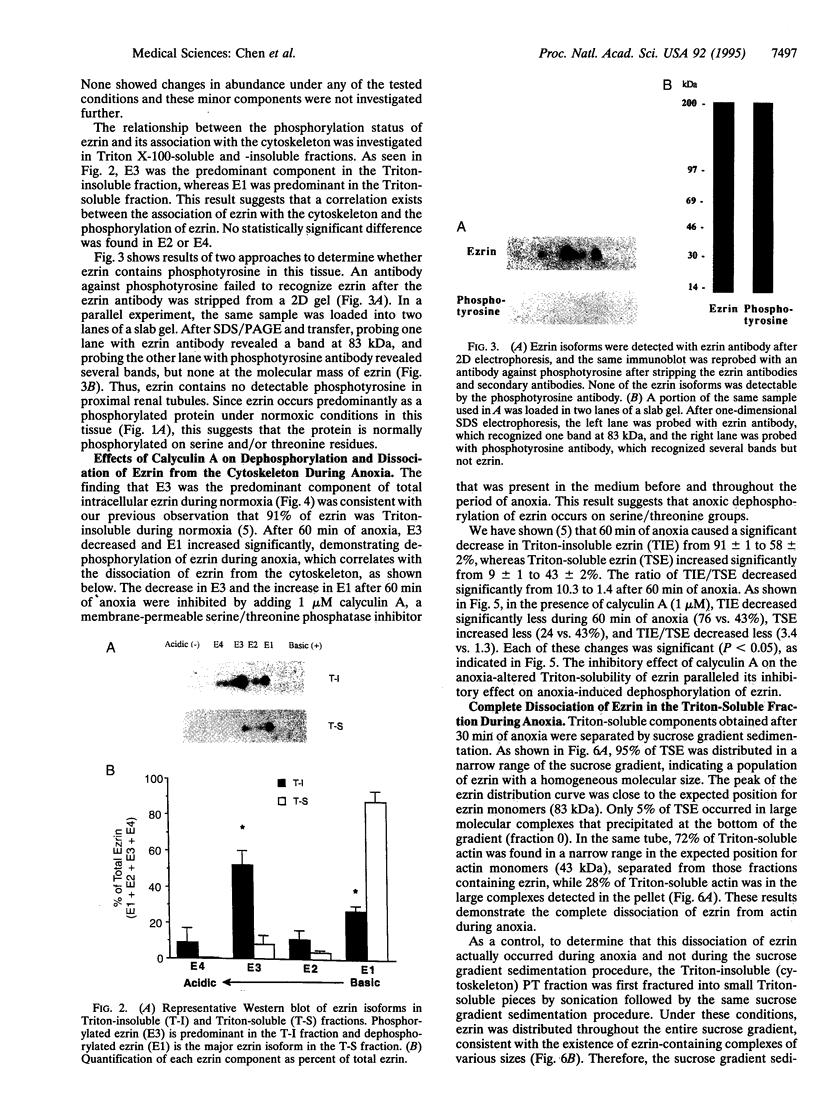
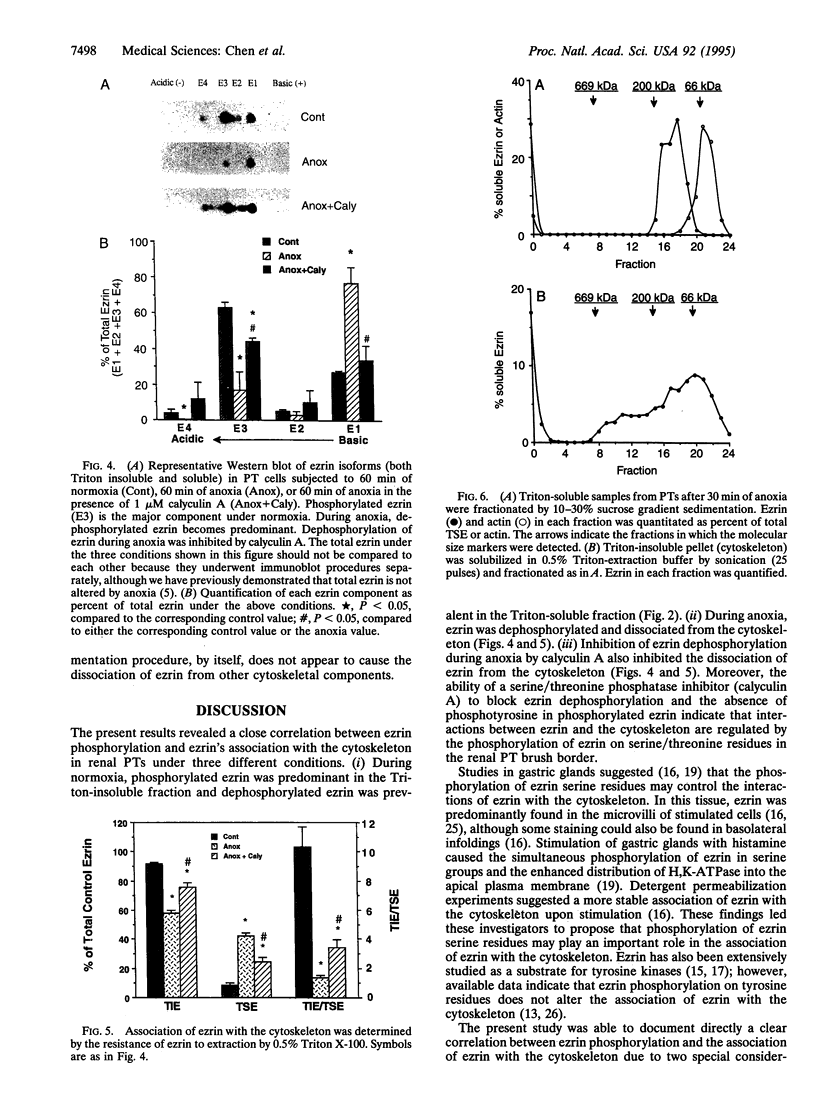
Abstract
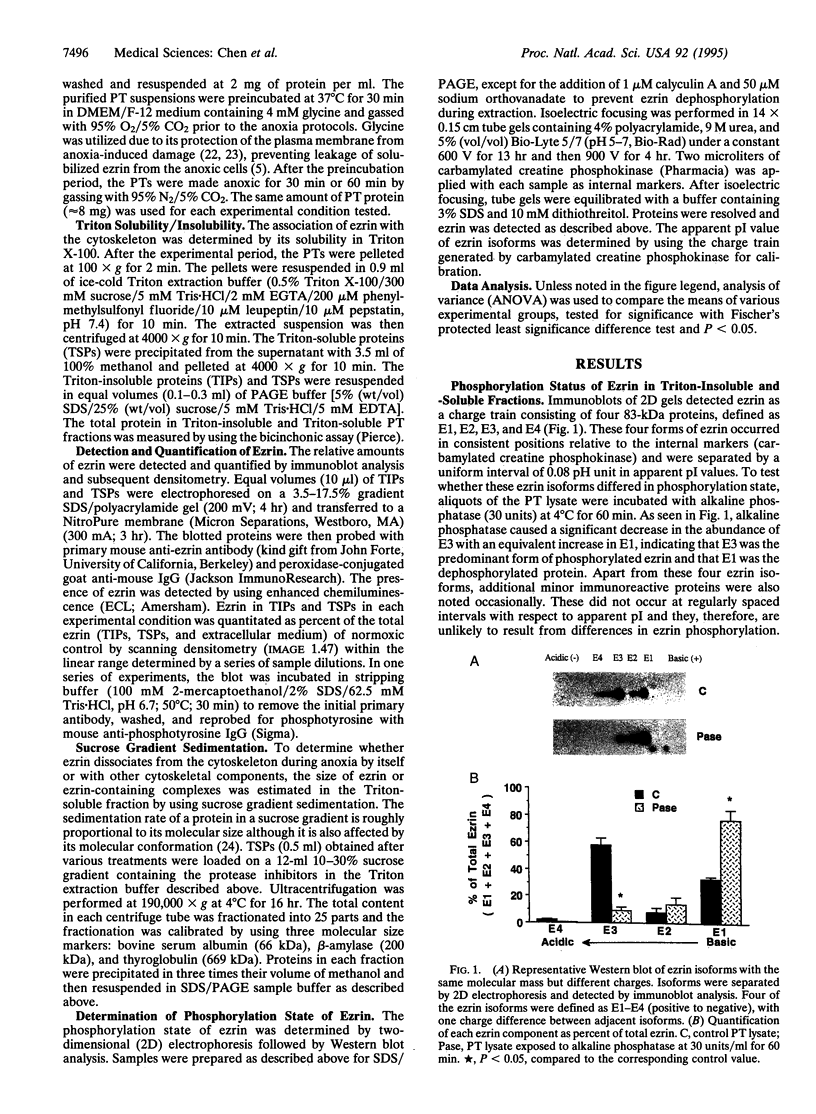
Disruption of the renal proximal tubule (PT) brush border is a prominent early event during ischemic injury to the kidney. The molecular basis for this event is unknown. Within the brush border, ezrin may normally link the cytoskeleton to the cell plasma membrane. Anoxia causes ezrin to dissociate from the cytoskeleton and also causes many cell proteins to become dephosphorylated in renal PTs. This study examines the hypothesis that ezrin dephosphorylation accompanies and may mediate the anoxic disruption of the rabbit renal PT. During normoxia, 73 +/- 3% of the cytoskeleton-associated (Triton-insoluble) ezrin was phosphorylated, but 88 +/- 6% of dissociated (Triton-soluble) ezrin was dephosphorylated. Phosphorylation was on serine/threonine resides, since ezrin was not detectable by an antibody against phosphotyrosine. After 60 min of anoxia, phosphorylation of total intracellular ezrin significantly decreased from 72 +/- 2% to 21 +/- 9%, and ezrin associated with the cytoskeleton decreased from 91 +/- 2% to 58 +/- 2%. Calyculin A (1 microM), the serine/threonine phosphatase inhibitor, inhibited the dephosphorylation of ezrin during anoxia by 57% and also blocked the dissociation of ezrin from the cytoskeleton by 53%. Our results demonstrate that (i) the association of ezrin with the renal microvillar cytoskeleton is correlated with phosphorylation of ezrin serine/threonine residues and (ii) anoxia may cause disruption of the renal brush border by dephosphorylating ezrin and thereby dissociating the brush border membrane from the cytoskeleton.