Significance
The mammalian target of rapamycin complex 1 (mTORC1) controls cell growth and metabolism in response to nutrients, growth factors, and cellular energy. Aberrant mTORC1 signaling is implicated in human diseases such as diabetes, obesity, and cancer. Our results reveal that ectopic mTORC1 activation in the liver controls the stress hormone fibroblast growth factor 21 (FGF21) in a peroxisome proliferator–activated receptor γ coactivator-1α (PGC-1α)–dependent manner via glutamine depletion, which in turn affects whole-body behavior and metabolism. mTORC1 signaling correlates with FGF21 expression in human liver tumors, suggesting that our findings in mice may have physiological relevance in glutamine-addicted human cancers. Thus, treatment with the anticancer drug rapamycin may have beneficial effects by blocking tumor growth and by preventing deregulation of whole-body physiology due to FGF21 expression.
Keywords: TSC, hepatocellular carcinoma, metabolic stress, behavior
Abstract
The liver is a key metabolic organ that controls whole-body physiology in response to nutrient availability. Mammalian target of rapamycin (mTOR) is a nutrient-activated kinase and central controller of growth and metabolism that is negatively regulated by the tumor suppressor tuberous sclerosis complex 1 (TSC1). To investigate the role of hepatic mTOR complex 1 (mTORC1) in whole-body physiology, we generated liver-specific Tsc1 (L-Tsc1 KO) knockout mice. L-Tsc1 KO mice displayed reduced locomotor activity, body temperature, and hepatic triglyceride content in a rapamycin-sensitive manner. Ectopic activation of mTORC1 also caused depletion of hepatic and plasma glutamine, leading to peroxisome proliferator–activated receptor γ coactivator-1α (PGC-1α)–dependent fibroblast growth factor 21 (FGF21) expression in the liver. Injection of glutamine or knockdown of PGC-1α or FGF21 in the liver suppressed the behavioral and metabolic defects due to mTORC1 activation. Thus, mTORC1 in the liver controls whole-body physiology through PGC-1α and FGF21. Finally, mTORC1 signaling correlated with FGF21 expression in human liver tumors, suggesting that treatment of glutamine-addicted cancers with mTOR inhibitors might have beneficial effects at both the tumor and whole-body level.
The atypical Ser/Thr kinase target of rapamycin (TOR) is a central controller of cell growth and metabolism, conserved from yeast to human. TOR exists in two structurally and functionally distinct complexes, TORC1 and TORC2 (1-4). Mammalian TOR complex 1 (mTORC1) consists of mTOR, raptor, and mLST8. mTORC1 is activated by nutrients, growth factors, and cellular energy and is acutely inhibited by rapamycin. Growth factors activate mTORC1 via the PI3K-PDK1-Akt signaling pathway. Akt phosphorylates and inhibits the tuberous sclerosis complex (TSC) heterodimer TSC1-TSC2. The TSC complex is a GTPase activating protein (GAP) toward the small GTPase ras-homolog enriched in brain (Rheb) that directly binds and activates mTORC1. Thus, deletion of either Tsc1 or Tsc2 causes ectopic activation of mTORC1. mTORC1 promotes anabolic processes such as protein, lipid, and nucleotide synthesis and ribosome biogenesis and inhibits catabolic processes such as autophagy (4–8). The best-characterized substrates of mTORC1 are 4E-BP and S6 kinase (S6K). Deregulation of the mTOR signaling network is associated with aging and several diseases, including diabetes, obesity, and cancer (9–11). In the tumor syndromes tuberous sclerosis complex and lymphangioleiomyomatosis (LAM), mTORC1 is deregulated due to mutations in the tumor suppressor gene Tsc1 or -2. Rapamycin and rapamycin analogs (rapalogs) are currently used as immunosuppressive agents and as anticancer drugs (12, 13).
In mammals, behavior and physiology display 24-h oscillations controlled by environmental cues such as light and feeding. Light activates the suprachiasmatic nucleus (SCN) in the hypothalamus. The SCN synchronizes cells in other brain regions and peripheral organs, such as the liver, either by humoral and neuronal signals (14) or by regulating body temperature and the fasting/feeding cycle (15–18). The fasting/feeding cycle is a particularly important synchronizer (Zeitgeber) of the liver (19, 20). Expression of several hepatic enzymes and hormones involved in carbohydrate, lipid, cholesterol, and xenobiotic metabolism are regulated over a 24-h cycle in response to nutrient availability (21–28). However, the molecular mechanism(s) by which nutrients control behavior and metabolism is poorly understood but may involve TORC1, a nutrient sensor and key regulator of metabolism. In Drosophila, neuronal TORC1 signaling affects circadian behavior (29). In the SCN, mTORC1 signaling is activated by light and controls behavior in a circadian manner (30–32). In the liver, fasting/feeding cycles regulate the energy sensor AMPK and mTORC1 (24, 33–36). Hepatic mTORC1 and the NAD+-dependent deacetylase SIRT1 are active during the night whereas AMPK is active during the day (34, 35, 37, 38). Moreover, in the liver and other tissues, AMPK (35) and SIRT1 (37, 38) control the core clock machinery. The core clock machinery, composed of the transcription factors CLOCK, BMAL1, PER, CRY, ROR, and REV-ERB, determines circadian oscillations. Disruption of core clock components in the liver strongly alters the regulation of hepatic as well as whole-body glucose and lipid metabolism (39–41). Importantly, time of feeding profoundly affects hepatic gene expression as well as mTORC1 and AMPK signaling (24, 33). Thus, time-restricted feeding improves metabolic rhythms and protects against obesity and liver diseases (33). However, the role of hepatic mTORC1 in the control of whole-body behavior and metabolism has not been investigated.
FGF21 is a hormone produced mainly in the liver (42) and whose expression oscillates over a 24-h cycle (43–45). Upon fasting, peroxisome proliferator-activated receptor α (PPARα) (46–48) and possibly PPARγ coactivator (PGC)-1α (49) activate FGF21 expression in the liver. FGF21 in turn controls behavior and whole-body metabolism by acting on the nervous system and peripheral organs (46, 47, 50–52). Via the central nervous system, FGF21 decreases locomotor activity and body temperature. In the liver, FGF21 stimulates fatty-acid oxidation and gluconeogenesis. Thus, the liver modulates glucose and lipid metabolism, locomotor activity, and body temperature, at least in part, through FGF21.
