Significance
Loss of function mutations in the potassium channel KCNJ10 causes a salt-wasting syndrome. The phenotype resembles Gitelman syndrome, which results from loss of sodium chloride transport along the distal convoluted tubule (DCT), but the mechanisms involved are not clear. Here, we perform experiments in the kidney from Kcnj10 knockout mice and demonstrate that Kcnj10 is a main contributor to the basolateral potassium conductance in the DCT and that the potassium channel activity regulates the expression of ste20-related proline–alanine-rich kinase (SPAK) and determines Na-Cl cotransporter (NCC) expression. Our study suggests the possibility that the regulation of basolateral Kcnj10 activity in the DCT may be the first step in response to a variety of physiological stimuli for initiating SPAK-WNK-dependent modulation of NCC expression in the kidney.
Keywords: SeSAME/EAST syndrome, Kir.5.1, SPAK, WNK
Abstract
The renal phenotype induced by loss-of-function mutations of inwardly rectifying potassium channel (Kir), Kcnj10 (Kir4.1), includes salt wasting, hypomagnesemia, metabolic alkalosis and hypokalemia. However, the mechanism by which Kir.4.1 mutations cause the tubulopathy is not completely understood. Here we demonstrate that Kcnj10 is a main contributor to the basolateral K conductance in the early distal convoluted tubule (DCT1) and determines the expression of the apical Na-Cl cotransporter (NCC) in the DCT. Immunostaining demonstrated Kcnj10 and Kcnj16 were expressed in the basolateral membrane of DCT, and patch-clamp studies detected a 40-pS K channel in the basolateral membrane of the DCT1 of p8/p10 wild-type Kcnj10+/+ mice (WT). This 40-pS K channel is absent in homozygous Kcnj10−/− (knockout) mice. The disruption of Kcnj10 almost completely eliminated the basolateral K conductance and decreased the negativity of the cell membrane potential in DCT1. Moreover, the lack of Kcnj10 decreased the basolateral Cl conductance, inhibited the expression of Ste20-related proline–alanine-rich kinase and diminished the apical NCC expression in DCT. We conclude that Kcnj10 plays a dominant role in determining the basolateral K conductance and membrane potential of DCT1 and that the basolateral K channel activity in the DCT determines the apical NCC expression possibly through a Ste20-related proline–alanine-rich kinase-dependent mechanism.
The distal convoluted tubule (DCT) of the kidney is responsible for the absorption of 5% of the filtered NaCl load and plays an important role in the reabsorption of Ca2+ and Mg2+ (1–3). The DCT has been functionally divided into early DCT (DCT1) and late DCT (DCT2). Whereas Na-Cl cotransporter (NCC) expression was detected throughout DCT, epithelial Na channel and renal outer medullary K channel have been shown to be expressed in DCT2 (4−6). In DCT1, Na enters the cell through the apical Na-Cl cotransporter (NCC) and leaves the basolateral membrane via the Na-K-ATPase while Cl leaves the cells through basolateral Cl channels or the KCl cotransporter (4, 7, 8). The K channels in the basolateral membrane participate in generating the cell membrane potential and are also responsible for K recycling, which is important for sustaining the Na-K-ATPase activity (9, 10). It is well established that the main basolateral K channel in the DCT is a 40-pS inwardly rectifying K channel which is a heterotetramer composed of Kcnj10 and Kcnj16 (11−15). In humans, loss-of-function mutations in the KCNJ10 gene have been shown to cause epilepsy, ataxia, sensorineural deafness and tubulopathy (SeSAME/EAST) syndrome (16–18). The renal manifestation of the disease is a reminiscence of Gitelman’s syndrome characterized by salt wasting, hypomagnesemia, metabolic alkalosis, and hypokalemia. This suggests that the membrane transport in the DCT is compromised by KCNJ10 mutations. However, the molecular mechanism by which KCNJ10 channel activity modulates the membrane transport in the DCT is not completely understood. Studies performed in Kcnj16 knockout mice demonstrated that the disruption of Kcnj16 did not recapitulate the phenotypes of SeSAME/EAST syndrome except for hypokalemia (19). Moreover, the basolateral K conductance of the DCT was increased in Kcnj16 knockout mice, indicating that Kir4.1 could compensate for the loss of Kir5.1 and sustains the transport in the DCT. Thus, it is essential to conduct the study in Kcnj10 knockout mice to understand the role of Kcnj10 in regulating the membrane transport in DCT. However, as described previously (20, 21), the growth of Kcnj10−/− mice was significantly retarded at 7 d (p7) after birth, and the body weight of the knockout mice was significantly lower than that of heterozygous (het) and WT littermates (Fig. S1). Moreover, homozygous Kcnj10−/− mice were not able to survive more than 2 wk after birth. Thus, the present study was carried out in p7/p10 neonatal littermates. The first aim of the present study is to examine whether Kir4.1 is essential for the basolateral K conductance in DCT1 and for generating the cell membrane potential. The second aim of the study is to examine whether the disruption of basolateral K channel activity impairs the activity of the apical NCC and the basolateral Cl channel in the DCT.
