Significance
Prevailing theory suggests that episodic memory encoding is subserved by early remodeling of hippocampal circuits, whereas remodeling of the neocortex occurs weeks to months later to promote long-term memory storage and recall. Herein, we show that episodic memory encoding elicits early remodeling of neocortical circuits in mouse brain. Specifically, memory encoding triggered a transcriptomic program in the medial prefrontal cortex (mPFC) that was accompanied by rapid structural and functional plasticity of local synaptic circuits. Using optogenetics to examine the real-time contribution of the mPFC to memory encoding, we found that activity of excitatory mPFC neurons is required for hippocampal activation and formation of long-term memory. These data have important implications for understanding memory processing in healthy and diseased brain states.
Keywords: learning, transcriptome, neuroplasticity, optogenetics, hippocampus
Abstract
Understanding the mechanisms by which long-term memories are formed and stored in the brain represents a central aim of neuroscience. Prevailing theory suggests that long-term memory encoding involves early plasticity within hippocampal circuits, whereas reorganization of the neocortex is thought to occur weeks to months later to subserve remote memory storage. Here we report that long-term memory encoding can elicit early transcriptional, structural, and functional remodeling of the neocortex. Parallel studies using genome-wide RNA sequencing, ultrastructural imaging, and whole-cell recording in wild-type mice suggest that contextual fear conditioning initiates a transcriptional program in the medial prefrontal cortex (mPFC) that is accompanied by rapid expansion of the synaptic active zone and postsynaptic density, enhanced dendritic spine plasticity, and increased synaptic efficacy. To address the real-time contribution of the mPFC to long-term memory encoding, we performed temporally precise optogenetic inhibition of excitatory mPFC neurons during contextual fear conditioning. Using this approach, we found that real-time inhibition of the mPFC inhibited activation of the entorhinal–hippocampal circuit and impaired the formation of long-term associative memory. These findings suggest that encoding of long-term episodic memory is associated with early remodeling of neocortical circuits, identify the prefrontal cortex as a critical regulator of encoding-induced hippocampal activation and long-term memory formation, and have important implications for understanding memory processing in healthy and diseased brain states.
Long-term memory is essential for cognition and its disruption is central to myriad neurological disorders. Therefore, a deeper understanding of the mechanisms by which long-term memories are formed in the brain and lost in neurological disease states represents a critical objective in neuroscience. To this end, a wealth of studies has demonstrated that encoding of long-term memory involves early and rapid enhancements in neuronal activation, synaptic plasticity-related gene expression, structural synaptic plasticity, and synaptic efficacy within hippocampal circuits (1–5). Accordingly, lesions of the hippocampus or surrounding medial temporal lobe structures elicit severe memory impairment (6, 7). Over the weeks to months that follow the initial memory encoding event, repeated hippocampal–neocortical interactions slowly reorganize the synaptic architecture in the neocortex and gradually instantiate the memory trace within neocortical circuits, particularly the medial prefrontal cortex (mPFC) (5, 8–10). Collectively, these observations have given rise to the prevailing view that long-term memory encoding requires early and rapid plasticity within the hippocampus, whereas reorganization of the neocortex is limited to the late stages of memory processing to subserve remote memory storage and recall (11, 12).
Intriguingly however, neuroimaging studies from mouse to man demonstrate that the hippocampus and prefrontal cortex are coactivated during memory encoding (13–20). Indeed, the magnitude of prefrontal activation during memory encoding has been shown to be predictive of the ability to later recall the experience (13), and prefrontal lesions are associated with impaired memory formation (15–17, 21–25). Nonetheless, the mechanisms by which the prefrontal cortex contributes to long-term memory encoding remain poorly understood. Herein, we provide evidence that long-term associative memory encoding activates a synaptic plasticity-related transcriptional program in the mPFC that is accompanied by rapid structural and functional plasticity of mPFC synaptic circuits. Using an in vivo optogenetic approach to examine the real-time contribution of mPFC activation to long-term memory encoding, we further show that excitatory mPFC neurons drive activation of the entorhinal–hippocampal circuit and regulate the formation of long-term memory. These results suggest a critical role for the prefrontal cortex in long-term memory encoding and have implications for understanding memory function in healthy and diseased brain states.
