Significance
This study investigates the role of epigenetics as a predisposing factor that modifies inborn emotional reactivity and the vulnerability or resilience to affective disorders, such as major depression or substance abuse. It tests the hypothesis that specific modified histones contribute to basal differences in temperament that bias emotional responses to the environment. It further describes the role of a growth factor that can modify these epigenetic patterns as it alters affective behavior.
Keywords: MR, H3K9me2, H4K20me3, plus maze
Abstract
Posttranslational modifications of histone tails in chromatin template can result from environmental experiences such as stress and substance abuse. However, the role of epigenetic modifications as potential predisposing factors in affective behavior is less well established. To address this question, we used our selectively bred lines of high responder (bHR) and low responder (bLR) rats that show profound and stable differences in affective responses, with bLRs being prone to anxiety- and depression-like behavior and bHRs prone to addictive behavior. We first asked whether these phenotypes are associated with basal differences in epigenetic profiles. Our results reveal broad between-group differences in basal levels of trimethylated histone protein H3 at lysine 9 (H3K9me3) in hippocampus (HC), amygdala, and nucleus accumbens. Moreover, levels of association of H3K9me3 at Glucocorticoid Receptor (GR) and Fibroblast growth Factor 2 (FGF2) promoters differ reciprocally between bHRs and bLRs in these regions, consistent with these genes’ opposing levels of expression and roles in modulating anxiety behavior. Importantly, this basal epigenetic pattern is modifiable by FGF2, a factor that modulates anxiety behavior. Thus, early-life FGF2, which decreases anxiety, altered the levels of H3K9me3 and its binding at FGF2 and GR promoters of bLRs rendering them more similar to bHRs. Conversely, knockdown of HC FGF2 altered both anxiety behavior and levels of H3K9me3 in bHRs, rendering them more bLR-like. These findings implicate FGF2 as a modifier of epigenetic mechanisms associated with emotional responsiveness, and point to H3K9me3 as a key player in the regulation of affective vulnerability.
Chromatin remodeling is a mediator of lasting neural changes in response to experience, such as exposure to stress and drugs of abuse (1–7). Indeed, the interaction of certain modified histones with specific gene promoters has been shown to be an important mechanism of experience-dependent neuroplasticity (8–13). Although numerous studies have examined the impact of the environment on neural epigenetic profiles, relatively few studies have focused on preexisting differences in epigenetic profiles as potential predisposing factors in emotional reactivity. Such studies require the availability of an animal model where difference in “temperament” or propensity for specific affective responses can be reliably predicted and altered. Our laboratory has generated such an animal model that captures vulnerability for “internalizing disorders” vs. “externalizing disorders.” Selectively bred high responder (bHR) rats exhibit greater responsiveness to novelty and to drug seeking behavior (externalizing behaviors), whereas selectively bred low responders (bLR) exhibit greater anxiety and depression-like responses (internalizing behaviors) (14, 15). These genetically bred phenotypes amplify behavioral traits observed in outbred animals (16–20).
Several genes have been implicated in modifying these phenotypes, both in the bred and outbred lines (14). In particular, a key gene in stress regulation, the glucocorticoid receptor (GR) showed higher levels of mRNA expression in the hippocampus (HC) of outbred LRs relative to outbred HRs, and has been implicated in increased anxiety behavior in these animals. Thus, administration of a GR antagonist into the HC reduced anxiety behavior in outbred LR rats. Importantly, bLRs also exhibit significantly higher levels of hippocampal GR mRNA compared with bHRs, whereas the mineralocorticoid receptor (MR) showed no differences between the lines (17). Moreover, our mouse genetic studies have demonstrated that GR is an early-life modifier of emotional reactivity, with its overexpression before weaning enhancing anxiety throughout life (21). This then suggests that GR a critical molecular organizer of stable differences in affective reactivity.
A countervailing modifier of anxiety behavior is the Fibroblast Growth Factor-2 (FGF2). This molecular organizer plays a critical role in brain development and hippocampal neurogenesis (22, 23). Moreover, FGF2 has been proposed as an endogenous anxiolytic and antidepressant that is depleted in the brain of depressed humans (24, 25). Its direct chronic administration was anxiolytic and antidepressant in rodents (25) and the silencing of endogenous hippocampal FGF2 using short-hairpin RNA increased anxiety-like behavior in outbred rats (26, 27). Our bLRs, which exhibit higher anxiety and depression behaviors, have lower basal levels of FGF2 mRNA in HC and nucleus accumbens (NAcc) (28, 29), and an environmental manipulation during adulthood that decreases anxiety behavior induces FGF2 expression selectively in these bLRs (30). Moreover, early-life FGF2 administration selectively decreases anxiety in bLRs throughout life (28).
