Significance
Allergic asthma represents an increasingly common public health problem. Here, we provide preclinical evidence for the efficacy of active tolerization using Helicobacter pylori components as a viable strategy for asthma prevention. We use a mouse model of allergic asthma to show that regular treatment with H. pylori extract effectively alleviates all hallmarks of the disease. Successful treatment depends on the regulatory cytokine IL-10 and on basic leucine zipper ATF-like 3 (BATF3)-dependent dendritic cell lineages. H. pylori extracts lacking the γ-glutamyl-transpeptidase GGT or the vacuolating cytotoxin VacA fail to protect against asthma; conversely, both factors can be administered in purified form to achieve protection. In conclusion, the immunomodulatory properties of the common infectious agent H. pylori can be exploited for therapeutic purposes in an allergy model.
Keywords: bacterial immunomodulation, allergy and asthma prevention, tolerogenic dendritic cells, bacterial persistence determinants
Abstract
The prevalence of allergic asthma and other atopic diseases has reached epidemic proportions in large parts of the developed world. The gradual loss of the human indigenous microbiota has been held responsible for this trend. The bacterial pathogen Helicobacter pylori is a constituent of the normal gastric microbiota whose presence has been inversely linked to allergy and asthma in humans and experimental models. Here we show that oral or i.p. tolerization with H. pylori extract prevents the airway hyperresponsiveness, bronchoalveolar eosinophilia, pulmonary inflammation, and Th2 cytokine production that are hallmarks of allergen-induced asthma in mice. Asthma protection is not conferred by extracts from other enteropathogens and requires a heat-sensitive H. pylori component and the DC-intrinsic production of IL-10. The basic leucine zipper ATF-like 3 (BATF3)-dependent CD103+CD11b− dendritic cell lineage is enriched in the lungs of protected mice and strictly required for protection. Two H. pylori persistence determinants, the γ-glutamyl-transpeptidase GGT and the vacuolating cytotoxin VacA, are required and sufficient for asthma protection and can be administered in purified form to prevent asthma. In conclusion, we provide preclinical evidence for the concept that the immunomodulatory properties of H. pylori can be exploited for tolerization strategies aiming to prevent allergen-induced asthma.
The prevalence of asthma and other allergic diseases has increased steadily in the course of the second half of the 20th century in both adult and pediatric, developed and developing populations (1). The lack of early childhood infections or microbial exposure due to improved sanitation, and the gradual loss of the indigenous microbiota have alternately been proposed to account for this major public health trend (2, 3). Epidemiological and experimental studies have consistently shown a strong inverse association of chronic infection with the human gastric bacterial pathogen Helicobacter pylori with the risk of developing allergic asthma (4–9). Chronic infection with H. pylori is less common in allergic individuals presenting with asthma, hay fever, or eczema than in the general population; this is especially true in children and in patients with early-onset disease (4–8). We have reported earlier that experimental infection of C57/BL6 mice with a mouse-colonizing human isolate of H. pylori confers robust protection against allergen-induced asthma, with particularly strong protective effects observed upon early-life exposure (9). Asthma protection could be attributed to H. pylori-specific tolerogenic reprogramming of dendritic cells in vitro and in vivo and to the induction of highly suppressive regulatory T cells (9, 10). Despite its striking immunomodulatory properties (11) and remarkable inverse link to various allergic diseases, the use of live H. pylori as a therapeutic intervention or preventive measure is unattractive due to the well-documented carcinogenic potential of chronic infection with this organism. H. pylori induces gastric and duodenal ulcers (12), and is also widely accepted to be the leading cause of gastric adenocarcinoma (13). Here, we have devised a strategy of H. pylori-specific tolerization that harnesses the bacteria’s immunomodulatory properties for the prevention of asthma while avoiding the risks associated with live infection and have elucidated several key determinants of asthma protection in both the bacteria and the host.
