Significance
In normal cells, quiescent nuclear factor κB (NFκB) is activated by inflammatory stimuli. In most cancers, the abnormal constitutive activation of NFκB contributes to malignant progression and resistance to therapy. Overexpression or constitutive activation of the EGF receptor (EGFR) in many cancers contributes to their proliferation and survival. We find that the constitutive activation of NFκB in several cancer cell lines is decreased by EGFR knockdown or by the EGFR inhibitor erlotinib. We used insertional mutagenesis to find that overexpression of Son of Sevenless 1 (SOS1), a component of EGF-dependent pathways that facilitate cell growth and survival, causes erlotinib resistance and increases NFκB activation. SOS1 is required for EGF-dependent activation of NFκB but its GDP–GTP exchange activity is not, revealing a novel function for this protein.
Abstract
Activation of nuclear factor κB (NFκB) is a central event in the responses of normal cells to inflammatory signals, and the abnormal constitutive activation of NFκB is important for the survival of most cancer cells. In nonmalignant human cells, EGF stimulates robust activation of NFκB. The kinase activity of the EGF receptor (EGFR) is required, because the potent and specific inhibitor erlotinib blocks the response. Down-regulating EGFR expression or inhibiting EGFR with erlotinib impairs constitutive NFκB activation in several different types of cancer cells and, conversely, increased activation of NFκB leads to erlotinib resistance in these cells. We conclude that EGF is an important mediator of NFκB activation in cancer cells. To explore the mechanism, we selected an erlotinib-resistant cell line in which the guanine nucleotide exchange factor Son of Sevenless 1 (SOS1), well known to be important for EGF-dependent signaling to MAP kinases, is overexpressed. Increased expression of SOS1 increases NFκB activation in several different types of cancer cells, and ablation of SOS1 inhibits EGF-induced NFκB activation in these cells, indicating that SOS1 is a functional component of the pathway connecting EGFR to NFκB activation. Importantly, the guanine nucleotide exchange activity of SOS1 is not required for NFκB activation.
Nuclear factor κB (NFκB), an important mediator of the normal response to inflammatory signals, is abnormally constitutively activated in most cancer cells, promoting resistance to apoptosis and contributing to tumorigenesis by driving cell proliferation and metastasis (1, 2). The five members of the mammalian NFκB family, RelA (p65), RelB, cRel, p105/p50 (NFκB1), and p100/p52 (NFκB2), form a variety of homo- and heterodimers. In normal unstimulated cells, NFκB dimers are kept inactive as cytoplasmic complexes, bound to a member of the inhibitor of κB (IκB) family. Many pathways that release NFκB from IκB use IκB kinase (IKK), which phosphorylates IκB, leading to its ubiquitination and proteasome-mediated degradation, liberating NFκB dimers, which then translocate to the nucleus where they activate the transcription of target genes (3). The activation of NFκB is regulated by many different stimuli in virtually all cell types, with many different functional consequences (4, 5). Specific and highly regulated control of NFκB is critical for its normal transient activation in response to stress and proinflammatory signals. Aberrant constitutive activation of NFκB in cancer (6, 7) contributes to malignant progression and therapeutic resistance, both in cell lines and in tumors (1, 8, 9).
The EGF receptor (EGFR/HER-1/ErbB1) is a member of the ErbB family of receptor tyrosine kinases. Upon stimulation, EGFR undergoes homodimerization or heterodimerization with other family members (HER-2/neu/ErbB2, HER-3/ErbB3 and HER-4/ErbB4) (10), leading to autophosphorylation and association with a set of intracellular signaling proteins that have been intensively studied for many years (11). Activation of downstream pathways facilitates cell growth, survival, and proliferation. Activation and mutation of EGFR have been observed in up to 30% of many different solid tumors, including head and neck, colorectal, breast, nonsmall cell lung, pancreatic, and gastric cancers, and usually correlate with a poor prognosis (12, 13). Thus, there is great interest in EGFR as a target for anticancer therapies that use small molecule inhibitors of its tyrosine kinase activity (14).
In contrast to the intensively studied pathways of NFκB activation as a part of the inflammatory response, the mechanisms underlying its activation in cancer are diverse and have not been well defined. For example, Lu et al. (6) showed that different cancer cell lines secrete several different cytokines and growth factors, each of which is capable of activating NFκB, including some for which this activity was not anticipated, such as transforming growth factor β and fibroblast growth factor 5. Furthermore, it is well known that some genes encoding cytokines that activate NFκB are themselves NFκB targets, including IL-1β and TNF-α, revealing positive feedback loops. NFκB can also be activated by EGF (15, 16), and different laboratories have indicated roles for several different proteins in this pathway, including RIP (17), NIK (18), GRB2-associated binder 1 (19), mTORC2 (20), CARMA3 (21), and FER (22). Although EGFR-dependent activation of NFκB has been reported before, its importance as a means of constitutive NFκB activation in cancer is unclear, and it is fair to say that the pathway has not yet been well defined.
To address these issues, we examined the ability of EGF/EGFR to activate NFκB in both nonmalignant and malignant human cell lines. Inhibition of the kinase activity of EGFR by the specific inhibitor erlotinib or knockdown of EGFR expression impaired the activation of NFκB. Conversely, activating NFκB with IL-1β increased resistance to erlotinib in cancer cells. To explore the EGFR-NFκB connection in an unbiased way, we used insertional mutagenesis to up-regulate the expression of proteins that mediate resistance to erlotinib, selecting a resistant cell line in which the guanine nucleotide exchange factor, Son of Sevenless 1 (SOS1), is overexpressed. SOS1 promotes RAS and RAC activation (23) downstream of a wide variety of receptor tyrosine kinases (24). In response to EGF, SOS1 interacts with activated EGFR through the adaptor protein GRB2, leading to the activation of RAS through the juxtaposition of SOS and RAS at the membrane (25, 26). We now show that, in addition to these well-known activities, SOS1 is required for the activation of NFκB in response to EGF.
Results
EGFR-Driven NFκB Activation in Human Mammary Epithelial Cells.
