Significance
The essential micronutrient zinc is known to modulate adaptive immune responses and dysregulated zinc homeostasis leads to immunodeficiency. However, the molecular mechanisms underlying this zinc-mediated modulation are unknown. We show that the zinc transporter ZIP10 plays an important role in B-cell receptor (BCR) signaling. Zip10-deficiency in mature B cells attenuated both T-cell–dependent and –independent immune responses. Zip10-deficient mature B cells proliferated poorly in response to BCR cross-linking, as a result of dysregulated BCR signaling. Our data establish that ZIP10 functions as a cellular regulator to modulate BCR signaling in humoral immune responses.
Keywords: B lymphocyte, acquired immunity, germinal center, antigen-presenting cell, zinc signaling
Abstract
The humoral immune response, also called the antibody-mediated immune response, is one of the main adaptive immune systems. The essential micronutrient zinc (Zn) is known to modulate adaptive immune responses, and dysregulated Zn homeostasis leads to immunodeficiency. However, the molecular mechanisms underlying this Zn-mediated modulation are largely unknown. Here, we show that the Zn transporter SLC39A10/ZIP10 plays an important role in B-cell antigen receptor (BCR) signal transduction. Zip10-deficiency in mature B cells attenuated both T-cell–dependent and –independent immune responses in vivo. The Zip10-deficient mature B cells proliferated poorly in response to BCR cross-linking, as a result of dysregulated BCR signaling. The perturbed signaling was found to be triggered by a reduction in CD45R phosphatase activity and consequent hyperactivation of LYN, an essential protein kinase in BCR signaling. Our data suggest that ZIP10 functions as a positive regulator of CD45R to modulate the BCR signal strength, thereby setting a threshold for BCR signaling in humoral immune responses.
The humoral immune response is a major arm of the adaptive immune systems, in which B cells play a key role (1, 2). In the bone marrow (BM), the initial commitment to pro-B cells occurs, followed by their differentiation into pre-B cells and then into immature (IMM) B cells, which express the B-cell antigen receptor (BCR) on their surface. The IMM B cells then migrate to the spleen as transitional B cells, and further differentiate into follicular (FO) or marginal zone (MZ) mature B cells. FO B cells are a highly recirculating population, and are essential for T-cell–dependent (TD) immune responses, in which BCR-activated B cells enter the germinal center (GC), where they undergo massive expansion and Ig class-switch recombination (CSR). In contrast, MZ B cells are noncirculating and mediate rapid T-cell–independent (TI) immune responses against blood-borne pathogens. In addition to the conventional B-2 cell subset described above, a distinct B-1 cell subset resides mainly in the peritoneal cavity and produces natural antibodies (1, 2).
The BCR is composed of membrane-bound Ig and associated Igα and Igβ subunits. Following BCR binding to its cognate antigen, the Igα and Igβ subunits are phosphorylated on tyrosines within their immunoreceptor tyrosine-based activation motifs (ITAMs) by SRC family kinases, including LYN (3). The SYK tyrosine kinase is then recruited to the phosphorylated ITAMs, resulting in SYK’s activation, and the subsequent activation of downstream molecules, such as ERK, PI3K, and NF-κB. BCR activation not only drives adaptive immune responses, but also mediates a “tonic” signal together with B-cell–activating factor (BAFF) receptor (BAFFR) signaling to help maintain immunocompetent mature B-cell pools in the steady state (4, 5). Thus, BCR signaling critically regulates the activation status and fate decisions of B cells.
Zinc (Zn) deficiency leads to lymphopenia and to attenuations of both cellular and humoral immunity, resulting in an increased susceptibility to various pathogens (6, 7). Zn is reported to function as a signaling factor (8–11) and its homeostasis is tightly controlled by Zn transporters, the SLC39/ZIP and SLC30/ZnT family members, which contribute to Zn influx and efflux, respectively (12, 13). Notably, it was shown that Zn transferred by a specific Zn transporter can selectively fine-tune distinct intracellular signaling events (14) by targeting specific signaling molecules (15–20). Moreover, the disruption of a given Zn signaling axis can have pathogenic consequences in the absence of redundant machinery (21). However, the specific mechanisms involved in Zn and Zn transporter modulation of the immune system—in particular, the humoral immune response—are not well understood.
In this study, we showed that the Zn transporter, ZIP10 (Zrt- and Irt-like protein 10), is required for proper antibody responses following BCR activation. Mice with a conditional knockout of ZIP10 in mature B cells showed dramatic attenuations of TD and TI antibody responses. In addition, GC development failed in these mice, resulting in a marked reduction in antigen-specific IgG1 responses. Moreover, ZIP10 deficiency led to hyperactivated BCR signaling, which reduced cell proliferation because of decreased CD45R protein tyrosine phosphatase (PTPase) activity. Our results establish a link between ZIP10 and humoral immunity, in which ZIP10 controls the BCR signal strength as a positive regulator of CD45R, thereby setting a threshold for B-cell signaling.
Results
Conditional Ablation of ZIP10 in Cells Involved in the Humoral Immune Response.
We first investigated the role of ZIP10 (22, 23), a Zn transporter whose physiological functions were unknown, in immune cells involved in the humoral immune response. Zip10 was ubiquitously but differentially expressed in various tissues, including immune tissues (24). Among the splenic immune cell populations, B cells expressed the highest level of Zip10 mRNA (SI Appendix, Fig. S1). To generate mice with the conditional deletion of ZIP10 in antigen-presenting cells (APCs), which regulate the humoral immune response, we used invariant chain (Cd74; Ii)-Cre transgenic mice, in which transgene-encoded Cre recombinase is expressed concurrently with the Ii locus, which is constitutively activated in MHCII+ APCs, such as the B-cell and dendritic cell (DC) populations (SI Appendix, Fig. S2 A–C). Zip10 was reduced by 70–80% in the splenic B cells and BM-derived DCs from the Ii-Cre/Zip10-conditional knockout (Ii-Cre-cKO) mice (SI Appendix, Fig. S2D).
ZIP10 Deficiency Reduces the Mature B-Cell Populations.
