of BlaC and explored its chemistry to design BlaC-specific fluorogenic substrates. The replacement of R164 in TEM-1 Bla by Ala results in the loss of stabilizing salt bridges in BlaC and thus an increase in the flexibility of the BlaC substrate-specificity loop. Therefore, it is more probable that the active site of BlaC accommodates chemical modifications on the lactam structure. Based on this structural insight, we explored substitutions at the 7-C position of the latcam ring. The introduced methoxy group is accommodated well in the pocket of BlaC, as revealed by the acyl intermediate structural complex. In this study, we examined the effect of modifications only on the 7-C position of the lactam ring, and it remains possible that other positions on the lactam structure may be modified to generate specificity for BlaC.
CDC-OMe showed excellent selectivity for BlaC over TEM-1 Bla, but low catalytic efficiency for BlaC (kcat/Km = 2.1 × 104 s−1 M−1), which requires long co-incubation times for a positive result. CDG-OMe has a much improved kinetic efficiency (an 11-fold increase of kcat/Km, 2.3 × 105 s−1 M−1) and still maintains high stability and selectivity for BlaC over TEM-1 Bla. The use of green fluorophore further enhanced its sensitivity for detecting live mycobacteria in solution.
Using a simple handmade device and a cell phone, we detected as low as 10 c.f.u. of BCG in human sputum, which not only demonstrates excellent sensitivity, but also suggests high specificity, because the cystic fibrosis patients from whom these samples were obtained commonly have chronic infection of S. aureus, Haemophilus influenza and P. aeruginosa34, which can express β-lactamase activity. Indeed, we found high levels of ampicillin-resistant bacteria in our sputum samples (Supplementary Fig. S9), but their presence did not impact selectivity or sensitivity.
In summary, we have developed a series of fluorogenic probes specific for Mtb by taking advantage of the uniquely flexible substrate-specificity loop of the BlaC enzyme expressed by tubercle bacilli. These probes are chemically modified cephalosporin lactams with a 7α-methoxy substitution. The acyl intermediate complexes of the E166A BlaC mutant and the probes were co-crystalized successfully for X-ray structure determination to reveal structural insights into the observed specificity of the probes for BlaC. A green fluorescent probe, CDG-OMe, enabled the successful detection and imaging, with high specificity, of live pathogen present at very low levels in patient sputum. With such a good sensitivity and specificity, it is highly promising that CDG-OMe could be developed easily into a rapid, low-cost TB diagnostic tool to meet the needs of people who live in remote, resource-limited areas. The success of this strategy may greatly contribute to decreasing Mtb-associated death rates and transmission.
Methods
Synthetic procedures and chemical characterizations of all the probes are given in the Supplementary Section. Fluorescence spectra were collected on a Fluoromax-3 spectrafluorometer (Jobin Yvon). Kinetic experiments were conducted in a M1000 microplate reader (TECAN, Research Triangle Park, North Carolina).
Cloning, expression and purification of BlaC
Wild-type BlaC was cloned from Mtb H37Rv genomic DNA as described previously23. The deacylation deficient E166A mutant was generated using the Quickchange site-directed mutagenesis kit (Stratagene no. 200519). Mutant and wild-type protein expression and purification were performed as described previously23.
Enzymatic kinetics
To a series of different concentrations of the probe (20, 40, 60, 80, 100, 120 and 150 μM)in1 × PBS (pH 7.4)in a 96-well plate (black and flat bottomed) was added TEM-1 Bla or BlaC. PBS was added to adjust the total volume to 100 μl. The fluorescence intensity at 454 nm was measured immediately in a microplate reader (excitation wavelength of 400 nm) over a period of 20-minutes at 22 °C. The values of the kinetic parameters (Km and kcat) were determined from the double-reciprocal plot of the hydrolysis rate versus the substrate concentration (Lineweaver–Burk plot). To determine the spontaneous hydrolysis rate of the probes, the fluorescence intensity was monitored over five days without the addition of enzyme. The rate was calculated from the plot of ln([S]0/([S]0 − [P])) versus time (Supplementary Fig. S4).