Glutamine, the most abundant amino acid in the body, plays an important role in growth and metabolism. A TSC1 or TSC2 deficiency causes metabolic/energetic stress by increasing anabolic processes and thereby increasing energy consumption (53, 54). Glucose-limited TSC-deficient cells are addicted to glutamine as an alternative carbon source (54). In addition to being a carbon source used for energy generation, glutamine is also a precursor for nucleotides and other amino acids. Finally, glutaminolysis both promotes mTORC1 signaling (55) and is activated by mTORC1 (56). mTORC1 activates glutaminolysis through repression of the glutamate dehydrogenase inhibitor SIRT4 (56). The above accounts, at least in part, for the observation that cancer cells are often addicted to glutamine (57–59).
Here, we investigate the role of hepatic mTORC1 in the regulation of whole-body physiology. Our results demonstrate that hepatic mTORC1 activation, due to Tsc1 knockout specifically in the liver, causes glutamine depletion and thereby PGC-1α–dependent FGF21 expression. This in turn leads to decreased locomotor activity, body temperature, and hepatic lipid content. Thus, hepatic mTORC1 controls behavior and lipid metabolism through FGF21. Furthermore, our findings suggest that glutamine-addicted tumors deregulate whole-body behavior and metabolism.
Results
Hepatic mTORC1 Controls Locomotor Activity, Body Temperature, and Lipid Metabolism.
To investigate the role of hepatic mTORC1 in whole-body physiology, we generated mice lacking Tsc1 exclusively in hepatocytes (L-Tsc1 KO mice). The L-Tsc1 KO mice displayed reduced levels of TSC1 and TSC2 specifically in the liver (Fig. 1A and Fig. S1A). The decrease in TSC2 is consistent with previous reports indicating that TSC1 stabilizes TSC2 (60, 61). Knockout mice fed ad libitum exhibited unchanged body weight and composition, compared with controls (Fig. S1 B and C). Mice were also subjected to fasting and refeeding to evaluate the effect of Tsc1 knockout on mTORC1 signaling. The L-Tsc1 KO mice displayed constitutively active mTORC1 signaling in the liver, as indicated by high levels of S6 phosphorylation upon both fasting and feeding. Akt phosphorylation was significantly reduced in refed L-Tsc1 KO mice, as expected due to both the S6K-mediated negative feedback loop (62–64) and ER stress (65, 66) (Fig. 1A). We next examined the effect of constitutive hepatic mTORC1 signaling on glucose and lipid homeostasis. Blood-glucose levels and hepatic expression of the gluconeogenic gene G6Pase were increased in fasted L-Tsc1 KO mice (Fig. S1 D and E). Triglyceride content was reduced in the liver of L-Tsc1 KO mice upon both fasting and refeeding (Fig. 1B). Thus, constitutive hepatic mTORC1 signaling disrupts glucose and lipid homeostasis. As shown by Yecies et al., the decreased hepatic triglyceride content observed in refed L-Tsc1 KO mice is due to attenuation of Akt signaling by the negative feedback loop (67) (Fig. 1A). To better understand the decrease in hepatic triglyceride content observed in fasted L-Tsc1 KO mice, we measured expression of Pgc-1α involved in mitochondrial oxidation and/or biogenesis. Consistent with the observed decrease in triglyceride content, expression of Pgc-1α and the PGC-1α target gene CD36 was increased twofold specifically in fasted knockout mice (Fig. S1E). This suggests that the decrease in triglyceride levels in fasted mice was due to an increase in fatty acid oxidation. The above results are in agreement with previous studies (68, 69), thereby confirming our L-Tsc1 knockout.
Fig. 1.
Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism. (A) Immunoblots of liver extracts from L-Tsc1 KO and control mice fasted overnight or refed for 4 h. Each lane consists of a mixture of liver extracts obtained from three animals. (B) Representative images of hematoxylin/eosin (H&E) and Oil red O staining of liver sections from L-Tsc1 KO and control mice fasted overnight (n = 8 for control and n = 7 for L-Tsc1 KO) or refed for 4 h (n = 9 for control and n = 7 for L-Tsc1 KO). [Original magnification: 40× (Upper).] Hepatic triglyceride content was measured from L-Tsc1 KO and control mice fasted overnight (n = 8 for control and n = 7 for L-Tsc1 KO) or refed for 4 h (control n = 9 and L-Tsc1 KO n = 7) (Lower). (C–H) Food was removed at ZT0. ZT is Zeitgeber time within a 24-h light/dark cycle, with ZT0 and ZT12 corresponding to the appearance and disappearance of light, respectively. (C and D) Locomotor activity was measured by the Comprehensive Laboratory Animal Monitoring System (CLAMS) (C), and body temperature was measured by rectal thermometer every 4 h over a 24-h cycle (D) (n = 8 per group). (E) Hepatic triglyceride content from L-Tsc1 KO and control mice was measured (n = 6 per time point and per genotype). (F) Locomotor activity was measured during the dark phase in L-Tsc1 KO and control mice. Animals were treated with rapamycin (2 mg/kg) or vehicle at ZT11.5. Data are presented as total counts from ZT12 to ZT24 (n = 6 per group). (G) Body temperature was measured by rectal thermometer at ZT24 in L-Tsc1 KO and control mice. Animals were treated with rapamycin (2 mg/kg) or vehicle at ZT18 (n = 6 per group). (H) Hepatic triglyceride content from L-Tsc1 KO and control mice at ZT20. Animals were treated with rapamycin (2 mg/kg) or control vehicle at ZT14 (n = 6 per group). White bars or squares represent control mice and black bars or squares represent L-Tsc1 KO mice. Values are expressed as mean ± SEM; the * indicates a statistical significant difference between the indicated groups (*P < 0.05, **P < 0.01, ***P < 0.001).
The above results taken together indicate that loss of TSC confers a phenotype mainly under fasted conditions. This is expected because the TSC complex inhibits mTORC1 under fasted, but not fed, conditions. Because loss of TSC confers a phenotype mainly under starvation conditions, all subsequent experiments designed to investigate the effect of mTORC1 activation on whole-body physiology were performed in fasted mice. Furthermore, examining only fasted mice circumvents complications due to the negative feedback loop that inhibits Akt during feeding.