Results
We first used the fluorescence microscope to examine the expression of Kcnj10 in the kidney of 4-wk-old and p8 WT mouse. We confirmed the previous report that Kcnj10 is expressed in the basolateral membrane of DCT, connecting tubule (CNT), and initial cortical collecting duct (CCD) of both adult and p8 mouse kidneys (22). Because the present study is focused on the DCT, we only presented the results regarding the expression of Kcnj10 in DCT. Fig. S1 is immunostaining images demonstrating that Kcnj10 is highly expressed in the cortex region of WT mice but is absent in the knockout mice. Fig. 1A is an image showing Kcnj10 immunostaining in the kidney of a 4-wk-old WT mouse while Fig. 1B shows immunostaining of parvalbumin, a Ca2+ binding protein that is exclusively expressed in the DCT (23). Fig. 1 C and D shows merged images of Kcnj10 and parvalbumin at a low and high magnification, respectively. It is apparent that Kcnj10 staining is extensively located in the basolateral membrane of the DCT. Kcnj10 has been shown to interact with Kcnj16 to form a 40-pS inwardly rectifying K channel in the basolateral membrane of mouse DCT1 (11, 19). Thus, we used the patch-clamp technique to examine whether this 40-pS K channel was also present in the basolateral membrane of DCT1 of p7/p10 mice. Fig. S2 shows the kidney size and isolated DCT from both WT and Kcnj10 knockout mice. It is apparent that the isolated DCTs in the Kcnj10−/− mice had a similar length and appearance to those of WT mice. Fig. 2A is a typical channel recording showing the 40-pS K channel activity in the basolateral membrane of DCT1 of a p8 WT mouse. We detected the 40-pS K channels in 23 patches from all 32 experiments and the mean channel open probability was 0.5 ± 0.05 (n = 10). Thus, the probability of finding the 40-pS K channel and the channel open probability in DCT1 of p7/p10 WT mice was similar to that in adult mice (15). However, the probability of finding the 40-pS K channel decreased in Kcnj10+/− mice (9 patches from all 22 patches) and was zero from all 32 experiments in homozygous Kcnj10−/− mice. Moreover, we failed to detect any K channel activity in these 32 patches in the basolateral membrane of DCT1 of the knockout mice. Thus, the results strongly suggest that Kcnj10 is absolutely required for forming the 40-pS K channel in the DCT1 and that this 40-pS K channel should play a dominant role in generating the basolateral K conductance in the DCT1.
Fig. 1.
Kcnj10 is expressed in the basolateral membrane of the DCT. The experiments were performed in the kidney slice obtained from a 4-wk-old WT mouse. (A) Kcnj10 immunostaining in the mouse kidney with low magnification. (B) Parvalbumin immunostaining in the mouse kidney with low magnification. Fluorescence microscope image showing the merged staining of Kcnj10 (red) and parvalbumin (green) with the low magnification (C) and a high magnification (D).
Fig. 2.
Kcnj10 forms a 40-pS K channel in the basolateral membrane of DCT1. (A) A patch-clamp recording shows the 40-pS K channel activity in the basolateral membrane of DCT1. The experiments were performed in a cell-attached patch of DCT1 in the p8 WT mice. The DCT was bathed in a 140 mM NaCl+5 mM KCl solution, and the pipette solution contains 140 mM KCl. The holding potentials are indicated on the top of each trace, and the channel closed levels are labeled by “C.” (B) (Upper) A whole-cell recording showing the Ba2+-sensitive K currents and NPPB-sensitive Cl currents in a DCT1 cell of p8 WT mouse. Ba2+ (1 mM) and NPPB (10 μΜ) were directly added to the bath. (Lower) The bar graph summarizes the results from 10 measurements at −60 mV on DCT1 of p7/p10 mice. The K and Cl currents were measured with the perforated whole-cell recording with 140 mM KCl in the pipette and in the bath.