In the present study, we evaluated the basal levels of various modified histone proteins (H3 and H4) in the HC, amygdala, and NAcc in the bHR and bLR rats. We then focused on a repressive trimethylated histone protein H3 at lysine 9 (H3K9me3) which is one of the most widely studied repressed modified histones (31), and which showed reliable bHR vs. bLR differences. Using chromatin immunoprecipiation (ChIP) assays, we asked whether some of the basal variations in GR and FGF2 expression between the bred lines might be the result of differences in the association of this histone at their promoter. Finally, we asked whether manipulating FGF2 either via early-life administration or via virally mediated knockdown can modify the epigenetic patterns observed in the bred lines.
Results
Endogenous Levels of Methylated Repressive Histone Protein H3K9me3 Are Altered in bHR-LR Rats.
Because HC, amygdala, and NAcc are brain regions implicated in anxiety and addiction, we evaluated repressive modified H3 in these areas. In general, the levels of H3K9me3 differed across the three regions studied (Fig. 1A). More importantly, within each of the three brain areas, we observed a decrease in the levels of H3K9me3 (P < 0.001; Fig. 1C) in bHRs relative to bLRs. Because modified histone H4K20me3 has been identified to play a role in responsiveness to stress and addiction, we tested its levels in the same three brain regions and found no differences in the levels of H4K20me3 between bHRs and bLRs (Fig. 1 B and D). Thus, decreased levels of the repressive H3K9me3 are distinctive and may play a role in regulation of stress and anxiety-related genes in the bLRs compared with bHRs. Therefore, we further investigated the association of H3K9me3 with the promoters of specific genes implicated in our behavioral phenotypes.
Fig. 1.
Differential regulation of repressive methylated histones in various brain regions of bLR and bHR rats. (A and C) bHRs showed a significant decrease in the levels of repressive methylated histone protein H3K9me3 in the HC, amygdala, and NAcc in comparison with bLR (***P < 0.001, n = 6 per group). (B and D) However, the levels of repressive methylated histone protein H4K20me3 did not differ between bHRs and bLRs (***P < 0.001, n = 6 per group).
Association of Methylated Repressive Histones at Stress- and Anxiety-Related Genes Are Altered.
GR and MR are both ligand-modulated transcription factors that transduce the actions of the circulating stress hormones corticosterone in rodents and cortisol in humans (32) and modulate anxiety behavior (17, 33, 34). Given the differences in anxiety and stress responsiveness between bHR and bLR rats (15, 35), we evaluated the association of these genes with the modified forms of histones using ChIP assays. The association levels of H3K9me3 at the GR promoter were significantly increased in bHRs HC (P < 0.001), amygdala, and NAcc (P < 0.002) compared with bLRs (Fig. 2 A and C), consistent with the lower level of GR expression in the less anxious bHRs relative to the more anxious bLRs. By contrast, the association of H3K9me3 at the MR promoter did not show any alterations in HC, amygdala, and NAcc of bHR versus bLR rats (Fig. 2 A and D), indicating that the differences between the bred lines is specific to particular stress genes.
Fig. 2.
bLR and bHR rats show differential association of methylated histone H3K9me3 at FGF2, GR, and MR promoters in various brain regions. (A and B) bHRs showed a significant (n = 6) decrease in the levels of association of repressive methylated histone protein H3K9me3 at FGF2 (***P < 0.001, n = 6 per group) (A and C) and a significant increase at GR in the HC, amygdala, and NAcc compared with bLRs (***P < 0.001, **P < 0.002, n = 6 per group). (A and D) Note the unaltered association of histone protein of H3K9me3 at MR promoter in both bHR and bLRs.
Because FGF2 plays a key role as an endogenous anxiolytic and shows higher expression levels in the less anxious bHRs (25, 28), we used a ChIP assay to investigate the association levels of various modified histone at the FGF2 gene. Compared with bLRs, negligible association was observed for the repressive H3K9me3 (P < 0.001) at the FGF2 gene promoter in bHR rat HC, amygdala, and NAcc (Fig. 2 A and B). This decreased association of the repressive methylated histone with FGF2 in HC may be critical for the up-regulated gene expression of FGF2 in HC of bHRs.
Effects of Early-Life FGF2.
Differential alteration of the basal levels of modified histone and its association at FGF2 promoter in bHR rats.
We have previously shown that the injection of a single dose of FGF2 (20 ng/g, s.c.) on the day after birth (PND2) produced a lifelong effect on emotionality, enhanced drug self-administration in outbred rats (36) and decreased anxiety-like behavior especially in bLR rats (28). In essence, early-life FGF2 shifts the behavioral phenotype from bLR toward bHR. To determine the possible mediation of this behavioral effect by epigenetic mechanisms, we used early-life FGF2 administration and evaluated the levels of repressive methylated histone protein and its association with FGF2 promoter in HC, amygdala, and NAcc. Detailed statistical analyses are provided in SI Results. In HC and NAcc, we observed that there is a significant decrease in the overall level of H3K9me3 in the bLRs following FGF2 injection compared with the bLR vehicle treated group (P < 0.001; post hoc analysis). By contrast, in the amygdala, the bLRs showed no alterations in H3K9me3 levels, whereas in the bHRs neonatal FGF2 increased H3K9me3 levels relative to vehicle-treated controls only in amygdala (Fig. 3 A and B).