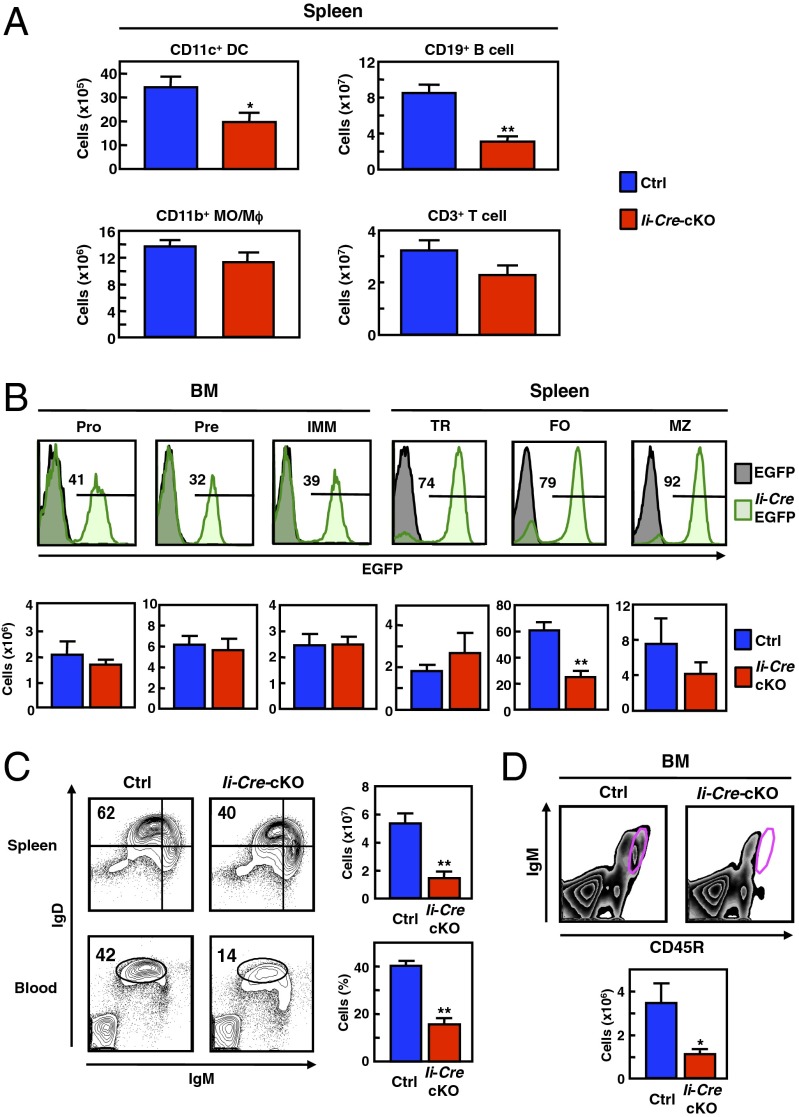
The Ii-Cre-cKO mice exhibited reduced numbers of splenic DCs and B cells (Fig. 1A). Among the B-cell subsets, most of the splenic, but not BM B-cell populations expressed Ii promoter-driven Cre recombinase (Fig. 1B). The number of FO B cells was significantly reduced in the Ii-Cre-cKO mice (Fig. 1B and SI Appendix, Fig. S3). The reduction in FO B cells was confirmed by examining the population of IgDhiIgMlo cells in the spleen and blood (Fig. 1C), and the recirculating mature B-cell pool in the BM from Ii-Cre-cKO mice (Fig. 1D). Similar data were obtained using Cd21-Cre transgenic mice (5), in which Zip10 was specifically deleted in mature B cells and follicular DCs, but not using Cd11c-Cre transgenic mice (25), in which Zip10 was deleted in DCs (SI Appendix, Fig. S4). Thus, the B-cell production appeared to be normal in Ii-Cre-cKO mice, but the homeostasis of conventional B-2 cells in the periphery was impaired. Furthermore, the number of B-1 cells and IgG3 natural antibody level were also significantly reduced in the Ii-Cre-cKO mice (SI Appendix, Fig. S5).
Fig. 1.
ZIP10 deficiency leads to the loss of mature B cells. (A) The numbers of CD11c+ DC, CD19+ B cell, CD11b+ MO/Mϕ, and CD3+ T cell in the spleen (11- to 12-wk-old males, n = 4 for each). Data represent the mean ± SEM (*P < 0.05, **P < 0.01). (B, Upper) EGFP reporter expression driven by Cre recombinase activity in B-cell subsets. (Lower) Bar charts representing the cell numbers in each B-cell subset (11- to 12-wk-old males, n = 4 for each). Data represent the mean ± SEM (**P < 0.01). (C) IgDhiIgMlo FO B-cell number and population in the spleen and blood, respectively (spleen; 11- to 12-wk-old males, n = 4 for each, blood; 17-wk-old males, n = 5 for each). Data represent the mean ± SEM (**P < 0.01). (D) CD45RhiIgM+ recirculating mature B-cell number in the BM (11- to 12-wk-old males, n = 4 for each). Data represent the mean ± SEM (*P < 0.05).
Zip10-Deficient Mature B Cells Have a Shortened Lifespan.
