Significance
Intrinsic bursts and rhythmic burst discharges are elicited by activation of T-type Ca2+ channels in the thalamic reticular nucleus (TRN). TRN bursts are believed to be critical for generation and maintenance of thalamocortical oscillations, leading to spike-and-wave discharges (SWDs) on the cortical electroencephalogram, which are the hallmarks of absence seizures. Using knockout mice for T-type Ca2+ channels that completely lack TRN bursts, however, we show that increased tonic firing in the TRN seems sufficient for drug-induced SWD generation. These results call into question the role of burst firing in TRN neurons in the genesis of SWDs, calling for a rethinking of the mechanism for absence seizure induction.
Abstract
Intrinsic burst and rhythmic burst discharges (RBDs) are elicited by activation of T-type Ca2+ channels in the thalamic reticular nucleus (TRN). TRN bursts are believed to be critical for generation and maintenance of thalamocortical oscillations, leading to the spike-and-wave discharges (SWDs), which are the hallmarks of absence seizures. We observed that the RBDs were completely abolished, whereas tonic firing was significantly increased, in TRN neurons from mice in which the gene for the T-type Ca2+ channel, CaV3.3, was deleted (CaV3.3−/−). Contrary to expectations, there was an increased susceptibility to drug-induced SWDs both in CaV3.3−/− mice and in mice in which the CaV3.3 gene was silenced predominantly in the TRN. CaV3.3−/− mice also showed enhanced inhibitory synaptic drive onto TC neurons. Finally, a double knockout of both CaV3.3 and CaV3.2, which showed complete elimination of burst firing and RBDs in TRN neurons, also displayed enhanced drug-induced SWDs and absence seizures. On the other hand, tonic firing in the TRN was increased in these mice, suggesting that increased tonic firing in the TRN may be sufficient for drug-induced SWD generation in the absence of burst firing. These results call into question the role of burst firing in TRN neurons in the genesis of SWDs, calling for a rethinking of the mechanism for absence seizure induction.
Absence seizures are generalized, nonconvulsive seizures characterized by the appearance of bilaterally synchronous spike-and-wave discharges (SWDs) on the electroencephalogram (EEG). The frequency of the SWDs is variable among different models and is usually higher (4–12 Hz) in rodents than in humans (3 Hz) (1). SWDs represent synchronized oscillations of the thalamocortical network (2–4), a network that includes neurons of the cerebral cortex, thalamocortical nucleus (TC), and thalamic reticular nucleus (TRN) (5). This thalamocortical circuitry is a key CNS structure for gating the flow of sensory information from the periphery to the cortex (6, 7). Both thalamocortical and corticothalamic connections are mainly glutamatergic (8). The TRN is a shell-like structure that covers most of the rostral, lateral, and ventral parts of the thalamus (5) and is composed exclusively of GABAergic interneurons that provide massive inhibitory input to TC neurons (9). The most distinctive feature of thalamocortical circuitry is its intrinsic ability to generate oscillations via the reciprocal circuits between TC and TRN neurons (10–12).
Both TC and TRN neurons are able to generate two distinctive patterns of action potential firing: tonic and burst (13, 14). Burst firing is mediated by low-voltage–activated (LVA) T-type Ca2+ channels (15). There are three subtypes of T-type Ca2+channels, called CaV3.1, CaV3.2, and CaV3.3, each with distinctive expression patterns and kinetic properties (16). Within the thalamocortical circuit, CaV3.1 channels are predominantly expressed in TC neurons, whereas CaV3.2 and CaV3.3 channels are expressed only in TRN neurons (17). Unlike high-voltage–activated Ca2+ channels, T-type Ca2+ channels are inactivated at membrane potentials around −60 to −50 mV (15). However, when the membrane potential is hyperpolarized for longer than 100 ms, these channels are de-inactivated and can then initiate a burst of action potentials once the membrane potential is repolarized (18). TC neurons with a relatively depolarized resting membrane potential exhibit a tonic mode of firing associated with conventional, Na/K-dependent action potentials upon receiving excitatory inputs. However, when the neurons are hyperpolarized by inhibitory inputs, high-frequency burst firing can be triggered by CaV3.1 channels. TRN neurons, on the other hand, have a relatively hyperpolarized resting potential around −70 mV and most of their T-type Ca2+ channels are available for activation. These channels, especially CaV3.3, enable TRN neurons to generate rhythmic bursts, even in response to excitatory inputs (12, 19). These oscillatory bursts of action potentials in the TRN provide a barrage of hyperpolarizing inhibitory postsynaptic potentials (IPSPs) to the TC neurons, which in turn respond with action potential bursts due to the activation of CaV3.1. These TC bursts provide rhythmic excitatory drive to the cortex, eventually resulting in SWDs.