Results
H. pylori Whole Cell Extract Protects Against Allergen-Induced Asthma.
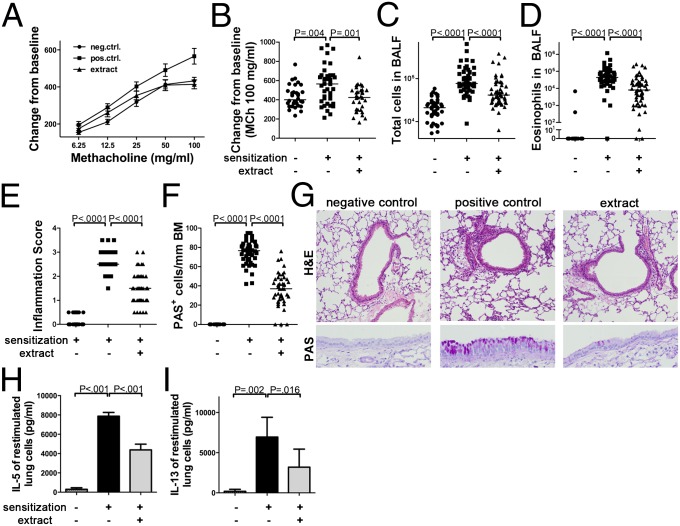
To assess whether regular administration of H. pylori extract protects against allergen-induced asthma and thus recapitulates the effects of live infection, we treated mice with weekly doses of intragastrically administered whole cell extract from age day 7 onwards before subjecting them to ovalbumin sensitization and challenge. Control mice that had received ovalbumin but no H. pylori extract developed airway hyperresponsiveness to methacholine (Fig. 1 A and B and Fig. S1 A–D) and bronchoalveolar immune cell infiltration and eosinophilia (Fig. 1 C and D), as well as histologically evident lung inflammation and goblet cell metaplasia (Fig. 1 E–G). The restimulation of single cell lung preparations with ovalbumin induced the production of high levels of the Th2 cytokines IL-5 and IL-13 (Fig. 1 H and I). In contrast, mice that had received H. pylori extract were protected against airway hyperresponsiveness (Fig. 1 A and B and Fig. S1 A–D), and exhibited significantly lower levels of bronchoalveolar and pulmonary inflammation, eosinophilia and goblet cell metaplasia (Fig. 1 C–G). Th2 cytokine production upon allergen restimulation of lung preparations was also reduced (Fig. 1 H and I). The failure of extract-treated mice to develop allergen-induced symptoms of asthma was not due to an impaired primary response to the allergen, as the levels of ovalbumin-specific serum IgE were similar in all sensitized mice (Fig. S1E).
Fig. 1.
Experimentally induced asthma is alleviated by treatment with H. pylori extract. (A–I) Mice were sensitized i.p. with alum-adjuvanted ovalbumin at 8 and 10 wk of age and challenged with aerosolized ovalbumin 2 wk after the second sensitization to induce asthma-like symptoms. Mock-sensitized mice served as negative controls. One group received once-weekly doses of 200 μg H. pylori extract intragastrically from day 7 of age until the second sensitization. (A and B) Airway hyperresponsiveness in response to increasing doses of methacholine and the highest dose of 100 mg/mL, respectively. (C and D) Total cells and eosinophils contained in 1 mL of BALF. (E–G) Tissue inflammation and goblet cell metaplasia as assessed on H&E and PAS-stained tissue sections; representative micrographs taken at 100× (H&E) and 400× (PAS) original magnification are shown in G. Pooled data from five independent studies are shown in A–F. (H and I) IL-5 and IL-13 secretion by single cell lung preparations restimulated with ovalbumin, as assessed by ELISA. Pooled data from two studies are shown in H and I. In scatter plots, each symbol represents one mouse; horizontal lines indicate the medians.