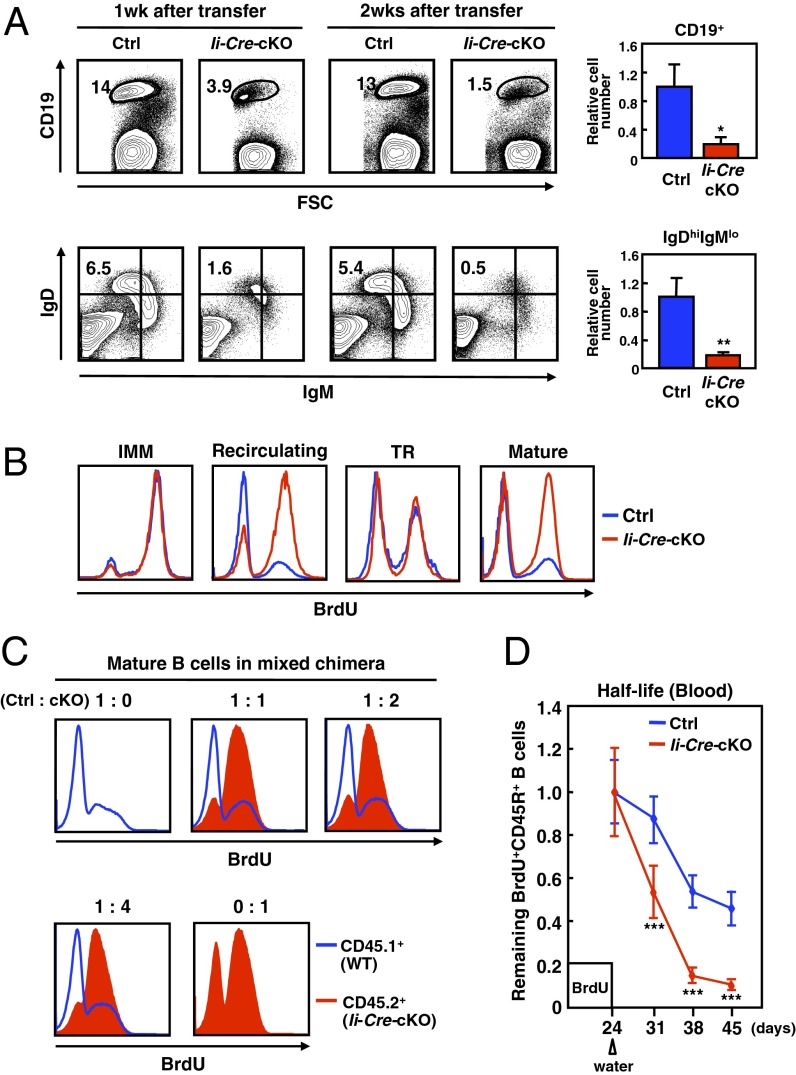
To determine whether the lymphopenic phenotype in Ii-Cre-cKO mice was the result of a cell-autonomous defect, we performed the adoptive transfer of splenic B cells into Rag1-KO mice. The resident IgDhiIgMlo B-cell population in the recipient spleen remained constant after the transfer of control cells, but decreased over time after the transfer of Ii-Cre-cKO B cells (Fig. 2A), suggesting that the decreased number of mature B cells is a B-cell–autonomous defect, and that ZIP10 is required for the persistence of mature B cells in the spleen.
Fig. 2.
Zip10-deficient mature B cells have a shortened lifespan. (A) Resident IgDhiIgMlo FO B-cell population in the spleen from Rag-1 KO mice transferred purified splenic B cells. Bar charts representing the relative cell numbers 10 d posttransfer (n = 3 for each). Data represent the mean ± SD (*P < 0.05, **P < 0.01). (B) BrdU incorporation in B-cell subsets in the BM and spleen. IMM: CD45RloIgM+; recirculating: CD45RhiIgM+; TR: CD45R+AA4.1+; mature: CD45R+AA4.1−. (C) BrdU incorporation in mature (CD45R+AA4.1−) B cells from mixed BM chimeras. (D) Lifespan of peripheral B cells. The relative percentage of CD45R+BrdU+ cells in the blood is shown (8 wk, n = 4 for Ctrl, n = 9 for Ii-Cre-cKO). Data represent the mean ± SD (***P < 0.001).
Next, we examined the possibility that a substantial portion of the newly generated peripheral B cells failed to enter the long-lived B-cell pool in the spleen, which would be reflected in an increased rate of B-cell turnover. Mice were fed BrdU, and the BrdU incorporation was measured in their B cells. Higher levels of BrdU were incorporated into the mature B cells of the spleen and BM from Ii-Cre-cKO than from control mice (Fig. 2B). It was possible that the higher BrdU incorporation rate in Zip10-deficient mature B cells was caused by the activation of the homeostatic proliferation machinery, because naïve B cells introduced into a lymphopenic host undergo antigen-independent proliferation (26). To exclude this possibility, we generated a mixed BM chimera by injecting wild-type (CD45.1) and Ii-Cre-cKO (CD45.2) BM cells at various ratios into lethally irradiated CD45.1 host mice. High levels of BrdU were incorporated into most of the reconstituted Zip10-deficient mature B cells compared with the reconstituted wild-type B cells, independent of the ratio of cells transferred (Fig. 2C), suggesting that the Zip10-deficient mature B cells were not influenced by homeostatic regulation mechanisms; rather, they failed to enter the long-lived pool and showed rapid turnover. To confirm that the Ii-Cre-cKO B cells underwent increased turnover, we measured the turnover rate of the blood-circulating B-cell pool, which mostly consists of recirculating mature B cells. As expected, BrdU-labeled Ii-Cre-cKO cells rapidly disappeared from the blood (Fig. 2D).
Mature B-cell homeostasis depends on BAFFR- (4) and BCR-mediated signaling (5, 27). Although BAFFR was expressed at lower levels (∼20%) in the Ii-Cre-cKO compared with control B cells, there was no significant difference in the living B-cell population compared with control cells at 4 d after BAFF treatment (SI Appendix, Fig. S6), suggesting that the loss of ZIP10 did not critically affect the BAFF dependency.
Impaired TD and TI Responses in the Absence of ZIP10.