To examine the role of hepatic mTORC1 in behavior and lipid metabolism, L-Tsc1 KO and control mice were monitored for locomotor activity, body temperature, and hepatic triglyceride content over a 24-h cycle. The mice were fasted for the entire 24 h starting at ZT0. ZT is Zeitgeber time within a 24-h light/dark cycle, with ZT0 and ZT12 corresponding to the appearance and disappearance of light, respectively. We note that, in our experimental conditions, ZT24 corresponds to 24 h of fasting and is thus different from ZT0. L-Tsc1 KO mice displayed reduced locomotor activity and body temperature during the dark phase, compared with controls (Fig. 1 C and D). Body temperature decreased by ZT4 in both L-Tsc1 KO and control mice but dropped further during the dark phase in knockout mice (Fig. 1D). Plasma levels of thyroid (T4) hormone and adipose-secreted leptin, key regulators of locomotor activity and thermogenesis, were similar in L-Tsc1 KO and control mice (Fig. S1F). Thus, mTORC1 appears to control locomotor activity and body temperature independently of leptin and thyroid hormone. Similar to locomotor activity and body temperature, triglyceride levels were reduced during the dark phase in the knockout mice (Fig. 1E), suggesting that mTORC1 also controls the daily levels of hepatic lipid metabolism.
To determine whether the observed decrease in locomotor activity, body temperature, and triglycerides was indeed due to mTORC1, animals were treated with rapamycin. The i.p. injection of rapamycin abolished hepatic mTORC1 signaling in L-Tsc1 KO mice (Fig. S1G) and restored normal levels of locomotor activity, body temperature, and hepatic triglycerides (Fig. 1 F–H). Collectively, these data demonstrate that hepatic mTORC1 controls the daily levels of locomotor activity, body temperature, and lipid metabolism.
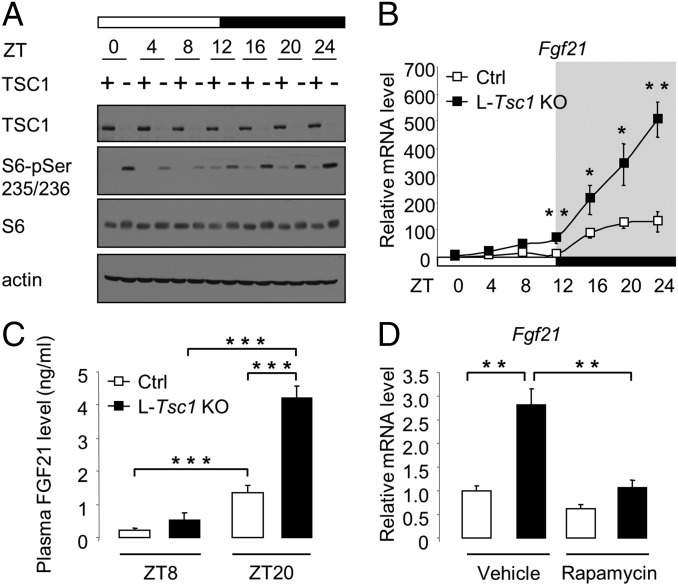
To investigate whether mTORC1 signaling itself oscillates, we examined S6 phosphorylation in the liver of wild-type mice killed every 4 h over a 24-h cycle. S6 phosphorylation was low during the light phase and high during the dark phase, indicating that mTORC1 signaling oscillates daily, even in the absence of food (Fig. 2A). This is in agreement with previous findings (34). To determine the role of the TSC complex in the daily regulation of mTORC1 signaling, we next examined hepatic S6 phosphorylation in L-Tsc1 KO mice. S6 phosphorylation displayed the similar diurnal rhythm, but at higher absolute levels at all time points, compared with the wild-type control (Fig. 2A and Fig. S1H). Thus, mTORC1 signaling oscillates daily, and this regulation is independent of the TSC complex.
Fig. 2.
Hepatic mTORC1 controls FGF21 expression. (A and B) Mice were fasted at ZT0 and killed every 4 h over a 24-h cycle. (A) Immunoblots of total liver extracts from L-Tsc1 KO and control mice. Each lane consists of a mixture of total liver extracts obtained from six animals. (B) Expression of Fgf21 mRNA in the liver of L-Tsc1 KO and control mice was measured by quantitative reverse transcription PCR (qRT-PCR). Total liver RNA was extracted. White squares represent control mice, and black squares represent L-Tsc1 KO mice (n = 6 per time point and per genotype). (C) Plasma FGF21 levels in L-Tsc1 KO and control mice. Food was removed at ZT0, and plasma FGF21 levels were measured at ZT8 and ZT20. White bars represent control mice, and black bars represent L-Tsc1 KO mice (n = 6 per time point and per genotype). (D) Expression of Fgf21 mRNA at ZT20 in the liver of L-Tsc1 KO and control mice was measured by qRT-PCR. Food was removed at ZT0. Animals were treated with rapamycin (2 mg/kg) or vehicle at ZT14. Total liver RNA was prepared from L-Tsc1 KO and control animals. White bars represent control mice, and black bars represent L-Tsc1 KO mice (n = 6 per condition and per genotype). Values are expressed as mean ± SEM; the * indicates a statistical significant difference between the indicated groups (*P < 0.05, **P < 0.01, ***P < 0.001).
Hepatic mTORC1 Controls FGF21 Expression.
How does hepatic mTORC1 control locomotor activity, body temperature, and lipid metabolism? Several observations suggest that mTORC1 may control behavior and lipid metabolism via FGF21. The hormone FGF21, expressed mainly in the liver, decreases locomotor activity, body temperature, and hepatic lipid accumulation (46, 47, 51). Furthermore, mTORC1 activates PGC-1α (Fig. S1E), and PGC-1α has been shown to promote FGF21 expression (49). To determine whether mTORC1 controls FGF21 expression, we examined Fgf21 mRNA levels in the liver and FGF21 protein levels in plasma in L-Tsc1 KO and control mice killed every 4 h over a 24-h cycle. Similar to mTORC1 activity (Fig. 2A), FGF21 expression was low during the light phase and high during the dark phase in both L-Tsc1 KO and control mice, but the increase during the dark phase was significantly higher in the knockout mice (Fig. 2 B and C). The i.p. injection of rapamycin restored normal Fgf21 mRNA levels in L-Tsc1 KO mice (Fig. 2D). The above results suggest that hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism via FGF21 expression.