Fig. 3.
Early-life FGF2 alters the levels of H3K9me3 in various brain regions of bLR rats. Immunoblot (A) and graph (B) show a significant (n = 6) decrease in the levels of H3K9me3 in HC and NAcc of bLR rats and increased in the levels of H3K9me3 in amygdala of bHR rats treated with FGF2 compared with vehicle treatment (***P < 0.001, n = 6 per group).
Likewise, the association of H3K9me3 with the FGF2 promoter also significantly decreased (P < 0.001; two-way ANOVA with post hoc analysis) in HC and NAcc in bLRs following early-life FGF2 injection and this was not observed in the amygdala of bLRs. By contrast, the bHRs showed no impact of the FGF2 neonatal treatment on the H3K9me3 association to FGF2 in any of the brain regions. This result demonstrates that early-life FGF2 reduced the difference in association of repressive methylated histone H3K9me3 at FGF2 promoter between bHR and bLR in HC and NAcc (Fig. 4 A and B). Conversely, the association of H3K9me3 with GR promoter was increased in HC (P < 0.001; post hoc analysis) in bLRs following early-life FGF2 injected without altering the NAcc or amygdala (Fig. 4 A and C) and without affecting the bHRs. Thus, the early-life intervention by FGF2 regulates modified histones differentially in various brain regions related to anxiety and addiction, primarily modifying H3K9me3 levels and associations in the bLRs to render them more similar to bHRs.
Fig. 4.
Early-life FGF2 alters the association of H3K9me3 at FGF2 and GR promoters. (A and B) The association of H3K9me3 at FGF2 decreased significantly in HC and NAcc bLR rats following neonatal FGF2 exposure (***P < 0.001, n = 6 per group). (A and C) However, the association of H3K9me3 at GR increased significantly only in HC following neonatal FGF2 exposure. Note that the amygdala remained unaltered in the association pattern of H3K9me3 at FGF2 and GR (***P < 0.001, n = 6 per group).
Alteration of methylation level of the FGF2 promoter.
DNA methylation typically leads to transcriptional silencing and H3K9 methylation has been linked with DNA methylation (37, 38), with H3K9me3 inducing DNA methylation via activation of various DNA methyltransferases (39, 40). As we have observed an alteration in the association of H3K9me3 at the FGF2 promoter, we further assessed the methylation levels at that promoter following neonatal FGF2 exposure. In untreated animals, we observed a significant increase in the methylation levels of the FGF2 promoter in bLR hippocampus relative to bHRs (P < 0.001; post hoc analysis) consistent with the lower expression level of FGF2 in the bLRs. However, following early-life FGF2 the methylation levels of FGF2 significantly decreases in bLRs compared with vehicle treated bLRs (P < 0.001; post hoc analysis) (Fig. S1). Thus, in the FGF2-treated groups, we observed no differences in the levels of methylation in the hippocampus between bHR and bLR rats. These results demonstrated that the basal repression of FGF2 in bLRs can be manipulated by early-life FGF2 interventions, with neonatal FGF2 modifying the epigenetic signature at its own promoter into adulthood.
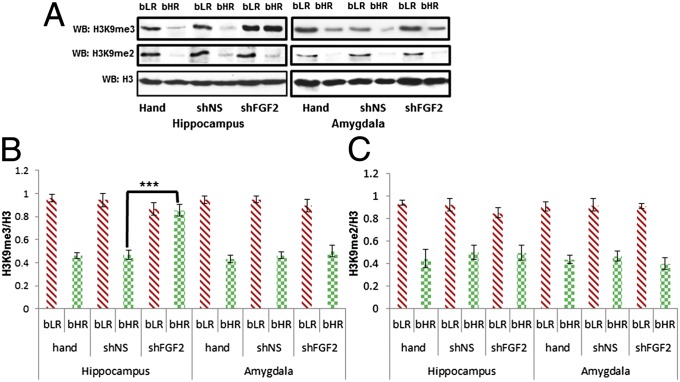
FGF2 Knockdown in Adult Hippocampus Alters the Behavior and Levels of Modified Histone (H3K9me3) in the bHRs.