To address the specificity of the observed effects and elucidate key prerequisites of protection, we examined various administration routes and regimens, ages at treatment onset, and extracts from other gastrointestinal pathogens. Interestingly, the systemic (intraperitoneal) administration of H. pylori extract was as efficient as the intragastric route at conferring protection against allergen-induced asthma (Fig. S1 F–I). Intragastric treatment was less effective when initiated in adult mice as opposed to neonates, and four consecutive doses of extract administered to young mice before weaning were insufficient to induce full protection (Fig. S1 F–I). Heat-inactivated H. pylori extract, as well as identical amounts of extracts generated from cultures of Escherichia coli or Salmonella typhimurium, failed to confer protection against the examined hallmarks of allergic airway disease (Fig. S1 F–I). In conclusion, the beneficial effects of extract treatment are specific to H. pylori and require a heat-sensitive component of the bacteria, and are most pronounced if the treatment is initiated in young mice.
Successful Tolerization Against Allergen-Induced Asthma Requires IL-10 and IL-18, but Not Regulatory T Cells.
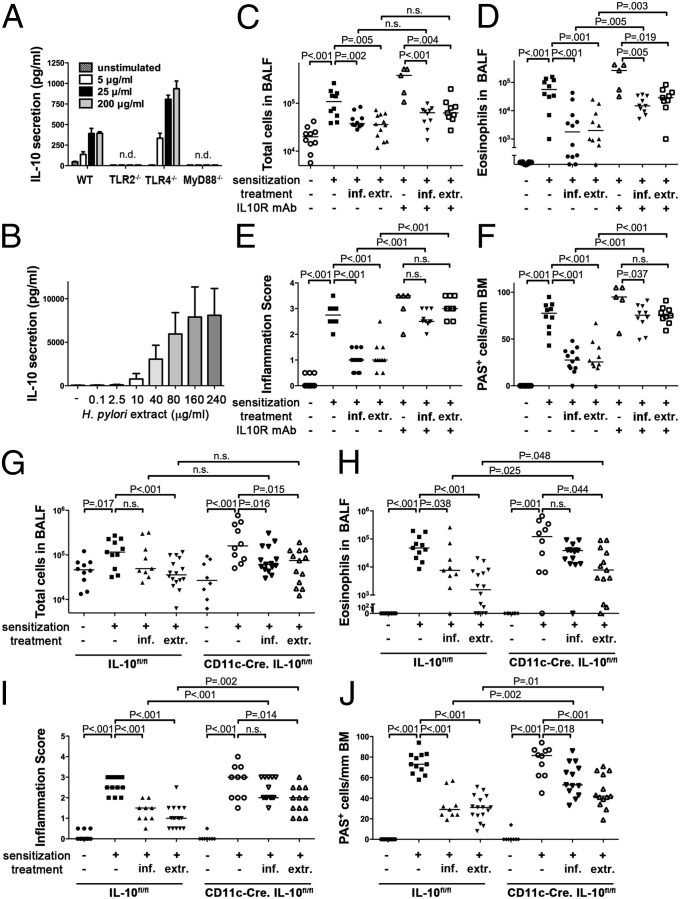
H. pylori is known to induce the production of IL-10 in various immune cell compartments (14, 15) and high gastric levels of IL-10 ensure H. pylori persistence and promote H. pylori-specific immune tolerance (16, 17). We have shown previously that dendritic cells (DCs) play a critical role in immune tolerance to live H. pylori; the depletion of CD11c-positive DCs breaks tolerance and promotes clearance of the bacteria (10). To assess whether DCs produce IL-10 not only in response to live infection as shown (10), but also in response to H. pylori extract, cultured murine bone marrow-derived (BM) DCs were treated with increasing concentrations of extract. Indeed, BM-DCs produced and secreted large amounts of IL-10, and this was dependent on TLR2 and MyD88 signaling, but independent of TLR4 (Fig. 2A). A clear dose-dependent secretion of IL-10 could also be observed in human blood-derived DCs from six independent donors cultured with H. pylori extract (Fig. 2B). To address whether IL-10 is required for asthma protection conferred by extract tolerization or live infection, we administered two doses of IL-10 receptor (IL-10R)-neutralizing antibody during the challenge phase of the protocol to mice that had either received extract from the neonatal period onwards or had been infected as neonates. IL-10 signaling was required for protection against asthma in both scenarios (Fig. 2 C–F). We further examined the effects of extract tolerization and live infection in mice that are deficient for IL-10 production specifically in the CD11c+ immune cell compartment. Although not entirely resistant to extract treatment or the beneficial effects of live infection, CD11c-Cre.IL-10fl/fl mice were less well protected than their Cre-negative littermates, i.e., exhibited significantly higher eosinophil counts, lung inflammation and goblet cell metaplasia (Fig. 2 G–J). The overall secretion of IL-10 by allergen-restimulated lung cells was reduced in CD11c-Cre.IL-10fl/fl mice (Fig. S2A), implying that CD11c+ cells represent a major source of pulmonary IL-10 in this setting. In summary, we conclude that H. pylori extract induces IL-10 production in both murine and human DCs and that IL-10 produced by CD11c+ DCs/mononuclear phagocytes, in the lungs and/or at other sites, contributes critically to protection.