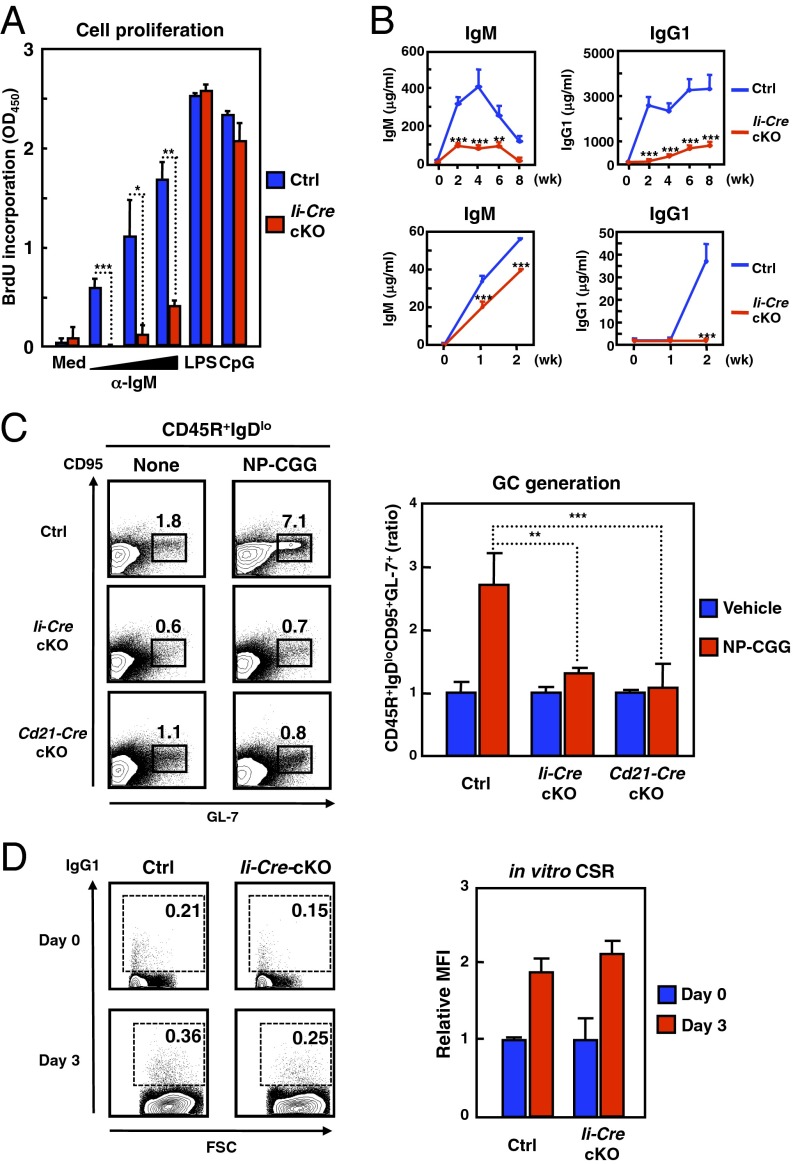
We next asked whether BCR signaling was impaired in Zip10-deficient B cells. We found that the BCR- but not Toll-like receptor-mediated proliferative activity was severely diminished in these cells (Fig. 3A), indicating that ZIP10 selectively regulates BCR signaling in vitro. Ii-Cre-cKO mice immunized with a TD antigen, 4-hydroxy-3-nitrophenylacetyl-conjugated chicken γ-globulin (NP-CGG), showed the dramatic reduction of anti-NP–specific IgM and IgG1 productions in a cell-intrinsic manner (Fig. 3B). Intriguingly, the secretion of IgG1 was considerably decreased (Fig. 3B), suggesting that GC formation, which is required for the generation of high-affinity IgG1 antibodies (28), might have been abrogated in the Ii-Cre-cKO mice. In fact, the generation of GC B cells was severely impaired in these mice (Fig. 3C), although the capacity for CSR was unaffected (Fig. 3D). These data collectively suggest that ZIP10 controls the TD immune response by regulating the persistence of GC B cells.
Fig. 3.
Impaired antibody production in the absence of ZIP10. (A) Cell proliferation assay. Data represent the mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001). (B, Upper) TD response in mice (n = 8 for each) immunized intraperitoneally with NP-CGG in alum. Data represent the mean ± SEM (**P < 0.01, ***P < 0.001). (Lower) TD response in Rag1-KO mice transferred purified wild-type or Ii-Cre-cKO CD43− splenic B cells with wild-type splenic CD4+ T cells, and immunized with NP-CGG. Data represent the mean ± SEM (***P < 0.001). (C) GC (CD45R+IgDloCD95+GL-7+) B-cell formation. Bar charts representing the relative GC B-cell populations (Nontreated control; n = 6, NP-CGG-treated control; n = 5, Nontreated Ii-Cre-cKO; n = 4, NP-CGG-treated Ii-Cre-cKO; n = 4, Nontreated Cd21-Cre-cKO; n = 3, NP-CGG-treated Cd21-Cre-cKO; n = 4). Data represent the mean ± SEM (**P < 0.01, ***P < 0.001). (D) CSR in vitro. Bar charts representing the relative mean fluorescence intensity of surface IgG1 expression. Data represent the mean ± SD.
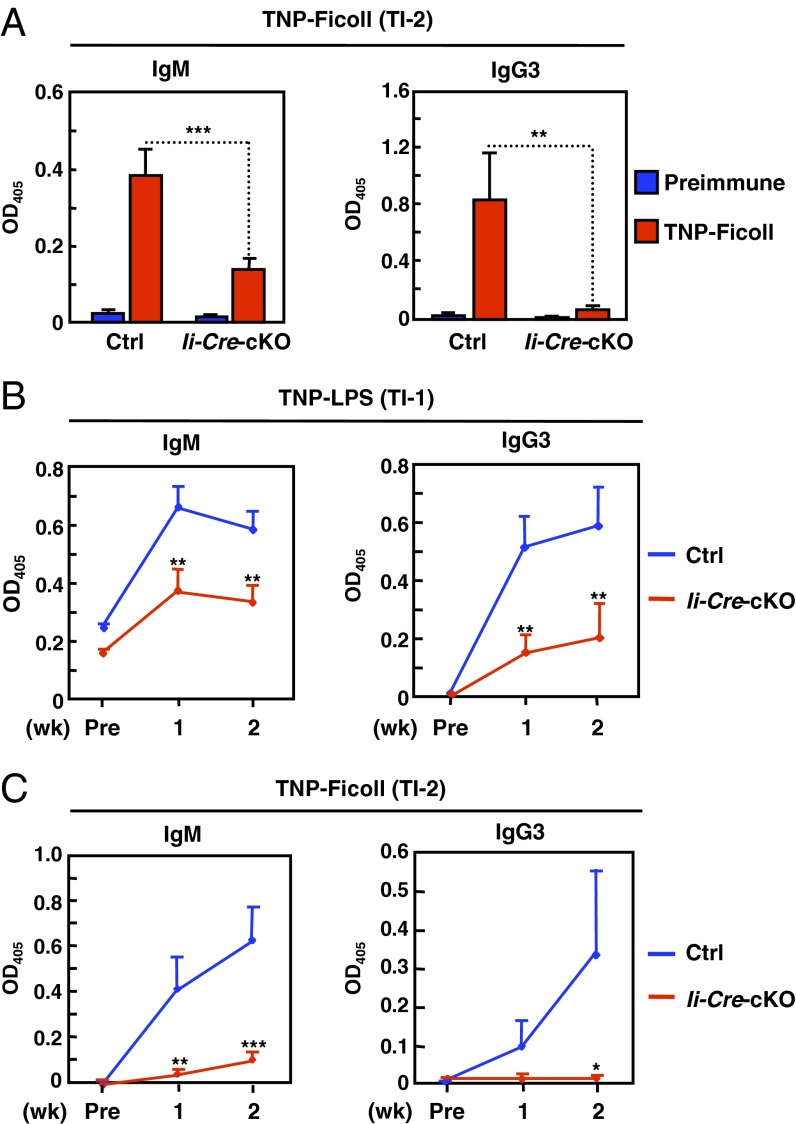
TI antigens are classified into type 1 (TI-1) and type 2 (TI-2). TI-1 antigens stimulate antibody production in all B cells in a polyclonal manner, whereas TI-2 ones primarily promote MZ B-cell activation, leading to robust IgM and IgG3 antibody productions. Immunization with 2, 4, 6-trinitrophenyl (TNP)-Ficoll (TI-2) or TNP-LPS (TI-1) resulted in anti-TNP IgM and IgG3 secretions that were both severely reduced in Ii-Cre-cKO mice (Fig. 4 A and B), and the impaired TI-2 response was because of a cell-intrinsic defect of Zip10-deficient B cells (Fig. 4C). Thus, ZIP10 contributes to both TD and TI immune responses, and its absence may result in reduced BCR-dependent cellular activity.