Results
To investigate the mechanisms by which the prefrontal cortex contributes to long-term episodic memory encoding, we used contextual fear conditioning, in which mice learn an association between a novel context and an event (foot shock) that occurs in that context. Single-trial contextual fear conditioning generates a temporally defined, long-lasting associative memory trace and is thus well suited for investigating the mechanisms that mediate long-term memory encoding in the brain (26). Moreover, previous studies suggest that associative fear learning increases neuronal activity in the mPFC (15) and that the mPFC is critical for long-term storage and recall of associative fear memory (9). To verify that this paradigm increases neuronal activity in the mPFC, we examined expression of the neuronal activity-dependent immediate early gene, early growth response 1 (zif268/egr-1), in the mPFC 1 h following contextual fear conditioning (27). In accord with previous data (15), we found that fear conditioning significantly increased immediate early gene expression in the mPFC compared with control animals exposed to either the context or foot shock alone, suggesting that the mPFC exhibits associative learning-specific neural activation (Fig. S1). Additionally, as immediate early gene expression in the mPFC was significantly greater in context-exposed control animals relative to control animals exposed only to foot shock, context-exposed mice served as controls in subsequent experiments (Fig. S1). Together, these data suggest that associative memory encoding increases neuronal activity in the mPFC and further validate contextual fear conditioning as a useful paradigm by which to examine the role of the prefrontal cortex in long-term associative memory encoding.
Next, we sought to determine whether the memory encoding-induced increase in mPFC neuronal activity was associated with a shift in the mPFC transcriptome. To this end, we performed genome-wide RNA sequencing (RNA-seq) of mouse mPFC 1 h following contextual fear conditioning. Strikingly, we found that 342 genes (121 up-regulated, 221 down-regulated) were differentially expressed in the mPFC of mice that underwent fear conditioning compared with control animals (Fig. 1A and Table S1). Gene ontology and gene network analyses of differentially expressed genes revealed that genes up-regulated in the mPFC during associative memory encoding form a highly interconnected network involved in biological processes that promote synaptic plasticity and memory formation, including synthesis of cAMP, neuritogenesis, long-term potentiation, and axon guidance (28) (Fig. 1 B and C and Table S2). Conversely, genes down-regulated in the mPFC during associative memory encoding are implicated in biological processes that have been shown to suppress synaptic plasticity and memory function, such as proliferation of microglia, activation of phagocytes, and apoptosis (29, 30) (Fig. 1 B and C and Table S2). Quantitative RT-PCR analysis of independent samples confirmed these expression changes (Fig. 1D and Table S3). These results suggest that the mPFC transcriptome is reprogrammed rapidly during associative memory encoding and that memory encoding primes the mPFC transcriptome for neuroplasticity.
Fig. 1.
Associative memory encoding primes the mPFC transcriptome for neuroplasticity. (A) Heatmap depicting 342 differentially expressed genes (DEGs) determined by genome-wide RNA sequencing (RNA-seq) of the mPFC upon control exposure (CTL) or contextual fear conditioning (FC). Rows represent DEGs, columns represent transcriptomic profiles of individual animals. Blue and red represent low and high levels of gene expression, respectively (n = 4 mice per group). (B) Ingenuity Gene Ontology Analysis depicting to which biological processes the DEGs contribute (red, up-regulated upon FC; blue, down-regulated upon FC). (C) Ingenuity Gene Network Analyses of DEGs up-regulated (Cell Signaling; P = 1 × 10−50) or down-regulated (Immune Cell Trafficking; P = 1 × 10−34) in the mPFC upon FC. Colored and uncolored nodes represent DEGs identified by RNA-seq and the Ingenuity network generation algorithm, respectively. (D) Expression of a subset of DEGs revealed by RNA-seq was confirmed in independent mPFC samples using quantitative RT-PCR (n = 5–10 mice per group). Values are normalized to expression levels of Gapdh. *P ≤ 0.05; **P ≤ 0.01. Values represent mean ± SEM.