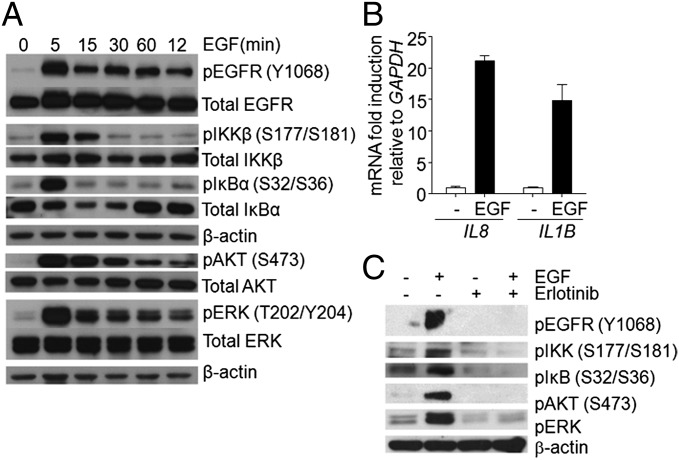
To investigate the pathway for EGF-dependent activation of NFκB without complications from a variety of genetic changes in different cancer cell lines, we began by studying nonmalignant human mammary epithelial (HME) cells. When EGF-starved HME cells were stimulated with EGF for different times (Fig. 1A), robust phosphorylation of EGFR, IKK, and IκB and substantial degradation and resynthesis of IκB were observed. Because the IκB gene NFKBIA is a highly specific NFκB target, the resynthesis of the IκB protein makes it clear that NFκB-dependent gene expression is driven very well by EGF in these cells. Because ERK and AKT are major downstream targets of EGF, we also analyzed their phosphorylation, which was increased by EGF, as expected (Fig. 1A). Quantitative real-time PCR showed a significant increase in the mRNA levels of the NFκB-targeted genes IL8 and IL1B in response to EGF (Fig. 1B). To determine whether the kinase activity of EGFR is required for signaling to NFκB, we treated EGF-starved cells with the potent and specific inhibitor erlotinib for 1 h and then stimulated them with EGF for 5 min (Fig. 1C). As expected, erlotinib blocks the phosphorylation of AKT and ERK, and it also blocks the phosphorylation of IKK and IκB. These results reveal robust EGF- and EGFR-dependent activation of NFκB in nonmalignant human epithelial cells.
Fig. 1.
EGFR-dependent activation of NFκB in HME cells. (A) The cells were EGF starved for 24 h and then stimulated with 10 ng/mL EGF for the indicated times. Stimulation of the known EGF-activated pathways that activate PI3K/ATK and MAPK, as well as the NFκB signaling components IKK and IκB, was detected with phospho-specific antibodies. Anti-IκB antibody detected the degradation and resynthesis of this protein. (B) Analysis by real-time PCR of the activation of the IL8 and IL1B genes in response to EGF. (C) Effects of erlotinib on EGF-treated HME cells. The cells were deprived of EGF for 24 h and then treated with EGF (100 ng/mL) for 10 min, with or without pretreatment with erlotinib (50 μM for 45 min). The levels of phosphorylated and total proteins were analyzed by the Western method. The experiments above were repeated three times, with very similar results.
EGFR Mediates NFκB Activation in Several Cancer Cell Lines.
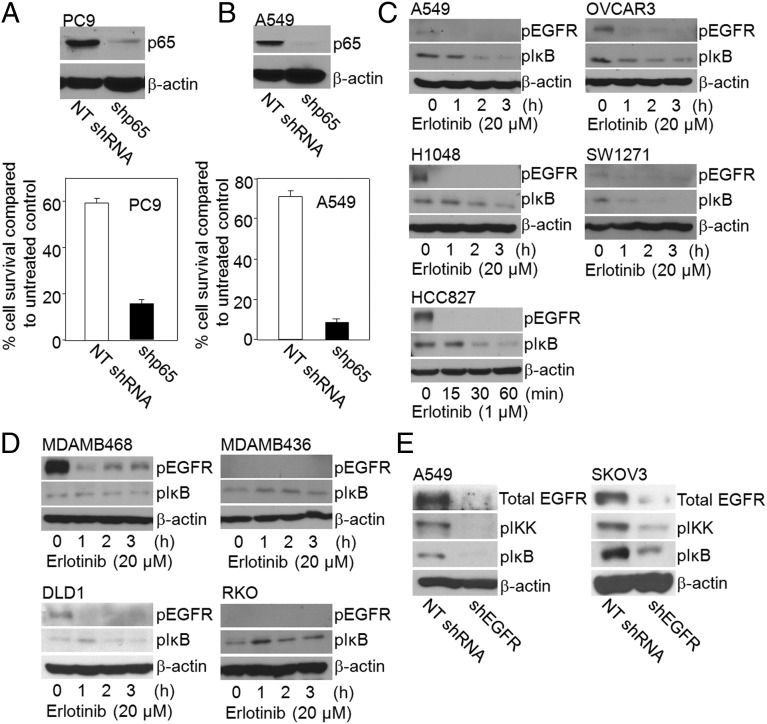
NFκB is constitutively activated in many cancers (6, 7) and thus, as expected, shRNA-mediated depletion of p65/RELA leads to greatly increased cell death in the nonsmall cell lung cancer (NSCLC) cell lines PC9 and A549 (Fig. 2 A and B). As noted above, several different mechanisms of NFκB activation in response to EGF have been reported in cell lines derived from several different tumor types. To investigate the role of EGFR in constitutive NFκB activation in cancer cells further, we treated the NSCLC cell lines A549 and HCC827, the ovarian cancer cell line OVCAR3, and the small cell lung cancer (SCLC) cell lines H1048 and SW1271 with erlotinib. Note that these cells were not pretreated with EGF or any other NFκB activator. A549, OVCAR3, H1048, and SW1271 cells have wild-type EGFR (27–30), whereas HCC827 cells carry an activating EGFR mutation (31). As expected, erlotinib inhibits EGFR phosphorylation in all of these cells and, importantly, it also decreases greatly the phosphorylation of IκB, within 2–3 h (Fig. 2C). However, the activation of NFκB was not inhibited by erlotinib in the breast cancer cell lines MDAMB468 and MDAMB436, or the colon cancer cell lines DLD1 and RKO (Fig. 2D). To confirm a role of EGFR in activating NFκB in some cancer cells, we down-regulated its expression in A549 and SKOV3 cells with an EGFR-specific shRNA. Consistent with the effects of erlotinib, the phosphorylations of IKK and IκB were decreased in the EGFR knockdown cells (Fig. 2E). In summary, EGFR is an important activator of NFκB in some cancer cells, but in others alternative mechanisms must be responsible.
