Significance
Learning is one of the most basic phenomena in the behavioral sciences. Animals learn some things better than others, and understanding what constrains this basic process is fundamental to our understanding of learning. Our paper applies an evolutionary approach to this question. We offer a simple model that considers the fitness of value of “prepared learning,” and we test this model using experimental evolution. In doing so, we created different lines of Drosophila that are prepared to learn from different experiences. To the best of our knowledge this is the first mathematical model explaining why some associations are learned more easily than others and to our knowledge is the first time that the evolution of prepared learning has been demonstrated experimentally.
Abstract
Animals learn some things more easily than others. To explain this so-called prepared learning, investigators commonly appeal to the evolutionary history of stimulus–consequence relationships experienced by a population or species. We offer a simple model that formalizes this long-standing hypothesis. The key variable in our model is the statistical reliability of the association between stimulus, action, and consequence. We use experimental evolution to test this hypothesis in populations of Drosophila. We systematically manipulated the reliability of two types of experience (the pairing of the aversive chemical quinine with color or with odor). Following 40 generations of evolution, data from learning assays support our basic prediction: Changes in learning abilities track the reliability of associations during a population’s selective history. In populations where, for example, quinine–color pairings were unreliable but quinine–odor pairings were reliable, we find increased sensitivity to learning the quinine–odor experience and reduced sensitivity to learning quinine–color. To the best of our knowledge this is the first experimental demonstration of the evolution of prepared learning.
For animals to learn, they must form associations among various stimuli. However, in a world full of potential stimuli, why does a special relationship form between a given stimulus and consequence in a way that actually allows the animal to predict future events? Animals seem to solve this problem by being born better able to learn some things than others. The most notable example of this special learning is the Garcia effect, published in one of the most influential papers in the history of animal learning (1). This paper showed that rats are prepared to learn some associations (e.g., taste and gastric illness) and less well prepared to learn others (e.g., light–sound combinations and gastric illness). In its day, this evidence was seen as both important and controversial, because it challenged the prevailing claims about the generality of the learning process [specifically the idea of equipotentiality (e.g., 2–6)]. We now have many examples of preparedness in learning (e.g., 5–8), although the terms used to describe this phenomenon have varied widely. Investigators have called this “belongingness” (9), species-specific defense reactions (10), biological constraints (e.g., 5, 11), adaptive specializations (8), and “preparedness” (4, 12). In response, learning theorists have advocated more general theories of learning that acknowledge an element of biological preparedness in nearly all learning (13–17).
Investigators seem to agree that the explanation of preparedness must flow from evolution. Evolution by natural selection, the argument goes, has prepared animals to learn from some associations better than others because these associations had predictive power in the animal’s evolutionary past. However, within this agreed framework, explanations of specific examples of prepared learning tend to be post hoc and glib, in that we identify the “predictive power” of specific associations only after investigators have identified an example of prepared learning. Taste obviously predicts the onset of gastric illness more reliably than flashing lights, after we have Garcia's result in hand. In response to this unsatisfying situation, several authors have argued that the study of preparedness needs a clear-cut predictive theory (3, 18, 19). Without such a predictive theory to guide them, investigators seem to have lost interest in further empirical studies of preparedness (see refs. 20–23 for possible exceptions to this pattern).
Even with an agreed conceptual framework in hand, studies of the evolution of preparedness face a significant empirical hurdle. The problem is that any model of the evolution of preparedness will flow from properties of the environment that the animal’s ancestors experienced during the course of evolution. How can we know empirically whether tastes have reliably predicted gastric illness since the Paleozoic? A meaningful test of claims like this would seem to require a time machine.
This paper addresses these two problems directly. First, it offers a simple model that formalizes existing ideas about the evolution of prepared learning in terms of measureable and experimentally accessible variables. Second, and most important, it uses the techniques of experimental evolution to create a selective environment in which stimuli, actions, and consequences have specific and controlled statistical relationships as our model of preparedness requires.
Experimental Preparation and Model
This section outlines the experimental selection regime and then offers a simple model that predicts the effects of selection on prepared learning in this situation. Our preparation creates an environment in which female Drosophila must choose between two places to lay their eggs which we call A and B (media types). This choice has fitness consequences because half the time the investigator only rears eggs from A, and the investigator only rears eggs from B half the time. Before the flies must make this choice, the procedure provides them with an experience that can signal the correct action. The investigator engineers this by exposing the flies to A and B in a preexposure or experience phase. In this experience phase, the investigator pairs one of the media types with the aversive chemical quinine (Q). In this experience phase the flies can experience Q paired with A and B alone or A alone and Q paired with B. If the pairing with quinine reliably indicates the fitness consequences of egg-laying in the second stage, then selection should favor aversion learning, such that flies avoid in stage two the medium type that was paired with quinine in stage one (see refs. 24, 25, and 26 for examples of this preparation).