The notion that Kcnj10 is an indispensable component for the basolateral K conductance is further confirmed by the experiments in which we used the perforated whole-cell recording to measure the K currents in the DCT1. Two unique features of DCT1 cell make it possible to assess the basolateral K conductance by measuring the whole-cell K currents: (i) there is no apical K channel in the DCT1 (15) and (ii) the DCT1 cells lack the cell coupling as evidenced by the fact that carboxyflurescein dye injected into the clamped cells did not spread into neighboring cells (Fig. S3). Fig. 2B is a typical recording demonstrating the whole-cell currents of DCT1 of WT mice with symmetrical 140 mM KCl in the bath and in the pipette. From the inspection of Fig. 2B, it is apparent that adding 1 mM Ba2+ and 10 μΜ 5-Nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) almost completely abolished both inward and outward currents. This suggests that the Ba2+-sensitive K currents and NPPB-sensitive Cl currents are the main membrane conductance in DCT1. The mean K and Cl currents of DCT1 at −60 mV were −2650 ±190 pA/cell (n = 10) and −1670 ± 140pA/cell (n = 10), respectively. However, the Ba2+-sensitive K currents decreased in the DCT1 cells of heterozygous Kcnj10+/− and were largely abolished in homozygous Kcnj10−/− mice. Fig. 3A is a whole-cell recording showing that K currents in Kcnj10+/− mice were reduced to −1500 ± 90 pA (n = 11) whereas they were only −280 ± 60 pA/per cell at −60 mV (n = 11) in Kcnj10−/− mice. In contrast, K currents in their corresponding WT littermates were 2850 ± 180 pA per cell (n = 5). This finding strongly indicates that Kcnj10 plays a key role in determining the basolateral K conductance in the DCT1.
Fig. 3.
The disruption of Kir4.1 abolished the basolateral K conductance and caused a depolarization in DCT1. (A) (Left) A whole-cell recording showing Ba2+-sensitive K currents in DCT1 cells of p8/p10 WT, heterozygous (het), and knockout (KO) mice. (Right) A bar graph summarizes the results measured at −60 mV. The K currents were measured with perforated whole-cell recording with symmetrical 140 mM KCl in the bath and in the pipette. (B) (Left) A whole-cell recording showing K reversal potential in DCT1 cells of p8/p10 WT and knockout mice. (Right) The mean value of K reversal potential from eight measurements is summarized in a bar graph. For measurement of K reversal potential, the bath solution contains 140 mM NaCl+ 5 mM KCl while the pipette solution has 140 mM KCl.
Because the basolateral K conductance participates in generating the cell membrane potential, a decrease in the K conductance induced by the disruption of Kcnj10 is expected to reduce the negativity of DCT1 membrane potential. This possibility was examined by measuring the K reversal potential (an index of the cell membrane potential) with 140 mM Na/5 mM K in the bath and 140 mM K in the pipette. Fig. 3B is a typical recording showing the currents measured in the DCT1 cells, which were clamped from −100 mV to 100 mV with a ramp protocol. The K reversal potential was −65 ± 2 mV (n = 8) in the DCT1 of WT mice but it depolarized to −39 ± 3 mV (n = 8) in the homozygous Kcnj10−/− mice. The K reversal potential in DCT1 of Kcnj10+/− mice was also lower (−57 ± 3 mV, n = 4) than that in WT. Thus, the results strongly indicate that Kcnj10 plays a key role in generating the negative membrane potential in DCT1. Also, our finding suggests that Kcnj16 alone was not able to form a functional K channel in the basolateral membrane in the DCT1. This notion was further suggested by immunocytochemical experiments in which the Kcnj16 immunostaining was examined with fluorescence microscope in the WT and Kcnj10−/− mice, respectively. The positive Kcnj16 staining was detected in the DCT, CNT, and CCD of both WT and knockout mice. Fig. 4A is an immunostaining image showing that Kcnj16 is expressed in the parvalbumin-positive DCT. Moreover, Kcnj16 immunostaining is highly located in the basolateral membrane of the DCT in WT mice (Fig. 4B). The disruption of Kcnj10 did not affect the overall expression of Kcnj16 in parvalbumin-positive DCT and other distal nephron (Fig. 4C). However, it largely abolished the membrane staining of Kcnj16 not only in the DCT but also in the parvalbumin-negative tubules, presumably in the CNT or CCD (Fig. 4D). Thus, our results further confirmed the previous finding that homotetramer of Kcnj16 alone was not able to form a functional K channel and are also in consistence with the observation that the basolateral K conductance was largely abolished in Kcnj10−/− mice despite of the presence of Kcnj16 (24).
Fig. 4.
The disruption of Kcnj10 affects the membrane expression of Kcnj16. Immunostaining of Kir5.1 (Kcnj16) (red) and parvalbumin (green) in p8 Kcnj10+/+ (A and B) and p8 Kcnj10−/− mice (C and D). A and B are merged images showing Kir5.1 and parvalbumin staining with low magnification while C and D show the double staining with a high magnification. Arrow indicates the intracellular staining of Kcnj16 in the knockout mice.