Fig. 2.
IL-10 is required for H. pylori-induced protection against allergic asthma. (A and B) IL-10 secretion by murine bone-marrow-derived DCs of the indicated genotypes and human monocyte-derived DCs from six healthy volunteers after exposure to H. pylori extract. One representative experiment of three is shown in A, and pooled data for all six donors is shown in B. (C–F) Mice were treated as described in Fig. 1 or were neonatally infected with H. pylori; the indicated groups received 2 doses of anti-IL-10R antibody during ovalbumin challenge. (G–J) CD11c-Cre.IL-10fl/fl mice and their IL-10fl/fl littermates were either neonatally infected or received H. pylori extract before being subjected to ovalbumin sensitization and challenge as described in Fig. 1. Total cells (C and G) and eosinophils (D and H) contained in 1 mL of BALF. Tissue inflammation (E and I) and goblet cell metaplasia (F and J).
Having shown previously that DC-derived IL-18 is a critical mediator of H. pylori-induced immune tolerance (10), we next examined the effects of H. pylori extract and live infection on IL-18R−/− mice. IL-18 signaling was absolutely required for the protective effects of live bacteria as well as extract treatment (Fig. S2 B–E), underscoring the tolerance-promoting role of this cytokine in the context of the H. pylori/host interaction. To further address whether Tregs were required for extract-mediated protection (as they are for live infection; ref. 9), we depleted CD25+ Tregs (>90% depletion efficiency in the lungs, Fig. S2F) by applying two doses of a CD25-specific antibody before ovalbumin challenge. Treg depletion had no effect on the protection from allergic asthma conferred by H. pylori extract (Fig. S1 G–J); this result was consistent with a lack of protective activity of CD25+ Tregs that were adoptively transferred from extract-treated mice to naive recipients (Fig. S1 G–J). H. pylori extract treatment of BM-DCs, in contrast to live infection, further failed to promote the expression of the Treg lineage-defining transcription factor FoxP3 in cocultured naive T cells (Fig. S2K), suggesting that H. pylori extract exerts its protective activity through the DC-intrinsic production of IL-10 (and IL-18), but independently of Tregs.
BATF3-Dependent DC Lineages Are Required for H. pylori-Induced Protection Against Allergic Airway Inflammation.