In this case, however, we want to generate selection that will favor learning from some associations but not others. To achieve this, we imagine that our two media types (labeled A and B above) vary in two distinct dimensions, so that, for example, the two media types differ in both an odor and a color. In this case, we can imagine that in some situations the pairing of quinine with color might be the best predictor of which type to avoid, whereas in others the pairing of quinine with odor might be most effective. We can represent this algebraically by introducing two conditional probabilities. Let C be the reliability of the color–quinine association, which we define as the probability that the investigator will rear eggs from the substrate with the color that was not paired with quinine in the experience phase, and similarly, let O be the reliability of the odor–quinine association.
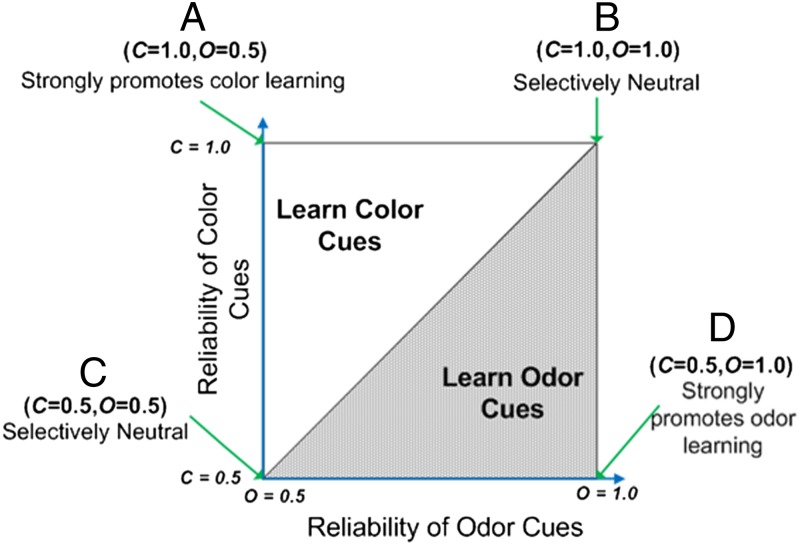
Now consider the fitness consequences of two alternative learning strategies: (i) learning in response to the color–quinine association (“color learning” for short) or (ii) learning in response to the odor–quinine association (“odor learning”). Recall that by color learning, we mean avoiding in stage two the color that was paired with quinine in stage one, whereas odor learning would imply avoiding the odor that was previously paired with quinine. It is a relatively simple, if tedious matter, to calculate the geometric mean fitness of these two learning tactics, but the results of these calculations are simple and intuitive (details and tables in SI Appendix). If the reliability of odor exceeds the reliability of color (O > C), then learning to odor will produce the higher fitness. Fig. 1 shows this result diagrammatically. To be specific, then we would predict the learning to color but not odor when the reliability of color is high (C = 1.0) and the reliability of odor is low (O = 0.5).
Fig. 1.
Predictions from the model. Whenever the reliability of odor cues is greater than the reliability of color cues (O > C), then learning about odor is favored. Reliability is the probability that the quinine pairing with a cue predicts fitness consequences (specifically, where not to lay eggs). We tested the points at the four corners of the graph. Two of these points fall on the line between color learning favored and odor learning favored, and thus either or both could be favored. Intuitively, we predict that when both modalities of stimuli predict equally well, learning about both should be favored, whereas learning about neither should be favored when neither modality of stimuli predict the best environment.
In the experiment presented here, we create four distinct selective environments as suggested by Fig. 1. These environments are the obvious factorial combination of high and low color reliability combined with high and low odor reliability. We predict that selection will favor sensitivity to learning experienced associations that reliably predict fitness consequences and not to those that are unreliable. We tested the predictions of the extreme values on the figure, points A through D, by assigning 10 populations of flies to each type of world and allowing them to evolve for 40 generations.
Results
Dependent Measures.