Our previous work in outbred rats demonstrated that the knockdown of FGF2 in the hippocampus results in an anxiogenic effect (26). In the present study, we compared the selectively bred lines and observed that in the elevated plus-maze, FGF2 knockdown in the hippocampus eliminated differences between bHR and bLR behavior (Fig. 5A). Specifically, bHRs administered a lentiviral vector (LV) expressing a short-hairpin RNA containing a nonsilencing control sequence, LVshNS, spent significantly more time in the open arms than the bLRs administered LVshNS (post hoc Bonferroni test: P < 0.001; Fig. 5A). However, administration of a lentiviral vector expressing a short-hairpin sequence targeted to FGF2, LVshFGF2, abolished these differences in anxiety behavior between the bred lines (Fig. 5B, P = 0.38). Interestingly, hippocampal FGF2 knockdown impacted open arm behavior differentially: bHRs administered LVshFGF2 spent significantly less time in the open arms of the maze than did bHRs administered LVshNS (P = 0.016); by contrast there were no differences between bLRs administered LVshNS and LVshFGF2 (P = 0.454). Thus, FGF2 knockdown selectively enhanced anxiety behavior in the typically less anxiety-prone bHRs.
Fig. 5.
Hippocampal knockdown of FGF2 eliminates characteristic differences in anxiety-like behavior between bHR and bLR rats. (A) Nonsilencing control virus-injected animals show prototypical behavioral differences on the elevated plus maze: bHRs spent significantly more time on the open arms and less time in the closed arms than do bLRs (*P < 0.05, n = 6 per group). (B) In contrast, hippocampal knockdown of FGF2 eliminated the behavioral differences between bHR and bLR animals (n = 6 per group).
We then assessed the levels of repressive modified histone H3K9me2 and H3K9me3 in both the HC and the amygdala of bHR/bLR rats from this study. Following LVshFGF2 in the HC, there was an increase in the levels of H3K9me3 in bHRs compared with bLRs LVshNS or compared with the handled animals without any lentivirus in the HC (Fig. 6 A and B; P < 0.001; detailed statistical analyses are described in the SI Results). In other words, the basal difference between the bHRs and the bLRs was significantly reduced in the HC following FGF2 knockdown. The alteration in epigenetic signature was not observed in the amygdala of the same brain. Moreover, the level of another repressive modified histone, H3k9me2, was not altered by the lentivirus either in HC or amygdala, demonstrating both the regional and target specificity of the FGF2 knockdown (Fig. 6 A and C).
Fig. 6.
Hippocampal knockdown of FGF2 differentially regulates repressive methylated histones various brain regions of bLR and bHR rats. Immunoblot (A) and graph (B) show that knockdown FGF2 significantly (n = 6) increased the levels of H3K9me3 only in the bHR HC (***P < 0.001, n = 6 per group). Immunoblot (A) and graph (C) show that the levels of H3K9me2 remained unaltered in both the HC and the amygdala of bHRs and bLRs following hippocampal knockdown of FGF2.
Discussion
This series of studies demonstrates three phenomena relating to the role of chromatin modifications in the control of affective behavior: (i) There are basal and pervasive differences in the levels of the modified histone protein H3K9me3 between two lines of rats that were derived from common outbred stocks and selectively bred for differences in emotional reactivity. Overall, levels of H3K9me3 are higher in the more behaviorally inhibited animals, the bLRs. (ii) Specific genes implicated in responsiveness to stress and anxiety, namely GR and FGF2, show differential association with H3K9me3 that are in line with differences in their gene expression levels across the two lines. Thus, the association of the inhibitory H3K9me3 with GR is lower in bLRs, consistent with the higher levels of GR expression in these more anxiety-prone animals; by contrast, the association of H3K9me3 with FGF2 in bLRs is higher, consistent with the low expression of FGF2 in these animals. (iii) FGF2 is not only a target of epigenetic modification by H3K9me3, it is also a trigger of epigenetic changes that can impact this modified histone protein and its association with various gene promoters.
These results are summarized in Fig. S2, and their implications for the roles of FGF2, GR, and H3K9me3 are depicted in Fig. 7. Together, they implicate H3K9me3 as a key player in the control of affective responsiveness, especially anxiety behavior. They also demonstrate that FGF2 partners with H3K9me3 to modulate the neural phenotype at the epigenetic level, both broadly (overall level of modified histone across several brain regions) and very specifically (opposing patterns of association with specific promoters that exert opposing control on anxiety). Below, we discuss each of these three principal findings.
Fig. 7.
FGF2 is both a target and a modifier of H3K9me3: Overall relationship between molecular organizers and anxiety behavior. In general, higher overall levels of H3K9me3 are associated with increased anxiety behavior. Moreover, FGF2 enhancement is anxiolytic and GR activation is anxiogenic. In high anxiety states, H3K9me3 shows lower association with GR and higher association with FGF2. Enhancing FGF2 decreases H3K9me3 levels, decreases its association with the FGF2 promoter, and increases its association with GR.