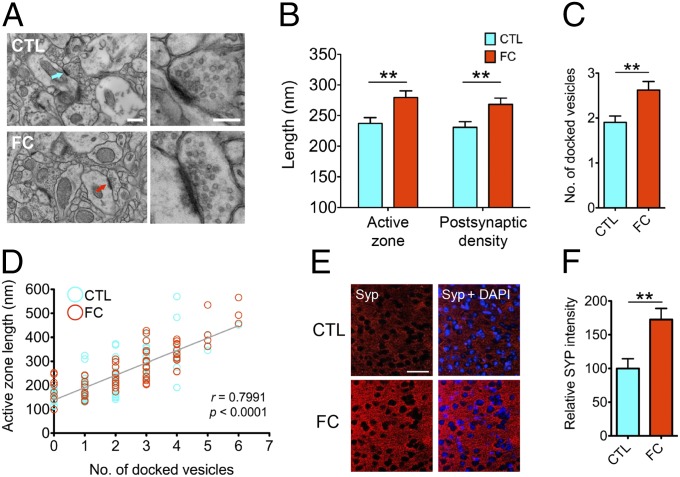
Given that changes in gene expression are required to drive long-lasting forms of synaptic plasticity (28), we reasoned that the memory encoding-induced gene expression changes we observed in the mPFC might be associated with early remodeling of the local synaptic architecture. To test this hypothesis, we performed transmission electron microscopy (TEM) to analyze the ultrastructure of individual mPFC synapses 1 h following contextual fear conditioning. Specifically, we assessed the length of the active zone and postsynaptic density as well as the number of docked synaptic vesicles per synapse, as these metrics represent structural indices of synaptic strengthening and are increased under conditions associated with memory formation (31, 32). We found that associative memory encoding led to a rapid induction of ultrastructural synaptic plasticity in the mPFC, as contextual fear conditioning significantly expanded the size of the active zone and postsynaptic density (Fig. 2 A and B and Fig. S2 A and B) and increased the number of docked vesicles within individual mPFC synapses relative to controls (Fig. 2C and Fig. S2C). In accord with previous studies (33, 34), we found that active zone length, postsynaptic density length, and docked synaptic vesicle number were highly correlated at the level of individual synapses (Fig. 2D and Fig. S2 D and E). Consistent with these observations, we found that expression of the presynaptic marker, synaptophysin, was significantly increased in the mPFC 1 h following contextual fear conditioning compared with controls (Fig. 2 E and F and Fig. S3).
Fig. 2.
Early remodeling of mPFC synapse ultrastructure upon associative memory encoding. (A) Representative transmission electron micrographs depicting mPFC synapse ultrastructure upon CTL or FC. (Scale bar, 500 nm.) Arrows indicate magnified synapses shown at Right. (Scale bar, 200 nm.) (B and C) Quantification of active zone and postsynaptic density length (B; n = 77–92 synapses from four mice per group) and docked synaptic vesicle number (C; n = 69–92 synapses from four mice per group) in mPFC synapses upon CTL or FC. (D) Active zone length was correlated with docked synaptic vesicle number across treatment groups. Blue and red circles indicate synapses in CTL and FC groups, respectively (Pearson r = 0.7991; P ≤ 0.0001). (E) Representative immunohistochemical images depicting synaptophysin immunoreactivity in the mPFC upon CTL or FC. (Scale bar, 50 μm.) (F) Quantification of E (n = 6 mice per group). **P ≤ 0.01. Values represent mean ± SEM.