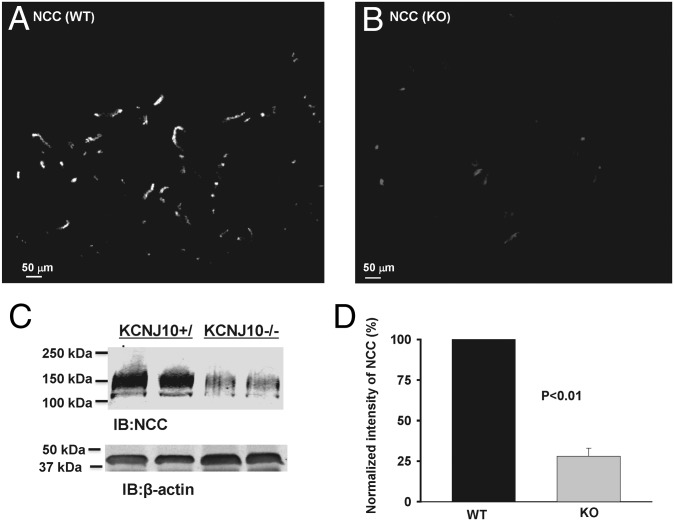
The basolateral K channel plays a key role in providing the driving force for Cl exit across the basolateral membrane through Cl channels. We speculate that the lack of the basolateral K channel may also inhibit the Cl channel. Thus, we used the whole-cell recording to examine NPPB-sensitive Cl currents in WT and homozygous mice. Fig. 5A is a recording showing NPPB-sensitive Cl currents in WT and homozygous Kcnj10−/− mice. It is apparent that Cl currents in the DCT1 of Kcnj10−/− mice (−460 ± 100 pA per cell at −60 mV, n = 8) were significantly lower than those of WT mice (−1750 ± 180 pA per cell at −60 mV, n = 8). Thus, the lack of Kcnj10 decreased Cl channel activity in DCT1. A decrease in the basolateral Cl conductance and basolateral membrane potential is expected to increase the intracellular Cl concentration in the DCT. A high intracellular Cl concentration has been shown to inhibit with-no-lysine kinase (WNK) autophosphorylation and its kinase activity and it also impairs WNK-ste20-related proline–alanine-rich kinase (SPAK) interaction (25, 26). Therefore, we next examined the possibility that the disruption of Kcnj10 may suppress the SPAK expression by examining the expression of SPAK in WT, heterozygous, and knockout mice. Fig. 5B is a Western blot showing that the expression of the full-length SPAK was significantly decreased in both Kcnj10+/− (52 ± 6% of the control value) and Kcnj10−/− mice (26 ± 4% of the control value) in comparison with those in WT mice (n = 4). Thus, the inhibition of the basolateral K conductance decreased full-length SPAK expression. Because full-length SPAK stimulates NCC activity through the phosphorylation (27–29), it is conceivable that the disruption of Kcnj10-induced inhibition of SPAK should suppress the NCC activity. Moreover, because Kcnj10 is the major type of K channel contributing to the basolateral K recycling, loss-of-function of Kcnj10 is expected to jeopardize K recycling across the basolateral membrane that is important to sustain the Na-K-ATPase activity and the transepithelial transport (30). Therefore, we examined the NCC expression in the DCT using immunostaining and Western blot. Fig. 6 A and B shows immunostaining images of NCC with a low magnification in WT mice and in homozygous Kcnj10−/− mice, respectively. It is apparent that the intensity of NCC immunostaining in Kcnj10−/− mice was lower than those of WT mice. The low expression of NCC in Kcnj10−/− mice was also confirmed by the Western blot analysis, demonstrating that the total NCC expression in Kcnj10−/− mice was only 28 ± 5% (n = 6) of the control value (Fig. 6 C and D). The notion that Kcnj10 expression affected NCC expression was also suggested by examining the expression of NCC in Kcnj10+/− mice. Western blot analysis demonstrated that the total renal NCC expression in Kcnj10+/− mice was also significantly reduced to 45 ± 8% (n = 4) of the control value but was higher than those of Kcnj10−/− mice (26 ± 5% of the control) (Fig. 7A). Fluorescence images further demonstrated that NCC staining was mainly localized in the apical membrane of WT mice (Fig. 7B) and also in Kcnj10+/− mice (Fig. 7C). However, not only was the total expression of NCC low in homozygous Kcnj10−/− mice, but also the intensity of NCC immunostaining in the apical membrane of DCT was decreased, and it was diffused into the intracellular space (Fig. 7 D and E). Thus, the results strongly suggest that the basolateral K channel activity plays an important role in the regulation of total NCC expression in the DCT.
Fig. 5.