Fig. 4.
ZIP10 deficiency results in impaired TI responses. (A) TI-2 response in mice (n = 5 for each) immunized intraperitoneally with TNP-Ficoll. Data represent the mean ± SEM (**P < 0.01, ***P < 0.001). (B) TI-1 response in mice (n = 5 for each) immunized intraperitoneally with TNP-LPS. Data represent the mean ± SEM (**P < 0.01). (C) TI-2 response in Rag1-KO mice transferred with purified CD19+ splenic B cells and immunized intraperitoneally with TNP-Ficoll. Data represent the mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001).
ZIP10 Controls the BCR Signal Transduction Pathway Through CD45R PTPase Activity.
We next examined the molecular mechanisms involved in the ZIP10-mediated modulation of BCR signaling. In B cells, ZIP10 was predominantly localized to the plasma membrane and was expressed with modifications such as glycosylation and truncation (SI Appendix, Figs. S7 A–C and S8), as previously described (23). Reflecting these observations, Zn uptake capacity was significantly lower in Zip10-deficient B cells (SI Appendix, Fig. S7D). However, intriguingly, inductively coupled plasma-atomic emission spectroscopy (ICP-AES) and flow cytometric analysis with Zn indicators showed little alteration in the total intracellular Zn content in the Zip10-deficient B cells (SI Appendix, Fig. S7 E–G), suggesting that ZIP10 may transport Zn from extracellular fluid under spatio-temporally restricted conditions, rather than affecting the overall intracellular Zn homeostasis in these cells.
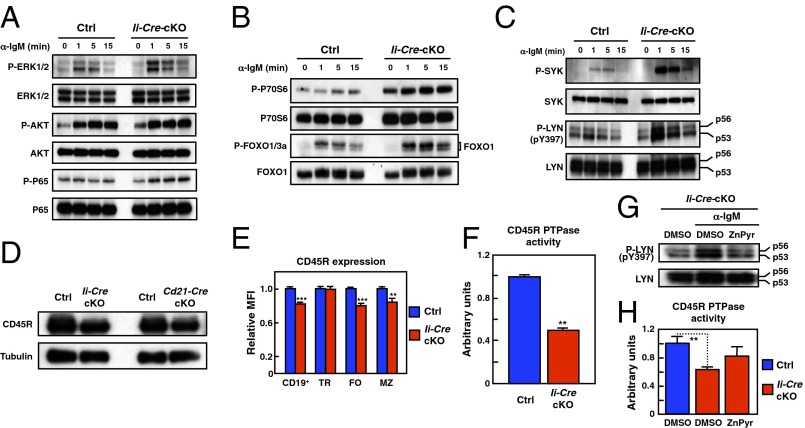
Because the BCR-induced cell proliferation was impaired in Zip10-deficient B cells (Fig. 3A), we speculated that BCR signaling is dysregulated in the absence of ZIP10. Indeed, Ii-Cre-cKO B cells showed hyperactivations of ERK, AKT, and NF-κB pathways after BCR cross-linking (Fig. 5 A and B). Ig stimulation also led to augmented activation of the upstream kinase, SYK (Fig. 5C). The induction of the activity of SRC-family kinases, such as LYN, via BCR is essential for coupling BCR stimulation to the activation of downstream pathways (29, 30). The phosphorylation of the stimulatory tyrosine residue (Y397) of LYN was up-regulated in Zip10-deficient B cells (Fig. 5C and SI Appendix, Fig. S9).
Fig. 5.
ZIP10 deficiency dysregulates BCR signaling. (A) BCR-induced ERK, AKT, and NF-κB P65 activation. (B) BCR-induced P70S6 and FOXO activation. (C) BCR-induced SYK and LYN activation. (D) Immunoblot for CD45R expression in purified splenic B cells. (E) Relative mean fluorescence intensity of CD45R surface expression in splenic B-cell subsets. Data represent the mean ± SD (**P < 0.01, ***P < 0.001). (F) Measurement of CD45R PTPase activity. Data represent the mean ± SD (**P < 0.01). (G) Effect of Zn introduction on LYN activation. The cells were stimulated with anti-IgM F(ab′)2 with or without Zn plus pyrithione for 5 min. (H) Effect of Zn on CD45 PTPase activity assayed ex vivo. The cells were treated with Zn plus pyrithione for 5 min. Data represent the mean ± SD (**P < 0.01).
CD45R is proposed to exert a negative effect on LYN activity in the lipid rafts (31). BCR stimulation temporarily excludes CD45R from the lipid rafts, releasing CD45R’s inhibitory effect on LYN and initiating signaling, but CD45R immediately reassociates with the lipid rafts (31). Thus, the spatiotemporal positioning of CD45R after BCR cross-linking dictates the status of LYN activity. Although the CD45R expression was slightly decreased in the mature B-cell subsets from Ii-Cre-cKO mice (Fig. 5 D and E), its PTPase activity was reduced to nearly half the level of control cells (Fig. 5F), indicating that ZIP10 positively regulates CD45R activity in mature B cells.