To further examine whether memory encoding elicits early plasticity within postsynaptic mPFC circuit elements, we used Golgi–Cox impregnation to investigate the morphology of individual dendritic spines on mPFC pyramidal neurons. Dendritic spines represent the postsynaptic component of excitatory synapses and exhibit morphological and functional continua, ranging from thin spines with a small head (i.e., thin spines) that are highly plastic and are hypothesized to underlie experience-dependent rewiring of neural circuits, to spines with a large, mushroom-like head (i.e., mushroom spines) that are more stable and are hypothesized to represent the physical substrates of long-term memories (35). We found that the ratio of thin spines to mushroom spines in the mPFC was significantly increased 1 h following contextual fear conditioning compared with controls (Fig. 3 A and B), suggesting that associative memory encoding increases dendritic spine plasticity in mPFC circuits. Next, to determine whether memory encoding-induced structural plasticity in mPFC circuits is associated with functional alterations in excitatory synaptic strength, we performed ex vivo whole-cell patch-clamp recording of mPFC pyramidal neurons to examine miniature excitatory postsynaptic currents (mEPSCs) (36). We found that mEPSC frequency, but not amplitude, was significantly increased at excitatory mPFC synapses 1 h following contextual fear conditioning relative to controls (Fig. 3C), suggesting that associative memory encoding rapidly enhances the efficacy of excitatory presynaptic transmission in the mPFC. Finally, using parallel TEM (Fig. S4 A and B) and Golgi–Cox impregnation (Fig. S4C), we found that despite the observed structural and functional plasticity induced in existing mPFC circuits following associative memory encoding, total synapse density in the mPFC remained unchanged. Collectively, these data suggest that mPFC activation during long-term associative memory encoding is associated with early transcriptional, structural, and functional remodeling of the existing mPFC circuitry.
Fig. 3.
Associative memory encoding enhances dendritic spine plasticity and synaptic efficacy in the mPFC. (A) Representative Golgi–Cox impregnated dendritic spines present on layer II/III mPFC pyramidal neurons upon CTL or FC. Green and blue arrows indicate thin and mushroom-shaped spines, respectively. (Scale bar, 5 μm.) (B) Quantification of A (n = 716–753 spines from three to four mice per group). (C) Representative traces of miniature excitatory postsynaptic currents (mEPSCs) recorded from layer II/III mPFC pyramidal neurons upon CTL or FC. (Scale bar, 40 pA, 2.5 s. Quantification of mEPSC frequency and amplitude in mPFC pyramidal neurons upon CTL or FC (n = 10–11 neurons per group). *P ≤ 0.05; n.s., not significant. Values represent mean ± SEM.
Next, we sought to determine the causal role of early mPFC activation in long-term memory encoding. As the mPFC represents a hub of information processing in the brain (37–41), the mPFC may play a privileged role in regulating hippocampal-dependent memory encoding. However, whether the mPFC regulates hippocampal activation during the encoding of long-term memories is not known. To test this possibility, we first confirmed that contextual fear conditioning increases neuronal activity in the entorhinal cortex and hippocampus. Consistent with previous data (10, 42), we found that Zif268 expression was significantly increased in the entorhinal cortex (Fig. S5 A and C) and hippocampal area cornu ammonis 1 (CA1) (Fig. S5 B and D) 1 h following context exposure or contextual fear conditioning. Next, we used an in vivo optogenetic approach to permit real-time manipulation of excitatory mPFC neuronal activity in freely behaving mice. Specifically, mPFC excitatory neurons were transduced bilaterally with an adeno-associated virus serotype 5 (AAV5) encoding a third-generation halorhodopsin (eNpHR3.0) fused to enhanced yellow fluorescent protein (EYFP) under transcriptional regulation of the Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα) promoter (CaMKIIα-eNpHR3.0-EYFP), followed by implantation of a fiber optic in the mPFC to permit real-time optogenetic silencing of local excitatory neuronal activity (Fig. S6). Stereotaxic injection of the CaMKIIα-eNpHR3.0-EYFP vector into mPFC resulted in mPFC-specific expression (Fig. 4A), and within mPFC, eNpHR3.0-EYFP was expressed in CaMKIIα-positive excitatory neurons (Fig. 4B). Single-cell recordings from acute mPFC slices confirmed that optical (593 nm) inhibition dampened action potential spiking in excitatory neurons expressing eNpHR3.0-EYFP (Fig. 4C). Next, we found that real-time optogenetic inhibition of the mPFC during contextual fear conditioning (Fig. 4D) significantly reduced Zif268 expression in the mPFC of mice expressing eNpHR3.0-EYFP compared with mice expressing EYFP alone (Fig. 4 E and F), confirming that optogenetic inhibition prevented mPFC activation during long-term associative memory encoding. Interestingly, optical inhibition also reduced the expression of synaptophysin in the mPFC of mice expressing eNpHR3.0-EYFP, but not EYFP alone (Fig. 4 E and F), suggesting that early plasticity of mPFC circuits during associative memory encoding requires excitatory neuronal activity. To determine whether the mPFC regulates hippocampal circuit activation during memory encoding, we next examined the effect of real-time optogenetic mPFC inhibition on hippocampal area CA1, an area critical for long-term memory formation (7). Remarkably, we found that optogenetic inactivation of the mPFC during memory encoding significantly reduced the expression of Zif268 and synaptophysin in hippocampal area CA1 of mice expressing eNpHR3.0-EYFP compared with mice expressing EYFP alone (Fig. 4 G and H), suggesting that the mPFC is a critical regulator of hippocampal activation during long-term memory encoding. Consistent with its role as the major information gateway linking the mPFC and hippocampus, we further found that mice expressing eNpHR3.0-EYFP exhibited significantly decreased expression of Zif268 and synaptophysin in the entorhinal cortex compared with control mice expressing EYFP alone (Fig. 4 I and J). Together, these data suggest that activity of excitatory mPFC neurons is required for entorhinal–hippocampal circuit activation during long-term associative memory encoding.