The disruption of Kcnj10 decreased Cl conductance and SPAK expression in DCT. (A) (Upper) A recording showing NPPB-sensitive Cl currents in DCT1 of WT and knockout mice. The measurements were carried out with perforated whole-cell recording with symmetrical 140 mM KCl in the bath and pipette. (Lower) The results from eight experiments measured at -60 mV are summarized in a bar graph. (B) (Upper) A Western blot showing the expression of full-length SPAK in the kidney from WT, heterozygous, and homozygous Kcnj10−/− mice. (Lower) Results from four experiments are summarized in a bar graph.
Fig. 6.
The disruption of Kcnj10 inhibits NCC expression. Fluorescence microscope image with a low magnification shows overall NCC immunostaining in (A) p8 Kcnj10+/+ and (B) p8 Kcnj10−/− mice. (C) A Western blot showing the NCC expression in Kcnj10+/+ and Kcnj10−/− mice. (D) A bar graph summarizes results of experiments similar as those shown in C.
Fig. 7.
NCC expression is decreased in heterozygous and knockout mice. (A) (Upper) A Western blot showing the NCC expression in WT, heterozygous, and Kcnj10−/− mice. (Lower) Results from four experiments are summarized in a bar graph. Double immunostaining images show NCC expression (red) in parvalbumin-positive (green) DCT cell of p8 WT (B), p8 heterozygous (C), and p8 homozygous Kcnj10−/− mice (D and E).
Discussion
The first finding of the present study is that Kir.4.1 (Kcnj10) is a major contributor to the basolateral K conductance in the DCT1. Previous studies using single tubule RT-PCR, Western blot analysis, and immunocytochemistry demonstrated that Kir4.1, Kir.5.1, Kir7.1, and KCNQ1, a voltage-gated K channel, were expressed in the DCT (31–33). Moreover, Kir7.1 protein was detected as early as in p7 mice, and it was also expressed in the basolateral membrane of DCT (32). However, the observation that the basolateral K conductance in the DCT1 was largely abolished in Kcnj10−/− mice strongly indicates that Kir4.1 plays a dominant role in determining the basolateral K conductance. This conclusion was also supported by the finding that the disruption of Kcnj10 expression shifted the K reversal potential to a less negative value. Thus, although K channels other than Kir4.1 and Kir.5.1 are expressed in the basolateral membrane of DCT1, they play a minor role in determining the basolateral K conductance. However, because our experiments were performed in p7/p10 mice, we could not exclude the possibility that K channels other than Kir.4.1 may increase their contributions to the basolateral K conductance in DCT1 of the adult kidney. However, even if this occurs, it is unlikely that they would exceed the Kcnj10 because no K channels other than the 40-pS K channel were detected in the DCT1 from adult mice in over 300 patches (15).
It is well established that Kir.4.1 and Kir.5.1 form a heterotetramer 40-pS K channel in the DCT1 (11). This finding is confirmed by our present observation that the 40-pS K channel was completely absent in the basolateral membrane of DCT1 of the knockout mice. Although Kir5.1 (Kcnj16) was still expressed in the DCT and nephrons other than DCT in the knockout mice, no membrane staining in the basolateral membrane was visible in Kcnj10−/− mice. Moreover, the K channel activity was completely absent in the basolateral membrane of DCT1 of knockout mice, suggesting that Kcnj16 was not able to form homotetramer alone or heterotetramer with other inwardly rectifying K channel such as Kir.7.1. In contrast, Kcnj10 was able to form a 20-pS homotetramer in the basolateral membrane of DCT in Kcnj16−/− mice (19). Therefore, the present study suggests that Kcnj10 is essential for the basolateral K conductance and the cell membrane potential in the DCT1.
The second finding is that the full-length SPAK expression decreased in the kidneys of both heterozygous and knockout mice. We speculate that the membrane potential or basolateral K conductance may play an important role in the regulation of SPAK expression because the expression level of SPAK was closely correlated with the negativity of the membrane potential in the DCT1 of WT, heterozygous, and knockout mice. However, it is not known whether the cell membrane potential directly or the intracellular Cl concentration indirectly regulates SPAK expression. The basolateral K channels participate in generating the membrane potential and K recycling across the basolateral membranes (9). Because the membrane potential provides the driving force for the Cl exit across the basolateral membrane through Cl channels, a depolarization is expected to decrease Cl exit thereby increasing the intracellular Cl concentration. The observation that the basolateral Cl conductance was significantly decreased in Kcnj10−/− mice in comparison with those of WT mice supports the notion that the transcellular Cl transport in the DCT1 was compromised after the disruption of Kcnj10. Thus, a depolarization of the cell membrane potential with diminished Cl conductance is expected to cause an increase in intracellular Cl concentration in the DCT1, which has been shown to inhibit WNK autophosphorylation and WNK-SPAK interaction, thereby inhibiting the SPAK activity (25, 26).