Fig. 2.
EGFR mediates NFκB activation in cancer. (A and B) NFκB activation and cancer cell survival. (Upper) PC9 cells (A) and A549 cells (B) were infected with empty vector or with a vector encoding NFκB shRNA and analyzed by the Western method for expression of p65 and β-actin. (Lower) Cell survival assays were performed in control and p65 knockdown cells. The cells were lysed with 0.5 M NaOH and the A260 was measured as an indication of the total amount of nucleic acid. Means of three experiments are shown; each measurement was performed in triplicate; bars, SD. (C) Erlotinib inhibits constitutive NFκB activation in cancer cells. Cells without EGF pretreatment were treated with erlotinib for different times, and the levels of phosphorylated EGFR and IκB were determined by the Western method. β-Actin was the loading control. (D) Constitutive NFκB activity is not inhibited by erlotinib in some cancer cells. Cells without EGF pretreatment were treated with erlotinib and the levels of phosphorylated and total proteins were analyzed by the Western method. (E) EGFR knockdown inhibits constitutive NFκB activation in cancer cells. A549 and SKOV3 cells were infected with a vector encoding either a nontargeted (NT shRNA) or an EGFR shRNA and analyzed for total EGFR by the Western method. Phosphorylations of IKK and IκB were analyzed by the Western method. The experiments were repeated twice, with very similar results.
High Expression of SOS1 Increases Resistance to Erlotinib.
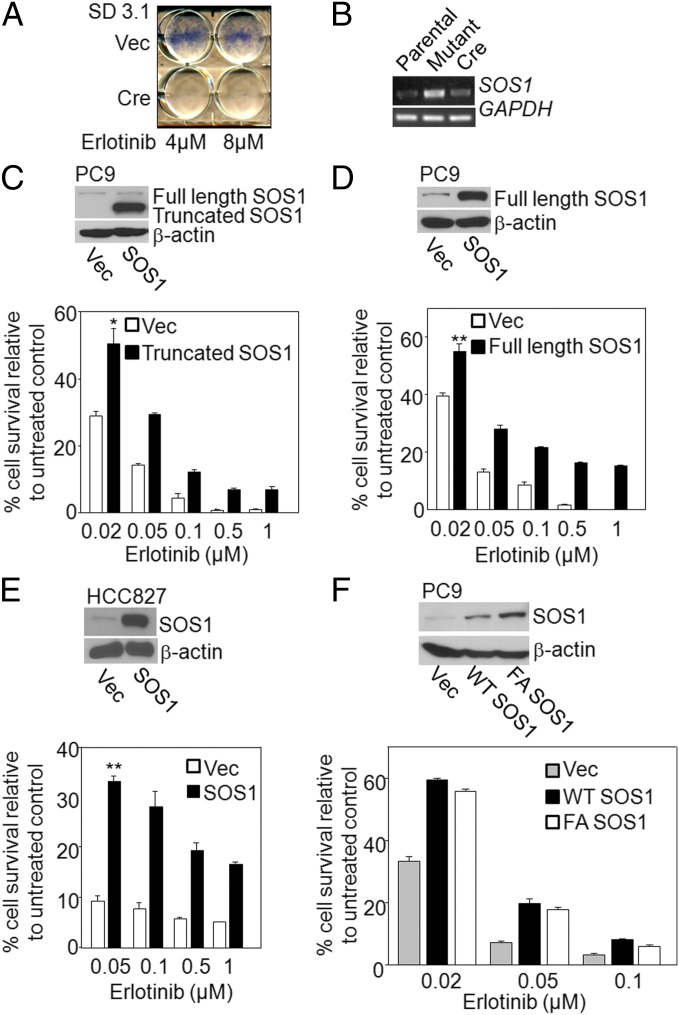
To begin to explore the mechanisms of EGFR-mediated NFκB activation in cancer cells, we isolated and characterized an erlotinib-resistant cell line. Using validation-based insertional mutagenesis (VBIM), which employs lentiviral vectors (32), we inserted the strong CMV promoter approximately randomly into the genome of the erlotinib-sensitive NSCLC cell line PC9 (33). The cells were treated with 4 μM erlotinib every 72 h. After 10 d, we isolated the erlotinib-resistant clone SD3.1, which was subsequently infected with a lentiviral vector encoding CRE recombinase, to excise the floxed CMV promoter (34). The cells were then treated with erlotinib to reveal reversion of the phenotype, providing genetic proof that the insertion caused resistance to this drug (Fig. 3A). To identify the mRNA responsible for resistance, we performed RNA-based cloning, using vector sequences present in the bicistronic mRNA expressed from the inserted promoter (32). The mRNA we identified encodes a major portion of the SOS1 protein. The full coding sequence of SOS1 translates to 1,333 amino acids, of which 355 residues of the N terminus were lost in the truncated protein driven by the inserted promoter. However, the portion of SOS1 remaining retains the catalytic domain (23). Using a 5′ VBIM-specific primer and a 3′ gene-specific primer, analysis by RT-PCR showed overexpression of the truncated SOS1 mRNA in erlotinib-resistant SD3.1 cells, and expression of CRE recombinase eliminated this expression (Fig. 3B). To determine whether both truncated and full-length SOS1 can mediate erlotinib resistance in unmutagenized cells, cDNAs encoding both forms of the protein were introduced into naive PC9 cells, resulting in stable pools of cells in which the two forms were expressed. Both pools were significantly more resistant to erlotinib than were the controls (Fig. 3 C and D). Similar results were obtained when full-length SOS1 was overexpressed in another EGFR mutant NSCLC cell line, HCC827 (Fig. 3E). Therefore, increased expression of SOS1 mediates erlotinib resistance. To determine whether the catalytic activity of SOS1 is required, the catalytically dead F929A mutant (35, 36) was stably expressed in PC9 cells. The resistance of these cells was similar to cells in which the wild-type SOS1 was overexpressed (Fig. 3F), indicating that, surprisingly, the catalytic function of SOS1 is not required to confer erlotinib resistance.