Core clock components, in addition to FGF21, are also key regulators of locomotor activity, body temperature, and hepatic glucose and lipid metabolism (40, 41). In agreement with previous studies, Cry1, Cry2, Per1, Per2, Rorα, and Rorγ mRNA levels and CRY2 and PER2 protein levels in the liver were low during the light phase and high during the dark phase in control animals (Fig. S2 A and B) (70–72). Also in agreement with previous studies, we observed that Rev-erbα and Rev-erbβ expression was the inverse of the above (Fig. S2 A and B) (70, 71, 73). L-Tsc1 KO mice displayed little-to-no change in (i) expression of the core clock components, as measured at the mRNA and protein levels (Fig. S2 A and B), (ii) interaction of circadian clock components (Fig. S2C), and (iii) binding of CLOCK to the fgf21 promoter (Fig. S3). Thus, mTORC1 appears not to control the core clock components, again suggesting that mTORC1 may control whole-body physiology through FGF21.
Hepatic mTORC1 Controls Locomotor Activity, Body Temperature, and Lipid Metabolism Through FGF21.
To investigate whether FGF21 is responsible for the decreased locomotor activity, body temperature, and triglycerides in L-Tsc1 KO mice, we examined the effect of FGF21 knockdown. L-Tsc1 KO and control mice were infected with adenovirus expressing shRNA against FGF21. The knockdown was confirmed by loss of FGF21 mRNA in the liver and reduced FGF21 protein in plasma (Fig. 3 A and B). Knockdown of FGF21 restored normal locomotor activity, body temperature, and hepatic triglycerides in L-Tsc1 KO mice (Fig. 3 C–E). Thus, hepatic mTORC1 inhibits locomotor activity, body temperature, and lipid metabolism through FGF21.
Fig. 3.
Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. (A–E) L-Tsc1 KO and control mice were infected with an adenovirus expressing shRNA against FGF21 or LacZ. Four days after infection, food was removed at ZT0, and animals were killed at ZT24 for further measurements. (A and B) Fgf21 mRNA (A) and plasma levels (B) were measured (n = 6 per condition and per genotype). (C) Locomotor activity was measured during the dark phase. Data are presented as total counts from ZT12 to ZT24 (n = 6 per condition and per genotype). (D) Body temperature was measured by rectal thermometer (n = 6 per condition and per genotype). (E) Representative images of hematoxylin/eosin (H&E) and Oil red O staining of liver sections. [Original magnification: 40× (Left).] Hepatic triglyceride content was measured (Right) (n = 6 per condition and per genotype). White bars represent control mice, and black bars represent L-Tsc1 KO mice. Values are expressed as mean ± SEM; the * indicates a statistical significant difference between the indicated groups (*P < 0.05, **P < 0.01, ***P < 0.001).
FGF21 controls carbohydrate metabolism in the liver (50) by inducing expression of gluconeogenic genes (74–76). L-Tsc1 KO mice display an increase in expression of gluconeogenic genes in the liver and increased blood glucose levels (Fig. S1 D and E). We investigated whether mTORC1 controls glucose metabolism through FGF21. Knockdown of FGF21 in the liver had no effect on blood glucose levels or expression of Pgc-1α, G6Pase, and PEPCK (Fig. S4 A and B). Thus, mTORC1 controls carbohydrate metabolism independently of FGF21.
Hepatic mTORC1 Controls FGF21 and Behavior and Lipid Metabolism Through PGC-1α.
How does hepatic mTORC1 control FGF21 expression? Purushotham et al. (49) reported that the transcriptional coactivator PGC-1α promotes FGF21 expression, and our results (Fig. S1E) indicate that Pgc-1α is up-regulated in L-Tsc1 KO mice. Furthermore, PGC-1α controls locomotor activity and body temperature (77) and promotes expression of genes involved in mitochondrial fatty acid oxidation, thereby preventing hepatic steatosis (78–80). Thus, we investigated whether hepatic mTORC1 controls FGF21 and whole-body physiology via PGC-1α. First, we examined PGC-1α mRNA and protein levels in the liver of L-Tsc1 KO and control mice killed every 4 h over a 24-h cycle. PGC-1α expression was low during the light phase and high during the dark phase, reaching a peak at ZT20 in both L-Tsc1 KO and control mice, but was significantly increased overall in the knockout compared with the control (Fig. 4 A and B). Expression of PGC-1α target genes, such as Fgf21 and the gluconeogenic genes G6Pase and PEPCK, was also low during the light phase and high during the dark phase (Figs. 2B and 4C). Second, we determined whether i.p. injection of rapamycin reduced expression of Pgc-1α and G6Pase in the liver of L-Tsc1 KO mice (Fig. 4D). As observed previously for Fgf21 expression (Fig. 2D), rapamycin restored normal expression of Pgc-1α and G6Pase. Third, we examined FGF21 expression upon knockout of Pgc-1α in the liver. Mice containing a floxed Pgc-1α allele were infected with Cre-expressing adenovirus. Hepatic deletion of Pgc-1α (L-Pgc-1α KO) was confirmed by loss of Pgc-1α mRNA in the liver (Fig. 4E). L-Pgc-1α KO mice displayed a strong decrease in FGF21 protein in the plasma (Fig. 4F), a twofold increase in triglyceride content in the liver (Fig. 4G), and a >1 °C increase in body temperature (Fig. 4H). Fourth, we examined the effect of PGC-1α knockdown in L-Tsc1 KO mice. Infection of L-Tsc1 KO mice with adenovirus expressing shRNA against PGC-1α strongly decreased hepatic Pgc-1α expression and reduced expression of G6Pase, PEPCK, and CD36 (Fig. 5A and Fig. S5). Importantly, knock down of Pgc-1α also decreased FGF21 expression, as measured at both the mRNA and plasma protein level (Fig. 5 A and B), and restored normal locomotor activity, body temperature, and hepatic triglyceride content in L-Tsc1 KO mice (Fig. 5 C–E). Collectively, the above results suggest that mTORC1 controls FGF21 and ultimately behavior and lipid metabolism via PGC-1α.
Fig. 4.