Crystallization
Crystals of wild-type and E166A BlaC were grown using the hanging-drop vapour-diffusion method35. BlaC was concentrated to 10 mg ml−1 and equilibrated overnight at 4 °C with the mother liquor (2.0 M NH4H2PO4, 0.1 M Tris buffer, pH 8.0) in a 1:1 ratio (protein:mother liquor). The solution was centrifuged for ten minutes at 13,000 revolutions per minute to remove insoluble precipitate. Hanging drops were set up and equilibrated against 1 ml of mother liquor. Microseeding with horsehair was sufficient to produce large diffraction-quality crystals. Crystals were transferred to a stabilization solution that contained 30% glycerol in the mother liquor and subsequently soaked with substrates for 2–4 hours. The concentration of substrate was increased slowly by transferring the crystals to successive drops to prevent cracking. Derivatized crystals were flash frozen in liquid nitrogen.
Data collection and processing
X-ray diffraction data were collected on beam lines 19ID and 23ID at the Advanced Photon Source, Argonne National Laboratory, Argonne, Illinois. Data sets were reduced using HKL3000 (ref. 36). Data were collected at a wavelength of 0.97 Å and a temperature of 120K.
Structure determination
Initial phases were obtained by molecular replacement using the CCP4 suite38 and 2GDN as a search model23. Each data set was refined against the resulting model, and iterative cycles of model building and refinement were performed with Coot 0.6.1 (ref. 39) and PHENIX (ref. 40). Protein Data Bank codes for BlaC–CDC-1 and BlaC–CDC-OMe acyl intermediate crystal structures are 3VFH and 3VFF, respectively.
Detection of mycobacteria in sputum
Mtb var. bovis strain BCG was cultured in 7H9 medium with a 10% oleic acid albumin dextrose complex (OADC) and 0.25% Tween-80 until it reached the log phase (optical density at 600 nm (OD600) of 0.5–1). E. coli, MRSA, P. aeruginosa strain PA01 and M. smegmatis were cultured in Luria–Bertani medium until OD600 = 0.5–1. After measuring the bacterial OD600, 107 c.f.u. of each bacterial strain was added into Eppendorf tubes. Bacteria were centrifuged, the supernatant removed and resuspended into the same medium (7H9 medium with 10% OADC) to normalize the autofluorescence from different media. A series of tenfold dilutions of BCG were made. Bacteria were then incubated at 37 °C to allow β-lactamase production for 0.3–0.5 bacterial generations, using a 20 hour calculated generation time for BCG, a two hour calculated generation time for M. smegmatis and a 20 minute calculated generation time for Pseudomonas, Staphylococcus and E. coli. Then 10 μl of bacteria, 40 μl of sputum and 50 μl of CDG-OMe (5 μM) were mixed in a 96-well plate, incubated at room temperature for 160 minutes and the fluorescence read with a spectrometer (Mithras LB 940, Berthold Technologies, Oakridge, Tennessee) at 490 nm excitation and 535 nm emission.
For cellular phone imaging, the handmade imaging box consisted of a light-tight box, true cyan colour light-emitting diode (LED) with a peak emission at 505–510 nm, an excitation filter (490±10 nm) between the LED and sample, and an emission filter (530 nm, bandpass 43 nm) between the sample and an imaging hole in the box (Supplementary Fig. S8). BCG (10 μl), sputum (90 μl) and CDG-OMe (10 μM, 100 μl) were mixed, and after ten minutes samples were photographed with an Apple iPhone 4S through a hole in the box. The photograph was then analysed for luminance (cd m−2) using iPhotoLux v.1.0 (Maxime Bombrun, ISIMA, France), a free iPhone application.
Supplementary Material
Acknowledgments
This work was supported by grant 48523 from the Bill and Melinda Gates Foundation, the Welch Foundation grant no. A-0015 and NIH P01 68135 for TB Structural Genomics. We thank Bob Fader in the Scott & White Memorial Hospital (Temple, Texas) for providing sputum samples from cystic fibrosis patients.
Footnotes
Author contributions: H.X. performed all the compound syntheses and characterizations, collected enzymatic kinetics and carried out the E. coli imaging. J.M. performed the crystallization and structural studies and analysed the data. Y.K., M.H.C. and H.A.H. performed the testing with BCG in human sputum. C.N.T. contributed the imaging box used for cellular phone imaging. H.X., J.M., Y.K., J.C.S., J.D.C. and J.R. conceived and designed the experiments. All authors discussed the results and commented on the manuscript. H.X., J.M., Y.K., J.C.S., J.D.C. and J.R. co-wrote the paper.
Additional information: Supplementary information and chemical compound information are available in the online version of the paper.
Reprints and permission information is available online at http://www.nature.com/reprints
Competing financial interests: Stanford and Texas A&M has filed patent protection on some of the work described in the manuscript, and Global BioDiagnostics Corp. has licensed this technology. J.D.C., C.N.T. and J.R. hold stocks or stock options with Global BioDiagnostics Corp.