We counted the number of eggs laid on each substrate type in the second or consequence phase in each generation of selection. Using these data we could calculate the extent to which the flies avoided the color or odor that had been paired with quinine in the first or “experience” phase. The results of these calculations are two dependent measures that we call P(Learn: Color) and P(Learn: Odor). We define P(Learn: Color) to be the proportion of eggs laid on the substrate with the color that was not paired with quinine in the experience phase. Similarly we define P(Learn: Odor) to be the proportion of eggs laid on the substrate with the odor that was not paired with quinine in the experience phase.
We have two sources of data. First, we collected data about the proportional choice of substrates during the selections. These data provide information about the changes that occurred between the starting and ending selections and reflect the variation inherent in differing combinations of stimuli and quinine pairings (see SI Appendix for details). Second, we conducted assays at the end of the experiment, following 40 generations of selections, in which we paired quinine with color and odor separately, allowing each line to be tested under identical conditions. We consider these two types of data in turn.
Selection Data.
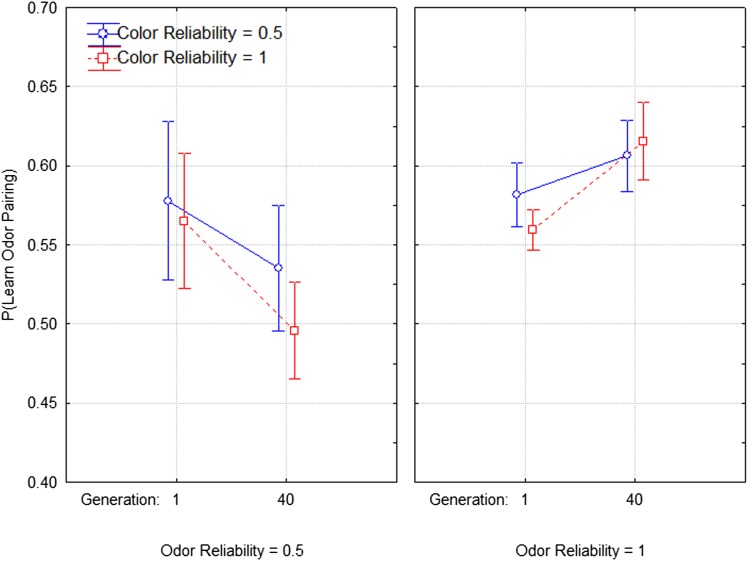
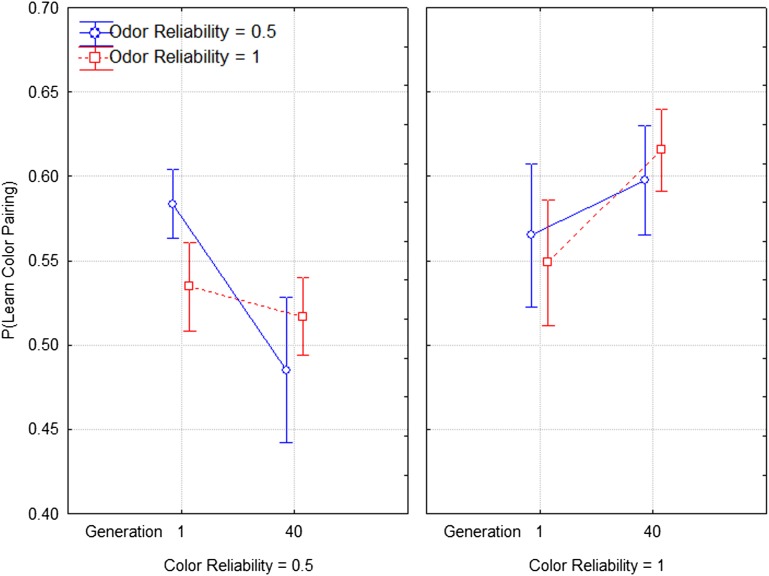
Figs. 2 and 3 show how the effect of the experience of quinine pairing changed from generation 1 to generation 40. Fig. 2 shows changes in the effect of a quinine–odor pairing [dependent measure P(Learn: Odor)]. The figure suggests the sensitivity to learning a quinine–odor pairing depends strongly on the reliability of the odor. In the first panel we see that P(Learn: Odor) declines when the quinine–odor pairing is unreliable, and in the second panel we see that P(Learn: Odor) increases when the quinine–odor pairing is reliable. A repeated measures analysis of variance confirms this interpretation by showing a significant interaction between time and quinine–odor reliability (F1,36 = 4.421, P = 0.042), as well as a statistically significant main effect of odor reliability (F1,36 = 4.153, P = 0.048). Similarly, Fig. 3 shows change in the effect of the quinine–color pairing [i.e., dependent measure P(Learn: Color)]. Again, we see that the reliability—here the reliability of the quinine–color pairing—is the key variable. P(Learn: Color) declines from the first to the last generation of selection when the quinine–color pairing is unreliable and increases when the quinine–color pairing is reliable. Again, a repeated measures ANOVA confirms this by showing a significant interaction between time (between the first and last generations) and the reliability of the quinine–color pairing (F1,36 = 4.378, P = 0.043) and color reliability alone (F1,36 = 7.177, P = 0.011). More details of these analyses can be found in SI Appendix.