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Algrain M., Turunen O., Vaheri A., Louvard D., Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol. 1993 Jan;120(1):129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman M., Franck Z., Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993 Aug;105(Pt 4):1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Microfilaments and membranes. Curr Opin Cell Biol. 1993 Aug;5(4):653–660. doi: 10.1016/0955-0674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol. 1983 Aug;97(2):425–432. doi: 10.1083/jcb.97.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol. 1989 Mar;108(3):921–930. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Doctor R. B., Mandel L. J. Cytoskeletal dissociation of ezrin during renal anoxia: role in microvillar injury. Am J Physiol. 1994 Sep;267(3 Pt 1):C784–C795. doi: 10.1152/ajpcell.1994.267.3.C784. [DOI] [PubMed] [Google Scholar]
- Coluccio L. M. Identification of the microvillar 110-kDa calmodulin complex (myosin-1) in kidney. Eur J Cell Biol. 1991 Dec;56(2):286–294. [PubMed] [Google Scholar]
- Dickman K. G., Mandel L. J. Glycolytic and oxidative metabolism in primary renal proximal tubule cultures. Am J Physiol. 1989 Aug;257(2 Pt 1):C333–C340. doi: 10.1152/ajpcell.1989.257.2.C333. [DOI] [PubMed] [Google Scholar]
- Egerton M., Burgess W. H., Chen D., Druker B. J., Bretscher A., Samelson L. E. Identification of ezrin as an 81-kDa tyrosine-phosphorylated protein in T cells. J Immunol. 1992 Sep 15;149(6):1847–1852. [PubMed] [Google Scholar]