Several models have been proposed to account for the mechanism of SWD generation in the thalamocortical circuit. One well-known model argues that intrinsic cortical oscillations drive the synchronized activity of the entire thalamocortical circuit to cause SWDs (20). The most widely accepted model suggests that rhythmic bursting activity in TRN neurons is a key element for initiation and maintenance of SWDs (4, 10, 12). This model has been indirectly supported by previous observations that lesions or blockade of voltage-gated Ca2+ channels in TRN disrupt SWDs (21), leading to the idea that the degree of the synchrony between TC and TRN is regulated by the burst firing of TRN neurons (10). However, there have been no direct experimental tests of the hypothesis that TRN bursts are indeed essential for SWDs in absence seizures. Here we have tested the role of TRN bursts in absence epilepsy and have surprisingly found that mice with complete genetic deletion of TRN burst activity exhibited enhanced SWDs with higher susceptibility to γ-butyrolactone (GBL), a widely used seizure-inducing drug. Apparently that enhanced TRN tonic firing is observed in the absence of bursts is sufficient to support SWDs.
Results
Generation of CaV3.3 Ca2+ Channel Knockout Mice.
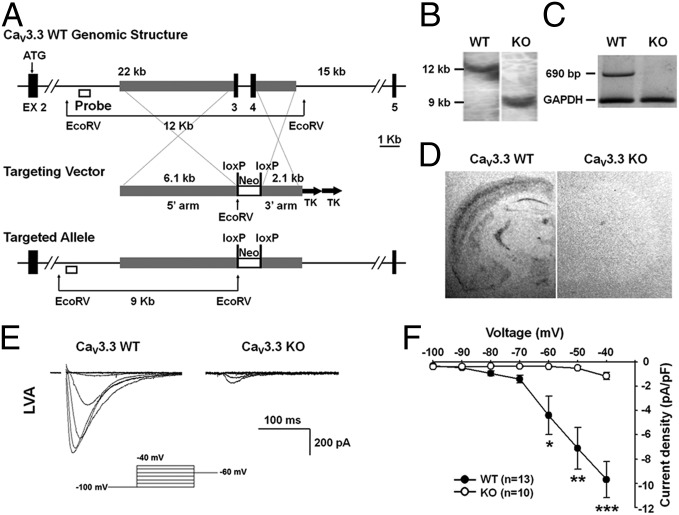
To explore the physiological function of CaV3.3, we carried out targeted disruption of the CaV3.3 gene in mice as shown in Fig. 1A. The targeting vector was designed to delete exon3 and exon4 that comprise the N terminus of the CaV3.3 protein (Amgen Co.; Fig. 1A). Following successful germ-line transmission, mice heterozygous for the targeting event were interbred to obtain homozygous CaV3.3 knockouts (KOs), as confirmed by PCR and/or Southern blot (Fig. 1B). No sex bias was observed in the offspring, and the expected Mendelian ratio was observed among wild-type, heterozygous, and homozygous mutant mice (data not shown). To assess whether disruption of the CaV3.3 gene was effective, we examined the level of CaV3.3 mRNA. RT-PCR analysis showed that no CaV3.3 mRNA was produced in the CaV3.3 brain, indicating that the gene targeting resulted in a null mutation for this locus (Fig. 1C). We also used in situ hybridization to check the expression and cellular localization of CaV3.3 in wild-type and mutant mouse brains (Fig. 1D).
Fig. 1.
Targeted disruption of the mouse CaV3.3 gene and decreased T-type current in CaV3.3 KO. (A) Schematic representations of the wild-type CaV3.3 allele, targeting vector, and mutant allele. Exons were represented by black boxes. Exon3 and exon4 were designed to be deleted to generate the mutant allele. (B) Southern blot analysis of genomic DNA isolated from tails of wild-type (WT) and homozygous KO mice. The restriction enzymes and probes used are shown in A. The 12 kb segment corresponds to the wild allele; the 9 kb, the targeted allele. (C) RT-PCR analysis was performed with mRNA derived from pooled samples of wild-type or the CaV3.3 mutant whole brain. PCR primers for RT-PCR were exon3–7. The 690 bp band indicates the PCR product of the wild type, whereas CaV3.3 mutant displays no band. (D) Quantitative analysis of the expression of the CaV3.3 mRNA with in situ hybridization. There is robust expression of CaV3.3 in the TRN of wild-type mouse but no expression in mutant TRN. Probes for in situ hybridization were exon3 and 4. (E) LVA Ca2+ currents were evoked by the depolarizing pulses ranging from −100 to −40 mV in wild-type and CaV3.3−/− neurons. (F) Current–voltage relationship displayed a significant reduction in the peak current density in CaV3.3−/− (open circle) compared with wild type (closed circle). Data are represented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed t test.
T-Type Currents and Oscillatory Burst Firing Are Impaired in CaV3.3 KO Mice.
The functional loss of CaV3.3 was examined by whole-cell voltage clamp analysis of TRN neurons in acute brain slices from ∼3–4-wk-old KO and wild-type mice. LVA inward currents were evoked from a holding potential of –100 mV to depolarizing levels ranging from –90 to –40 mV (Fig. 1E). The peak density of a fast-inactivating current, typical of T-type Ca2+ currents, evoked at appropriate test potentials were significantly reduced in CaV3.3−/− TRN neurons compared with wild-type controls (Fig. 1F). These results are consistent with those reported previously (22) and suggest that CaV3.3 is responsible for most T-type Ca2+ currents in TRN neurons. The small amount of residual current observed in CaV3.3−/− neurons at –40 mV is probably mediated by CaV3.2 and as observed previously (22).