Hepatic PGC-1α controls FGF21 expression. (A–C) Mice were fasted at ZT0 and killed every 4 h over a 24-h cycle. (A) Expression of Pgc-1α mRNA in the liver of L-Tsc1 KO and control mice was measured by qRT-PCR. Total liver RNA was extracted. White squares represent control mice, and black squares represent L-Tsc1 KO mice (n = 6 per time point and per genotype). (B) Immunoblots of liver nuclear extracts from L-Tsc1 KO and control mice. PGC-1α protein levels were analyzed. Each lane consists of a mixture of liver nuclear extracts obtained from six animals per genotype. (C) Expression of G6Pase and PEPCK mRNA in the liver of L-Tsc1 KO and control mice was measured by qRT-PCR. Total liver RNA was extracted. White squares represent control mice, and black squares represent L-Tsc1 KO mice (n = 6 per time point and per genotype). (D) Expression of Pgc-1α, G6Pase mRNA at ZT20 in the liver of L-Tsc1 KO and control mice was measured by qRT-PCR. Food was removed at ZT0, and animals were treated with rapamycin (2 mg/kg) or vehicle at ZT14. Total liver RNA was extracted. White bars represent control mice, and black bars represent L-Tsc1 KO mice (n = 6 per condition and per genotype). (E–H) Pgc-1α floxed mice were infected with a Cre (L-Pgc-1α KO) or null (Ctrl)-expressing adenovirus. Four days after infection, animals were fasted at ZT0 for 20 h before being killed. White bars represent control mice (n = 7), and black bars represent L-Pgc-1α KO mice (n = 8). (E) Expression of Pgc-1α mRNA in the liver was measured by qRT-PCR. (F) Plasma FGF21 levels were measured. (G) Hepatic triglyceride content was measured. (H) Body temperature was measured by rectal thermometer at ZT20. Values are expressed as mean ± SEM; the * indicates a statistical significant difference between the indicated groups (*P < 0.05, **P < 0.01, ***P < 0.001).
Fig. 5.
Hepatic mTORC1 controls FGF21 and behavior and lipid metabolism through Pgc-1α. (A–E) L-Tsc1 KO and control mice were infected either with an adenovirus expressing shRNA against PGC-1α or LacZ. Four days after infection, food was removed at ZT0. (A and B) Pgc-1α and Fgf21 mRNA (A) and plasma FGF-21 levels (B) were measured at ZT20 (n = 10 per condition and per genotype). (C) Locomotor activity was measured during the dark phase. Data are presented as total counts between ZT12 and ZT24 (n = 8 per condition and per genotype). (D) Body temperature was measured by rectal thermometer at ZT24 (n = 8 per condition and per genotype). (E) Representative images of hematoxylin/eosin (H&E) and Oil red O staining of liver sections. Animals were killed at ZT24. [Original magnification: 40× (Left).] Hepatic triglyceride content was measured (Right) (n = 8 per condition and per genotype). White bars represent control mice, and black bars represent L-Tsc1 KO mice. Values are expressed as mean ± SEM; the * indicates a statistical significant difference between the indicated groups (*P < 0.05, **P < 0.01, ***P < 0.001).
The transcriptional repressor E4BP4 inhibits FGF21 expression by binding a d-box in the Fgf21 promoter (45). Although E4BP4 expression increased in the dark phase, its expression was similar in L-Tsc1 KO and control mice (Fig. S6A). ER stress promotes FGF21 expression through ATF4 expression (81–83). ATF4 is a transcriptional effector of the PERK-branch of the unfolded protein response. L-Tsc1 KO mice displayed an early ER stress response as assessed by eIF2α phosphorylation (Fig. S6B) (84). However, ATF4 expression was not affected in L-Tsc1 KO mice, as measured at both the mRNA and protein level (Fig. S6C). Thus, mTORC1 does not appear to regulate FGF21 via E4BP4 or ATF4, further suggesting that mTORC1 activates FGF21 through PGC-1α. Of note, L-Tsc1 KO mice displayed similar PPARα mRNA levels compared with controls, suggesting that PPARα is not the limiting factor controlling FGF21 expression (Fig. S6D).
Hepatic mTORC1 Activates PGC-1α and FGF21 via Glutamine Depletion.
The hormone FGF21 is produced in the liver (42) in response to metabolic stress such as carbon depletion (46, 47, 83, 85). Glutamine, via glutaminolysis, can serve as an alternative carbon source to prevent metabolic/energetic stress in glucose-deprived cells (53, 54, 59). mTORC1 promotes glutaminolysis, and consequently mTORC1 hyperactivation in Tsc2-deficient cells leads to glutamine depletion (56). Thus, we investigated whether L-Tsc1 KO mice display glutamine depletion that may in turn induce FGF21. First, we examined glutamine levels in the liver and in plasma of L-Tsc1 KO mice. Glutamine levels were decreased during the dark phase in both L-Tsc1 KO and control mice, but the decrease in the dark phase was significantly more pronounced in the knockout mice (Fig. 6A). Asparagine is synthesized by amide transfer from glutamine to aspartate. Consistent with the reduced hepatic glutamine levels, asparagine levels were also reduced in the liver whereas aspartate levels were unaffected (Fig. S7A). The levels of branched-chain amino acids were also unchanged (Fig. S7A). Thus, L-Tsc1 KO mice are depleted specifically for glutamine. Second, to determine whether glutamine depletion was due to mTORC1 hyperactivity, animals were treated with rapamycin. The i.p. injection of rapamycin restored normal glutamine levels in L-Tsc1 KO mice (Fig. 6 B and C). Third, we examined whether glutamine depletion is responsible for PGC-1α and FGF21 expression. The i.p. injection of glutamine decreased Pgc-1α and Fgf21 mRNA levels in the liver, reduced FGF21 protein levels in plasma, and increased body temperature (Fig. 6 D–F). Interestingly, glutamine also increased hepatic mTORC1 signaling in both L-Tsc1 KO and control mice (Fig. 6G), consistent with previous in vitro studies (55, 86). Collectively, these data suggest that hepatic mTORC1 controls whole-body glutamine levels and thereby whole-body physiology through PGC-1α–dependent FGF21 expression.
Fig. 6.