- Everett A. W., Nichol K. A. Ezrin immunoreactivity in neuron subpopulations: cellular distribution in relation to cytoskeletal proteins in sensory neurons. J Histochem Cytochem. 1990 Aug;38(8):1137–1144. doi: 10.1177/38.8.2114439. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Bretscher A., Esch F. S., Hunter T. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J. 1989 Dec 20;8(13):4133–4142. doi: 10.1002/j.1460-2075.1989.tb08598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Cooper J. A., Bretscher A., Hunter T. The protein-tyrosine kinase substrate, p81, is homologous to a chicken microvillar core protein. J Cell Biol. 1986 Feb;102(2):660–669. doi: 10.1083/jcb.102.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzel D. K., Urushidani T., Usinger W. R., Smolka A., Forte J. G. Immunological localization of an 80-kDa phosphoprotein to the apical membrane of gastric parietal cells. Am J Physiol. 1989 Jun;256(6 Pt 1):G1082–G1089. doi: 10.1152/ajpgi.1989.256.6.G1082. [DOI] [PubMed] [Google Scholar]
- Hanzel D., Reggio H., Bretscher A., Forte J. G., Mangeat P. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli. EMBO J. 1991 Sep;10(9):2363–2373. doi: 10.1002/j.1460-2075.1991.tb07775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Rennke H., Levinsky N. G. Recovery of proximal tubular function from ischemic injury. Am J Physiol. 1984 Feb;246(2 Pt 2):F159–F166. doi: 10.1152/ajprenal.1984.246.2.F159. [DOI] [PubMed] [Google Scholar]
- Kobryn C. E., Mandel L. J. Decreased protein phosphorylation induced by anoxia in proximal renal tubules. Am J Physiol. 1994 Oct;267(4 Pt 1):C1073–C1079. doi: 10.1152/ajpcell.1994.267.4.C1073. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Schnellmann R. G., Jacobs W. R. Intracellular glutathione in the protection from anoxic injury in renal proximal tubules. J Clin Invest. 