Notably, under normal conditions, LYN was not constitutively activated in Zip10-deficient B cells (SI Appendix, Fig. S10), suggesting that the partial reduction of CD45R does not significantly affect LYN’s activity in the steady state; rather, it may preferentially abrogate the negative regulatory effect on LYN after BCR cross-linking, leading to the augmentation of LYN activity. Indeed, the forced introduction of Zn ex vivo revealed that the BCR-induced LYN activation was suppressed by Zn in Zip10-deficient B cells (Fig. 5G). Intriguingly, the reduced CD45R PTPase activity was inclined to recover by forced Zn introduction ex vivo in Zip10-deficient B cells (Fig. 5H). Furthermore, the activity of an active form of CD45R recombinant protein containing two PTP domains (amino acids 592–1291) was not up-regulated at any Zn concentrations, but was strongly suppressed only in the presence of an unphysiologically high concentration of Zn (1 mM), to the same extent as with orthovanadate (Na3VO4), a general PTPase inhibitor, in vitro (SI Appendix, Fig. S11). Thus, undefined mechanisms may exist by which ZIP10 positively and indirectly modulates CD45R PTPase activity at the restricted region, such as lipid rafts, where a small amount of CD45R dynamically interacts with LYN (31), perhaps through a Zn-requiring process.
Zn-Deficient Mice Display an Impaired TD Antibody Response.
Zn deficiency is known to attenuate the humoral immunity (6, 7). We finally asked how Zn is required for the antibody-mediated immune response in a mouse model. The mice were fed Zn-adequate (ZnA) or Zn-deficient (ZnD) chow for 4 wk, and were then immunized with NP-CGG in alum. The ZnD mice displayed a clear growth retardation phenotype and reduced serum Zn 2 wk after the start of Zn-deficient diet (SI Appendix, Fig. S12 A and B), as previously reported (32). Unexpectedly, the number and intracellular free Zn level of the FO B cells were not altered between the two groups (SI Appendix, Fig. S12 C and D). In ZnD splenic B cells, the expressions of some Zn transporters and metallothioneins were changed (SI Appendix, Fig. S13). These findings suggest that the unknown resistant system against ZnD condition by the alteration of Zn transporter expressions maintains Zn homeostasis and alleviates the effect of Zn deficiency on the cell death of this population.
This hypothesis is supported by the finding that the forced chelation of Zn in splenic B cells induced cell death with the induction of Zip10 transcription (SI Appendix, Fig. S14), suggesting that ZIP10 may be involved in cell survival in the critical ZnD environment. Although the previous report described that ZnD up-regulates ZIP10 expression (33), we could not observe it in splenic B cells from 4-wk ZnD mouse in vivo (SI Appendix, Fig. S13). In future studies, a more detailed time-course analysis will be required. Notably, we found that the GC B-cell population and antigen-specific IgG1 response were significantly reduced in the immunized ZnD-fed mice (SI Appendix, Fig. S12 E and F). Because GC B cells contained a higher amount of Zn than FO B cells (SI Appendix, Fig. S12G), they may be more susceptible to the ZnD condition. Thus, the ZnD-induced abrogation of GC formation and the antibody response partly mimicks the phenotypes in Zip10-deficient mice (Fig. 3 B and C), and suggests that Zn is a critical regulator in BCR-mediated cell activation process.
Discussion
In the present study, we found that a ZIP10 deficiency in mature B cells leads to their reduction (Fig. 1) and impaired TD and TI antibody responses (Figs. 3 and 4). Therefore, ZIP10 is important for both mature B-cell maintenance and humoral immune responses.
TD immune responses were considerably attenuated in Ii-Cre-cKO mice, and this defective response occurred in a cell-intrinsic manner (Fig. 3B). Notably, the secretion of antigen-specific IgG1 was almost completely abrogated because of the impaired GC formation in Ii-Cre- and Cd21-Cre-cKO mice (Fig. 3C). These findings suggest that the signals produced by ZIP10-deficient mature B cells are not sufficient for their proliferation, resulting in impaired antibody responses. This notion is supported by the impaired BCR-induced cell proliferation (Fig. 3A) and the marked attenuation of the TI response in Ii-Cre-cKO mice (Fig. 4). These data indicate that the reduced number of mature B cells (Fig. 1) cannot fully account for the suppressed TD and TI responses, and that impaired BCR signaling is likely to contribute to these attenuated responses in Ii-Cre-cKO mice. Thus, ZIP10 contributes appreciably to the humoral immune responses by regulating the cellular signaling after antigen exposure.
Because BCR-mediated signaling controls B-cell activation (3), we speculated that the loss of ZIP10 might affect this signaling cascade. Surprisingly, Zip10-deficient B cells exhibited enhanced overall BCR signaling (Fig. 5 A–C). Although this result seems to be paradoxical with the impaired proliferation of Zip10-deficient B cells (Fig. 3A), LYN actually plays a critical role not only in activating BCR-mediated signals, but also in simultaneously generating inhibitory signals mediated by FCγRIIB1, CD22, and paired immunoglobulin-like receptor-B (PIR-B), subsequently leading to recruitment of the SH2-containing inositol-5′-phosphatase 1 (SHIP-1) and SHP-1 PTPases, which down-regulate BCR signaling (30). Thus, the reduced Ig-stimulated proliferation of Zip10-deficient mature B cells may have been because of a rapid negative-feedback loop elicited by inhibitory signals, which led to a shortened signal that was insufficient to promote proliferation. This notion is supported by the observations that LYNup/up mice, which express a constitutively active form of LYN, display the spontaneous and simultaneous activation of positive (SYK) and negative (CD22, SHP-1, SHIP-1) regulators, and impaired cell proliferation upon BCR cross-linking (29).