Basal Differences in the Posttranslational Modifications at H3K9me3, a Histone Associated with Transcriptional Repression.
Relative to bLRs, the more active bHRs exhibit an overall decreased level of the repressive H3K9me3. This decrease was observed in all three brain regions examined: the HC, NAcc, and amygdala. It is notable that this epigenetic signature was stable across generations, as rats from three generations of our selectively bred lines (generations 31, 34, and 35) were used in the various studies and exhibited similar basal differences in levels of H3K9me3.
Both H3K9me3 and H4K20me3 are associated with transcriptional repression and silencing (41, 42). It is therefore notable that the differential pattern in modified histones is selective, as we observed no basal differences in levels of H4K20me3 between bHRs and bLRs in the three brain regions studied. However, this does not preclude differences in other modified histone proteins across the lines that play a role in transcriptional activation or inhibition. The functional role of this global difference in H3K9me3 is not currently well understood, but it is notable that manipulations that alter anxiety behavior also altered the global levels of this histone, as discussed below. It will be of interest to ascertain whether other animal models of basal differences in anxiety behavior reveal similar differences in H3K9me3. If so, this would implicate this modified histone as a target for altering the propensity for excessive anxiety.
Association of Modified Histones at the Promoters of FGF2 and GR.
Alteration in the association of modified histones at promoter sites is crucial for the regulation of transcriptional activity of specific genes, thereby regulating their expression levels. Despite the overall difference in the global pattern of H3K9me3 levels in the two selectively bred lines, the association with specific genes was quite specific and not necessarily reflective of the overall levels of the modified histone. Thus, GR, which we and others have implicated in greater basal anxiety (17, 21, 43) and which is elevated basally in the bLRs (17), exhibited a lower level of association with H3K9me3 which is consistent with the higher expression levels of that gene. This result was observed in all three brain regions studied: the HC, amygdala, and NAcc. Once again, these marks were specific: We saw no differences in the association of other histones, e.g., H4k20me3 with the GR gene promoter. Moreover, the effect was selective to GR but did not affect a closely related gene, MR, which showed no differences in to either modified histone across the two lines (data not shown).
By contrast, the association of H3K9me3 with FGF2 was higher in the bLRs, which was more reflective of the overall elevation of this modified histone in these animals. This finding is also consistent with the decrease in FGF2 mRNA levels in the bLR rats across several brain regions (28, 29). This set of results suggests that the differential levels of association of the modified histone H3 with susceptibility genes (FGF2 and GR) correlate with the differences in gene expression in HC and amygdala, along with differences in stress responsiveness and anxiety behavior.
Although this series of studies has focused on basal differences in H3K9me3, work by others has demonstrated the responsiveness of this modified histone to environmental manipulations, such stress and addiction further supporting its role in the control of affective behavior. In particular, acute cocaine exposure increased H3K9me3 levels in NAcc (44) and has an adaptive role in animal models of stress (7). In the NAcc, cocaine also alters the association between the modified histone H3 (acetylation, methylation, or phosphorylation) with various genes including long interspersed nuclear element-1 (44), H-Ras1 (7), mitogen and stress-activated protein kinase-1 MSK1, c-fos, and c-jun (45). Our findings that basal differences in H3K9me3 association affect regulating genes in animals with differing emotional reactivity, together with the responsiveness of the H3 histone to various manipulations that modify affective behavior, converge to suggest a critical role of this modified histone in regulating emotional behavior.
Role of FGF2 in Altering the Levels of Repressive H3K9me3 and Its Association at FGF2 and GR Promoters.
An emerging literature that began with studies in human depressed brains and was followed up by a range of studies in animals models strongly implicates FGF2 in the modulation of affect, especially in decreasing anxiety behavior (25, 27). Our own work has shown that neonatal FGF2 resulted in a long-term decrease in anxiety-like behavior in bLR which naturally exhibit high anxiety behavior (28). Studies have demonstrated that intracerebroventricular infusion of chronic FGF-2 can inhibit anhedonia-like behavior following chronic unpredictable stress. Also selective infusion of FGF-2 in the PFC, but not into dorsal striatum, shows antidepressant-like and anxiolytic-like effects following forced swim test and novelty suppressed feeding test (46). Moreover, knockdown of FGF2 using single microinjection in the hippocampus resulted in an anxiety-like effect in outbred animals (26). However, the mechanism whereby FGF2 exerts its long lasting effects (e.g., between neonatal administration and adulthood) remains unknown. The current study tested the possibility that chromatin remodeling was one of the mechanisms of these lasting effects of FGF2. Indeed, we show here that a brief exposure to FGF2 in early life resulted in a long-term decrease in the expression levels of H3k9me3 in adult bLRs relative to controls. This effect was not observed in the bHRs who exhibit high basal levels of FGF2. Other studies have also identified epigenetic factors to be influential in early developmental period for adaptation in understanding vulnerability and resilience (47). It is notable that this selectivity in H3K9me3 modulation parallels the behavioral effects of early-life FGF2, which decreased anxiety behavior in bLRs without affecting bHRs (28).