Fig. 3.
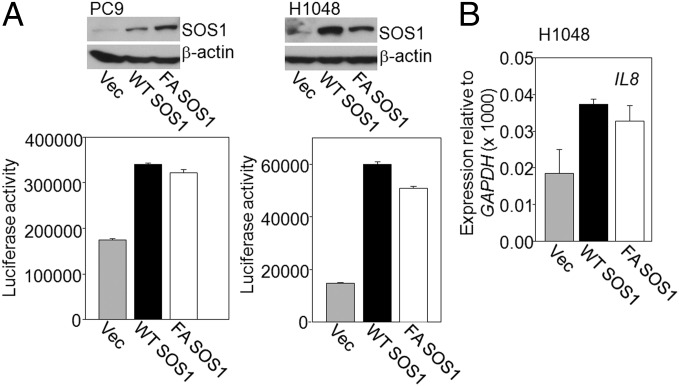
SOS1 overexpression increases erlotinib resistance. (A and B) Identification of SOS1 in an erlotinib-resistant clone. (A) Resistant SD3.1 cells were infected with an empty lentiviral vector or with a vector encoding CRE recombinase. The cells were plated into six-well plates at 200,000 cells per well. The next day, the cells were treated with erlotinib (4 or 8 μM) and stained with methylene blue 72 h later. (B) Truncated SOS1 mRNA levels were analyzed by RT-PCR, using 5′ VBIM-specific and 3′ gene-specific primers in parental, SD3.1 (mutant), or SD3.1CRE cells. GAPDH was used as a loading control. (C and D, Upper) PC9 cells were infected with empty vector (Vec) or a vector encoding truncated or full-length SOS1 and expression was examined by the Western method. (Lower) Control and SOS1-expressing cells were plated in triplicate at 200,000 cells per well in six-well plates and allowed to attach overnight. The cells were treated with erlotinib for 72 h and lysed with 0.5 M NaOH, and the A260 was measured. (E) The level of SOS1 in HCC827 cells over expressing the full-length protein was determined by the Western method (Upper). The cells were treated with erlotinib and cell survival was determined after 72 h (Lower). (F) The level of SOS1 in HCC827 cells expressing the empty vector (Vec), wild-type SOS1 (WT SOS1), or mutant F929A SOS1 (FA SOS1) protein was determined by the Western method (Upper). The cells were treated with erlotinib and cell survival was determined after 72 h (Lower). Means of three experiments are shown; each measurement was performed in triplicate; bars, SD. *P < 0.05; **P < 0.005.
SOS1 Overexpression Increases NFκB Activation in Cancer Cells.
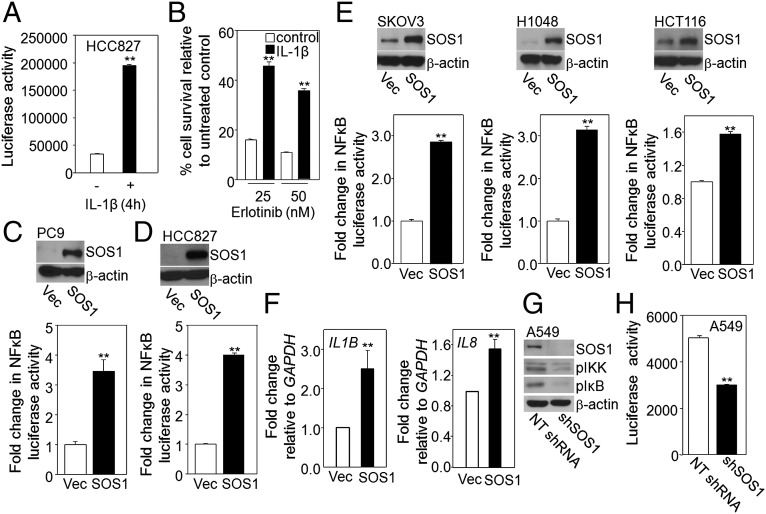
Because EGFR is an important activator of NFκB in cancer cells, we investigated whether increased activation of NFκB itself leads to erlotinib resistance. We used the NSCLC cell line HCC827, which carries an activating EGFR mutation, making it very sensitive to erlotinib (31). Stimulation of these cells with IL-1β for 4 h led to substantial increases both in NFκB activation (Fig. 4A) and in resistance to erlotinib (Fig. 4B). Because NFκB activation and SOS1 overexpression both mediate erlotinib resistance, we investigated whether SOS1 regulates NFκB. When PC9 and HCC827 cells with integrated NFκB-dependent luciferase reporters were transduced with a vector expressing SOS1 to generate stable pools of cells overexpressing this protein, we observed ∼3.5- to 4-fold increases in reporter activity (Fig. 4 C and D). To show that NFκB activation by SOS1 is not limited to NSCLC cell lines, the protein was overexpressed in several additional cancer cell lines (SKOV3, H1048, and HCT116) and NFκB activation was analyzed (Fig. 4E). SOS1 overexpression increased NFκB activation by ∼3-fold in SKOV3 and H1048 cells, and by ∼1.6-fold in HCT116 cells. Analysis by real-time PCR revealed that the levels of mRNAs expressed from the NFκB target genes IL1B and IL8 increased in PC9 cells in which SOS1 was overexpressed (Fig. 4F). The role of SOS1 in NFκB activation was further analyzed by downregulating its expression in A549 cells, leading to substantial decreases in the constitutive phosphorylation of IKK and IκB (Fig. 4G). Decreased NFκB activation upon SOS1 depletion was also seen in a reporter assay (Fig. 4H). In investigating whether the catalytic function of SOS1 is required for activating NFκB, we found that overexpression in PC9 and H1048 cells of the catalytically dead F929A mutant of SOS1 increased NFκB activation comparably to overexpression of wild-type SOS1 (Fig. 5A). An increased level of IL8 mRNA was also observed in H1048 cells overexpressing F929A SOS1 (Fig. 5B). These results indicate that the catalytic activity of SOS1 is not necessary for NFκB activation. As expected, the phosphorylation of ERK or AKT in HCC827 cells was abolished by the MEK inhibitor PD0325901 or the PI3K inhibitor GDC0941 (Fig. S1 A and B). However, there was only slight inhibition of SOS1-dependent NFκB activation by PD0325901 or GDC0941 (Fig. S1 A and B). Similarly, a RAF inhibitor GW5074 also did not abolish SOS1-dependent NFκB activation (Fig. S1C). We conclude that NFκB activation by SOS1 is not primarily dependent on the canonical pathway through which SOS1 promotes ERK activation.