References
- 1.Dye C, et al. Measuring tuberculosis burden, trends, and the impact of control programmes. Lancet Infect Dis. 2008;8:233–243. doi: 10.1016/S1473-3099(07)70291-8. [DOI] [PubMed] [Google Scholar]
- 2.Dinnes J, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess. 2007;11:1–196. doi: 10.3310/hta11030. [DOI] [PubMed] [Google Scholar]
- 3.Ling DI, Flores LL, Riley LW, Pai M. Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-regression. PLoS One. 2008;3:e1536. doi: 10.1371/journal.pone.0001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greco S, Girardi E, Navarra A, Saltini C. Current evidence on diagnostic accuracy of commercially based nucleic acid amplification tests for the diagnosis of pulmonary tuberculosis. Thorax. 2006;61:783–790. doi: 10.1136/thx.2005.054908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehme CC, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes R, Wonderling D, Li B, Higgins B. The cost effectiveness of nucleic acid amplification techniques for the diagnosis of tuberculosis. Respir Med. 2012;106:300–307. doi: 10.1016/j.rmed.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Flores AR, Parsons LM, Pavelka MS., Jr Genetic analysis of the betalactamases of Mycobacterium tuberculosis and Mycobacterium smegmatis and susceptibility to beta-lactam antibiotics. Microbiology. 2005;151:521–532. doi: 10.1099/mic.0.27629-0. [DOI] [PubMed] [Google Scholar]
- 8.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE, II, Blanchard JS. Meropenem–clavulanate is effective against extensively drug-resistant. Mycobacterium tuberculosis Science. 2009;323:1215–1218. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd DB, Lunn WH. Electronic structures of cephalosporins and penicillins. 9. Departure of a leaving group in cephalosporins. J Med Chem. 1979;22:778–784. doi: 10.1021/jm00193a006. [DOI] [PubMed] [Google Scholar]
- 10.Faraci WS, Pratt RF. Elimination of a good leaving group from the 3′ -position of a cephalosporin need not be concerted with beta-lactam ring-opening – TEM-2 beta-lactamase-catalyzed hydrolysis of pyridine-2-azo-4′-(N′,N′-dimethylaniline) cephalosporin (PADAC) and of cephaloridine. J Am Chem Soc. 1984;106:1489–1490. [Google Scholar]
- 11.Faraci WS, Pratt RF. Mechanism of inhibition of the PC1 beta-lactamase of Staphylococcus aureus by cephalosporins: importance of the 3′-leaving group. Biochemistry. 1985;24:903–910. doi: 10.1021/bi00325a014. [DOI] [PubMed] [Google Scholar]
- 12.Pratt RF, Faraci WS. Direct observation by proton NMR of cephalosporoate intermediates in aqueous solution during the hydrazinolysis and beta-lactamase-catalyzed hydrolysis of cephalosporins with 3′ leaving groups: kinetics and equilibria of the 3′ elimination reaction. J Am Chem Soc. 1986;108:5328–5333. [Google Scholar]
- 13.Zlokarnik G, et al. Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Science. 1998;279:84–88. doi: 10.1126/science.279.5347.84. [DOI] [PubMed] [Google Scholar]
- 14.Gao W, Xing B, Tsien RY, Rao J. Novel fluorogenic substrates for imaging beta-lactamase gene expression. J Am Chem Soc. 2003;125:11146–11147. doi: 10.1021/ja036126o. [DOI] [PubMed] [Google Scholar]
- 15.Xing B, Khanamiryan A, Rao J. Cell-permeable near-infrared fluorogenic substrates for imaging beta-lactamase activity. J Am Chem Soc. 2005;127:4158–4159. doi: 10.1021/ja042829+. [DOI] [PubMed] [Google Scholar]
- 16.Yao H, So MK, Rao J. A bioluminogenic substrate for in vivo imaging of beta-lactamase activity. Angew Chem Int Ed. 2007;46:7031–7034. doi: 10.1002/anie.200701931. [DOI] [PubMed] [Google Scholar]
- 17.Rukavishnikov A, Gee KR, Johnson I, Corry S. Fluorogenic cephalosporin substrates for beta-lactamase TEM-1. Anal Biochem. 2011;419:9–16. doi: 10.1016/j.ab.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Kong Y, et al. Imaging tuberculosis with endogenous beta-lactamase reporter enzyme fluorescence in live mice. Proc Natl Acad Sci USA. 2010;107:12239–12244. doi: 10.1073/pnas.1000643107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee S, Pieper U, Kapadia G, Pannell LK, Herzberg O. Role of the Omega-loop in the activity, substrate specificity, and structure of class A beta-lactamase. Biochemistry. 1998;37:3286–3296. doi: 10.1021/bi972127f. [DOI] [PubMed] [Google Scholar]