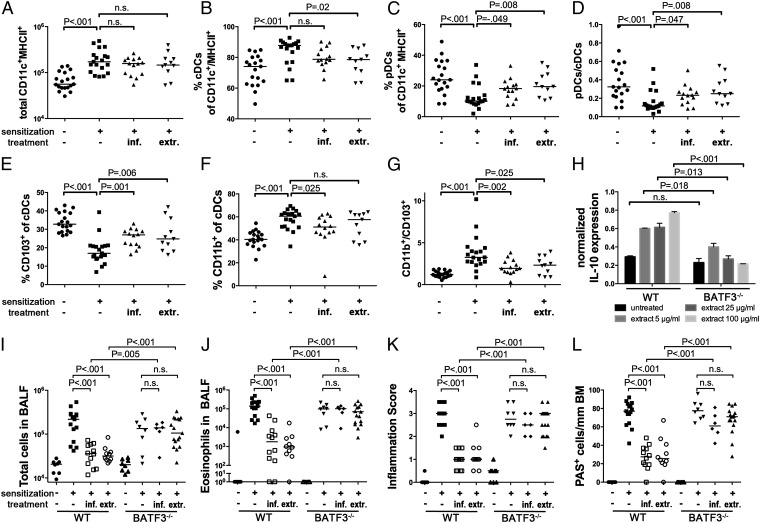
Having identified DCs as critical mediators of H. pylori-specific tolerance (10) and as key producers of protective IL-10 (Fig. 2), we next sought to dissect the role of specific DC subsets in the context of H. pylori infection and tolerization. To this end, we generated single cell lung preparations from extract-treated, infected, and positive as well as negative control mice and subjected them to quantitative flow cytometric analysis of various lung-infiltrating DC populations. Interestingly, despite the fact that the mice examined in this fashion exhibited very typical levels of protection (Fig. S3 A–D), their lungs were infiltrated with the same overall numbers of CD11c+ MHCII+ DCs as the lungs of asthmatic mice (Fig. 3A). However, when we distinguished between conventional and plasmacytoid DCs (cDCs, pDCs) based on their expression of B220 (also known as CD45R, a marker of the B-cell lineage that is also shared by pDCs, Fig. S3E), we found that CD11c+ MHCIIhi B220− cDCs were relatively more abundant in asthmatic mice, whereas CD11clo MHCIIlo B220+ pDCs were more abundant in the lungs of protected mice (Fig. 3 B–D). Furthermore, the total numbers of lung-infiltrating pDCs were higher than in nonsensitized negative controls (Fig. S3F), indicating that pDCs are actively recruited to the lungs of allergen-challenged mice that are either infected with H. pylori or treated with H. pylori extract. Another interesting difference was found among asthmatic and protected mice when we discriminated between CD11b+ and CD103+ cDC subsets (Fig. S3G). Strikingly, whereas the asthmatic lungs of positive control mice were predominantly infiltrated by CD11b+ cDCs, the lungs of protected mice were relatively more infiltrated by CD103+ cDCs (Fig. 3 E–G). Again, CD103+ cDCs appeared to be specifically recruited to the lungs of allergen-challenged mice either infected with H. pylori or treated with H. pylori extract (Fig. S3H).
Fig. 3.
CD103+ conventional DCs accumulate in the lungs of H. pylori-infected and extract-treated mice and are required for protection. (A–G) Groups of mice treated as described in Figs. 1 and 2 were analyzed with respect to lung infiltration by pDCs and two lineages of cDCs. Data are pooled from three independent studies. (A) Total infiltration of CD11c+MHC+ cells. (B and C) Frequencies of B220− cDCs and B220+ pDCs among all CD11c+MHC+ cells. (D) Ratios of pDCs to cDCs as calculated per mouse. (E and F) Frequencies of CD103+ and CD11b+ cells among all cDCs. (G) Ratios of CD11b+ and CD103+ cDCs as calculated per mouse. (H) IL-10 transcript levels normalized to GAPDH, of immunomagnetically isolated CD11c+ DCs from mesenteric lymph nodes of WT and BATF3−/− mice, treated with the indicated increasing doses of H. pylori extract. (I–L) Wild-type and BATF3−/− mice were treated or infected as described in Figs. 1 and 2 and subjected to ovalbumin sensitization and challenge. (I and J) Total cells and eosinophils contained in 1 mL of BALF. (K and L) Tissue inflammation and goblet cell metaplasia.