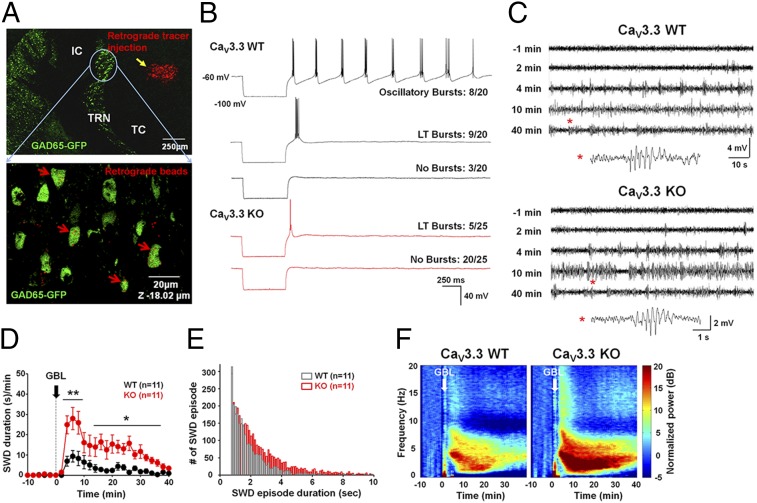
To identify GABAergic neurons that project from TRN to TC, we crossed the CaV3.3 mutant mice with glutamate decarboxylase 65 (GAD65)-GFP transgenic mice. Injection of a retrograde tracer into the TC region of these mice retrogradely labeled some of the GFP-positive GABAergic neurons in the dorsomedial region of TRN (Fig. 2A), allowing us to identify and characterize GABAergic projection neurons of TRN. In acute brain slices from wild-type mice, a brief hyperpolarizing current pulse that brought the membrane potential to −100 mV evoked subsequent oscillatory rebound bursting in ∼40% of projection neurons, whereas 45% showed only a single low-threshold (LT) burst, and 15% exhibited no LT bursting. In CaV3.3−/− mice, 80% of GFP-positive projection neurons showed no LT burst firing, whereas 20% exhibited a single weak LT burst and oscillatory bursts were never observed (Freeman–Halton extension of the Fisher’s exact probability test, P = 0.0000053) (Fig. 2B). Thus, the ability of TRN neurons to fire bursts of action potentials is severely impaired in CaV3.3−/− mice.
Fig. 2.
Altered firing properties of TRN neurons lacking CaV3.3 channels and increased susceptibility to GBL-induced SWD in CaV3.3 KO mice. (A) TRN neurons labeled with red retrograde tracer injected into TC of GAD65-GFP mice. Confocal image of TRN neurons colabeled with retrograde beads (red) and GFP (expanded image). (B) Firing characters of CaV3.3+/+ (upper three traces) and CaV3.3−/− (lower two traces) TRN neurons. (C) EEG traces of adult CaV3.3+/+ (Upper) and CaV3.3−/− (Lower) mice for 1 min before and various times after GBL injection. SWDs marked with asterisks are expended at the bottom. (D) SWD density calculated by total duration of SWD per min in CaV3.3+/+ (black circle) and CaV3.3−/− (red circle) mice. (E) Distribution of SWD episode duration after GBL injection. (F) Average EEG power spectrograms of CaV3.3+/+ and CaV3.3−/− mice during a 50-min recording. GBL was injected after 10 min of baseline recording. Data are represented as mean ± SEM; *P < 0.05, **P < 0.01.
Increased GBL-Induced Absence Seizures in CaV3.3 KO or Knockdown Mice.
Because CaV3.3 is abundantly expressed in TRN but not in TC (17), we could use CaV3.3−/− mice to determine the role of TRN neuron bursting in SWDs. Based on the current model (10), we predicted that SWDs should decrease or disappear in CaV3.3−/− mice. To test this prediction, we examined the susceptibility of adult CaV3.3−/− mice to the seizure-inducing drug GBL (Fig. 2C). Administration of GBL (70 mg/kg body weight, i.p.) to wild-type mice at the age of ∼10 wk induced typical paroxysmal SWDs (Fig. 2C) and behavioral arrest (Fig. S1A). Contrary to our expectations, in CaV3.3−/− mice, SWDs were not reduced; in fact, GBL more effectively induced SWDs both in density (s/min) and total duration in CaV3.3−/− mice than in wild-type mice [repeated-measures ANOVA (rmANOVA), group effect, F(1) = 15.67, P = 0.0008; TIME effect, F(57) = 14.46, P < 0.0001; Group × Time interaction, F(57) = 4.35, P < 0.0001] (Fig. 2D), with longer SWD episode duration (Kolmogorov–Smirnov test, P = 0.00003) (Fig. 2E). The relative EEG power in the 3–5 Hz range characteristic of SWDs was significantly higher in the CaV3.3−/− mice than wild-type for the entire recording duration after GBL treatment (Fig. 2F). Similar results were found in juvenile (age 3–4 wk) mutant mice [rmANOVA, group effect, F(1) = 1.94, P = 0.1807; time effect, F(57) = 15.2, P < 0.0001; Group × Time interaction, F(57) = 1.9, P < 0.0001] (Fig. S1 C and D). SWDs were analyzed based on the raw and filtered waveform of EEG recordings as described in detail in SI Materials and Methods (Fig. S1 A and B). The antiabsence drug ethosuximide (ETX) dramatically suppressed GBL-induced SWDs in mutant animals, which confirms that these were typical SWDs (Fig. S1B1).