Fig. 4.
Overexpression of SOS1 increases NFκB activation in cancer cells. (A and B) Increased activation of NFκB causes erlotinib resistance. (A) HCC827 cells were stimulated with IL-1β for 4 h, and NFκB activation was determined in a reporter assay. The results of triplicate luciferase assays are shown as means ± SD **P < 0.005. (B) The cells were plated in triplicate at 200,000 cells per well in six-well plates and allowed to attach overnight. They were then stimulated with IL-1β for 4 h or left unstimulated, then treated with erlotinib for 72 h and lysed with 0.5 M NaOH. The A260 was then determined, as a measure of the total amount of nucleic acid. The fraction of surviving cells was determined by normalizing the data from erlotinib-treated cells to untreated controls. Means of three experiments are shown; each measurement was performed in triplicate; bars, SD. **P < 0.005. (C–E) Stable pools of PC9 (C); HCC827 (D); and SKOV3, H1048, and HCT116 (E) cells expressing an NFκB luciferase reporter were infected with a lentivirus encoding SOS1 or with the empty vector (Vec). SOS1 expression was analyzed by the Western method. NFκB activity was determined by using a luciferase assay. The results of triplicate luciferase assays are shown as means ± SD **P < 0.005. (F) Analysis by real-time PCR of the activation of the IL1B and IL8 genes in PC9 cells overexpressing SOS1. Means of two experiments are shown; each measurement was performed in triplicate; bars, SD. (G and H) Decreased NFκB activation in SOS1 knockdown cells. The levels of phosphorylated IKK and IκB were detected by the Western method (G) and NFκB activity was measured in a luciferase assay (H). The results of triplicate luciferase assays are shown as means ± SD.
Fig. 5.
The catalytic activity of SOS1 is not required for NFκB activation in cancer cells. (A and B) Stable pools of PC9 and H1048 cells expressing an NFκB luciferase reporter were infected with a lentivirus encoding wild-type SOS1 (WT SOS1), F929A SOS1 (FA SOS1), or empty vector (Vec). SOS1 expression was analyzed by the Western method. NFκB activity was measured in a luciferase assay. The results of triplicate luciferase assays are shown as means ± SD. (B) Analysis by real-time PCR of the activation of the IL8 gene in H1048 cells expressing WT SOS1 and F929A SOS1. Means of two experiments are shown; each measurement was performed in triplicate; bars, SD.
SOS1 Mediates EGF-Dependent NFκB Activation.
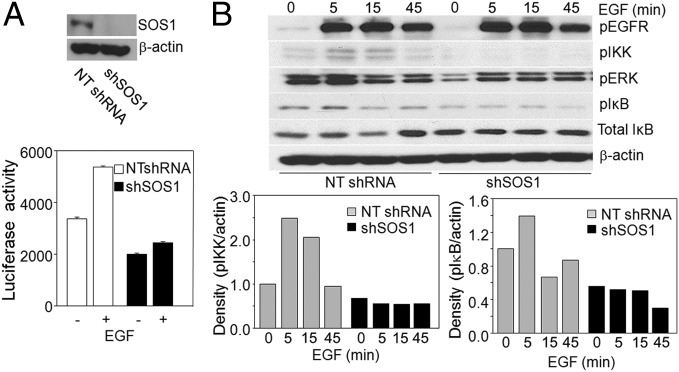
SOS1 is well known to be involved in EGF-dependent signaling pathways that facilitate cell growth and survival. Because EGFR plays an important role in NFκB activation in cancer cells and because SOS1 overexpression increases NFκB activation, it was logical to investigate whether SOS1 is on the pathway of EGFR-dependent NFκB activation. Ablation of SOS1 in A549 cells did impair EGF-induced activation of an NFκB reporter gene (Fig. 6A). Note that these cancer cells already have high constitutive levels of NFκB activation, limiting the ability of EGF to drive a further increase. To extend this result, we stimulated with EGF A549 and SKOV3 cells in which SOS1 expression was down-regulated and analyzed NFκB-dependent signaling. In cells with little SOS1 expression the basal levels of IKK and IκB phosphorylation were decreased, and EGF-dependent increases in the phosphorylation of IKK and IκB were not observed. The degradation and resynthesis of total IκB was also not observed upon EGF stimulation when SOS1 expression was ablated (Fig. 6B and Fig. S2). As expected, SOS1 down-regulation decreased the basal levels of phosphorylated ERKs and also inhibited EGF-stimulated ERK phosphorylation (Fig. 6B and Fig. S2). These results indicate that SOS1 is involved in the pathway of EGFR-mediated NFκB activation.
Fig. 6.