A large body of studies has demonstrated that WNK and SPAK interactions play a key role in the regulation of NCC phosphorylation and activity (34–39). It has been reported that a decrease in NCC phosphorylation leads to enhancing the ubiquitination and internalization of NCC (40). Thus, a decrease in the full-length SPAK expression in Kcnj10 knockout mice is expected to decrease NCC phosphorylation, thereby decreasing NCC expression in the DCT. The role of Kcnj10 in determining NCC expression was indicated by observations that the expression of NCC in Kcnj10−/− and Kcnj10+/− mice was significantly decreased in comparison with those of WT, although heterozygous mice had as normal growth and life span as those of WT. Relevant to our finding is the report that the lack of SPAK activity decreased NCC expression in SPAK knockout mice (41). Thus, the results suggest that the basolateral K channel activity or the cell membrane potential plays a role in the regulation of NCC expression possibly by SPAK-dependent pathways. The lack of SPAK has been reported to mimic the effect of the deletion of NCC on the epithelial structure, significantly decreasing the DCT volume (29, 42). Thus, it is conceivable that a decrease in DCT volume may be also involved in decreasing NCC expression in Kcnj10 knockout mice.
Fig. 8 is a scheme illustrating the mechanism by which the disruption of Kcnj10 decreases NCC expression. We hypothesize that the inhibition of the basolateral K conductance decreases Cl exit across the basolateral membrane, thereby suppressing SPAK activity in the DCT. A decrease in SPAK expression inhibits the NCC expression. Thus, our data suggest that the loss-of-function mutation of Kcnj10-induced salt wasting is the result of decreasing NCC expression in the DCT, thereby increasing Na delivery to the connecting tubule and cortical collecting duct. An enhanced Na absorption in the distal nephron causes excessive K wasting, thereby causing hypokalemia and metabolic alkalosis. In addition, a depolarization in the DCT reduces the driving force for transcellular Mg2+ absorption in the DCT and leads to hypomagnesemia (43). We conclude that Kcnj10 plays a predominant role in determining the basolateral K conductance in the DCT1 and that the disruption of Kcnj10 decreases the NCC expression.
Fig. 8.
A cell scheme illustrating the mechanism by which the basolateral K channel activity regulates the apical NCC expression in the DCT1. The solid and dotted lines, respectively, mean an enhanced or a diminished signaling.
Methods
Animals.
Kcnj10−/−, Kcnj10+/−, and Kcnj10+/+ mice were obtained through mating Kcnj10+/− mice, which were kindly provided by Paulo Kofuji at University of Minnesota (Minneapolis) to R. Lifton (20, 21). Primers used for genotyping were the following: Kcnj10, forward 5′-TGG ACG ACC TTC ATT GAC ATG CAA TGG-3′ and reverse 5′-CTT TCA AGG GGC TGG TCT CAT CTA CCA CAT -3′ (20). Although Kcnj10−/− mice were viable and had no obvious abnormality at birth in comparison with their WT littermates, they died within 2 wk (21). Thus, we carried out the experiments using 7- to 10-d-old homozygous Kcnj10−/−, heterozygous Kcnj10+/−, and Kcnj10+/+ mice. The animal-using protocol was approved by independent Institutional Animal Care and Use Committee at both Yale University and New York Medical College.
Preparation of DCT1.
The experiments were performed on DCT1, a nephron segment immediately adjacent to the glomerulus (Fig. S2B). The methods for dissecting DCT were described previously (15). Moreover, the appearance of DCT1 isolated from Kcnj10−/− mice was similar to those in WT mice (Fig. S2B). The isolated DCT was transferred onto a 5 mm × 5 mm cover glass coated with polylysine (Sigma) to immobilize the tubule, and the cover glass was placed in a chamber mounted on an inverted microscope (Nikon). The DCT was superfused with Hepes-buffered NaCl solution containing: 140 mM NaCl, 5 mM KCl, 1.8 mM MgCl2, 1.8 mM CaCl2, and 10 mM Hepes (pH = 7.4) at room temperature for the single-channel patches and for the measurement of K reversal potential.
Electrophysiology.
The methods for the single-channel patch-clamp experiments, whole-cell recording, and measurement of K reversal potentials were described previously and also discussed in the supplemental material and ref. 15.
Immunostaining.
