Abstract
Digitonin-permeabilized chromaffin cells secrete catecholamines by exocytosis in response to micromolar Ca2+ concentrations, but lose the ability to secrete in response to Ca2+ as the cells lose soluble proteins through the plasma membrane pores. We have previously shown [Morgan and Burgoyne (1992) Nature, 355, 833-836] that cytosol can retard this loss of secretory competence and that two distinct stimulatory activities (Exo1 and Exo2) are present in cytosol. Here we report that Exo2 behaved as a single peak of activity through purification on hydroxyapatite, ammonium sulfate precipitation and gel filtration and the activity correlated with a single polypeptide of approximately 44 kDa on SDS gels. Protein sequencing of this band revealed it to be the catalytic subunit of cyclic AMP-dependent protein kinase (PKA). Both cyclic AMP and the commercially available catalytic subunit of PKA stimulated exocytosis in a dose-dependent manner which was absolutely dependent on the presence of micromolar Ca2+. These data show that PKA (Exo2) regulates Ca(2+)-dependent exocytosis in bovine adrenal chromaffin cells.
Full text
PDF


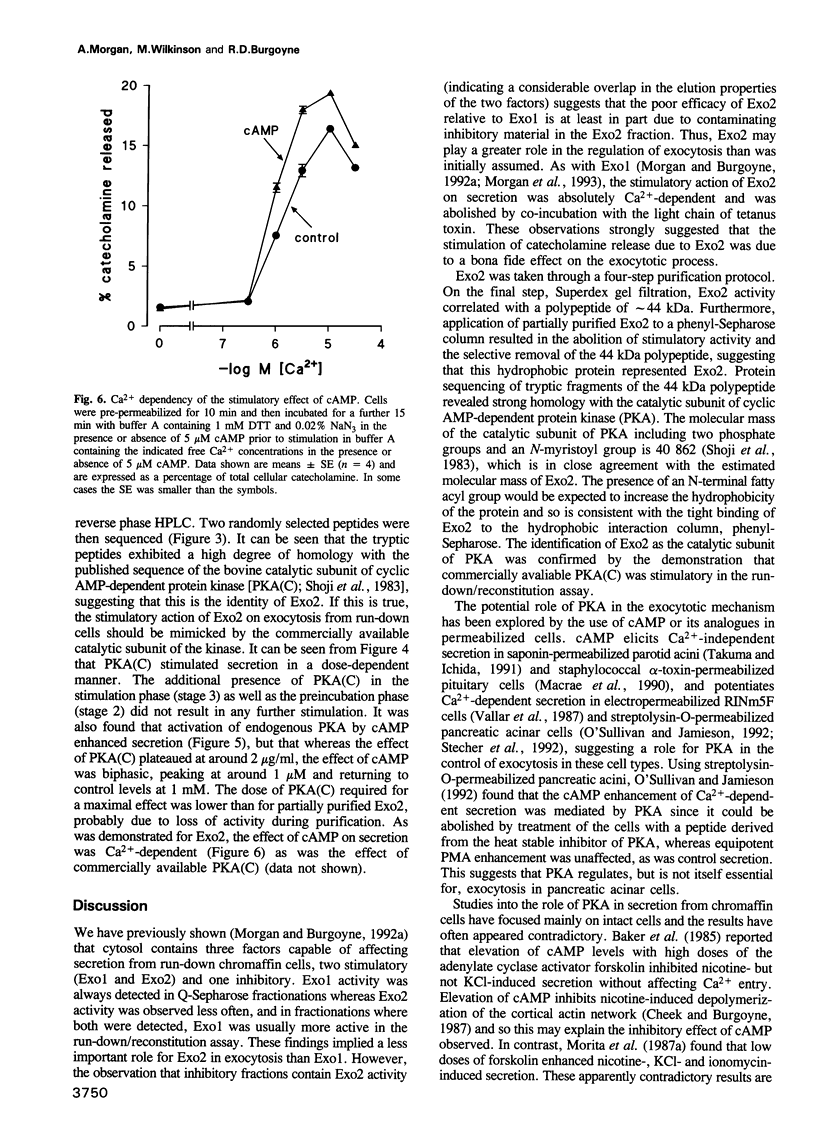


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. M., Burgoyne R. D. The stimulatory effect of calpactin (annexin II) on calcium-dependent exocytosis in chromaffin cells: requirement for both the N-terminal and core domains of p36 and ATP. Cell Signal. 1990;2(3):265–276. doi: 10.1016/0898-6568(90)90054-e. [DOI] [PubMed] [Google Scholar]
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Anderson K., Robinson P. J., Marley P. D. Cholinoceptor regulation of cyclic AMP levels in bovine adrenal medullary cells. Br J Pharmacol. 1992 Jun;106(2):360–366. doi: 10.1111/j.1476-5381.1992.tb14341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):83–103. doi: 10.1098/rstb.1981.0174. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 1978 Dec 7;276(5688):620–622. doi: 10.1038/276620a0. [DOI] [PubMed] [Google Scholar]
- Bittner M. A., Holz R. W., Neubig R. R. Guanine nucleotide effects on catecholamine secretion from digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Aug 5;261(22):10182–10188. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991 Jul 22;1071(2):174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. A major role for protein kinase C in calcium-activated exocytosis in permeabilised adrenal chromaffin cells. FEBS Lett. 1988 Sep 26;238(1):151–155. doi: 10.1016/0014-5793(88)80246-1. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Burgoyne R. D. Cyclic AMP inhibits both nicotine-induced actin disassembly and catecholamine secretion from bovine adrenal chromaffin cells. J Biol Chem. 1987 Aug 25;262(24):11663–11666. [PubMed] [Google Scholar]
- Cheek T. R., Burgoyne R. D. Effect of activation of muscarinic receptors on intracellular free calcium and secretion in bovine adrenal chromaffin cells. Biochim Biophys Acta. 1985 Jul 30;846(1):167–173. doi: 10.1016/0167-4889(85)90122-3. [DOI] [PubMed] [Google Scholar]