Within the thalamocortical pathway, CaV3.3 is expressed both in cortex and in TRN (17). To address possible cortical contributions to the seizure phenotype of CaV3.3−/− mice, we used virus-mediated gene silencing to knock down CaV3.3 predominantly in TRN. An adeno-associated viral (AAV) vector containing shRNA specific for CaV3.3 (Fig. S2 A and B) was injected bilaterally into the TRN of 8-wk-old GAD65-GFP mice (Fig. 3A, Upper). Three weeks later, both scrambled (control) and shRNA for CaV3.3 (shCaV3.3) visualized with red fluorescence (mCherry) colocalized with GAD65-positive (GFP expressing) neurons in TRN. Expression of virus was restricted to TRN neurons (Fig. 3A, Middle and Lower) and significantly reduced CaV3.3 mRNA expression by ∼62% in TRN compared with control (Fig. 3B). Such TRN-specific gene silencing of CaV3.3 reduced T-type Ca2+ currents in TRN neurons (Fig. S2 C and D) and enhanced sensitivity to GBL, similar to what we observed in the CaV3.3−/− mice. CaV3.3 knockdown caused a significantly shorter onset time and a higher density of SWD compared with mice injected with control virus [rmANOVA, group effect, F(1) = 4.52, P = 0.0468; time effect, F(57) = 9.21, P = 0; Group × Time interaction, F(57) = 1.12, P = 0.2514] (Fig. 3 C and D). An EEG power spectrum in the 3–5 Hz range also was significantly higher in CaV3.3 knockdown mice than in control mice (Fig. 3E). Therefore, these results suggest that an increased GBL-induced absence seizure in CaV3.3 KO was mediated by deletion of CaV3.3 at TRN, not cortex.
Fig. 3.
Enhanced susceptibility to GBL-induced SWD after microinjection of AAV-shCaV3.3 in the TRN of GAD65-GFP mice. (A) Confocal images showing expression of AAV-control and AAV-shCaV3.3 (yellow circles indicate the TRN region in Top panels). Virus-infected neurons are colabeled with GFP (green, GAD65-GFP–positive neurons; red, AAV-control–infected neurons in Middle panel; and AAV-shCaV3.3 in Bottom panel; yellow, merged). (B) Representative images of in situ hybridization (Upper, AAV-control; Lower, AAV-shCaV3.3). (C) EEG traces of AAV-control (Left) and AAV-shCaV3.3 mice (Right) for 1 min before and various time after GBL injection. (D) SWD density calculated by total duration of SWD per min in AAV-control– (black circle) and AAV-shCaV3.3– (red circle) injected mice. (E) Average EEG power spectrograms of AAV-control– and AAV-shCaV3.3–injected mice during a 50-min recording. GBL was injected after 10 min of baseline recording. Data are represented as mean ± SEM, *P < 0.05.
Complete Deletion of Burst Firing in TRN Enhances Absence Seizures in CaV3.3−/− KO and CaV3.2−/−/3.3−/− Double-KO Mice.
To sidestep the possible effects of residual T-type channel activity present in the TRN of CaV3.3 knockdown and CaV3.3−/− mice (Fig. 1F), we made double-KO (DKO) mice lacking both CaV3.2 and CaV3.3 (CaV3.2−/−/3.3−/− mice) (Fig. S3A). Although these mice had no bursting activity in their TRN neurons (Fisher’s exact probability, P = 0.0012) (Fig. 4A), the effect of GBL injection was similar to what we observed in CaV3.3−/− and CaV3.3 knockdown mice [rmANOVA, group effect, F(1) = 21.35, P = 0.0004; time effect, F(57) = 13.99, P < 0.0001; Group × Time interaction, F(57) = 5.43, P < 0.0001] (Fig. 4 B–F). The interspike interval within an SWD was not changed. Further, neither CaV3.3−/− KO nor CaV3.2−/−/3.3−/− DKO mice exhibited spontaneous SWD (data not shown). These findings indicate that SWD can be maintained in the complete absence of TRN burst firing and, in fact, is enhanced when bursts are abolished.
Fig. 4.
Altered firing properties of TRN neurons and increased susceptibility to GBL-induced SWD in CaV3.2−/−/3.3−/− mice. (A) Firing properties of CaV3.2+/+/3.3+/+ (Right) and CaV3.2−/−/3.3−/− (Left) TRN neurons. (B) EEG traces of adult CaV3.2+/+/3.3+/+ (Left) and CaV3.2−/−/3.3−/− (Right) mice for 1 min before and various times after GBL injection. (C) SWD density calculated by total duration of SWD per min in CaV3.2+/+/3.3+/+ (black circle) and CaV3.2−/−/3.3−/− (red circle) mice. (D) Average EEG power spectrograms of CaV3.2+/+/3.3+/+ and CaV3.2−/−/3.3−/− mice during a 50-min recording. GBL was injected after 10 min of baseline recording. (E) Onset time of GBL-induced SWDs in CaV3.3−/− (white) and CaV3.2−/−/3.3−/− (red) mice and their controls (black and gray, respectively). (F) Total duration of SWD after GBL injection. Data are represented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Tonic Firing Is Increased in CaV3.3−/− KO and CaV3.2−/−/3.3−/− DKO Mice.