SOS1 mediates EGF-dependent NFκB activation: (A, Upper) A549 cells were infected with vector only or SOS1 shRNA and immunoblotted for SOS1 and β-actin. (Lower) Cells were stimulated with EGF (100 ng/mL) for 5 h or left unstimulated, and NFκB-dependent luciferase activity was measured. The cell lysates were normalized for protein concentration. (B, Upper) The cells were serum starved for 24 h and then stimulated with 100 ng/mL EGF for the indicated times. Phosphorylated EGFR, ERK, IKK, and IκB were detected with phospho-specific antibodies. Anti-IκB antibody detected the degradation and resynthesis of this protein. β-Actin was used as a loading control. (Lower) Densitometric analyses are determined by the ratio of expression of phosphorylated IKK or IκB and β-actin, measured by using NIH software (ImageJ 1.48v). The experiments above were repeated twice, with very similar results.
Discussion
The EGF/EGFR Pathway Is Responsible for NFκB Activation in Some Cancers.
NFκB can be activated by EGF in nonmalignant cells, including the human embryonic kidney cell line 293 (7) and human kidney proximal tubule cells (37). We have now found that EGFR also mediates NFκB activation in nonmalignant human mammary epithelial cells and that this activation is inhibited by the EGFR tyrosine kinase inhibitor erlotinib. Whereas NFκB activation is tightly regulated as a component of the normal inflammatory response, deregulated constitutive activation of NFκB is a hallmark of most cancers, where it contributes to resistance to apoptosis, proliferation, and the propensity to metastasize (1). Therefore, understanding the causes of constitutive NFκB activation in cancer is an important issue.
EGFR is commonly overexpressed or constitutively activated in cancer cells and contributes to their uncontrolled proliferation and survival (38). Although treatment with EGF is known to activate NFκB (39), the basis of constitutive NFκB activation in cancer is complex (6) and the contribution of EGFR to NFκB activation in the absence of treatment with exogenous EGF is unknown. Many different specific mutations provide a variety of opportunities to connect EGFR to NFκB in different cancer cells. We have found that treatment with erlotinib or down-regulation of EGFR expression inhibits NFκB activation in several different types of cancer cells in tissue culture, indicating that this pathway is likely to be responsible for constitutive NFκB activation in some cancers. However, additional mechanisms are sure to be responsible for constitutive NFκB activation in cancer, because treatment with erlotinib had no effect on NFκB activation in other cancer cells, and indeed some of these cells showed little or no constitutive phosphorylation of EGFR. Consistent with our results, it has been reported recently that the dual tyrosine kinase inhibitor lapatinib, which interrupts both the HER2/neu and EGFR pathways, inhibits NFκB activation in breast cancer cells overexpressing HER2 (40).
Constitutive NFκB activation contributes importantly to resistance to therapies in many cancers (1, 41, 42), and we show here that increasing NFκB activation by treating cells with IL-1β greatly increased resistance to erlotinib. We conclude that there is an important connection between EGFR and NFκB activation in cancer cells. Patients treated with erlotinib may receive therapeutic benefit not only from the ability of this drug to suppress the growth stimulatory activation of the RAS/ERK pathway, but also from its ability to inhibit NFκB activation. Recently it has been reported that inhibiting NFκB sensitizes NSCLC cells to erlotinib-induced cell death (42) and, similarly, quinacrine, which inhibits NFκB (43, 44), has been combined with erlotinib in a phase I/II clinical trial to test the synergistic effect of this combination in patients with advanced or metastatic NSCLC (NCT01839955).
Mechanistic Insight.
Previously, we used the VBIM method of insertional mutagenesis to show that overexpression of kinesins mediates resistance to docetaxel (34) and that overexpression of FER confers resistance to quinacrine (22). We have now used this method to isolate an erlotinib-resistant cell line in which SOS1 is overexpressed. SOS1 is well known to participate in EGF-dependent signaling pathways that facilitate cell growth and survival (26, 45). SOS1 is a guanine nucleotide exchange factor that promotes RAS and RAC activation downstream of EGFR and other growth factor receptors. Stimulation of cells with growth factors leads to the association of SOS–GRB2 complexes with the activated receptors, leading to the activation of RAS through the juxtaposition of SOS and RAS at the membrane (25). Growth factor-induced phosphorylation of serine/threonine residues of SOS1, mediated by MAP kinases, alters its association with GRB2 and inhibits its function, providing a negative feedback mechanism (45–47). SOS1 has been reported to be involved in several different cancers including breast cancer, hematological malignancies, and skin cancer (23). Amplification of GRB2 and SOS1 has been reported in bladder cancer (48), and overexpression of SOS1 has been seen in prostate (49) and kidney cancer cells (50). The requirement of EGFR for SOS1-dependent skin tumor development has been shown in a transgenic mouse model (51). The ability of increased expression of SOS1 to confer resistance to erlotinib in cancer cells prompted us to explore its role in the EGFR–NFκB pathway. Suppression of SOS1 inhibits EGF–induced NFκB activation, indicating that SOS1 is indeed involved in this pathway. We have also shown that overexpression of SOS1 increases NFκB activation dramatically in cancer cells, and that constitutive NFκB activation is impaired by SOS1 depletion. These results underscore the importance of SOS1 in cancer cell survival, which is well known to be mediated by up-regulation of NFκB activation. To our knowledge ours is the first report showing that SOS1 is an activator of NFκB. The F929A mutation of SOS1 abrogates its ability to catalyze guanine nucleotide exchange (35, 36) but not its ability to activate NFκB. We demonstrate that F929A mutant SOS1 increases erlotinib resistance and NFκB activation with an efficiency similar to that of wild-type SOS1. Consistently, SOS1-dependent activation of NFκB is not inhibited by a MEK inhibitor, a PI3K inhibitor, or a RAF inhibitor (Fig. S1 A–C). These results indicate that SOS1-mediated EGFR–NFκB activation is independent of the catalytic activity of SOS1 and that a currently unknown pathway connects SOS1 to NFκB. It is interesting and relevant that a recent study has shown that SOS1 plays an adaptor role in RAC and p38 activation in which its catalytic activities are also dispensable (36).