- Greenberg A., Zinder O. Alpha- and beta-receptor control of catecholamine secretion from isolated adrenal medulla cells. Cell Tissue Res. 1982;226(3):655–665. doi: 10.1007/BF00214792. [DOI] [PubMed] [Google Scholar]
- Hay J. C., Martin T. F. Resolution of regulated secretion into sequential MgATP-dependent and calcium-dependent stages mediated by distinct cytosolic proteins. J Cell Biol. 1992 Oct;119(1):139–151. doi: 10.1083/jcb.119.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh R., Marley P. D. Regulation of cyclic AMP levels by calcium in bovine adrenal medullary cells. J Neurochem. 1991 Nov;57(5):1721–1728. doi: 10.1111/j.1471-4159.1991.tb06373.x. [DOI] [PubMed] [Google Scholar]
- Kish P. E., Ueda T. Calcium-dependent release of accumulated glutamate from synaptic vesicles within permeabilized nerve terminals. Neurosci Lett. 1991 Jan 28;122(2):179–182. doi: 10.1016/0304-3940(91)90852-k. [DOI] [PubMed] [Google Scholar]
- Koffer A., Gomperts B. D. Soluble proteins as modulators of the exocytotic reaction of permeabilised rat mast cells. J Cell Sci. 1989 Nov;94(Pt 3):585–591. doi: 10.1242/jcs.94.3.585. [DOI] [PubMed] [Google Scholar]
- Lomneth R., Martin T. F., DasGupta B. R. Botulinum neurotoxin light chain inhibits norepinephrine secretion in PC12 cells at an intracellular membranous or cytoskeletal site. J Neurochem. 1991 Oct;57(4):1413–1421. doi: 10.1111/j.1471-4159.1991.tb08308.x. [DOI] [PubMed] [Google Scholar]
- Macrae M. B., Davidson J. S., Millar R. P., van der Merwe P. A. Cyclic AMP stimulates luteinizing-hormone (lutropin) exocytosis in permeabilized sheep anterior-pituitary cells. Synergism with protein kinase C and calcium. Biochem J. 1990 Nov 1;271(3):635–639. doi: 10.1042/bj2710635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley P. D., Thomson K. A., Jachno K., Johnston M. J. Histamine-induced increases in cyclic AMP levels in bovine adrenal medullary cells. Br J Pharmacol. 1991 Dec;104(4):839–846. doi: 10.1111/j.1476-5381.1991.tb12515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott D., Adams M., Boarder M. R. Effect of forskolin and prostaglandin E1 on stimulus secretion coupling in cultured bovine adrenal chromaffin cells. J Neurochem. 1988 Feb;50(2):616–623. doi: 10.1111/j.1471-4159.1988.tb02955.x. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Walent J. H. A new method for cell permeabilization reveals a cytosolic protein requirement for Ca2+ -activated secretion in GH3 pituitary cells. J Biol Chem. 1989 Jun 15;264(17):10299–10308. [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Exo1 and Exo2 proteins stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. Nature. 1992 Feb 27;355(6363):833–836. doi: 10.1038/355833a0. [DOI] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Interaction between protein kinase C and Exo1 (14-3-3 protein) and its relevance to exocytosis in permeabilized adrenal chromaffin cells. Biochem J. 1992 Sep 15;286(Pt 3):807–811. doi: 10.1042/bj2860807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Dohi T., Kitayama S., Koyama Y., Tsujimoto A. Enhancement of stimulation-evoked catecholamine release from cultured bovine adrenal chromaffin cells by forskolin. J Neurochem. 1987 Jan;48(1):243–247. doi: 10.1111/j.1471-4159.1987.tb13154.x. [DOI] [PubMed] [Google Scholar]