To explain this unexpected observation, we considered the possibility that loss of T-type Ca2+ currents could have other effects on TRN neurons that enhance SWDs. In contrast to the loss of TRN bursts, the frequency of tonic firing evoked by depolarizing currents was significantly increased in TRN GFP-positive projection neurons of CaV3.3−/− mice compared with those in wild-type mice [two-way ANOVA, group effect, F(1,36) = 4.8, P = 0.035] (Fig. 5 A and B). Tonic firing was also increased in CaV3.2−/−/3.3−/− mice (Fig. S3 B and C). To examine the cause of the increased tonic firing, we analyzed the after-hyperpolarizations (AHPs) that are known to regulate action potential frequency. The amplitude of the fast AHP (fAHP), which plays an important role in repolarization of membrane potential after an action potential, was not significantly different between CaV3.3−/− and wild-type neurons (Fig. 5C). Coupling between T channels and Ca2+-activated K+ channels is known to be indispensable for oscillatory discharges of TRN neurons (23). Thus, we examined the amplitude of the medium AHP (mAHP) caused by activation of Ca2+-activated K+ channels and found that mAHPs were significantly reduced in the mutant mice (Fig. 5D). This was also observed in CaV3.2−/−/3.3−/− mice (Fig. S3 D and E). Therefore, an increased tonic firing of mutant TRN neurons might be at least partially due to a decreased mAHP. In contrast, other intrinsic electrophysiological properties of TC neurons were unaffected in mutant mice (Fig. S4 A–D). These neurons also exhibited no differences in the amplitude or frequency of spontaneous inhibitory postsynaptic currents (IPSCs) resulting from TRN input (Fig. S4 E–G), which is consistent with the lack of spontaneous SWDs in CaV3.3−/− mice.
Fig. 5.
Enhanced inhibitory synaptic inputs to TC neurons during tonic firing in TRN neurons of CaV3.3−/− mice. (A) Representative traces of tonic firings in CaV3.3+/+ (Left) and CaV3.3−/− TRN neurons (Right). (B) Average number of tonic firings with different current pulses in CaV3.3+/+ and CaV3.3−/− TRN neurons. (C) Average fAHPs in CaV3.3+/+ and CaV3.3−/− TRN neurons. (D) Average mAHPs in CaV3.3+/+ and CaV3.3−/− TRN neurons. (E) IPSC evoked by tonic-frequency (upper trace, five stimuli at 100 Hz) or burst-frequency (lower trace, five stimuli at 500 Hz) electrical stimulations in CaV3.3+/+ and CaV3.3−/− TC neurons. (F) Average IPSC area in CaV3.3+/+ and CaV3.3−/− TC neurons. (G) PPR in CaV3.3+/+ and CaV3.3−/− TC neurons. Data are represented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Enhanced TRN–TC Inhibitory Synaptic Connection in the Mutant.
To determine the downstream effect of increased tonic firing in TRN neurons, we next examined the TRN–TC inhibitory synaptic connection. Whole-cell patch clamp recordings from TC neurons of CaV3.3−/− mice, measured in the presence of the glutamate receptor blockers 6-cyano-7-nitroquinoxaline-2, 3-dione and 2-Amino-5-phosphonopentanoic acid, revealed monosynaptic IPSCs in response to electrical stimulation of TRN. There was no significant difference in the amplitude of IPSCs evoked in TC neurons by single stimuli (wild type, 274 ± 117 pA, n = 8; CaV3.3−/−, 321 ± 131 pA, n = 9, P = 0.8). This indicates that basal synaptic strength is unchanged in the mutant. However, differences were observed in response to multiple TRN stimuli. We used five stimuli at 100 Hz or 500 Hz to mimic tonic or burst firing, respectively (Fig. 5E). Previous in vivo observations show that TRN neurons fire bursts composed of 5–15 spikes at frequencies ranging from 250 to 550 Hz (24). Kim et al. (25) demonstrated that a 100 Hz tonic discharge or a 500 Hz burst frequency of perigeniculate nucleus generates IPSPs in postsynaptic TC cells in vitro. For tonic-frequency stimulation, synaptic strength, measured as the integrated charge of IPSCs, was significantly larger in CaV3.3−/− compared with wild-type mice (Fig. 5F). However, there was no difference between the two genotypes in their responses to burst-frequency stimulation. As a result, in the mutant IPSC, responses to tonic-frequency stimulation were significantly larger than responses to burst-frequency stimulation. To reveal how tonic-frequency stimulation increased inhibitory synaptic transmission in CaV3.3−/− mice, we analyzed the paired-pulse ratio (PPR; ratio of second/first response) of IPSCs. The PPR evoked by tonic-frequency stimulation was greater (P = 0.038) in CaV3.3−/− (0.74 ± 0.6) than in wild type (0.55 ± 0.75) (Fig. 5G). This indicates that the increase in inhibitory synaptic transmission in CaV3.3−/− mice is activity-dependent and presynaptic in origin, perhaps caused by the decreased mAHPs of TRN neurons. The change in PPR may reflect changes in presynaptic release probability.