Materials and Methods
The hTERT-HME1 cell line was purchased from Clontech. The cancer cell lines HCC827, A549, HCT116, H1048, and SW1271 were obtained from American Tissue Culture Collection. VBIM vector constructs were described previously (32, 34). Cell survival assays were done as described previously (34). Luciferase assays were done by using the NFκB reporter construct. Detailed materials and methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Maojing Yang for excellent technical assistance. This work was supported by National Institutes of Health Grant P01CA062220.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412390111/-/DCSupplemental.
References
- 1.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107(3):241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perkins ND. The diverse and complex roles of NF-κB subunits in cancer. Nat Rev Cancer. 2012;12(2):121–132. doi: 10.1038/nrc3204. [DOI] [PubMed] [Google Scholar]
- 3.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Perkins ND, Gilmore TD. Good cop, bad cop: The different faces of NF-kappaB. Cell Death Differ. 2006;13(5):759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 5.Baltimore D. NF-κB is 25. Nat Immunol. 2011;12(8):683–685. doi: 10.1038/ni.2072. [DOI] [PubMed] [Google Scholar]
- 6.Lu T, Sathe SS, Swiatkowski SM, Hampole CV, Stark GR. Secretion of cytokines and growth factors as a general cause of constitutive NFkappaB activation in cancer. Oncogene. 2004;23(12):2138–2145. doi: 10.1038/sj.onc.1207332. [DOI] [PubMed] [Google Scholar]
- 7.Sethi G, Ahn KS, Chaturvedi MM, Aggarwal BB. Epidermal growth factor (EGF) activates nuclear factor-kappaB through IkappaBalpha kinase-independent but EGF receptor-kinase dependent tyrosine 42 phosphorylation of IkappaBalpha. Oncogene. 2007;26(52):7324–7332. doi: 10.1038/sj.onc.1210544. [DOI] [PubMed] [Google Scholar]
- 8.Yu HG, et al. Increased expression of RelA/nuclear factor-kappa B protein correlates with colorectal tumorigenesis. Oncology. 2003;65(1):37–45. doi: 10.1159/000071203. [DOI] [PubMed] [Google Scholar]
- 9.Tang X, et al. Nuclear factor-kappaB (NF-kappaB) is frequently expressed in lung cancer and preneoplastic lesions. Cancer. 2006;107(11):2637–2646. doi: 10.1002/cncr.22315. [DOI] [PubMed] [Google Scholar]
- 10.Ladanyi M, Pao W. Lung adenocarcinoma: Guiding EGFR-targeted therapy and beyond. Mod Pathol. 2008;21(Suppl 2):S16–S22. doi: 10.1038/modpathol.3801018. [DOI] [PubMed] [Google Scholar]
- 11.Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284(1):99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 12.Wykosky J, Fenton T, Furnari F, Cavenee WK. Therapeutic targeting of epidermal growth factor receptor in human cancer: Successes and limitations. Chin J Cancer. 2011;30(1):5–12. doi: 10.5732/cjc.010.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W, et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;480(7375):118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammerman PS, Jänne PA, Johnson BE. Resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2009;15(24):7502–7509. doi: 10.1158/1078-0432.CCR-09-0189. [DOI] [PubMed] [Google Scholar]
- 15.Brown M, Cohen J, Arun P, Chen Z, Van Waes C. NF-kappaB in carcinoma therapy and prevention. Expert Opin Ther Targets. 2008;12(9):1109–1122. doi: 10.1517/14728222.12.9.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang W, et al. EGFR-induced and PKCε monoubiquitylation-dependent NF-κB activation upregulates PKM2 expression and promotes tumorigenesis. Mol Cell. 2012;48(5):771–784. doi: 10.1016/j.molcel.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habib AA, et al. The epidermal growth factor receptor engages receptor interacting protein and nuclear factor-kappa B (NF-kappa B)-inducing kinase to activate NF-kappa B. Identification of a novel receptor-tyrosine kinase signalosome. J Biol Chem. 2001;276(12):8865–8874. doi: 10.1074/jbc.M008458200. [DOI] [PubMed] [Google Scholar]
- 18.Chen D, et al. NIK is a component of the EGF/heregulin receptor signaling complexes. Oncogene. 2003;22(28):4348–4355. doi: 10.1038/sj.onc.1206532. [DOI] [PubMed] [Google Scholar]
- 19.Kapoor GS, Zhan Y, Johnson GR, O’Rourke DM. Distinct domains in the SHP-2 phosphatase differentially regulate epidermal growth factor receptor/NF-kappaB activation through Gab1 in glioblastoma cells. Mol Cell Biol. 2004;24(2):823–836. doi: 10.1128/MCB.24.2.823-836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka K, et al. Oncogenic EGFR signaling activates an mTORC2-NF-κB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1(6):524–538. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang T, et al. CARMA3 is crucial for EGFR-Induced activation of NF-κB and tumor progression. Cancer Res. 2011;71(6):2183–2192. doi: 10.1158/0008-5472.CAN-10-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo C, Stark GR. FER tyrosine kinase (FER) overexpression mediates resistance to quinacrine through EGF-dependent activation of NF-kappaB. Proc Natl Acad Sci USA. 2011;108(19):7968–7973. doi: 10.1073/pnas.1105369108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierre S, Bats AS, Coumoul X. Understanding SOS (Son of Sevenless) Biochem Pharmacol. 2011;82(9):1049–1056. doi: 10.1016/j.bcp.2011.07.072. [DOI] [PubMed] [Google Scholar]
- 24.Buday L, Downward J. Many faces of Ras activation. Biochim Biophys Acta. 2008;1786(2):178–187. doi: 10.1016/j.bbcan.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Jorge R, et al. HSos1 contains a new amino-terminal regulatory motif with specific binding affinity for its pleckstrin homology domain. J Biol Chem. 2002;277(46):44171–44179. doi: 10.1074/jbc.M204423200. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D. Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat Cell Biol. 2007;9(6):706–712. doi: 10.1038/ncb1594. [DOI] [PubMed] [Google Scholar]