To assess the functional relevance of CD103+ lung-infiltrating DCs in asthma protection in our model, we examined mice lacking the transcription factor basic leucine zipper ATF-like 3 (BATF3), which has previously been shown to direct the development of CD8α+ lymphoid tissue DCs as well as CD103+ CD11b− DCs in the lungs, intestine and skin (18). We were able to confirm that the lungs of BATF3−/− mice are entirely devoid of CD103+ DCs, and exhibit normal and higher frequencies of pDCs and CD11b+ DCs, respectively (Fig. S3 I and J). Interestingly, pure populations of mesenteric lymph node-derived DCs from BATF3−/− mice failed to express IL-10 upon treatment with increasing doses of H. pylori extract ex vivo (Fig. 3H). BATF3−/− mice were significantly less protected than wild-type mice against allergen-induced asthma upon infection with H. pylori, and upon treatment with H. pylori extract (Fig. 3 I–L), despite being colonized at comparable levels (Fig. S3K). In summary, BATF3-dependent CD103+ DC lineages infiltrate the lungs of protected mice, and are required for the H. pylori-driven, IL-10-mediated protection from allergic asthma.
The H. pylori Persistence Determinants γ-Glutamyl Transpeptidase and Vacuolating Cytotoxin Are Required and Sufficient for Protection Against Allergic Airway Inflammation.
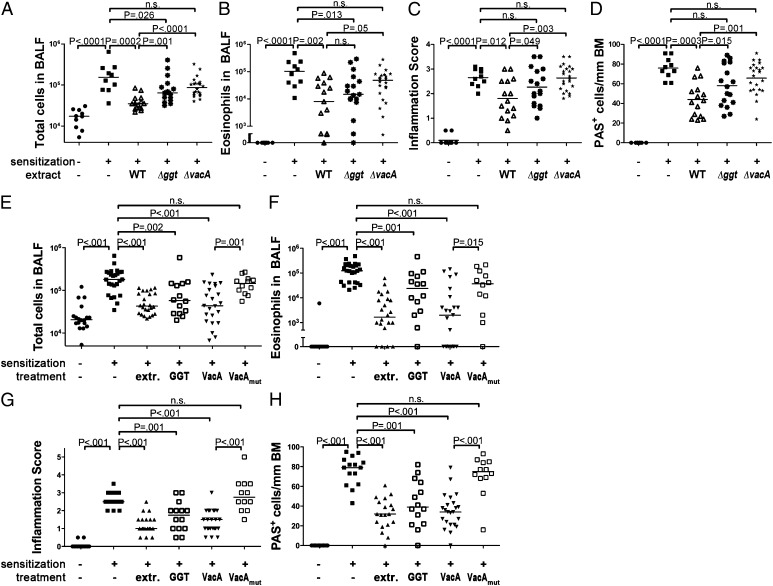
We have shown recently that two H. pylori virulence determinants encoded by all clinical isolates investigated to date, the γ-glutamyl transpeptidase GGT and the vacuolating cytotoxin VacA, promote persistence through tolerogenic reprogramming of DCs (19). To examine whether GGT and/or VacA contribute to asthma protection conferred by extract tolerization, we compared the protective properties of extracts from wild-type bacteria and from GGT- or VacA-deficient isogenic mutants. Interestingly, both mutant extracts were consistently less efficient than wild-type extract at protecting allergen-sensitized and -challenged mice against bronchoalveolar and pulmonary inflammation, eosinophilia, and goblet cell metaplasia (Fig. 4 A–D). To examine whether either factor alone is sufficient to provide protection, we intraperitoneally administered either recombinant GGT or oligomeric VacA purified from culture supernatants of H. pylori once weekly from day 7 of age onwards. No adverse effects were observed in any of the mice, despite their young age at the time of the first doses. Strikingly, both VacA and GGT provided a level of protection against asthma that was comparable to the protection conferred by parallel whole cell extract treatment (Fig. 4 E–H). VacA was somewhat more protective than GGT at identical concentrations. VacA lacking an amino-terminal hydrophobic region of three tandem GXXXG motifs that is essential for VacA‘s cytotoxic activity (20) failed to protect against asthma (Fig. 4 E–H). Wild-type, but not mutant, VacA had similar effects on pulmonary Th2 cytokine production and DC infiltration (Fig. S4 A–E) as live H. pylori or whole cell extract (Figs. 1 and 3). IL-10R neutralization during ovalbumin challenge abrogated the protective activity of VacA, which was observed not only upon intraperitoneal administration, but also upon oral administration, and in adult as well as neonatally treated mice (Fig. S4 F–I). We conclude that GGT and VacA are key determinants of H. pylori-induced asthma protection and may be administered in purified form to prevent allergic asthma.