Discussion
TRN neurons are thought to be pacemakers for the initiation of thalamocortical oscillations (3, 11). Both pharmacological and lesion studies have suggested that rhythmic bursts in the TRN are a key element in generating these oscillations (21, 26). However, our results show that tonic firing of TRN neurons in the absence of TRN burst firing is sufficient to generate SWDs, which we believe is a novel observation.
The Role of TRN in the Enhancement of SWDs.
Our results raise the possibility that tonic firing in the TRN has a function in regulating the thalamocortical rhythms in certain pathological states, such as absence epilepsy. Both genetic elimination and viral-mediated, TRN-specific knockdown of the T-type Ca2+ current caused similar and significant increases in both tonic firing and GBL-induced SWDs. This shows that the level of tonic firing is highly correlated with the degree of SWDs and with susceptibility to absence seizures. Moreover, the results from the CaV3.2−/−/3.3−/− mice, which completely lack burst firing in TRN neurons, indicate that SWDs can be initiated in the complete absence of burst firing. Therefore, we propose that the excitability of TRN neurons can play an important modulatory role in maintaining SWDs. This proposal is based on the facts that both CaV3.3−/− and CaV3.2−/−/3.3−/− mice lacked burst firing, yet had enhanced tonic firing and enhanced SWDs. Tonic firing in the TRN neurons might be a more potent regulator of the thalamocortical circuit than burst firing. Indeed, we observed that stimulation of TRN neurons at a tonic frequency evoked stronger summed inhibition of TC neurons than stimulation at burst frequency. This suggests that tonic firing might even be more efficient in mediating the TRN-to-TC signals and initiating thalamocortical rhythms. On the other hand, the gain of CaV3.2 channel function has been reported in the mutated channel identified in absence epilepsy patients (27). However, there is still a controversy on the role of CaV3.2 in absence epilepsy because no consistent sequence variant has been found in CaV3.2 in absence patients (28, 29).
It is important to note that there were no spontaneous SWDs in the mutant mice with the increased tonic firing of TRN neurons. In contrast, genetic enhancement or elimination of the T current in TC neurons is known to induce or abolish spontaneous SWDs, respectively (30, 31), which provides convincing evidence that CaV3.1 in TC neurons plays a key role in the genesis of absence seizure. Until the current studies, however, effects of manipulations of TRN neurons have not been tested before.
Pharmacological Model of Absence Seizure.
GBL is a precursor of the GABA analog, gamma-hydroxybutyric acid (GHB), and is widely used as a pharmacological model of absence seizures in mice. The established target of GBL (or GHB) action is the presynaptic GABAB receptor that regulates corticothalamic excitatory synapses onto TC neurons. We used a GBL concentration (70 mg/kg) that is known to preferentially influence the presynaptic GABAB receptors on cortical and TRN neurons, rather than the postsynaptic GABAB receptors of TC (32). Therefore, we did not bypass the TC GABAB activation. It is also known that the dose we used does not induce sleep or coma in mice (33). To test the role of burst firing in TRN in the generation of absence seizures, we applied GBL to both CaV3.3−/− KO and CaV3.2−/−/3.3−/− DKO mice. Because the enhancement of TRN excitability in both mice was insufficient to induce spontaneous SWDs, additional increases in TC excitability might be required for GBL to cause the thalamocortical oscillations that underlie SWDs. Consistent with our current observations, it has been reported that TRN neurons in epileptic genetic absence epileptic rats from Strasbourg (GAERS) rats displayed higher excitability, higher firing frequency, and regularity (34). In parallel with these results from GAERS rat, stargazer mice (another genetic model for absence seizures) showed hyperexcitable TRN neurons due to an enhanced NMDA receptor (35). Thus, several gene mutation models of absence seizures show altered TRN excitability.
Increased Tonic Firing in TRN in the Absence of CaV3.3 T-Type Channels.
The maximum frequency of tonic firing in neurons is determined by AHPs that arise from the interplay of K+ and Ca2+ channels. Our data showed increased mAHPs but not fAHPs in TRN neurons of CaV3.3 KO and CaV3.2/3.3 DKO mice. Previous papers have reported that CaV3 channels are coupled to Kv4 channels (LVA A-type potassium channels), which is essential to influence firing properties in cerebellar stellate cells. Also Kv4 and CaV3 channels are linked by an accessory calcium-sensing protein, K+ channel-interacting protein 3 (KChIP3), but not KChIP1 or KChIP2, in stellate cells (36). However, KChIP3 proteins are not expressed in TRN neurons (37). Therefore, it is unlikely that Kv4 is involved in mAHPs in TRN neurons.