We found that CD45R, which negatively regulates LYN activity, exhibited reduced PTPase activity in Zip10-deficient B cells (Fig. 5F). However, the partial reduction of CD45R PTPase activity did not affect the LYN activity in resting Zip10-deficient B cells (SI Appendix, Fig. S10), in agreement with a previous report that the stimulatory tyrosine of LYN has a normal phosphorylation level in B cells carrying mutant CD45R alleles that reduce CD45R’s expression (34). Given the BCR-dependence of the dynamic behavior of CD45R (31), the BCR-induced augmentation of LYN activity in Zip10-deficient B cells may result from an impairment of the poststimulatory negative feedback on LYN by CD45R. In support of this scenario, Zip10-deficient B cells showed lower uptake of Zn from extracellular space (SI Appendix, Fig. S7D), and Zn plus pyrithione treatment ex vivo suppressed the LYN activation after BCR stimulation with the up-regulation of CD45R PTPase activity in Zip10-deficient B cells (Fig. 5 G and H). In addition, the coincubation of Zn with CD45 protein in vitro did not up-regulate the PTPase activity, but rather suppressed it at the unphysiologically high Zn concentration (SI Appendix, Fig. S11). Thus, regarding how ZIP10 positively regulates the CD45R PTPase activity, our results suggest that ZIP10 acts through a Zn-requiring process rather than by directly affecting PTPase, as previously reported for the receptor PTPase-β (35). In general, PTPase activity is inhibited by oxidants (36), and the Zn’s ability to function as an antioxidant is well established (37). Thus, it is possible that the decrease in CD45R PTPase activity in Zip10-deficient mature B cells was because of reduced Zn-mediated antioxidant effects in redox signaling. Based on the finding that the cell membrane-localized ZIP10 transports Zn (SI Appendix, Figs. S7 A–D and S8) (22), ZIP10 may positively regulate the CD45R PTPase activity through Zn uptake from the extracellular space to participate in the negative feedback of BCR signaling.
Notably, neither conventional ICP-AES nor a fluorescent method could detect a difference in intracellular Zn content between the control and Zip10-deficient B cells (SI Appendix, Fig. S7 E–G). Furthermore, we could not observe the significant changes of expression patterns of other Zn transporters between the two groups (deposited microarray data in RefDIC; see SI Appendix). These findings suggest that ZIP10 is not a major contributor to the overall intracellular Zn homeostasis, but may dynamically affect BCR signaling in a local manner by transporting a subtle amount of Zn from the extracellular fluid. This could indeed be the case. ZIP10 is expressed at a rather low level in splenic B cells and even in its ectopically expressed-293T cells (SI Appendix, Figs. S7 A–C and S15), implicating its rapid protein turnover and spatiotemporal expression. Nevertheless, ZIP10 deficiency leads to a striking loss of FO B cells and marked impairment of the antibody response. Given that a redundant system does not appear to be functional in Zip10-deficient B cells, these data collectively suggest that ZIP10 has a more efficient Zn-transporting ability than other Zn transporters, and is critically involved in mature B-cell functions. Therefore, ZIP10 may function as a Zn importer in restricted regions, such as the lipid rafts required for BCR signaling, rather than broadly over the entire cell.
In light of the enhanced BCR signaling described above, it appears that the Zip10-deficient phenotype is a partial phenocopy of the LYNup/up phenotype, in which a substantial fraction of resting mature B cells is deleted, probably because of enhanced signaling above a certain threshold (29). Anergic B cells have a shortenend half-life in the presence of competitive B cells, and exhibit impaired proliferation in response to antigen-induced BCR aggregation (38). Thus, B-cell anergy may account for the increased B-cell turnover in Ii-Cre-cKO mice. However, we could not detect an up-regulation of LYN activation in Zip10-deficient B cells in the steady state (SI Appendix, Fig. S10), suggesting that ZIP10 controls mature B-cell maintenance by a LYN-independent mechanism.
How similar is the immunological abnormalities in Zip10-deficient mice to those in animals under Zn deficiency? Zn deficiency attenuates the Th1 response, which promotes Ig CSR to noncytophilic IgG2, such as IgG2a (IgG2c in C57BL/6), without affecting the Th2 response, which does promote CSR toward cytophilic IgG1 and IgE (39). Given that ZIP10 deficiency significantly attenuated the level of IgG2c but not IgG1 and IgE in steady state (SI Appendix, Fig. S5), it would be interesting to hypothesize that the loss of ZIP10 may also affect signal transduction mediated by Th1 cytokines, such as IFN-γ, while remaining intact with one by Th2 cytokines in resting B cells. Nevertheless, our data showed that both Zip10-deficient and ZnD mice displayed the reduced GC B-cell number followed by the attenuated antigen-specific IgG1 response upon antigen exposure (Fig. 3C and SI Appendix, Fig. S12). Because membrane Zn concentrations are strongly influenced in dietary Zn deficiency (40), these findings collectively suggest that the spatiotemporal Zn uptake by cell membrane-localized ZIP10 plays more important role in the activated B-cell function via BCR signaling, rather than in that of the resting B cells.
In conclusion, this study provides novel insight into the function of the Zn transporter ZIP10 in antibody-mediated immune response. Our findings establish that ZIP10 functions as a previously unidentified regulator of BCR signaling by setting its threshold, and is thus required for regulating the humoral immune response. Notably, we recently found that ZIP10 also has an important role in preventing apoptosis at the pro–B-cell stage (24), suggesting that ZIP10 plays distinct roles in the early and late B-cell developmental stages by regulating different signaling cascades. In this regard, Zip10-deficient mice may be a useful animal model for studying B-cell homeostasis and function in vivo. Future studies investigating the molecular details involving ZIP10 will improve our understanding of Zn’s role in lymphocyte biology.
Materials and Methods
Cell Sorting.
The primary splenic B-cell, MO/Mϕ, DC, CD4+ T-cell, and CD8+ T-cell populations were sorted by autoMACS or LS columns (Miltenyi Biotech), using CD19 or CD43, CD11b, CD11c, CD4, and CD8 microbeads (Miltenyi Biotech), respectively. BM and splenic B-cell subsets were sorted by FACSAria II.
Statistical Analysis.
Differences among multiple groups were compared by one-way ANOVA followed by a post hoc comparison using Fisher’s protected least-significant difference test. The two-tailed Student t test was used to analyze the difference between two groups. Detailed descriptions of all of the materials and methods are provided in the SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Toshio Hirano, Masaru Taniguchi, Shigeo Koyasu, Toshitada Takemori, Keigo Nishida, Kohei Kometani, Rika Ouchida, and Koji Tokoyoda for helpful advice; Drs. Sidonia Fagarasan and Tasuku Honjo for CH12 mouse B-cell lymphoma; Ms. Norie Takeuchi for her secretarial assistance; and GENOSTAFF and Shino-Test. Co. Ltd for excellent technical assistance. The plasmid containing the mouse Ii promoter pDOI-6 was a gift from Dr. Diane Mathis, and the Cd21-Cre transgenic and CAG-CAT-EGFP mice were kind gifts from Dr. Klaus Rajewsky and Dr. Jun-ichi Miyazaki, respectively. This study was supported by KAKENHI Grants 25860371 (to S. Hojyo) and 23592239 (to T.F.), a RIKEN Junior Research Associate Program (T.M.), and the Mishima Kaiun Memorial Foundation (T.F.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The microarray analysis data are available from RefDIC, http://refdic.rcai.riken.jp (accession nos. RSM07992–RSM07995).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323557111/-/DCSupplemental.