- 27.Tracy S, et al. Gefitinib induces apoptosis in the EGFRL858R non-small-cell lung cancer cell line H3255. Cancer Res. 2004;64(20):7241–7244. doi: 10.1158/0008-5472.CAN-04-1905. [DOI] [PubMed] [Google Scholar]
- 28.Bianco C, et al. Antitumor activity of combined treatment of human cancer cells with ionizing radiation and anti-epidermal growth factor receptor monoclonal antibody C225 plus type I protein kinase A antisense oligonucleotide. Clin Cancer Res. 2000;6(11):4343–4350. [PubMed] [Google Scholar]
- 29.Ogino H, et al. E7080 suppresses hematogenous multiple organ metastases of lung cancer cells with nonmutated epidermal growth factor receptor. Mol Cancer Ther. 2011;10(7):1218–1228. doi: 10.1158/1535-7163.MCT-10-0707. [DOI] [PubMed] [Google Scholar]
- 30.Ohashi K, et al. Characteristics of lung cancers harboring NRAS mutations. Clin Cancer Res. 2013;19(9):2584–2591. doi: 10.1158/1078-0432.CCR-12-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pao W, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu T, et al. Validation-based insertional mutagenesis identifies lysine demethylase FBXL11 as a negative regulator of NFkappaB. Proc Natl Acad Sci USA. 2009;106(38):16339–16344. doi: 10.1073/pnas.0908560106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okabe T, et al. Differential constitutive activation of the epidermal growth factor receptor in non-small cell lung cancer cells bearing EGFR gene mutation and amplification. Cancer Res. 2007;67(5):2046–2053. doi: 10.1158/0008-5472.CAN-06-3339. [DOI] [PubMed] [Google Scholar]
- 34.De S, Cipriano R, Jackson MW, Stark GR. Overexpression of kinesins mediates docetaxel resistance in breast cancer cells. Cancer Res. 2009;69(20):8035–8042. doi: 10.1158/0008-5472.CAN-09-1224. [DOI] [PubMed] [Google Scholar]
- 35.Jeng HH, Taylor LJ, Bar-Sagi D. Sos-mediated cross-activation of wild-type Ras by oncogenic Ras is essential for tumorigenesis. Nat Commun. 2012;3:1168. doi: 10.1038/ncomms2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jun JE, Yang M, Chen H, Chakraborty AK, Roose JP. Activation of extracellular signal-regulated kinase but not of p38 mitogen-activated protein kinase pathways in lymphocytes requires allosteric activation of SOS. Mol Cell Biol. 2013;33(12):2470–2484. doi: 10.1128/MCB.01593-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Häussler U, von Wichert G, Schmid RM, Keller F, Schneider G. Epidermal growth factor activates nuclear factor-kappaB in human proximal tubule cells. Am J Physiol Renal Physiol. 2005;289(4):F808–F815. doi: 10.1152/ajprenal.00434.2003. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Stiegler AL, Boggon TJ, Kobayashi S, Halmos B. EGFR-mutated lung cancer: A paradigm of molecular oncology. Oncotarget. 2010;1(7):497–514. doi: 10.18632/oncotarget.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alberti C, et al. Ligand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NFkB pathway in advanced-stage epithelial ovarian cancer. Oncogene. 2012;31(37):4139–4149. doi: 10.1038/onc.2011.572. [DOI] [PubMed] [Google Scholar]
- 40.Ma C, et al. Lapatinib inhibits the activation of NF-κB through reducing phosphorylation of IκB-α in breast cancer cells. Oncol Rep. 2013;29(2):812–818. doi: 10.3892/or.2012.2159. [DOI] [PubMed] [Google Scholar]
- 41.Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805(2):167–180. doi: 10.1016/j.bbcan.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Bivona TG, et al. FAS and NF-κB signalling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471(7339):523–526. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurova KV, et al. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-kappaB-dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci USA. 2005;102(48):17448–17453. doi: 10.1073/pnas.0508888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jani TS, DeVecchio J, Mazumdar T, Agyeman A, Houghton JA. Inhibition of NF-kappaB signaling by quinacrine is cytotoxic to human colon carcinoma cell lines and is synergistic in combination with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or oxaliplatin. J Biol Chem. 2010;285(25):19162–19172. doi: 10.1074/jbc.M109.091645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douville E, Downward J. EGF induced SOS phosphorylation in PC12 cells involves P90 RSK-2. Oncogene. 1997;15(4):373–383. doi: 10.1038/sj.onc.1201214. [DOI] [PubMed] [Google Scholar]
- 46.Buday L, Warne PH, Downward J. Downregulation of the Ras activation pathway by MAP kinase phosphorylation of Sos. Oncogene. 1995;11(7):1327–1331. [PubMed] [Google Scholar]
- 47.Orton RJ, Sturm OE, Gormand A, Wolch W, Gilbert DR. Computational modelling reveals feedback redundancy within the epidermal growth factor receptor/extracellular-signal regulated kinase signalling pathway. IET Syst Biol. 2008;2(4):173–183. doi: 10.1049/iet-syb:20070066. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe T, et al. Significance of the Grb2 and son of sevenless (Sos) proteins in human bladder cancer cell lines. IUBMB Life. 2000;49(4):317–320. doi: 10.1080/15216540050033195. [DOI] [PubMed] [Google Scholar]
- 49.Timofeeva OA, et al. Enhanced expression of SOS1 is detected in prostate cancer epithelial cells from African-American men. Int J Oncol. 2009;35(4):751–760. [PMC free article] [PubMed] [Google Scholar]
- 50.Guerrero C, et al. Expression of alternative forms of Ras exchange factors GRF and SOS1 in different human tissues and cell lines. Oncogene. 1996;12(5):1097–1107. [PubMed] [Google Scholar]
- 51.Sibilia M, et al. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell. 2000;102(2):211–220. doi: 10.1016/s0092-8674(00)00026-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.