Hepatic mTORC1 activates PGC-1α and FGF21 via glutamine depletion. (A) Hepatic and plasma glutamine levels in L-Tsc1 KO and control mice were quantified by LC-MS/MS. 8–9-wk-old mice were fasted at ZT0 and killed at ZT8 and ZT20 (n = 6 per time point and per genotype). (B) Hepatic glutamine levels in L-Tsc1 KO and control mice were quantified by LC-MS/MS. 12-wk-old animals were fasted at ZT0, treated with rapamycin (2 mg/kg) or control vehicle and killed at ZT20 (n = 6 per condition and per genotype). (C) Plasma glutamine levels in L-Tsc1 KO and control mice were quantified by LC-MS/MS. Twelve-week-old animals were fasted at ZT0, treated with rapamycin (2 mg/kg) or control vehicle and killed at ZT20 (n = 6 per condition and per genotype). (D–G) Food was removed at ZT0 and L-Tsc1 KO mice and controls were treated with l-Glutamine (1 g/kg) or saline solution, 2 h before sacrifice at ZT20. (D) Expression of Fgf21 and Pgc-1α mRNA in the liver of L-Tsc1 KO and control mice was measured by qRT-PCR. Total liver RNA was extracted (n = 6 per condition and per genotype). (E) Plasma FGF21 levels in L-Tsc1 KO and control mice were measured (n = 6 per condition and per genotype). (F) Body temperature was measured by rectal thermometer at ZT20 (n = 6 per condition and per genotype). (G) Immunoblots of liver extracts from L-Tsc1 KO and control mice injected with glutamine or saline solution. (H) FGF21 and phospho-S6 staining in human HCC. Representative images of an HCC showing little-to-no staining of either (a) FGF21 or (b) phospho-S6. Images of HCC presenting diffuse staining of both (c) FGF21 and (d) phospho-S6. (e–h) Examples of HCCs exhibiting medium to high staining of both (e and g) FGF21 and (f and h) phospho-S6. White bars represent control mice, and black bars represent L-Tsc1 KO mice. Values are expressed as mean ± SEM; the * indicates statistical significant difference between the indicated groups (*P < 0.05, **P < 0.01, ***P < 0.001).
mTORC1 Signaling Correlates with FGF21 Expression in Human Liver Tumors.
Several observations suggest that mTORC1, often deregulated in cancer, may lead to FGF21 expression in tumors. First, as described in Fig. 6 A–C and in the literature (56), mTORC1 hyperactivation causes glutamine depletion. Second, tumors are often glutamine-addicted, causing cancer patients to display glutamine depletion (57–59). Third, FGF21 is expressed in response to metabolic stress such as carbon limitation (46, 47, 83, 85). To determine whether mTORC1 signaling correlates with FGF21 expression in tumors, we examined S6 phosphorylation and FGF21 protein levels in human hepatocellular carcinoma (HCC) biopsies. Histological examination of HCC from 10 separate patients revealed a strong correlation between mTORC1 signaling and FGF21 expression. Four HCCs expressed both phospho-S6 and FGF21 whereas six expressed neither (Fig. 6H and Fig. S7B). Thus, our findings in L-Tsc1 KO mice may be physiologically relevant in tumors and, furthermore, suggest that treatment of glutamine-addicted cancers with mTOR inhibitors might have beneficial effects at both the tumor and whole-body level.
Discussion
To obtain insight on how nutrient availability controls whole-body behavior and metabolism, we investigated the role of the nutrient sensor mTORC1 on locomotor activity, body temperature, and glucose and lipid homeostasis. In particular, we examined the effects of hepatic mTORC1 activation and inhibition on whole-body physiology using L-Tsc1 KO or rapamycin-treated mice, respectively. We observed that mTORC1 hyperactivity in the liver induced glutamine depletion, which in turn activated the transcriptional coactivator PGC-1α and thereby the stress hormone FGF21. FGF21 expression reduced locomotor activity, body temperature, and lipid metabolism. Our results suggest that mTORC1 in the liver controls hepatic metabolism and whole-body behavior (Fig. 7). Finally, we found that mTORC1 signaling in human HCC correlates with FGF21 expression. This suggests that our findings in mice may have physiological relevance in glutamine-addicted human cancers. Glutamine-addicted tumors could deregulate whole-body physiology through FGF21. Accordingly, treatment with the anticancer drug rapamycin may have beneficial effects at both the tumor and whole-body level. In addition to blocking growth of the tumor, rapamycin may prevent glutamine depletion and thereby FGF21 expression.
Fig. 7.
Model of hepatic mTORC1 controlling whole-body physiology. Hepatic mTORC1 causes glutamine depletion and thereby PGC-1α-dependent FGF21 expression, which in turn affects whole-body physiology.
An important observation in this study is that L-Tsc1 KO mice display whole-body glutamine depletion, and this in turn leads to behavioral and metabolic alterations. The glutamine depletion observed in L-Tsc1 KO mice and its prevention by rapamycin treatment is consistent with a recent study showing that mTORC1 stimulates glutamine-dependent anaplerosis and that mTORC1 inhibition increases intracellular glutamine levels in cultured cells (56). Moreover, confirming previous in vitro studies (55, 86, 87), we show that glutamine increases hepatic mTORC1 signaling. Thus, our findings indicate that the role of mTORC1 in glutamine metabolism has consequences at the whole-body level in addition to the cellular level. mTORC1 promotes glutamine catabolism that decreases locomotor activity, body temperature, and lipid metabolism. mTOR inhibitors or a glutamine supplement may have a beneficial effect in countering disruption of whole-body physiology due, for example, to disease.
We report that L-Tsc1 KO mice display an increase in PGC-1α expression. This is consistent with previous studies in skeletal muscle showing a reduction in PGC-1α expression upon inactivation of mTORC1 (88, 89). Although mTORC1 may promote expression of the transcriptional coactivator PGC-1α in both muscle and liver, the molecular mechanism appears to be different in the two tissues. In muscle cells, mTORC1 controls PGC-1α expression through the transcription factor YY1 and under conditions where glutamine is likely not limiting (89). In liver, mTORC1 activates PGC-1α expression in response to glutamine depletion. The mechanism through which glutamine depletion activates Pgc-1α remains unknown but might be independent of YY1.
How does PGC-1α activate Fgf21 in L-Tsc1 KO mice? PGC-1α is a transcriptional coactivator with RORα/γ or PPARα (77, 79) which have been proposed to be direct activators of Fgf21 (46, 90, 91). We found that RORγ expression is slightly increased in the liver of L-Tsc1 KO mice during the dark phase whereas RORα and PPARα were reduced and unchanged, respectively (Figs. S2A and S6D). This suggests that PGC-1α may activate Fgf21 via RORγ but does not rule out PPAR-α.
Our finding that PGC-1α promotes FGF21 expression is consistent with the previous observation that PGC-1α promotes FGF21 through PPARα (49). However, Estall et al., showed that PGC-1α indirectly represses FGF21 through the transcriptional repressor Rev-erbα (90). These seemingly conflicting findings on the role of PGC-1α in the regulation of Fgf21 may be due to different experimental conditions. We (Fig. S2 A and B) and others (70, 71, 73) observed that Rev-erbα is expressed only during the light phase whereas glutamine depletion increases PGC-1α and FGF21 expression only during the dark phase. PGC-1α may inhibit FGF21 expression via Rev-erbα during the light phase.