- Morita K., Dohi T., Kitayama S., Koyama Y., Tsujimoto A. Stimulation-evoked Ca2+ fluxes in cultured bovine adrenal chromaffin cells are enhanced by forskolin. J Neurochem. 1987 Jan;48(1):248–252. doi: 10.1111/j.1471-4159.1987.tb13155.x. [DOI] [PubMed] [Google Scholar]
- Nishizaki T., Walent J. H., Kowalchyk J. A., Martin T. F. A key role for a 145-kDa cytosolic protein in the stimulation of Ca(2+)-dependent secretion by protein kinase C. J Biol Chem. 1992 Nov 25;267(33):23972–23981. [PubMed] [Google Scholar]
- O'Sullivan A. J., Jamieson J. D. Protein kinase A modulates Ca(2+)- and protein kinase C-dependent amylase release in permeabilized rat pancreatic acini. Biochem J. 1992 Oct 15;287(Pt 2):403–406. doi: 10.1042/bj2870403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J., Capdevielle J., Guillemot J. C., Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992 May 15;203(1):173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Sarafian T., Aunis D., Bader M. F. Loss of proteins from digitonin-permeabilized adrenal chromaffin cells essential for exocytosis. J Biol Chem. 1987 Dec 5;262(34):16671–16676. [PubMed] [Google Scholar]
- Sarafian T., Pradel L. A., Henry J. P., Aunis D., Bader M. F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol. 1991 Sep;114(6):1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Ericsson L. H., Walsh K. A., Fischer E. H., Titani K. Amino acid sequence of the catalytic subunit of bovine type II adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1983 Jul 19;22(15):3702–3709. doi: 10.1021/bi00284a025. [DOI] [PubMed] [Google Scholar]
- Sontag J. M., Thierse D., Rouot B., Aunis D., Bader M. F. A pertussis-toxin-sensitive protein controls exocytosis in chromaffin cells at a step distal to the generation of second messengers. Biochem J. 1991 Mar 1;274(Pt 2):339–347. doi: 10.1042/bj2740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B., Ahnert-Hilger G., Weller U., Kemmer T. P., Gratzl M. Amylase release from streptolysin O-permeabilized pancreatic acinar cells. Effects of Ca2+, guanosine 5'-[gamma-thio]triphosphate, cyclic AMP, tetanus toxin and botulinum A toxin. Biochem J. 1992 May 1;283(Pt 3):899–904. doi: 10.1042/bj2830899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma T., Ichida T. Cyclic AMP antagonist Rp-cAMPS inhibits amylase exocytosis from saponin-permeabilized parotid acini. J Biochem. 1991 Aug;110(2):292–294. doi: 10.1093/oxfordjournals.jbchem.a123573. [DOI] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]
- Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J Biol Chem. 1987 Apr 15;262(11):5049–5056. [PubMed] [Google Scholar]
- Walent J. H., Porter B. W., Martin T. F. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992 Sep 4;70(5):765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- Wu Y. N., Vu N. D., Wagner P. D. Anti-(14-3-3 protein) antibody inhibits stimulation of noradrenaline (norepinephrine) secretion by chromaffin-cell cytosolic proteins. Biochem J. 1992 Aug 1;285(Pt 3):697–700. doi: 10.1042/bj2850697. [DOI] [PMC free article] [PubMed] [Google Scholar]