Fig. 4.
Optogenetic silencing of excitatory mPFC neurons impairs entorhinal–hippocampal circuit activation during associative memory encoding. (A) AAV5-CaMKIIα-eNpHR3.0-EYFP was injected into bilateral mPFC to selectively transduce mPFC excitatory neurons and permit optogenetic inhibition thereof. (Scale bar, 500 μm.) (B) Representative immunohistochemical images depicting CaMKIIα expression in mPFC neurons transduced with CaMKIIα-eNpHR3.0-EYFP. (Scale bar, 10 μm.) (C) Ex vivo optical (593 nm) inhibition of action potential spiking (50 pA current injection) in a representative mPFC pyramidal neuron expressing eNpHR3.0-EYFP. (Scale bar, 20 mV, 100 ms.) (D) Schematic of the experimental paradigm used for in vivo mPFC optogenetic inhibition experiments shown in E–J. Animals injected with AAV5-CaMKIIα-eNpHR3.0-EYFP or AAV5-CaMKIIα-EYFP control virus received continuous optogenetic inhibition of excitatory mPFC neurons during FC. One hour later, tissue was harvested for analysis. (E–J) Representative immunohistochemical images depicting Zif268 and synaptophysin immunoreactivity in the mPFC (E and F), hippocampal area CA1 (CA1; G and H), and entorhinal cortex (EC; I and J) of mice expressing eNpHR3.0-EYFP or EYFP alone following continuous optogenetic inhibition of mPFC during FC. (Scale bars, 50 μm.) (F) Quantification of E (n = 3–4 mice per group). (H) Quantification of G (n = 3–4 mice per group). (J) Quantification of I (n = 3–4 mice per group). *P ≤ 0.05; **P ≤ 0.01. Values represent mean ± SEM.
Finally, to directly examine the effect of real-time optogenetic mPFC silencing on long-term memory formation, separate cohorts of mice were injected bilaterally with either the eNpHR3.0-EYFP or EYFP vector, implanted with a fiber optic in the mPFC, and received continuous optogenetic inhibition of mPFC excitatory activity during contextual fear conditioning as described above. One day and 30 d after training, mice were returned to the experimental context to assess recent and remote long-term memory, respectively (Fig. 5A). Interestingly, we found that mice expressing eNpHR3.0-EYFP spent significantly less time freezing during both the recent (Fig. 5B) and remote (Fig. 5C) memory tests compared with EYFP controls, suggesting that optogenetic inhibition of excitatory mPFC neurons during memory encoding impaired long-term memory formation. Importantly, neither basal locomotor activity (Fig. 5D) nor sensitivity to foot shock (Fig. 5E) differed significantly between the groups. These results suggest that real-time silencing of mPFC excitatory neurons during associative memory encoding is sufficient to impair the formation of long-term associative memory.
Fig. 5.