We and others observed that hepatic mTORC1 activity is circadian (24, 33, 34, 36). Two previous studies showed that hepatic mTORC1 rhythmicity is controlled by the circadian clock (34, 36). Furthermore, Jouffe et al. (34) speculated that the circadian clock controls mTORC1 signaling via autophagy. Interestingly, hepatic mTORC1 signaling remains rhythmic in L-Tsc1 KO mice. It is well established that loss of the TSC complex prevents autophagy, as a consequence of mTORC1 activation, and autophagy is indeed inhibited in L-Tsc1 KO mice (92). Thus, at least in the absence of the TSC complex, mTORC1 rhythmicity appears not to be regulated by autophagy. Recently, Khapre et al. showed that Bmal1 negatively regulates mTORC1 signaling over a 24-h cycle by affecting the expression of mtor and deptor in the liver (36). This provides a mechanism through which the transcription factors of the circadian clock impinge on mTORC1 signaling. However, further investigation is required to determine the role of other circadian clock components in the regulation of mTORC1 signaling. The rhythmicity of mTORC1 signaling accounts for the rhythmicity of glutamine depletion and PGC-1α–dependent FGF21 expression. Thus, disruption of the circadian clock frequently observed in cancer or upon jet lag might alter mTORC1 signaling and thereby whole-body physiology. Finally, we note that, although the circadian clock affects mTORC1 signaling (34, 36), mTORC1 itself appears not to control the core clock components (Fig. S2 A and B).
In conclusion, our findings underscore the importance of hepatic mTORC1 in the regulation of whole-body physiology. Hyperactivation of mTORC1 in the liver causes glutamine depletion, which leads to FGF21 expression and, in turn, changes in whole-body physiology. Moreover, mTORC1 signaling correlates with FGF21 expression in human liver tumors. This latter observation is relevant because human cancers often exhibit hyperactive mTORC1 signaling and glutamine addiction (9–11, 59). Thus, treatment of glutamine-addicted cancers with mTOR inhibitors might have beneficial effects at both the tumor and whole-body level.
Materials and Methods
Mice.
Generation of liver-specific Tsc1 knockout (L-Tsc1 KO) mice has been described previously (6). Where indicated, mice were intraperitoneally injected with rapamycin (LC Laboratories) at 2 mg/kg or vehicle. Rapamycin solvent was composed of 5% (vol/vol) PEG-400, 4% (vol/vol) ethanol, and 5% (vol/vol) Tween 80. Additionally, where indicated, animals were intraperitoneally injected with l-glutamine (Sigma) at 1 g/kg or with saline solution. For details see SI Materials and Methods.
Other Methods.
For other methods, see SI Materials and Methods. The primer sequences used in this study are listed in Table S1.
Statistical Analyses.
Statistical significance was measured using a Student’s unpaired t test to determine differences among two groups. The differences were considered to be significant if P < 0.05. Data are presented as mean ± SEM. Immunoblot quantitation was assessed using Image J software.
Supplementary Material
Acknowledgments
We thank Christoph Handschin for reagents and Annette Roulier for the illustration. This work was supported by the Swiss National Science Foundation, the Canton of Basel, the Louis Jeantet Foundation (M.N.H.), the Société Francophone du Diabète-Association de Langue Française pour l’Etude du Diabète et des Maladies Métaboliques (M.C.), and the Werner Siemens Foundation (A.M.R. and V.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412047111/-/DCSupplemental.
References
- 1.Loewith R, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10(3):457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 2.Jacinto E, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6(11):1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 3.Sarbassov DD, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 4.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robitaille AM, et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science. 2013;339(6125):1320–1323. doi: 10.1126/science.1228771. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339(6125):1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimobayashi M, Hall MN. Making new contacts: The mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15(3):155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 9.Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23(6):744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013;23(1):53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: A new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10(11):868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Kim SG, Blenis J. Rapamycin: One drug, many effects. Cell Metab. 2014;19(3):373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cailotto C, et al. Effects of nocturnal light on (clock) gene expression in peripheral organs: A role for the autonomic innervation of the liver. PLoS ONE. 2009;4(5):e5650. doi: 10.1371/journal.pone.0005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 16.Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: From gene expression to physiology. Chromosoma. 2004;113(3):103–112. doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- 17.Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12(18):1574–1583. doi: 10.1016/s0960-9822(02)01145-4. [DOI] [PubMed] [Google Scholar]
- 18.Saini C, Morf J, Stratmann M, Gos P, Schibler U. Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes Dev. 2012;26(6):567–580. doi: 10.1101/gad.183251.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291(5503):490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 21.Gachon F, Olela FF, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006;4(1):25–36. doi: 10.1016/j.cmet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Gachon F, Firsov D. The role of circadian timing system on drug metabolism and detoxification. Expert Opin Drug Metab Toxicol. 2011;7(2):147–158. doi: 10.1517/17425255.2011.544251. [DOI] [PubMed] [Google Scholar]
- 23.Sun Z, Lazar MA. Dissociating fatty liver and diabetes. Trends Endocrinol Metab. 2013;24(1):4–12. doi: 10.1016/j.tem.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollmers C, et al. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA. 2009;106(50):21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121(6):2133–2141. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schibler U, Naef F. Cellular oscillators: Rhythmic gene expression and metabolism. Curr Opin Cell Biol. 2005;17(2):223–229. doi: 10.1016/j.ceb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Eckel-Mahan KL, et al. Coordination of the transcriptome and metabolome by the circadian clock. Proc Natl Acad Sci USA. 2012;109(14):5541–5546. doi: 10.1073/pnas.1118726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamovich Y, et al. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19(2):319–330. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng X, Sehgal A. AKT and TOR signaling set the pace of the circadian pacemaker. Curr Biol. 2010;20(13):1203–1208. doi: 10.1016/j.cub.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao R, Anderson FE, Jung YJ, Dziema H, Obrietan K. Circadian regulation of mammalian target of rapamycin signaling in the mouse suprachiasmatic nucleus. Neuroscience. 2011;181:79–88. doi: 10.1016/j.neuroscience.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao R, Lee B, Cho HY, Saklayen S, Obrietan K. Photic regulation of the mTOR signaling pathway in the suprachiasmatic circadian clock. Mol Cell Neurosci. 2008;38(3):312–324. doi: 10.1016/j.mcn.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao R, et al. Translational control of entrainment and synchrony of the suprachiasmatic circadian clock by mTOR/4E-BP1 signaling. Neuron. 2013;79(4):712–724. doi: 10.1016/j.neuron.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatori M, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jouffe C, et al. The circadian clock coordinates ribosome biogenesis. PLoS Biol. 2013;11(1):e1001455. doi: 10.1371/journal.pbio.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamia KA, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326(5951):437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khapre RV, et al. BMAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging (Albany, NY Online) 2014;6(1):48–57. doi: 10.18632/aging.100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asher G, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134(2):317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 38.Nakahata Y, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13(2):125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Feng D, Lazar MA. Clocks, metabolism, and the epigenome. Mol Cell. 2012;47(2):158–167. doi: 10.1016/j.molcel.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93(1):107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492(1):203–206. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 43.Kornmann B, Schaad O, Reinke H, Saini C, Schibler U. Regulation of circadian gene expression in liver by systemic signals and hepatocyte oscillators. Cold Spring Harb Symp Quant Biol. 2007;72:319–330. doi: 10.1101/sqb.2007.72.041. [DOI] [PubMed] [Google Scholar]