Compared with fAHPs, which are mediated by voltage-gated K+ channels, mAHPs occur when Ca2+-activated K+ channels are activated by Ca2+ influx through T-type Ca2+ channels (38). This is consistent with our results of no change in fAHP in the TRN of CaV3.3 KO and CaV3.2/3.3 DKO mice. The mAHPs prevent an excessive increase in axon fiber excitability and contribute to the gradual decrease in tonic firing rate during a continuous activity. In TRN neurons, the mAHP is largely dependent on CaV3.3 T-type channels that are the major T-type Ca2+ channel in this region (22). In CaV3.3−/− and CaV3.2−/−/3.3−/− mice, the mAHP is greatly reduced due to the lack of T-type channels. This has a significant effect on signal transfer between TRN and TC neurons. Two possible effects of reduced mAHP include (i) increased axon fiber excitability in the TRN neurons of the CaV3.3−/− mice, enabling recruitment of abgreater number of axon fibers from the surrounding TRN neurons, and (ii) increased probability of release during the tonic-frequency activity in CaV3.3−/− mice. Consequent increases in GABA release onto TC fibers will lead to an augmentation of signals from the TRN, which might account for the increased SWDs in CaV3.3−/− mice. The current model for SWD generation postulates that inhibitory inputs from the TRN onto the TC hyperpolarize TC neurons, which leads to the activation of LT T-type Ca2+ channels and subsequent burst firing in the TC. Our current results agree with the hypothesis that TRN inputs to TC are critical for SWD generation. However, our study indicates that TRN tonic firing, in absence of burst firing, is also quite capable of delivering effective inhibitory synaptic input onto TC neurons. Another potential explanation could be that enhanced inhibitory inputs onto the TC may influence tonic inhibition in the TC, which has been observed in typical absence epilepsy (39, 40).
Enhanced understanding of the detailed pathophysiological mechanism of SWD generation in the thalamocortical circuit will help improve the treatment of absence epilepsy. Our findings about the novel function of tonic firing in the TRN neurons indicates a need to revise the current model of thalamocortical circuit function and opens up a previously unidentified venue for the development of remedies for absence epilepsy.
Materials and Methods
Animals.
CaV3.3 KO was generated on a hybrid background of 129/SvJ and Black Swiss strains and backcrossed to the desired inbred C57BL/6 or 129S4/SvJae for 10 generations. Then, each background line was used to generate desired homozygotes for different experiments. Double heterozygotes of CaV3.2+/−/3.3+/− were first generated by intercrossing CaV3.2 heterozygotes and CaV3.3 heterozygotes, and then double heterozygous outcomes were interbred to generate CaV3.2−/−/3.3−/− DKO mice. For details, see SI Materials and Methods.
Electrophysiology.
For whole-cell patch clamp recordings in TRN cells, horizontal slices 280 μm thick were prepared from 3- to 4-wk-old B6129CaV3.3−/−, B6129CaV3.3−/−/GAD65GFPtg, and wild-type littermates. Details of electrophysiological recording conditions, data acquisition, and analysis are given in SI Materials and Methods.
AAV-shRNA Vector Design.
To design the AAV-shCaV3.3 vector, we digested out the U6 promoter, CaV3.3 shRNA, and mCherry fragment from the pSicoR lentiviral vector and cloned it into the transfer plasmid AAV containing multiple cloning site (Agilent Technologies cat. no. 240071). The scrambled shRNA-containing AAV (AAV-ctrl) construct was used as the control. For details, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Kevin P. Campbell for providing CaV3.2 mouse line. This work was supported by grants from Institute for Basic Science (IBS-R001-D1-2014-a00), Korea Institute of Science and Technology, and the National Honor Scientist Program of Korea. This work was also supported by the World Class Institute (WCI) Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (MSIP) (NRF Grant WCI 2009-003) and National Agenda Project (NAP) of the Korea Research Council of Fundamental Science & Technology (NAP-09-04) (to C.J.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408609111/-/DCSupplemental.