Real-time activity of excitatory mPFC neurons is critical for long-term associative memory formation. (A) Schematic of the experimental paradigm used for in vivo mPFC optogenetic inhibition experiments shown in B–E. Animals injected with AAV5-CaMKIIα-eNpHR3.0-EYFP or AAV5-CaMKIIα-EYFP received continuous optogenetic inhibition of excitatory mPFC neurons during FC. Mice were returned to the experimental context 1 d and 30 d later to assess recent and remote long-term memory, respectively. (B and C) Real-time optogenetic inhibition of excitatory mPFC neurons during FC significantly impaired the formation of recent (B) and remote (C) long-term associative memory in mice expressing eNpHR3.0-EYFP compared with EYFP controls (n = 6 mice per group). (D and E) Optogenetic inhibition of the mPFC did not alter basal locomotor activity (D) or response to foot shock (E) during the conditioning period (n = 6 mice per group). *P ≤ 0.05; **P ≤ 0.01. Values represent mean ± SEM.
Discussion
Pioneering work on the mechanisms of memory suggests that episodic memory encoding requires early and rapid alterations in gene expression and synaptic efficacy within the hippocampus, whereas reorganization of neocortical circuits occurs weeks to months later to subserve long-term memory storage and recall (12). Intriguingly however, neuroimaging studies from mouse to man consistently report coactivation of the hippocampus and prefrontal cortex during memory encoding (13–17), thus raising the possibility that the neocortex may play a heretofore undefined role in the early stages of memory processing in the brain. In the present work, genome-wide RNA-seq analysis revealed that long-term associative memory encoding triggers a synaptic plasticity-related transcriptional program in the mPFC. Consistent with these gene expression changes, analysis of synapse ultrastructure, dendritic spine morphology, and synaptic transmission showed that memory encoding induced early remodeling of the mPFC synaptic architecture and enhanced local synaptic efficacy. Finally, using in vivo optogenetic silencing, we found that real-time inactivation of mPFC excitatory neurons during associative memory encoding impaired entorhinal–hippocampal circuit activation and the formation of long-term associative memory. Collectively, our results provide, to our knowledge, the first evidence that episodic memory encoding can induce early transcriptional, structural, and functional remodeling of the neocortex and identify the mPFC as a critical regulator of entorhinal–hippocampal circuit activation and the formation of long-term memory.
Whereas decades of investigation in humans and animal models have established a central role for the hippocampus in episodic memory encoding (1), a large body of literature suggests that the prefrontal cortex may also represent an integral component of the neural memory system. For instance, positron emission tomography and functional magnetic resonance imaging studies in humans and nonhuman primates demonstrate that the prefrontal cortex is activated during working memory and associative memory-encoding paradigms and that the magnitude of prefrontal activation at the time of encoding is predictive of the ability to subsequently remember an event (13–20). In accord with these findings, prefrontal lesions have been shown to impair episodic memory formation across species (15–17, 21–25). Interestingly however, mechanistic investigation of the role of the prefrontal cortex in memory processing has largely focused on its role in memory consolidation and retrieval. In this regard, data in animal models suggest that glucose utilization (8), activity-dependent immediate early gene expression (9, 10), and dendritic spine density (5) are increased in the prefrontal cortex following consolidation of remote, but not recent, long-term memory and that blockade of mPFC neuronal activity during memory recall impairs recall of remote, but not recent, long-term memory (7, 9, 10). Thus, whereas previous reports have established the importance of the mPFC in late-stage memory consolidation and storage, the mechanisms by which the mPFC regulates memory encoding remain unclear. To this end, our present results suggest that the mPFC exhibits early transcriptional, structural, and functional plasticity upon long-term associative memory encoding and that activation of excitatory neurons in the mPFC drives hippocampal activation and long-term memory formation. Importantly, our findings are consistent with recent reports suggesting that neurons in the prefrontal cortex are “tagged” during long-term memory encoding (16) and required for the incorporation of newly learned information into existing neural schemas (17) and thus suggest that early remodeling of the neocortical architecture may represent a fundamental feature of episodic memory encoding in the brain.