1990 Feb;85(2):316–324. doi: 10.1172/JCI114440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkanen R., von Bonsdorff C. H., Turunen O., Wahlström T., Vaheri A. Redistribution of Mr 75,000 plasma membrane protein, cytovillin, into newly formed microvilli in herpes simplex and Semliki Forest virus infected human embryonal fibroblasts. Eur J Cell Biol. 1988 Aug;46(3):435–443. [PubMed] [Google Scholar]
- Pestonjamasp K., Amieva M. R., Strassel C. P., Nauseef W. M., Furthmayr H., Luna E. J. Moesin, ezrin, and p205 are actin-binding proteins associated with neutrophil plasma membranes. Mol Biol Cell. 1995 Mar;6(3):247–259. doi: 10.1091/mbc.6.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Funayama N., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992 Sep;103(Pt 1):131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Nairn A. C. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- Turunen O., Wahlström T., Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol. 1994 Sep;126(6):1445–1453. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushidani T., Hanzel D. K., Forte J. G. Characterization of an 80-kDa phosphoprotein involved in parietal cell stimulation. Am J Physiol. 1989 Jun;256(6 Pt 1):G1070–G1081. doi: 10.1152/ajpgi.1989.256.6.G1070. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Bernard D. B., Donohoe J. F., Levinsky N. G. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978 Jul;14(1):31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Abarzua M., Rajan T. Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. J Clin Invest. 1987 Nov;80(5):1446–1454. doi: 10.1172/JCI113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M. Oxygen deprivation-induced injury to isolated rabbit kidney tubules. J Clin Invest. 1985 Sep;76(3):1193–1208. doi: 10.1172/JCI112075. [DOI] [PMC free article] [PubMed] [Google Scholar]