References
- 1.Crunelli V, Leresche N. Childhood absence epilepsy: Genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3(5):371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- 2.Steriade M, Deschênes M, Domich L, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985;54(6):1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- 3.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 4.Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283(5401):541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- 5.Jones EG. Thalamic circuitry and thalamocortical synchrony. Philos Trans R Soc Lond B Biol Sci. 2002;357(1428):1659–1673. doi: 10.1098/rstb.2002.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 7.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357(1428):1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonnum F, Storm-Mathisen J, Divac I. Biochemical evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibres in rat brain. Neuroscience. 1981;6(5):863–873. doi: 10.1016/0306-4522(81)90168-8. [DOI] [PubMed] [Google Scholar]
- 9.Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980;200(2):341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- 10.Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30(7):350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 11.von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261(5119):361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- 12.McCormick DA, Bal T. Sleep and arousal: Thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 13.Jahnsen H, Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: An in vitro study. J Physiol. 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contreras D, Curró Dossi R, Steriade M. Electrophysiological properties of cat reticular thalamic neurones in vivo. J Physiol. 1993;470:273–294. doi: 10.1113/jphysiol.1993.sp019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83(1):117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Reyes E, et al. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391(6670):896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 17.Talley EM, et al. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19(6):1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol. 1995;483(Pt 3):641–663. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deschêenes M, Hu B. Electrophysiology and pharmacology of the corticothalamic input to lateral thalamic nuclei: An intracellular study in the cat. Eur J Neurosci. 1990;2(2):140–152. doi: 10.1111/j.1460-9568.1990.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 20.Manning JP, Richards DA, Leresche N, Crunelli V, Bowery NG. Cortical-area specific block of genetically determined absence seizures by ethosuximide. Neuroscience. 2004;123(1):5–9. doi: 10.1016/j.neuroscience.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Avanzini G, Vergnes M, Spreafico R, Marescaux C. Calcium-dependent regulation of genetically determined spike and waves by the reticular thalamic nucleus of rats. Epilepsia. 1993;34(1):1–7. doi: 10.1111/j.1528-1157.1993.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 22.Astori S, et al. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci USA. 2011;108(33):13823–13828. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cueni L, et al. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci. 2008;11(6):683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- 24.Steriade M, Domich L, Oakson G. Reticularis thalami neurons revisited: Activity changes during shifts in states of vigilance. J Neurosci. 1986;6(1):68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim U, Sanchez-Vives MV, McCormick DA. Functional dynamics of GABAergic inhibition in the thalamus. Science. 1997;278(5335):130–134. doi: 10.1126/science.278.5335.130. [DOI] [PubMed] [Google Scholar]
- 26.Meeren HK, Veening JG, Möderscheim TA, Coenen AM, van Luijtelaar G. Thalamic lesions in a genetic rat model of absence epilepsy: Dissociation between spike-wave discharges and sleep spindles. Exp Neurol. 2009;217(1):25–37. doi: 10.1016/j.expneurol.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Eckle VS, et al. Mechanisms by which a CACNA1H mutation in epilepsy patients increases seizure susceptibility. J Physiol. 2014;592(Pt 4):795–809. doi: 10.1113/jphysiol.2013.264176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chioza B, et al. Evaluation of CACNA1H in European patients with childhood absence epilepsy. Epilepsy Res. 2006;69(2):177–181. doi: 10.1016/j.eplepsyres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, et al. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol. 2003;54(2):239–243. doi: 10.1002/ana.10607. [DOI] [PubMed] [Google Scholar]
- 30.Ernst WL, Zhang Y, Yoo JW, Ernst SJ, Noebels JL. Genetic enhancement of thalamocortical network activity by elevating alpha 1g-mediated low-voltage-activated calcium current induces pure absence epilepsy. J Neurosci. 2009;29(6):1615–1625. doi: 10.1523/JNEUROSCI.2081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song I, et al. Role of the alpha1G T-type calcium channel in spontaneous absence seizures in mutant mice. J Neurosci. 2004;24(22):5249–5257. doi: 10.1523/JNEUROSCI.5546-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snead OC., 3rd The gamma-hydroxybutyrate model of absence seizures: Correlation of regional brain levels of gamma-hydroxybutyric acid and gamma-butyrolactone with spike wave discharges. Neuropharmacology. 1991;30(2):161–167. doi: 10.1016/0028-3908(91)90199-l. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee PK, Snead OC., 3rd Presynaptic gamma-hydroxybutyric acid (GHB) and gamma-aminobutyric acidB (GABAB) receptor-mediated release of GABA and glutamate (GLU) in rat thalamic ventrobasal nucleus (VB): A possible mechanism for the generation of absence-like seizures induced by GHB. J Pharmacol Exp Ther. 1995;273(3):1534–1543. [PubMed] [Google Scholar]
- 34.Tóth TI, Bessaïh T, Leresche N, Crunelli V. The properties of reticular thalamic neuron GABA(A) IPSCs of absence epilepsy rats lead to enhanced network excitability. Eur J Neurosci. 2007;26(7):1832–1844. doi: 10.1111/j.1460-9568.2007.05800.x. [DOI] [PubMed] [Google Scholar]
- 35.Lacey CJ, Bryant A, Brill J, Huguenard JR. Enhanced NMDA receptor-dependent thalamic excitation and network oscillations in stargazer mice. J Neurosci. 2012;32(32):11067–11081. doi: 10.1523/JNEUROSCI.5604-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson D, et al. Regulation of the KV4.2 complex by CaV3.1 calcium channels. Channels (Austin) 2010;4(3):163–167. doi: 10.4161/chan.4.3.11955. [DOI] [PubMed] [Google Scholar]
- 37.Xiong H, Kovacs I, Zhang Z. Differential distribution of KChIPs mRNAs in adult mouse brain. Brain Res Mol Brain Res. 2004;128(2):103–111. doi: 10.1016/j.molbrainres.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 38.Engbers JD, et al. Intermediate conductance calcium-activated potassium channels modulate summation of parallel fiber input in cerebellar Purkinje cells. Proc Natl Acad Sci USA. 2012;109(7):2601–2606. doi: 10.1073/pnas.1115024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cope DW, et al. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med. 2009;15(12):1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker MC, Kullmann DM. Jasper’s Basic Mechanisms of the Epilepsies. 2012. Tonic GABAA receptor-mediated signaling in epilepsy. eds Noebels JL, Massimo A, Rogawski M, Olsen R, Delgado-Escueta A (Oxford Univ Press, New York), 4th Ed. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.