Fig. 2.
Selection data of following the quinine pairing with olfactory cues. The x axis represents the starting and end points of the experiment, in means of two-generation blocks (consistent with the randomization scheme of the experiment). Error bars are SEs.
Fig. 3.
Selection data of following the quinine pairing visual cues. The x axis represents the starting and end points of the experiment, in means of two-generation blocks (consistent with the randomization scheme of the experiment). Error bars are SEs.
Final Assays.
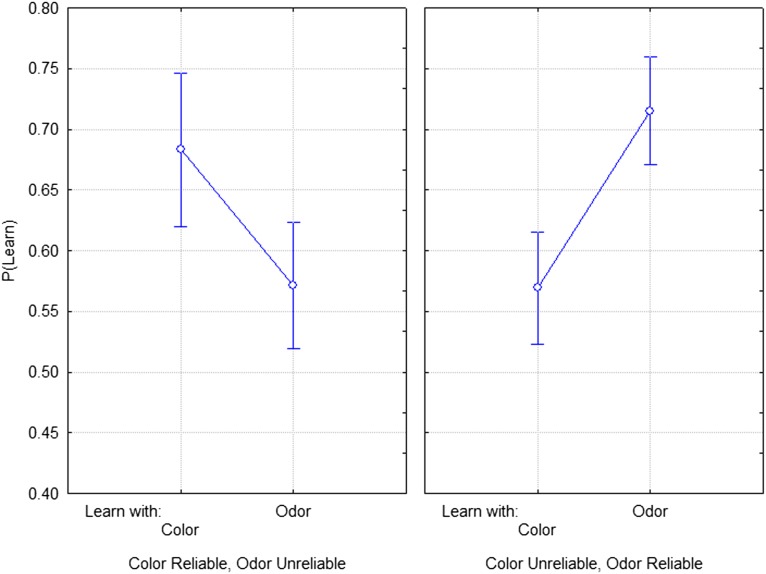
The end-of-selection assays give a very similar picture of the results (Figs. 4 and 5). Fig. 4 shows flies’ learning to quinine–color pairing in terms of our experiment’s two treatment variables (color reliability and odor reliability). We observed higher levels of P(Learn: Color) in those treatments where color was reliable (F1,36 = 4.189, P = 0.048). The effect of olfactory cue reliability was not significant, and did not differ across visual cue reliability levels. The figure suggests an interaction between color and odor reliability, because it looks as if the effect of color reliability is greatly reduced when odor is also reliable, but this interaction is not quite significant (F1,36 = 3.435, P = 0.072). This is an interesting result because it reasonably suggests that sensitivity to learning about color–quinine pairing is not selected for when odor–quinine pairs are reliable. A more powerful experimental design might reveal this effect. Fig. 5 shows the flies’ sensitivity to learning quinine–odor pairing measured in our final assays. The result here is straightforward and clear. P(Learn: Odor) increases with the reliability of the quinine–odor pairing (F1,36 = 7.88, P = 0.008). The differences between the odor and color results probably occur because of preexisting sensitivities to learning about these two sensory modes. Wild-type flies learn odor associations more readily than color, and so it is reasonable to expect that odor learning could reduce selection on color learning, but not vice versa. More details of these analyses can be found in SI Appendix.
Fig. 4.
Here we show the results of learning scores from end of experiment assays testing learning about color stimuli alone (without the presence of odor stimuli). This figure presents the data in the factorial form of the experiment’s design. P(Learn: Color) for each line is the mean of the two learning scores when tested separately with Q+aqua and with Q+blue. In each case, we calculate the proportion of eggs laid on the substrate that had not been paired with quinine previously. Learning about visual cues was enhanced when visual cues were a reliable predictor of the best environment across evolutionary time. The interaction between visual cue and olfactory cue reliability nears significance. The interaction suggests that learning about visual cues is best with visual cues are reliable, but olfactory cues are not.
