The cerebellum is a neuronal machine that is composed of numerous uniform modules called “microcomplexes.” The cerebellum plays an essential role for adaptive motor learning by providing the motor control system with an “internal model.” The internal models are formed by integration of calculations performed by microcomplexes in which the input-output relationship is adaptively modified by the “error signals” conveyed into Purkinje cells by climbing fibers (1, 2). Despite the long history of the cerebellum research, much of the mechanism on how each microcomplex works and how the calculations of microcomplexes are integrated to form internal models still remains to be studied as this research has been hampered by the difficulty in observing and manipulating a too large number of microcomplexes either at once or individually. One possible way to overcome this difficulty is to take a reductionist approach, i.e., to study the “smallest” cerebellum. In PNAS, the report by Matsui et al. (3), together with recently published works by others (4–7), demonstrates that the tiny cerebellum of the larva of teleost zebrafish (Danio rerio) can be a suitable model for this purpose, because it is much smaller in size with much smaller number of microcomplexes than in mammals and is still amenable to various genetic manipulations. Transparency of larva enables the whole-brain neural activity imaging with single-cell resolution by use of modern cellular activity imaging technology such as two-photon microscopy in combination with various intracellular calcium indicators (3–7).
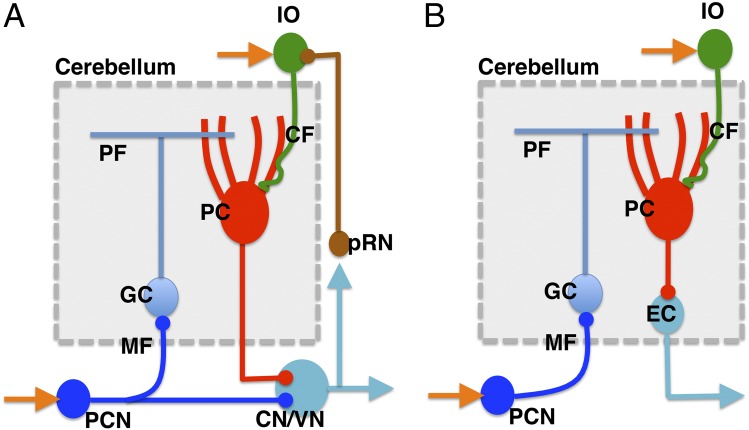
The cerebellum is evolutionarily a relatively new addition to the brain structure, which has been brought about as an evolutionary innovation in gnathostomes, possibly by exaptation of the brain structures and genes already present in the vertebrate common ancestor (8). Recently, the structure and development of the cerebellum in larval and adult zebrafish have been well characterized (9, 10). In the mammalian cerebellum (Fig. 1A), each microcomplex receives major input signals via mossy fibers (MFs) into granule cells (GCs), which transmit the signals to Purkinje cells (PCs) by way of parallel fibers (PFs), and the signals are ultimately conveyed to the cerebellar or vestibular nuclear neurons (CNs/VNs) that send commands as the “controller” to downstream “controlled objects” such as motor neurons. Each microcomplex also receives climbing fiber (CF) signals encoding errors for learning. CFs originate from the inferior olive (IO) and pass the cerebellar cortex to supply strong excitatory synapses to PCs. Collaterals of MFs and CFs also innervate CNs/VNs (1, 2). The structure of the zebrafish cerebellar microcomplex is largely the same as that of mammals except that euridendroid cells (ECs), which lie inside of the cerebellum, receive signals from the PCs in contrast to the CNs/VNs of mammals, which lie outside of the cerebellum (Fig. 1B). Some ECs also receive direct inputs from PFs, although they are not shown in Fig. 1B (9, 11).
Fig. 1.
(A) A microcomplex of the mammalian cerebellum. (B) A microcomplex of the zebrafish cerebellum. Adapted from refs. 2 and 9. CN/VN, cerebellar nucleus/vestibular nucleus; GC, granule cell; IO, inferior olive; MF, mossy fiber; PC, Purkinje cell; PCN, precerebellar nucleus; PF, parallel fiber; pRN, parvocellular red nucleus.
Recent studies have accumulated evidence that the cerebellum of larval zebrafish is also critically involved in regulation or learning of various adaptive motor behaviors such as the classical fear conditioning, the optokinetic reflex (OKR), and optomotor reflex (OMR) as the mammalian cerebellum regulates the classical eye blink conditioning, OKR, and vestibule-ocular reflex (VOR). A classical conditioning in larvae develops an enhanced motor response to a visual stimulus [conditioned stimulus (CS)] when it is paired with touch [unconditioned stimulus (US)]. In vivo calcium imaging revealed that CS and US activate different subsets of neurons in the cerebellum and the activities of CS-responsive neurons were enhanced after conditioning, and an ablation experiment demonstrated that the cerebellum is involved in acquisition and extinction, but not the retention, of this memory (4). The responses of the cerebellar neurons were also examined by calcium imaging for fictive OMR in which a larva was put in a closed-loop environment where a paralyzed larva was presented with black and white alternating stripes streaming in the caudal to rostral direction, and the speed of the stream was readjusted depending on the levels of motor output for a larval fictive swim bout to catch up to the stream of stripes. A decrease in the gain setting of a feedback loop resulted in adaptive increase of motor output and activated neurons in the cerebellum and the IO (5, 6). The cerebellum receives OKR-related sensory and motor signals through mossy fibers and, in particular, retinal image motion signals via climbing fibers from the contralateral IO, which in turn receives direct input from the pretectum. The whole-brain imaging also revealed notable temporal gradients of activity timing in the olivo-cerebellar pathway and the pretectum during OKR (7).