- 20.Knox JR. Extended-spectrum and inhibitor-resistant TEM-type beta-lactamases – mutations, specificity, and 3-dimensional structure. Antimicrob Agents Chemother. 1995;39:2593–2601. doi: 10.1128/aac.39.12.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrecht HA, et al. Cephalosporin 3′-quinolone esters with a dual mode of action. J Med Chem. 1990;33:77–86. doi: 10.1021/jm00163a013. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin JE, Urban FJ, Cooper RDG, Jose FL. Direct 6-methoxylation of penicillin derivatives – convenient pathway to substituted beta-lactam antibiotics. J Am Chem Soc. 1973;95:2401–2403. doi: 10.1021/ja00788a071. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Cassidy C, Sacchettini JC. Crystal structure and activity studies of the Mycobacterium tuberculosis beta-lactamase reveal its critical role in resistance to beta-lactam antibiotics. Antimicrob Agents Chemother. 2006;50:2762–2771. doi: 10.1128/AAC.00320-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minasov G, Wang XJ, Shoichet BK. An ultrahigh resolution structure of TEM-1 beta-lactamase suggests a role for Glu166 as the general base in acylation. J Am Chem Soc. 2002;124:5333–5340. doi: 10.1021/ja0259640. [DOI] [PubMed] [Google Scholar]
- 25.Urano Y, et al. Evolution of fluorescein as a platform for finely tunable fluorescence probes. J Am Chem Soc. 2005;127:4888–4894. doi: 10.1021/ja043919h. [DOI] [PubMed] [Google Scholar]
- 26.McNerney R, Daley P. Towards a point-of-care test for active tuberculosis: obstacles and opportunities. Nature Rev Microbiol. 2011;9:204–213. doi: 10.1038/nrmicro2521. [DOI] [PubMed] [Google Scholar]
- 27.Backus KM, et al. Uptake of unnatural trehalose analogs as a reporter for Mycobacterium tuberculosis. Nature Chem Biol. 2011;7:228–235. doi: 10.1038/nchembio.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioerger TR, et al. The non-clonality of drug resistance in Beijing-genotype isolates of Mycobacterium tuberculosis from the Western Cape of South Africa. BMC Genomics. 2010;11:670. doi: 10.1186/1471-2164-11-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon HH, Tomioka H, Saito H. Distribution and characterization of beta-lactamases of mycobacteria and related organisms. Tuber Lung Dis. 1995;76:141–148. doi: 10.1016/0962-8479(95)90557-x. [DOI] [PubMed] [Google Scholar]
- 30.Majiduddin FK, Materon IC, Palzkill TG. Molecular analysis of beta-lactamase structure and function. Int J Med Microbiol. 2002;292:127–137. doi: 10.1078/1438-4221-00198. [DOI] [PubMed] [Google Scholar]
- 31.Petrosino J, Cantu C, III, Palzkill T. β-Lactamases: protein evolution in real time. Trends Microbiol. 1998;6:323–327. doi: 10.1016/s0966-842x(98)01317-1. [DOI] [PubMed] [Google Scholar]
- 32.Hugonnet JE, Blanchard JS. Irreversible inhibition of the Mycobacterium tuberculosis beta-lactamase by clavulanate. Biochemistry. 2007;46:11998–12004. doi: 10.1021/bi701506h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremblay LW, Fan F, Blanchard JS. Biochemical and structural characterization of Mycobacterium tuberculosis beta-lactamase with the carbapenems ertapenem and doripenem. Biochemistry. 2010;49:3766–3773. doi: 10.1021/bi100232q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell RS, Kumar V, Robbins SL, Abbas AK, Fausto N. Robbins Basic Pathology. Saunders/Elsevier; 2007. [Google Scholar]
- 35.McPherson A. Preparation and Analysis of Protein Crystals. Waverly: 1982. [Google Scholar]
- 36.Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: the integration of data reduction and structure solution – from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- 37.Mccoy AJ, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey S. The CCP4 suite – programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 39.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.