Fig. 5.
Scores from end of experiment assays testing learning about odor stimuli alone (without the presence of color stimuli). Learning to olfactory cues alone is enhanced in both treatments for which olfactory cues are reliable. P(Learn: Odor) is the mean of the learning scores for each line for tests of Q+amyl acetate and Q+benzaldehyde. The effect of olfactory cue reliability is statistically significant (F1,36 = 7.8829; P = 0.008); neither the effect of visual cue reliability nor the interaction between visual and olfactory cue reliability are statistically significant.
Discussion
Significance of Results.
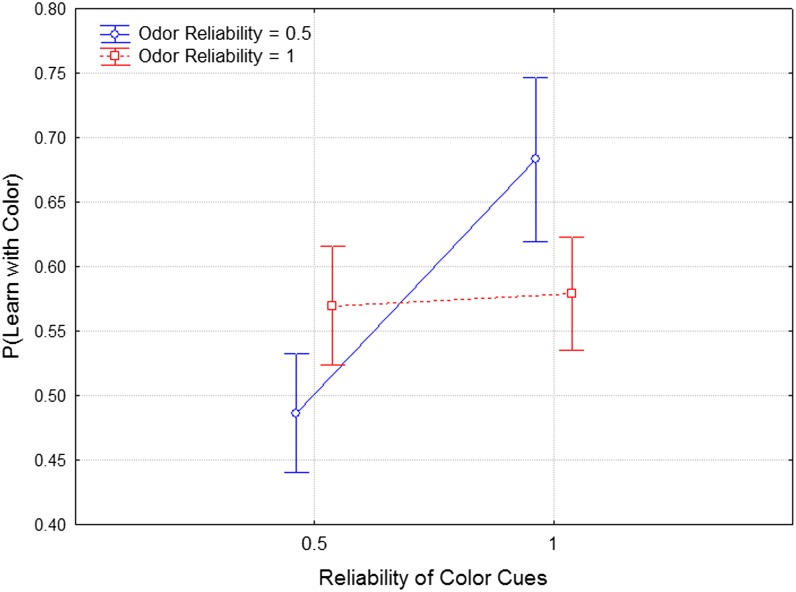
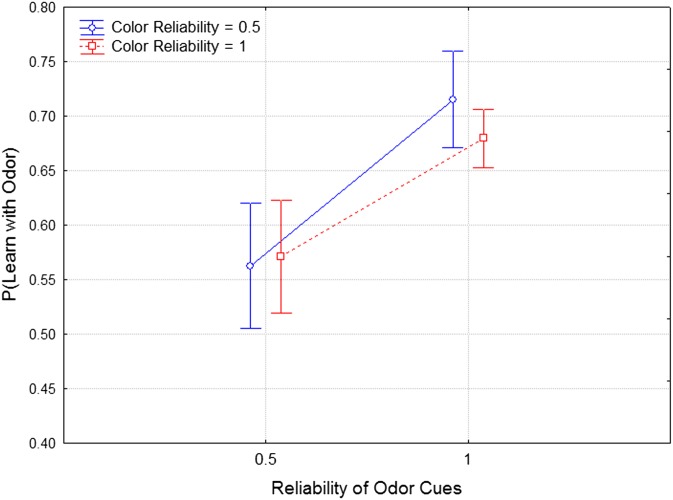
To explain Garcia and Koelling’s famous result, we—like others—hypothesize that during the course of evolution taste–food associations have reliably predicted food quality, whereas light–food associations have not. Although this seems perfectly reasonable, it is hard to see it as anything but a just-so story, because we have no measurements of taste–food quality associations across evolutionary time. Consider in contrast the difference between our two most extreme treatments (Fig. 6). In one treatment, we created a situation in which color–quinine associations were reliable across 40 generations of selection (C = 1.0), but odor–quinine associations were unreliable (O = 0.5), and this should select for learning in response to color but not odor, as we in fact observe. A second treatment tested the opposite extreme in which odor–quinine associations were reliable across 40 generations (O = 1.0), and color–quinine associations were unreliable; again we observe enhanced learning to odor and a reduction in learning to color.
Fig. 6.
Data redrawn from Figs. 4 and 5 to show the treatments where one stimulus modality is reliable, whereas the other is not. Here we directly compare the performance of lines in these treatments in learning about color cues alone and learning about odor cues alone.
Reliability Effect.