References
- 1.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20(2):149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26(6):703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Kurosaki T. Genetic analysis of B cell antigen receptor signaling. Annu Rev Immunol. 1999;17:555–592. doi: 10.1146/annurev.immunol.17.1.555. [DOI] [PubMed] [Google Scholar]
- 4.Schneider P, Tschopp J. BAFF and the regulation of B cell survival. Immunol Lett. 2003;88(1):57–62. doi: 10.1016/s0165-2478(03)00050-6. [DOI] [PubMed] [Google Scholar]
- 5.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117(6):787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Shankar AH, Prasad AS. Zinc and immune function: The biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68(2) Suppl:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 7.Prasad AS. Zinc in human health: Effect of zinc on immune cells. Mol Med. 2008;14(5-6):353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J Biol Inorg Chem. 2011;16(7):1123–1134. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirano T, et al. Roles of zinc and zinc signaling in immunity: Zinc as an intracellular signaling molecule. Adv Immunol. 2008;97:149–176. doi: 10.1016/S0065-2776(08)00003-5. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura H, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7(9):971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 11.Yamasaki S, et al. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177(4):637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukada T, Kambe T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics. 2011;3(7):662–674. doi: 10.1039/c1mt00011j. [DOI] [PubMed] [Google Scholar]
- 13.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci. 2004;61(1):49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukada T, Hojyo S, Bin B. Zinc signal in growth control and bone diseases. In: Fukada T, Kambe T, editors. Zinc Signals in Cellular Functions and Disorders. Tokyo: Springer; 2014. , in press. [Google Scholar]
- 15.Fukada T, et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; Its involvement in BMP/TGF-beta signaling pathways. PLoS ONE. 2008;3(11):e3642. doi: 10.1371/journal.pone.0003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hojyo S, et al. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS ONE. 2011;6(3):e18059. doi: 10.1371/journal.pone.0018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu MJ, et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-κB. Cell Reports. 2013;3(2):386–400. doi: 10.1016/j.celrep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishida K, et al. Zinc transporter Znt5/Slc30a5 is required for the mast cell-mediated delayed-type allergic reaction but not the immediate-type reaction. J Exp Med. 2009;206(6):1351–1364. doi: 10.1084/jem.20082533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita S, et al. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature. 2004;429(6989):298–302. doi: 10.1038/nature02545. [DOI] [PubMed] [Google Scholar]
- 20.Chowanadisai W, Graham DM, Keen CL, Rucker RB, Messerli MA. Neurulation and neurite extension require the zinc transporter ZIP12 (slc39a12) Proc Natl Acad Sci USA. 2013;110(24):9903–9908. doi: 10.1073/pnas.1222142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukada T, Hojyo S, Furuichi T. Zinc signal: A new player in osteobiology. J Bone Miner Metab. 2013;31(2):129–135. doi: 10.1007/s00774-012-0409-6. [DOI] [PubMed] [Google Scholar]
- 22.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98(5):692–697. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehsani S, et al. LIV-1 ZIP ectodomain shedding in prion-infected mice resembles cellular response to transition metal starvation. J Mol Biol. 2012;422(4):556–574. doi: 10.1016/j.jmb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyai T, et al. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc Natl Acad Sci USA. 2014;111:11780–11785. doi: 10.1073/pnas.1323549111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204(7):1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodland RT, Schmidt MR. Homeostatic proliferation of B cells. Semin Immunol. 2005;17(3):209–217. doi: 10.1016/j.smim.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zotos D, Tarlinton DM. Determining germinal centre B cell fate. Trends Immunol. 2012;33(6):281–288. doi: 10.1016/j.it.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Hibbs ML, et al. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J Exp Med. 2002;196(12):1593–1604. doi: 10.1084/jem.20020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishizumi H, Horikawa K, Mlinaric-Rascan I, Yamamoto T. A double-edged kinase Lyn: A positive and negative regulator for antigen receptor-mediated signals. J Exp Med. 1998;187(8):1343–1348. doi: 10.1084/jem.187.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrivastava P, Katagiri T, Ogimoto M, Mizuno K, Yakura H. Dynamic regulation of Src-family kinases by CD45 in B cells. Blood. 2004;103(4):1425–1432. doi: 10.1182/blood-2003-03-0716. [DOI] [PubMed] [Google Scholar]
- 32.Kawamura T, et al. Severe dermatitis with loss of epidermal Langerhans cells in human and mouse zinc deficiency. J Clin Invest. 2012;122(2):722–732. doi: 10.1172/JCI58618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichten LA, Ryu MS, Guo L, Embury J, Cousins RJ. MTF-1-mediated repression of the zinc transporter Zip10 is alleviated by zinc restriction. PLoS ONE. 2011;6(6):e21526. doi: 10.1371/journal.pone.0021526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zikherman J, Doan K, Parameswaran R, Raschke W, Weiss A. Quantitative differences in CD45 expression unmask functions for CD45 in B-cell development, tolerance, and survival. Proc Natl Acad Sci USA. 2012;109(1):E3–E12. doi: 10.1073/pnas.1117374108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson M, Hogstrand C, Maret W. Picomolar concentrations of free zinc(II) ions regulate receptor protein-tyrosine phosphatase β activity. J Biol Chem. 2012;287(12):9322–9326. doi: 10.1074/jbc.C111.320796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9(2):387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 37.Powell SR. The antioxidant properties of zinc. J Nutr. 2000;130(5S Suppl):1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 38.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3(6):691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 39.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18(6):263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 40.Chvapil M. New aspects in the biological role of zinc: A stabilizer of macromolecules and biological membranes. Life Sci. 1973;13(8):1041–1049. doi: 10.1016/0024-3205(73)90372-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.