Moreover, DNA methylation is often associated with transcriptional repression, possibly via the repression of retrotransposons and other foreign elements (48). Interestingly, we observed that methylation at FGF2 was basally higher in bLR hippocampus suggesting increased repression of FGF2 in bLRs, consistent with our H3K9me3 findings. However, early-life FGF2 decreased the methylation of FGF2 selectively in bLR hippocampus. This finding is consistent with the view that neonatal administration of exogenous FGF2 to animals with low basal levels of this growth factor (bLRs) resulted in epigenetic changes that enhanced their endogenous FGF2 function, resulting in an anxiolytic effect (28).
Because our previous FGF2 knockdown study was carried out in outbred animals (26) we asked whether the effect of knocking down FGF2 specifically in hippocampus would produce a differential effect on anxiety behavior between the selectively bred lines, and whether this differential behavioral effect would be associated with differences in H3K9me3. FGF2 knockdown, in the hippocampus increased anxiety in the bHRs who have higher basal levels of FGF2 and are typically less prone to anxiety. This manipulation led to a highly selective change in the levels of H3K9me3, only in the hippocampus and only in the bHRs.
Taken together, our findings suggest that FGF2 exerts its effects on anxiety behavior, at least in part, by modulating H3K9me3 to influence both its overall levels and its association with specific target genes such as GR as well as its own (FGF2) promoter. The impact on a critical modified histone protein likely represents a key feature of FGF2’s ability to trigger long-term changes in neuroplasticity and modulation of emotional reactivity.
It is notable that most of our effects were consistently seen in the hippocampus, but not necessarily in the amygdala. This may be due to the fact that this study has focused on unlearned, spontaneous anxiety behavior, which is regulated by the hippocampus, with a particular role of the ventral dentate gyrus (49). Other tests that rely on learned fear responses (which also differ between bHR and bLR animals- unpublished) and other manipulations that modify the reactivity to fear might uncover a role of the epigenetic signature of the amygdala in their mediation.
Given that the FGF system is altered in major depression (25) and given the current findings demonstrating that FGF2 is both a target and a trigger of changes in H3K9me3, it is reasonable to hypothesize that this modified histone protein is a key player in mood disorders, and that the H3K9me3-FGF2 interaction could represent an important target for treatment or prevention of major depression.
In sum, we have observed the existence of basal epigenetic differences that may function during development to determine long-term resilience or vulnerability to anxiety. However, these epigenetic differences are also intrinsically sensitive to intervention, thus enabling preventive strategies in vulnerable individuals. As importantly, we propose a particular molecule, FGF2, as being able to orchestrate epigenetic changes that affect a range of targets, including the FGF system itself and its functional opponent the GR/stress system. The bidirectional relation between FGF2 and epigenetic mechanisms has the potential to provide a number of novel molecular targets for resilience enhancement.
Materials and Methods
Selectively Bred Rats (bHR and bLR).
The bLR-HR rats were generated from our in-house breeding colony, where the bLR/bHR lines have been maintained for several generations (35). On the basis of the locomotor activity, bHR and bLR rats of our breeding colony were screened as described (30). Peripheral neonatal FGF2 administrations were performed as previously described on both bHRs and bLRs (28). Knockdown of FGF2 in the hippocampus was also performed as described (26) on bHRs and bLRs. Adult rats from both bHR/bLR lines were killed and several brain regions (HC, amygdala, and NAcc) were processed to be used for different experiments.
Western Blot Analysis.
The detailed procedure for Western blot is presented in SI Materials and Methods. Briefly, the tissues from three brain regions (HC, amygdala, and NAcc) were homogenized and resolved in 12.5% (wt/vol) SDS/PAGE and transferred to PVDF membrane (Millipore). The membranes were blocked in 10% (wt/vol) nonfat milk and incubated with various primary antibodies. Enhanced chemiluminescence (GE Healthcare) was used following manufacturer’s instructions for visualization of antigen-antibody binding. Quantification of the autoradiographs was done using Image J (NIH Software) after normalization.
Chromatin Immunoprecipitation Assay.