Our data support the hypothesis that prepared learning evolves in response to reliable associations experienced by a lineage during the course of evolution. Our data show this in two ways. First, when we consider how our flies’ sensitivity to experience changed over many generations, we see that reliability is the key variable, so that, for example, sensitivity to color–quinine pairings decreased when color was an unreliable indicator of fitness consequences, but increased when color was reliable (Figs. 2 and 3). Second, we see a similar pattern in our final assays that compare our four experimental treatments. Again we see that the reliability of quinine–stimulus associations is the key predictor of evolved differences in learning (Figs. 4 and 5). When color and odor are both reliable, an animal could, in theory, use either type of learning or some combination to achieve the same effect. Our model predicts that when reliabilities are equal, neither modality is better to attend to. Our data suggest that a preexisting bias in favor of odor learning may reduce selection for color learning in this situation (Fig. 4), perhaps creating a type of selective “blocking” that is analogous to the phenomena of blocking in Pavlovian conditioning. Further studies of these types of interactions between learning abilities could prove illuminating.
In previous work (26), we showed that the reliability of learned information interacts with the certainty of a best action (another component of environmental change) to influence the evolution of enhanced learning versus an unlearned preference. This paper confirms the importance of reliability in the adaptive value of learning, but specifically in the prepared learning about two forms of information. Here we showed that the differing reliabilities of alternate sources of information influence which source of information flies are most prepared to learn about.
Results in Context.
It is important to recognize the difference between artificial selection and experimental evolution. In artificial selection the investigator imposes selection directly on a trait such as bristle number or learning ability. In experimental evolution, as used here, we are instead testing hypotheses about how the properties of the environment generate selection. This paper has, for example, tested the hypothesis that patterns of reliability create selection for prepared learning. Experimental evolution offers us an important tool to address questions about the evolution of behavior that would otherwise be simple speculations (27).
The hypothesis that prepared learning reflects the properties of statistical relationships (among stimuli, responses, and outcomes) is widely accepted, even though the terminology used to describe these relationships varies greatly. Studies of prepared learning with both humans and nonhumans have frequently invoked evolution to explain their results (e.g., 23, 28, 29). We do not claim, therefore, that our model is conceptually novel. It simply reframes this long-standing idea in terms of the practical experimental situation in which we can manipulate the conceptually important statistical relationships, notably the reliability of stimulus–quinine associations.
This novel study tested specific predictions about the role of the stimulus reliability across evolutionary time in the formation of relationships between particular stimuli and actions. Our central hypothesis for the evolution of prepared learning is that some stimuli pairings have remained reliable throughout the lineage of a given organism, and natural selection has, therefore, favored learning of these types of associations. Our results throughout 40 generations of experimental evolution with fruit flies support this hypothesis. This work provides an important step forward in understanding both prepared learning and how inherited tendencies interact with information gained through experience.
Methods and Materials
Flies and Husbandry.
Our starting population was a mix of wild-caught, laboratory-adapted flies from four different locations in Minnesota and Wisconsin. We combined 400 male and 400 female adults from each population and maintained them in overlapping generations in a large population cage for 14 mo before the start of the experiment. We housed all flies at 24 °C and tested them at 14 d old (postegg). We reared all eggs in a common environment at a density of 80 eggs per vial and six vials per line per generation. For each generation, we moved flies to population test cages (33 cm L × 21 cm W × 12 cm H) upon eclosure as adults, setting up populations of 240 females and a comparable number of males per line. Each cage featured a removable tray on which we placed two fresh Petri dishes of standard cornmeal and molasses-based food, and after 3 d, we tested each cage of flies.
Aversion Learning and Selection on Populations.
We tested each generation of flies once, testing as groups in the population cages. As described in our model, each test consists of two phases: an experience phase and a consequence phase. In the first phase, the experience phase, we exposed flies to two Petri dishes of agar-based media in a single 3-h session (10 mL of agar placed into the bottom of each 100 × 15 mm Petri dish). We introduced color into the substrate by placing painted disks underneath the Petri dishes, using cobalt blue and aqua blue color (details of these colors in SI Appendix). We introduced odor by mixing amyl acetate and benzaldehyde into the agar (we first diluted each into a mixture of 35% odorant, 65% ethyl alcohol) and added each to agar (20 g sucrose, 10 g agar, 1 L water; 1 mL for amyl acetate and 0.1 mL for benzaldehyde). Before the experiment, we conducted pilot studies to demonstrate learning to the colors and to the odors chosen and tested that neither mode of stimuli completely overshadowed the other during learning trials. Finally, we added quinine at 4 g/1 L agar. To start the experience phase, we positioned the Petri dishes on a sliding tray at the bottom of each cage; we could replace these dishes without moving the flies. In the second phase, a consequence phase, we presented new plates of agar without quinine for 5 h. The pairing of color and odor could be different, depending on the assigned reliability of each (Fig. 1). We randomized the locations of the plates, with visual stimuli always remaining in the same location in both experience and consequence phases and the corresponding odors changing location (depending, again, on the assignment of reliability for each modality). We separated the experience and consequence phases with a 30-min period of no stimuli.