Mice were anesthetized with ketamine (100 mg/kg) and Xylazine (10 mg/kg) and the abdomens cut open for perfusion of kidneys with 2 mL PBS containing heparin (40 unit/mL) followed by 20 mL of 4% (vol/vol) paraformaldehyde. After perfusion, the kidneys were removed and subjected to postfixation with 4% (vol/vol) paraformaldehyde for 12 h. The kidneys were dehydrated and cut into 8- to 10-μM slices with Leica1900 cryostat (Leica). The tissue slices were dried at 42 °C for 1 h. The slides were washed with 1× PBS for 15 min, and permeablized with 0.3% Triton dissolved in 1× PBS buffer containing 1% BSA and 0.1% lysine (pH = 7.4) for 15 min. Kidney slices were blocked with 2% (vol/vol) horse serum for 30 min at room temperature and then incubated with primary antibodies (Kir.4.1, parvalbumin, Kir5.1 and NCC) for 12 h at 4 °C. Slides were thoroughly washed with 1× PBS and followed by addition of second antibodies mixture in 0.4% Triton dissolved in 1× PBS for 2 h at room temperature.
Preparation of Protein Samples and Western Blot.
The tissue of renal cortex was homogenized in an ice-cold solution containing 250 mM sucrose, 50 mM Tris⋅HCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and 1% protease and phosphatase inhibitor mixtures (Sigma) titrated to pH 7.6. After homogenization, the protein concentration was measured using the DC Protein Assay Kit (Bio-Rad). The proteins were separated by electrophoresis on 4–15% (vol/vol) SDS-polyacrylamide gels and transferred to nitrocellulose membrane. The membranes were blocked with LI-COR blocking buffer (PBS). An Odyssey infrared imaging system (LI-COR) was used to scan the membrane at a wavelength of 680 nM or 800 nM.
Experimental Materials and Statistics.
Kir5.1 and parvalbumin antibodies were purchased from Santa Cruz. We obtained Kir4.1 antibody from Millipore. Antibodies for NCC and SPAK were a gift from Robert Hoover (Emory University, Atlanta, GA), who used this antibody in his previous publication (44), and from J. McCormick and D. Ellison (Oregon Health & Science University, Portland, OR). The 5-Nitro-2-(3-phenylpropylamino)benzoic Acid (NPPB) was purchased from Sigma. The data are presented as mean ± SEM. We used the paired Student t test or one-way analysis of variance to determine the statistical significance.
Supplementary Material
Acknowledgments
The authors thank Drs. Robert Hoover, David Ellison, and James McCormick for their generosity in providing NCC and SPAK antibodies. The work is supported by National Institutes of Health Grant RO1 DK54983.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1411705111/-/DCSupplemental.
References
- 1.Monroy A, Plata C, Hebert SC, Gamba G. Characterization of the thiazide-sensitive Na(+)-Cl(-) cotransporter: A new model for ions and diuretics interaction. Am J Physiol Renal Physiol. 2000;279(1):F161–F169. doi: 10.1152/ajprenal.2000.279.1.F161. [DOI] [PubMed] [Google Scholar]
- 2.Ellison DH, Valazquez H, Wright FS. Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol Renal Physiol. 1987;253(3 Pt 2):F546–F554. doi: 10.1152/ajprenal.1987.253.3.F546. [DOI] [PubMed] [Google Scholar]
- 3.Simon DB, et al. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12(1):24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann S, et al. Expression of the thiazide-sensitive Na-Cl cotransporter by rabbit distal convoluted tubule cells. J Clin Invest. 1995;96(5):2510–2514. doi: 10.1172/JCI118311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubera I, et al. Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest. 2003;112(4):554–565. doi: 10.1172/JCI16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wade JB, et al. Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol. 2011;300(6):F1385–F1393. doi: 10.1152/ajprenal.00592.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lourdel S, Paulais M, Marvao P, Nissant A, Teulon J. A chloride channel at the basolateral membrane of the distal-convoluted tubule: A candidate ClC-K channel. J Gen Physiol. 2003;121(4):287–300. doi: 10.1085/jgp.200208737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liapis H, Nag M, Kaji DM. K-Cl cotransporter expression in the human kidney. Am J Physiol. 1998;275(6 Pt 1):C1432–C1437. doi: 10.1152/ajpcell.1998.275.6.C1432. [DOI] [PubMed] [Google Scholar]
- 9.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85(1):319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Hebert SC, Giebisch G. Renal K+ channels: Structure and function. Annu Rev Physiol. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- 11.Lourdel S, et al. An inward rectifier K(+) channel at the basolateral membrane of the mouse distal convoluted tubule: Similarities with Kir4-Kir5.1 heteromeric channels. J Physiol. 2002;538(Pt 2):391–404. doi: 10.1113/jphysiol.2001.012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams DM, et al. Molecular basis of decreased Kir4.1 function in SeSAME/EAST syndrome. J Am Soc Nephrol. 2010;21(12):2117–2129. doi: 10.1681/ASN.2009121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lachheb S, et al. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol. 2008;294(6):F1398–F1407. doi: 10.1152/ajprenal.00288.2007. [DOI] [PubMed] [Google Scholar]