The brain regions (HC, amygdala, and NAcc) were dissected from the frozen bHR-LR rat brain by a punching method. The detailed procedure has been mentioned in SI Materials and Methods. Briefly, the dissected regions were cross-linked, homogenized and centrifuged. The pellets were suspended in the nuclear lysis buffer and using the Sonicator (Branson), the extracted chromatin was sheared to 200–300 bp. The chromatin was then subjected to immunoprecipitation using antibodies against anti-H3K9me3 (Abcam) overnight at 4 °C. Protein–DNA–antibody complexes were precipitated with Dynabeads@protein A (Invitrogen) for 2 h at 4 °C. The precipitated protein–DNA complexes were eluted from the antibody and then reversed-cross-linking was done in 0.3 M NaCl at 65 °C overnight. Proteins were digested with proteinase K for 1 h at 45 °C. The DNA were extracted, purified (Qiagen), and quantified using PCR. Primers directed to the gene promoter used for amplification are listed in SI Materials and Methods.
DNA Methylation.
The brain regions (HC, amygdala, and NAcc) were dissected from the frozen bHR-LR rat brain using a 19-gauge syringe (C310GA/TW/SP). The genomic DNA was isolated from the dissected regions using PureLink Genomic DNA kit manufacturer’s protocol (Life Technologies). The isolated genomic DNA was further processed to extract unmethylated and methylated DNA using CpG MethylQuest DNA Isolation kit manufacturer’s protocol (Millipore). The detailed protocol for genomic DNA extraction and isolation of methylated DNA is presented in the SI Materials and Methods. The PCR product for total, methylated and unmethylated DNA was compared. Signal intensity was quantified using Image J (NIH Software).
FGF2 Knockdown Experiments.
All adult bLR-HR rats were housed 2–3 animals per cage for the duration of the study (35). Lentivirus (1 µL) containing either a short-hairpin RNA targeted against FGF2 (LVshFGF2) or a scrambled version of the targeted sequence (LVshNS) was infused (26). After 4 wk from surgery, animals were subjected to elevated plus maze test under dim light (30 lx). Detailed protocol has been illustrated in SI Materials and Methods for the knockdown study. After 24 h of the behavioral test, animals were killed and brains were stored at −80°C for Western blotting.
Statistical Analysis.
For Western blot and CHIP data analysis, a Student t test was used to analyze basal differences between bHR and bLR rats. Two-way ANOVAs were performed for Figs. 3–6. A three-way ANOVA was performed for Fig. S1. All detailed statistical analyses are presented in SI Materials and Methods and SI Results, and the most interesting significant findings are presented in the main text.
Supplementary Material
Acknowledgments
We thank Angela Koelsch for assistance in the breeding and maintenance in the bHR/bLR rat lines. This work was supported by National Institute on Drug Abuse Grant 5 P01 DA021633, National Institutes of Health Grant R01MH104261, National Institute of Mental Health Grant R01-MH-104261, Office of Naval Research Grants N00014-09-1-0598 and N00014-12-1-0366, the Pritzker Neuropsychiatric Disorders Research Consortium, and the Hope for Depression Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1411618111/-/DCSupplemental.
References
- 1.Levine AA, et al. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci USA. 2005;102(52):19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renthal W, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56(3):517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder FA, et al. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology. 2008;33(12):2981–2992. doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilang-Bleuel A, et al. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: Involvement in a glucocorticoid receptor-dependent behavioural response. Eur J Neurosci. 2005;22(7):1691–1700. doi: 10.1111/j.1460-9568.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- 5.Chandramohan Y, Droste SK, Reul JM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-D-aspartate receptor and the glucocorticoid receptor: Relevance for c-fos induction. J Neurochem. 2007;101(3):815–828. doi: 10.1111/j.1471-4159.2006.04396.x. [DOI] [PubMed] [Google Scholar]
- 6.Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci USA. 2009;106(49):20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covington HE, 3rd, et al. A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron. 2011;71(4):656–670. doi: 10.1016/j.neuron.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver IC. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: Let’s call the whole thing off. Epigenetics. 2007;2(1):22–28. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- 9.Weaver IC. Shaping adult phenotypes through early life environments. Birth Defects Res C Embryo Today. 2009a;87(4):314–326. doi: 10.1002/bdrc.20164. [DOI] [PubMed] [Google Scholar]