We imposed different selective regimes by rearing eggs from one substrate type (e.g., the one not paired with quinine in the experience phase) and discarding eggs from the other (e.g., the one paired with quinine in the experience phase). We removed eggs selected for propagation from the substrate using a needle and placed them in vials on standard cornmeal-based fly food for incubation, thus providing a common rearing environment for all flies.
Treatments and Lines.
We set up 40 lines of 240 females each and approximately the same number of males from the source population and then randomly assigned 10 lines to each of the four experimental treatments. The treatments were (i) both color and odor reliable (C = 1.0, O = 1.0), (ii) color but not odor reliable (C = 1.0, O = 0.5), (iii) odor but not color reliable (C = 0.5, O = 1.0), and (iv) neither color nor odor reliable (C = 0.5, O = 0.5). At the start of the experiment, we randomized the reliability of the quinine cue for each stimulus modality for each generation within blocks of two generations each and did this separately for each line, according to its assigned treatment. SI Appendix gives details on these assignments.
Data Collection and Final Assays.
Following 40 generations of selections, we then performed a series of assays. We tested each line for learning about each stimulus modality alone. To test learning for color alone, a subset of each line was tested for learning the Q+aqua pairing, and a second subset was tested for the Q+blue pairing. To test learning for odor alone, we tested subsets with Q+benzaldehyde and with Q+amyl acetate. These tests are analogous to the selection procedure and to classic aversion discrimination learning. For example, flies are given experience with Q+aqua and plain blue and then tested with plain aqua and plain blue; we calculate the eggs laid during the test on the substrate consistent with learning (in this case, laying eggs on blue is consistent with learning). We tested each case (e.g., Q+aqua and Q+blue) to control for the effect of potential evolved preferences and then averaged these two scores for each line. In rearing the flies for these tests, we collected all eggs from plates containing standard cornmeal agar food and then reared these eggs under identical conditions (the same as used during the selections). The Petri dishes of agar from the selections as well as the end of experiment assays were scanned with a photo scanner at 600 dots per inch, allowing counting of eggs at later dates and the ability to confirm that counts were consistent between workers. Each Petri dish contained a serial number etched into the bottom, allowing for identification of each plate throughout the testing, scanning, and counting process. All counting was blinded, with counts reassigned to lines and tests using a database query.
Supplementary Material
Acknowledgments
We thank Tyler Blazey, Will Blanco, Nick Stephens, and Juho Lehtilä for help with laboratory tasks and Aziz Khazaeli and Charles Rodell for providing the initial populations. This research was supported by a Frank McKinney fellowship, Department of Ecology, Evolution and Behavior, the Graduate School, and the Center for Cognitive Sciences (NIH T32 HD007151) at the University of Minnesota, by the Center for Insect Science at the University of Arizona (NIH 2 K12 GM000708), and by a National Science Foundation Grant to the authors (NSF IOS-1021183).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404176111/-/DCSupplemental.