According to the theoretical modeling work of Marr (43) and the standard model of systems-level memory consolidation (11), the hippocampus is believed to integrate input conveyed from distributed neocortical circuits with self-generated spatial information at the time of memory encoding to generate a unified, spatially contextualized memory trace. However, the neocortical inputs that drive hippocampal activation during memory encoding, and thus regulate hippocampal-dependent memory formation, remain unclear. In this regard, our present data suggest that the activity of excitatory mPFC neurons is required for hippocampal activation and long-term memory formation and thus suggest that excitatory mPFC neurons may represent a critical source of hippocampal input during episodic memory encoding. As previous data indicate that late-stage memory consolidation requires hippocampus-driven reorganization of neocortical circuits (5, 16), our data support a stage-dependent directionality model of memory-relevant information flow, in which neocortex-driven hippocampal activity is critical for memory encoding and formation, whereas hippocampus-driven neocortical reorganization mediates subsequent memory consolidation and storage.
Neuroimaging data in humans and animal models indicate that the mPFC serves as a hub of information processing in the brain and exhibits functional connectivity with the hippocampal formation (37, 40, 41). Moreover, a recent study suggests that the mPFC exhibits significantly elevated resting-state aerobic glycolysis (44), suggesting that the mPFC may be uniquely suited to respond to learning-induced biosynthetic demands. Indeed, elevated aerobic glycolysis in the mPFC is closely associated with enhanced expression of genes related to synaptic remodeling and memory formation (45). Taken together with our present data suggesting that the mPFC exhibits rapid encoding-induced plasticity and represents an integral source of neocortical input to the hippocampus during memory encoding, the mPFC may indeed represent a critical node of memory processing in the brain. However, as memory encoding elicits the coordinated activity of widely distributed neural circuits (14), future studies delineating the relative contributions of various nodes of the neocortical network to hippocampal-dependent memory formation will likely provide critical insights into systems-level memory processing in the brain.
Converging evidence from human and animal studies suggests that metabolic demands render the prefrontal cortex preferentially vulnerable to Alzheimer’s disease (AD)-related neuropathology (40, 46–49). For instance, the mPFC is among the first brain regions to develop amyloid-β (Aβ) plaque deposition, an early pathological hallmark of AD (50). However, the mechanisms by which pathological alterations in the prefrontal cortex may contribute to memory impairment in AD remain unclear. Based on the present results, one possibility is that Aβ pathology in the prefrontal cortex may disrupt prefrontal-dependent activation of the entorhinal–hippocampal circuitry and thereby impair encoding of new long-term memories. In support of this view, human neuroimaging data suggest that functional connectivity between the prefrontal cortex, entorhinal cortex, and hippocampus is substantially decreased in the AD-affected brain (51). Finally, it is noteworthy that the pattern of gene expression we observed in the mPFC following memory encoding (i.e., up-regulation of synaptic plasticity-related genes, down-regulation of immune-related genes) is the inverse of the gene expression profile characteristic of AD (52) (i.e., up-regulation of immune-related genes, down-regulation of synaptic plasticity-related genes). As emerging evidence suggests that microglia, the resident CNS immune cells, induce synaptic pruning under conditions of dampened neural activity (53), dissection of the interplay between neuronal activity, synaptic plasticity, and immune cell activation is likely to provide novel insights into the mechanisms of memory function in the healthy brain and its impairment in a variety of neurological disorders.
Materials and Methods
Wild-type (B6SJL; Taconic Farms) male mice were used at 2.5 ± 0.5 mo of age for experimentation. All animal work was approved by the Committee for Animal Care of the Division of Comparative Medicine at the Massachusetts Institute of Technology. Materials and methods regarding behavioral analysis, immunohistochemistry, genome-wide RNA-seq, quantitative RT-PCR, electron microscopy, Golgi–Cox impregnation, electrophysiology, optogenetics, and statistical analysis are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank J. Gräff, R. Madabhushi, and T. X. Phan for reading and commenting on the manuscript; M. Taylor for mouse colony maintenance; and the Massachusetts Institute of Technology (MIT) BioMicro Center for technical assistance. This work was supported by BrightFocus Foundation Research Fellowship A2013026F (to A.W.B.), the MIT Department of Brain and Cognitive Sciences (R.G.C.), and the MIT Picower Institute Innovation Fund (L-H.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE58510).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408378111/-/DCSupplemental.