- 14.Cha SK, et al. Calcium-sensing receptor decreases cell surface expression of the inwardly rectifying K+ channel Kir4.1. J Biol Chem. 2011;286(3):1828–1835. doi: 10.1074/jbc.M110.160390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, et al. Src family protein tyrosine kinase regulates the basolateral K channel in the distal convoluted tubule (DCT) by phosphorylation of KCNJ10 protein. J Biol Chem. 2013;288(36):26135–26146. doi: 10.1074/jbc.M113.478453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bockenhauer D, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360(19):1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reichold M, et al. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci USA. 2010;107(32):14490–14495. doi: 10.1073/pnas.1003072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scholl UI, et al. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA. 2009;106(14):5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulais M, et al. Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome. Proc Natl Acad Sci USA. 2011;108(25):10361–10366. doi: 10.1073/pnas.1101400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kofuji P, et al. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: Phenotypic impact in retina. J Neurosci. 2000;20(15):5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci. 2001;21(15):5429–5438. doi: 10.1523/JNEUROSCI.21-15-05429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandulik S, et al. The salt-wasting phenotype of EAST syndrome, a disease with multifaceted symptoms linked to the KCNJ10 K+ channel. Pflugers Arch. 2011;461(4):423–435. doi: 10.1007/s00424-010-0915-0. [DOI] [PubMed] [Google Scholar]
- 23.Belge H, et al. Renal expression of parvalbumin is critical for NaCl handling and response to diuretics. Proc Natl Acad Sci USA. 2007;104(37):14849–14854. doi: 10.1073/pnas.0702810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J. 1996;15(12):2980–2987. [PMC free article] [PubMed] [Google Scholar]
- 25.Piala AT, et al. 2014. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7:ra41.
- 26.Ponce-Coria J, et al. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA. 2008;105(24):8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.San-Cristobal P, et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA. 2009;106(11):4384–4389. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J Biol Chem. 2002;277(52):50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 29.McCormick JA, et al. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab. 2011;14(3):352–364. doi: 10.1016/j.cmet.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz SG. Homocellular regulatory mechanisms in sodium-transporting epithelia: Avoidance of extinction by “flush-through.”. Am J Physiol. 1981;241(6):F579–F590. doi: 10.1152/ajprenal.1981.241.6.F579. [DOI] [PubMed] [Google Scholar]
- 31.Zheng W, et al. Cellular distribution of the potassium channel KCNQ1 in normal mouse kidney. Am J Physiol Renal Physiol. 2007;292(1):F456–F466. doi: 10.1152/ajprenal.00087.2006. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki Y, et al. Expression of the K+ channel Kir7.1 in the developing rat kidney: Role in K+ excretion. Kidney Int. 2003;63(3):969–975. doi: 10.1046/j.1523-1755.2003.00806.x. [DOI] [PubMed] [Google Scholar]
- 33.Ookata K, et al. Localization of inward rectifier potassium channel Kir7.1 in the basolateral membrane of distal nephron and collecting duct. J Am Soc Nephrol. 2000;11(11):1987–1994. doi: 10.1681/ASN.V11111987. [DOI] [PubMed] [Google Scholar]
- 34.Lalioti MD, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38(10):1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 35.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest. 2003;111(7):1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozansky DJ, et al. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest. 2009;119(9):2601–2612. doi: 10.1172/JCI38323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castañeda-Bueno M, et al. Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA. 2012;109(20):7929–7934. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci USA. 2013;110(19):7838–7843. doi: 10.1073/pnas.1304592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyden LM, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482(7383):98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenbaek LL, Kortenoeven MLA, Aroankins TS, Fenton RA. Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis. J Biol Chem. 2014;289(19):13347–13361. doi: 10.1074/jbc.M113.543710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang SS, et al. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol. 2010;21(11):1868–1877. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loffing J, et al. Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol. 2004;15(9):2276–2288. doi: 10.1097/01.ASN.0000138234.18569.63. [DOI] [PubMed] [Google Scholar]
- 43.Houillier P. Mechanisms and regulation of renal magnesium transport. Annu Rev Physiol. 2014;76(1):411–430. doi: 10.1146/annurev-physiol-021113-170336. [DOI] [PubMed] [Google Scholar]
- 44.Ko B, et al. A new model of the distal convoluted tubule. Am J Physiol Renal Physiol. 2012;303(5):F700–F710. doi: 10.1152/ajprenal.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.