References
- 1.Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychon Sci. 1966;4(1):123–124. [Google Scholar]
- 2.Logue AW. Taste aversion and the generality of the laws of learning. Psychol Bull. 1979;86(2):276–296. [Google Scholar]
- 3.Domjan M. Biological constraints on instrumental and classical conditioning: Implications for general process theory. Psychol Learn Motiv. 1983;17:215–277. [Google Scholar]
- 4.Seligman MEP. On the generality of the laws of learning. Psychol Rev. 1970;77(5):406–418. [Google Scholar]
- 5.Shettleworth SJ. Constraints on learning. Adv Stud Behav. 1972;4:1–68. [Google Scholar]
- 6.Rozin P, Kalat JW. Specific hungers and poison avoidance as adaptive specializations of learning. Psychol Rev. 1971;78(6):459–486. doi: 10.1037/h0031878. [DOI] [PubMed] [Google Scholar]
- 7.Dobrzecka C, Szejkowska G, Konorski J. Qualitative versus directional cues in two forms of differentiation. Science. 1966;153(3731):87–89. doi: 10.1126/science.153.3731.87. [DOI] [PubMed] [Google Scholar]
- 8.LoLordo VM. Selective associations. In: Dickinson A, Boakes RA, editors. Mechanisms of Learning and Motivation: A Memorial Volume to Jerzy Konorski. Hillsdale, NJ: Lawrence Erlbaum Assoc; 1979. pp. 367–398. [Google Scholar]
- 9.Thorndike EL. The Fundamentals of Learning. New York: Teachers College Press; 1932. [Google Scholar]
- 10.Bolles RC. Species-specific defense reactions and avoidance learning. Psychol Rev. 1970;77(1):32–48. [Google Scholar]
- 11.Shettleworth SJ. Constraints on conditioning in the writings of Konorski. In: Dickinson A, Boakes RA, editors. Mechanisms of Learning and Memory: A Memorial Volume to Jerzy Konorski. Hillsdale, NJ: Lawrence Erlbaum Assoc; 1979. pp. 399–441. [Google Scholar]
- 12.Seligman MEP, Hager JL. Biological Boundaries of Learning. New York: Appleton Century Crofts; 1972. [Google Scholar]
- 13.Staddon JER. Adaptive Behavior and Learning. New York: Cambridge Univ Press; 1983. [Google Scholar]
- 14.Domjan M. Pavlovian conditioning: A functional perspective. Annu Rev Psychol. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- 15.Hollis KL. Contemporary research on Pavlovian conditioning. A “new” functional analysis. Am Psychol. 1997;52(9):956–965. doi: 10.1037//0003-066x.52.9.956. [DOI] [PubMed] [Google Scholar]
- 16.Beecher MD. Some comments on the adaptationist approach to learning. In: Bolles RC, Beecher MD, editors. Evolution and Learning. Hillsdale, NJ: Lawrence Erlbaum Assoc; 1988. pp. 239–248. [Google Scholar]
- 17.Bolles RC, Beecher MD, editors. Evolution and Learning. Hillsdale, NJ: Laurence Erlbaum Assoc; 1988. [Google Scholar]
- 18.Domjan M, Galef BG. Biological constraints on instrumental and classical conditioning: Retrospect and prospect. Anim Learn Behav. 1983;11(2):151–161. [Google Scholar]
- 19.Johnston TD. Contrasting approaches to a theory of learning. Behav Brain Sci. 1981;4(1):125–173. [Google Scholar]
- 20.Cook M, Mineka S. Selective associations in the observational conditioning of fear in rhesus monkeys. J Exp Psychol Anim Behav Process. 1990;16(4):372–389. [PubMed] [Google Scholar]
- 21.Cook M, Mineka S, Wolkenstein B, Laitsch K. Observational conditioning of snake fear in unrelated rhesus monkeys. J Abnorm Psychol. 1985;94(4):591–610. doi: 10.1037//0021-843x.94.4.591. [DOI] [PubMed] [Google Scholar]
- 22.Mineka S, Ohman A. Phobias and preparedness: The selective, automatic, and encapsulated nature of fear. Biol Psychiatry. 2002;52(10):927–937. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- 23.Ohman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol Rev. 2001;108(3):483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 24.Mery F, Kawecki TJ. Experimental evolution of learning ability in fruit flies. Proc Natl Acad Sci USA. 2002;99(22):14274–14279. doi: 10.1073/pnas.222371199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mery F, Kawecki TJ. The effect of learning on experimental evolution of resource preference in Drosophila melanogaster. Evolution. 2004;58(4):757–767. doi: 10.1111/j.0014-3820.2004.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 26.Dunlap AS, Stephens DW. Components of change in the evolution of learning and unlearned preference. Proc Biol Sci. 2009;276(1670):3201–3208. doi: 10.1098/rspb.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garland T, Jr, Rose MR, eds (2009) Experimental Evolution: Concepts, Methods, and Applications of Selection Experiments (Univ of Calif Press, Berkeley)
- 28.Dellarosa Cummins D, Cummins R. Biological preparedness and evolutionary explanation. Cognition. 1999;73(3):B37–B53. doi: 10.1016/s0010-0277(99)00062-1. [DOI] [PubMed] [Google Scholar]
- 29.Coelho CM, Purkis H. The origins of specific phobias: Influential theories and current perspectives. Rev Gen Psychol. 2009;13(4):335–348. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.