References
- 1.Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat Rev Neurosci. 2008;9(1):65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 2.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 3.Peleg S, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328(5979):753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 4.Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: A comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21(14):5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci. 2009;29(25):8206–8214. doi: 10.1523/JNEUROSCI.0966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goshen I, et al. Dynamics of retrieval strategies for remote memories. Cell. 2011;147(3):678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 8.Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400(6745):671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- 9.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304(5672):881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 10.Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305(5680):96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: A simple network model. Proc Natl Acad Sci USA. 1994;91(15):7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6(2):119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 13.Wagner AD, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281(5380):1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 14.Sperling RA, et al. Encoding novel face-name associations: A functional MRI study. Hum Brain Mapp. 2001;14(3):129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Fukushima H, Kida S. Induction and requirement of gene expression in the anterior cingulate cortex and medial prefrontal cortex for the consolidation of inhibitory avoidance memory. Mol Brain. 2011;4:4. doi: 10.1186/1756-6606-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesburguères E, et al. Early tagging of cortical networks is required for the formation of enduring associative memory. Science. 2011;331(6019):924–928. doi: 10.1126/science.1196164. [DOI] [PubMed] [Google Scholar]
- 17.Tse D, et al. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333(6044):891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- 18.Jonides J, et al. Spatial working memory in humans as revealed by PET. Nature. 1993;363(6430):623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 19.Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA. 1993;90(3):878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Exp Brain Res. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- 21.Zhao MG, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47(6):859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Einarsson EO, Nader K. Involvement of the anterior cingulate cortex in formation, consolidation, and reconsolidation of recent and remote contextual fear memory. Learn Mem. 2012;19(10):449–452. doi: 10.1101/lm.027227.112. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler MA, Stuss DT, Tulving E. Frontal lobe damage produces episodic memory impairment. J Int Neuropsychol Soc. 1995;1(6):525–536. doi: 10.1017/s1355617700000655. [DOI] [PubMed] [Google Scholar]
- 24.Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nat Neurosci. 1999;2(4):311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- 25.Petrides M. Monitoring of selections of visual stimuli and the primate frontal cortex. Proc Biol Sci. 1991;246(1317):293–298. doi: 10.1098/rspb.1991.0157. [DOI] [PubMed] [Google Scholar]
- 26.Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- 27.Jones MW, et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4(3):289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- 28.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 29.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628(1–2):227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka S, et al. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J Neurosci Res. 2006;83(4):557–566. doi: 10.1002/jnr.20752. [DOI] [PubMed] [Google Scholar]
- 31.Buchs PA, Muller D. Induction of long-term potentiation is associated with major ultrastructural changes of activated synapses. Proc Natl Acad Sci USA. 1996;93(15):8040–8045. doi: 10.1073/pnas.93.15.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21(12):4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci. 1997;17(15):5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holderith N, et al. Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat Neurosci. 2012;15(7):988–997. doi: 10.1038/nn.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17(3):381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285(5435):1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 37.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 38.Xu W, Südhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339(6125):1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wheeler AL, et al. Identification of a functional connectome for long-term fear memory in mice. PLOS Comput Biol. 2013;9(1):e1002853. doi: 10.1371/journal.pcbi.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu H, et al. Rat brains also have a default mode network. Proc Natl Acad Sci USA. 2012;109(10):3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3(6):533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- 43.Marr D. Simple memory: A theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262(841):23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 44.Vaishnavi SN, et al. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci USA. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19(1):49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckner RL, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vlassenko AG, et al. Spatial correlation between brain aerobic glycolysis and amyloid-β (Aβ ) deposition. Proc Natl Acad Sci USA. 2010;107(41):17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bero AW, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14(6):750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bero AW, et al. Bidirectional relationship between functional connectivity and amyloid-β deposition in mouse brain. J Neurosci. 2012;32(13):4334–4340. doi: 10.1523/JNEUROSCI.5845-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: The challenge of the second century. Sci Transl Med. 2011;3(77):sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blalock EM, et al. Incipient Alzheimer’s disease: Microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci USA. 2004;101(7):2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.