All these experiments explained above were performed under the condition where all neurons in the cerebellum expressed the calcium indicators indiscriminately. To study the dynamic property of microcomplex, the development of tools for labeling and manipulating different types of neurons specifically in the microcomplex has long been awaited. Matsui et al. (3) present a tour de force achievement toward this goal. First, they isolated a 258-bp PC-specific enhancer element from the zebrafish carbonic anhydrase-related protein VIII (Car8) gene and established transgenic fish expressing a transneuronal anterograde tracer, wheat germ agglutinin (WGA), under control of this enhancer to identify second-order PC efferents outside the cerebellum by way of ECs. Combined with the results obtained by stochastic labeling of single ECs, they concluded that ECs from the caudal (medial and lateral) cerebellum were mostly connected to the octaval nuclei or to anterior brain regions in the hypothalamus and the torus semicircularis in the midbrain. The authors also show that the PCs in the lateral part of the cerebellum also send the direct afferent to the octaval nuclei. In contrast, the ECs in the rostro-medial part of the cerebellum projected long ascending axons to the nucleus of the medial longitudinal fascicle (MLF), the red nucleus, and the thalamus and long descending axons to the reticular formation. Furthermore, by observation of GCaMP5G fluorescence during OKR performance, the authors show that significant changes of calcium levels were restricted to PCs only in the caudal part of the cerebellum, whereas the change in fluorescence in OMR was spatially restricted to the rostro-medial part of the cerebellar PC layer. All these data showed that, by connectivity and physiological activity, the caudal part of the cerebellum controls saccadic eye movements in OKR by sending projections to the octaval populations. In contrast, the rostro-medial part of the cerebellum controls swimming behavior in OMR and is connected to the locomotor-related system of the central nervous system. They further confirmed this hypothesis by optogenetic interrogation (activation or inactivation) of selected Purkinje cell regions during animal behaviors.
Recently, another group has systematically characterized the neural activities responsible for the rotational stimuli and translational stimuli that drive OKR and OMR in the pretectum of the larval zebrafish, respectively, which send projections to the IO as mentioned above (12). How the cerebellar neurons and the OKR- or OMR-related neurons outside of the cerebellum such as the pretectum interact is the interesting subject that can be addressed using the transgenic line introduced by Matsui et al.
What is highly desired as a natural extension of this work in the near future is the establishment of transgenic lines in which the major components of the cerebellum, i.e., PCs, GCs, and ECs, are differentially labeled so that their activities can be imaged simultaneously and distinctively. This would resolve the interactions among these neurons in real time and contribute immensely to confirming how much the cerebellum really behaves like a neuronal machine. For example, neural computation theory has proposed two types of internal models that the cerebellum would provide (Fig. 2) (1, 2). A forward model reproduces the dynamics of a controlled object, whereas an inverse model reproduces a reciprocal of these dynamics. A forward model provides an internal feedback that can replace the external feedback from the controlled object (Fig. 2A). An inverse model, by contrast, provides a controller that does not receive feedback (a feed-forward controller), which can replace the original controller (Fig. 2B). The whole-cerebellum imaging of neural activities with complete identification of all imaged neurons will solve long-standing issues such as whether these two types of models are used in separate parts of the cerebellum for different purposes or if they operate in combination.
Fig. 2.
(A) A forward-model control system. (B) An inverse-model control system. Adapted from ref. 1.
Supplementary Material
Footnotes
The author declares no conflict of interest.
See companion article on page 11846.
References
- 1.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9(4):304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 2.Ito M. Error detection and representation in the olivo-cerebellar system. Front Neural Circuits. 2013;7:1. doi: 10.3389/fncir.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsui H, Namikawa K, Babaryka A, Köster RW. Functional regionalization of the teleost cerebellum analyzed in vivo. Proc Natl Acad Sci USA. 2014;111:11846–11851. doi: 10.1073/pnas.1403105111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aizenberg M, Schuman EM. Cerebellar-dependent learning in larval zebrafish. J Neurosci. 2011;31(24):8708–8712. doi: 10.1523/JNEUROSCI.6565-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portugues R, Engert F. Adaptive locomotor behavior in larval zebrafish. Front Syst Neurosci. 2011;5:72. doi: 10.3389/fnsys.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahrens MB, et al. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature. 2012;485(7399):471–477. doi: 10.1038/nature11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portugues R, Feierstein CE, Engert F, Orger MB. Whole-brain activity maps reveal stereotyped, distributed networks for visuomotor behavior. Neuron. 2014;81(6):1328–1343. doi: 10.1016/j.neuron.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami Y, Uchida K, Rijli FM, Kuratani S. Evolution of the brain developmental plan: Insights from agnathans. Dev Biol. 2005;280(2):249–259. doi: 10.1016/j.ydbio.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Bae YK, et al. Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Dev Biol. 2009;343(2):406–426. doi: 10.1016/j.ydbio.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Wullimann MF, et al. The long adventurous journey of rhombic lip cells in jawed vertebrates: A comparative developmental analysis. Front Neuroanat. 2011;5:27. doi: 10.3389/fnana.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heap LA, Goh CC, Kassahn KS, Scott EK. Cerebellar output in zebrafish: An analysis of spatial patterns and topography in eurydendroid cell projections. Front Neural Circuits. 2013;7:53. doi: 10.3389/fncir.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubo F, et al. Functional architecture of an optic flow-responsive area that drives horizontal eye movements in zebrafish. Neuron. 2014;81(6):1344–1359. doi: 10.1016/j.neuron.2014.02.043. [DOI] [PubMed] [Google Scholar]