- 10.Weaver IC. Epigenetic effects of glucocorticoids. Semin Fetal Neonatal Med. 2009b;14(3):143–150. doi: 10.1016/j.siny.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: A novel mechanism of alcoholism. J Neurosci. 2008;28(14):3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 14.Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology. 2014;76(Pt B):425–436. doi: 10.1016/j.neuropharm.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stedenfeld KA, et al. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiol Behav. 2011;103(2):210–216. doi: 10.1016/j.physbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piazza PV, et al. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1(4):339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- 17.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: Differential expression of stress-related molecules. J Neurosci. 2000;20(18):6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabbaj M, Evans S, Watson SJ, Akil H. The search for the neurobiological basis of vulnerability to drug abuse: Using microarrays to investigate the role of stress and individual differences. Neuropharmacology. 2004;47(Suppl 1):111–122. doi: 10.1016/j.neuropharm.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Kabbaj M, et al. Social defeat alters the acquisition of cocaine self-administration in rats: Role of individual differences in cocaine-taking behavior. Psychopharmacology (Berl) 2001;158(4):382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- 20.Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2009;65(10):863–868. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Wei Q, et al. Glucocorticoid receptor overexpression in forebrain: A mouse model of increased emotional lability. Proc Natl Acad Sci USA. 2004;101(32):11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raballo R, et al. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000;20(13):5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6(5):474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 24.Evans SJ, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci USA. 2004;101(43):15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner CA, Watson SJ, Akil H. The fibroblast growth factor family: Neuromodulation of affective behavior. Neuron. 2012;76(1):160–174. doi: 10.1016/j.neuron.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eren-Koçak E, Turner CA, Watson SJ, Akil H. Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biol Psychiatry. 2011;69(6):534–540. doi: 10.1016/j.biopsych.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmaso N, Vaccarino FM. Toward a novel endogenous anxiolytic factor, fibroblast growth factor 2. Biol Psychiatry. 2011;69(6):508–509. doi: 10.1016/j.biopsych.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner CA, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proc Natl Acad Sci USA. 2011;108(19):8021–8025. doi: 10.1073/pnas.1103732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinton SM, et al. Neonatal fibroblast growth factor treatment enhances cocaine sensitization. Pharmacol Biochem Behav. 2012;103(1):6–17. doi: 10.1016/j.pbb.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29(19):6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng SS, Yue WW, Oppermann U, Klose RJ. Dynamic protein methylation in chromatin biology. Cell Mol Life Sci. 2009;66(3):407–422. doi: 10.1007/s00018-008-8303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Kloet ER. Hormones, brain and stress. Endocr Regul. 2003;37(2):51–68. [PubMed] [Google Scholar]
- 33.Wei Q, et al. Overexpressing the glucocorticoid receptor in forebrain causes an aging-like neuroendocrine phenotype and mild cognitive dysfunction. J Neurosci. 2007;27(33):8836–8844. doi: 10.1523/JNEUROSCI.0910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci USA. 2007;104(11):4688–4693. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stead JD, et al. Selective breeding for divergence in novelty-seeking traits: Heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36(5):697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- 36.Turner CA, et al. Neonatal FGF2 alters cocaine self-administration in the adult rat. Pharmacol Biochem Behav. 2009;92(1):100–104. doi: 10.1016/j.pbb.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamaru H, Selker EU. A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature. 2001;414(6861):277–283. doi: 10.1038/35104508. [DOI] [PubMed] [Google Scholar]
- 38.Rountree MR, Selker EU. DNA methylation and the formation of heterochromatin in Neurospora crassa. Heredity (Edinb) 2010;105(1):38–44. doi: 10.1038/hdy.2010.44. [DOI] [PubMed] [Google Scholar]
- 39.Jackson JP, Lindroth AM, Cao X, Jacobsen SE. Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature. 2002;416(6880):556–560. doi: 10.1038/nature731. [DOI] [PubMed] [Google Scholar]
- 40.Johnson LM, et al. The SRA methyl-cytosine-binding domain links DNA and histone methylation. Curr Biol. 2007;17(4):379–384. doi: 10.1016/j.cub.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447(7143):407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 42.Izzo A, Schneider R. Chatting histone modifications in mammals. Brief Funct Genomics. 2010;9(5-6):429–443. doi: 10.1093/bfgp/elq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eagle AL, et al. Single prolonged stress enhances hippocampal glucocorticoid receptor and phosphorylated protein kinase B levels. Neurosci Res. 2013;75(2):130–137. doi: 10.1016/j.neures.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maze I, et al. Cocaine dynamically regulates heterochromatin and repetitive element unsilencing in nucleus accumbens. Proc Natl Acad Sci USA. 2011;108(7):3035–3040. doi: 10.1073/pnas.1015483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brami-Cherrier K, Roze E, Girault JA, Betuing S, Caboche J. Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. J Neurochem. 2009;108(6):1323–1335. doi: 10.1111/j.1471-4159.2009.05879.x. [DOI] [PubMed] [Google Scholar]
- 46.Elsayed M, et al. Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biol Psychiatry. 2012;72(4):258–265. doi: 10.1016/j.biopsych.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38(9):1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: Reprogramming and beyond. Nat Rev Genet. 2008;9(2):129–140. doi: 10.1038/nrg2295. [DOI] [PubMed] [Google Scholar]
- 49.Eadie BD, et al. Fmr1 knockout mice show reduced anxiety and alterations in neurogenesis that are specific to the ventral dentate gyrus. Neurobiol Dis. 2009;36(2):361–373. doi: 10.1016/j.nbd.2009.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.