- 44.Oishi K, Uchida D, Ishida N. Circadian expression of FGF21 is induced by PPARalpha activation in the mouse liver. FEBS Lett. 2008;582(25-26):3639–3642. doi: 10.1016/j.febslet.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 45.Tong X, et al. Transcriptional repressor E4-binding protein 4 (E4BP4) regulates metabolic hormone fibroblast growth factor 21 (FGF21) during circadian cycles and feeding. J Biol Chem. 2010;285(47):36401–36409. doi: 10.1074/jbc.M110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inagaki T, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5(6):415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Badman MK, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5(6):426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Lundåsen T, et al. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360(2):437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 49.Purushotham A, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9(4):327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Potthoff MJ, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA. 2009;106(26):10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bookout AL, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19(9):1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emanuelli B, et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest. 2014;124(2):515–527. doi: 10.1172/JCI67353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 54.Choo AY, et al. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell. 2010;38(4):487–499. doi: 10.1016/j.molcel.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durán RV, et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47(3):349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 56.Csibi A, et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell. 2013;153(4):840–854. doi: 10.1016/j.cell.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischer JE, Chance WT. Total parenteral nutrition, glutamine, and tumor growth. JPEN J Parenter Enteral Nutr. 1990;14(Suppl 4):86S–89S. doi: 10.1177/0148607190014004101. [DOI] [PubMed] [Google Scholar]
- 58.Souba WW. Glutamine and cancer. Ann Surg. 1993;218(6):715–728. doi: 10.1097/00000658-199312000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Chong-Kopera H, et al. TSC1 stabilizes TSC2 by inhibiting the interaction between TSC2 and the HERC1 ubiquitin ligase. J Biol Chem. 2006;281(13):8313–8316. doi: 10.1074/jbc.C500451200. [DOI] [PubMed] [Google Scholar]
- 61.Meikle L, et al. A mouse model of tuberous sclerosis: Neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27(21):5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Um SH, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 63.Ueno M, et al. Regulation of insulin signalling by hyperinsulinaemia: Role of IRS-1/2 serine phosphorylation and the mTOR/p70 S6K pathway. Diabetologia. 2005;48(3):506–518. doi: 10.1007/s00125-004-1662-6. [DOI] [PubMed] [Google Scholar]
- 64.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14(18):1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 65.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 66.Appenzeller-Herzog C, Hall MN. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol. 2012;22(5):274–282. doi: 10.1016/j.tcb.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Yecies JL, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14(1):21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468(7327):1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 69.Kenerson HL, Yeh MM, Yeung RS. Tuberous sclerosis complex-1 deficiency attenuates diet-induced hepatic lipid accumulation. PLoS ONE. 2011;6(3):e18075. doi: 10.1371/journal.pone.0018075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Preitner N, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110(2):251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 71.Cho H, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485(7396):123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asher G, et al. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010;142(6):943–953. doi: 10.1016/j.cell.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 73.Feng D, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331(6022):1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoon JC, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 75.Puigserver P, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423(6939):550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 76.Rhee J, et al. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): Requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc Natl Acad Sci USA. 2003;100(7):4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447(7143):477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 78.Leone TC, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: Muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3(4):e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20(5):1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koo SH, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10(5):530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 81.Schaap FG, Kremer AE, Lamers WH, Jansen PL, Gaemers IC. Fibroblast growth factor 21 is induced by endoplasmic reticulum stress. Biochimie. 2013;95(4):692–699. doi: 10.1016/j.biochi.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 82.De Sousa-Coelho AL, Marrero PF, Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem J. 2012;443(1):165–171. doi: 10.1042/BJ20111748. [DOI] [PubMed] [Google Scholar]
- 83.Kim KH, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19(1):83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 84.Kang YJ, Lu MK, Guan KL. The TSC1 and TSC2 tumor suppressors are required for proper ER stress response and protect cells from ER stress-induced apoptosis. Cell Death Differ. 2011;18(1):133–144. doi: 10.1038/cdd.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang C, et al. Activation of Liver FGF21 in hepatocarcinogenesis and during hepatic stress. BMC Gastroenterol. 2013;13:67. doi: 10.1186/1471-230X-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim SG, et al. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol Cell. 2013;49(1):172–185. doi: 10.1016/j.molcel.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van der Vos KE, et al. Modulation of glutamine metabolism by the PI(3)K-PKB-FOXO network regulates autophagy. Nat Cell Biol. 2012;14(8):829–837. doi: 10.1038/ncb2536. [DOI] [PubMed] [Google Scholar]
- 88.Bentzinger CF, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8(5):411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Cunningham JT, et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450(7170):736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 90.Estall JL, et al. PGC-1alpha negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb(alpha) axis. Proc Natl Acad Sci USA. 2009;106(52):22510–22515. doi: 10.1073/pnas.0912533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Solt LA, Burris TP. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor alpha. J Biol Chem. 2010;285(21):15668–15673. doi: 10.1074/jbc.M110.102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Menon S, et al. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal. 2012;5(217):ra24. doi: 10.1126/scisignal.2002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.