Fig. 4.
H. pylori GGT and VacA are required and sufficient for protection against asthma. (A–D) Groups of mice were treated as described in Fig. 1 with H. pylori extract generated from either wild-type (WT) or Δggt or ΔvacA mutant bacteria, and were subjected to ovalbumin sensitization and challenge. (E–H) Mice were i.p. injected weekly with 25 μg per dose of either recombinant GGT or purified wild-type or mutant (Δ6–27) VacA starting on day 7 of age until the second sensitization. Total cells (A and E) and eosinophils (B and F) contained in 1 mL of BALF. Tissue inflammation (C and G) and goblet cell metaplasia (D and H).
Discussion
We have devised here a strategy of active tolerization for the prevention of allergic asthma that exploits the immunomodulatory properties of H. pylori without exposing to the risks associated with live infection. By orally or intraperitoneally administering H. pylori whole cell extract to allergen-sensitized mice, we were able to achieve a level of protection against asthma that was equivalent to the protection conferred by live infection (9). Extract-mediated protection was highly specific to H. pylori, i.e., was not conferred by extract from other Gram-negative enteropathogens such as E. coli or Salmonella typhimurium. The treatment was particularly successful when initiated in young mice, an observation that is in line with the superior protection afforded by experimental (live) infection of neonatal relative to adult mice (9). The differential susceptibility to successful tolerization of neonates and adults may be attributable to the general tolerogenic bias of the immature neonatal immune system, with its higher Treg/Teffector cell ratios and Treg-predominant responses to foreign antigens (21). Our results are in line with the epidemiological finding that children benefit more from harboring H. pylori than adults in terms of their asthma risk (6); similarly, early onset asthma in adolescents and young adults is more strongly inversely correlated with H. pylori seropositivity than adult-onset asthma (5). The data presented here thus imply that children at high risk of developing asthma are more likely than adults to benefit from H. pylori-specific tolerization strategies.
Having shown earlier that H. pylori-specific immune tolerance is a consequence of tolerogenic reprogramming of DCs by the bacteria (10), we set out to examine the contribution of specific DC lineages and their immunomodulators to immune tolerance and asthma protection in the settings of neonatal infection and neonatal-onset tolerization with H. pylori extract. A careful immunophenotypic analysis of the DC subsets infiltrating the lungs of protected mice revealed a preferential recruitment of CD103+CD11b− conventional DCs, and to a lesser extent of B220+ plasmacytoid DCs. The contribution of CD103+ DCs to asthma protection was further functionally assessed in mice lacking the BATF3 transcription factor, which drives the development of CD8α+ lymphoid tissue-resident DC lineages and of the closely related CD103+CD11b− DC lineages in various tissues including the lung, intestine and skin (18). We were able to confirm that CD103+CD11b− DCs are completely absent from the lungs of BATF3−/− mice, whereas all other examined subsets are present in normal numbers. BATF3−/− animals were equally susceptible to allergen-induced asthma as wild-type mice; however, neither regular extract treatment nor H. pylori colonization had any detectable beneficial effect on the examined hallmarks of asthma in this strain, indicating that BATF3-dependent DCs are strictly required for protection in both scenarios. We further found that lymph node-derived DCs from BATF3−/− mice fail to produce IL-10 upon treatment with H. pylori extract ex vivo. This observation is well in line with the requirement for IL-10 signaling proficiency and, more specifically, for the DC-intrinsic production of IL-10 for optimal H. pylori-mediated protection against allergic asthma. We conclude from the combined results that BATF3-dependent DC lineages suppress pulmonary allergen-specific immune responses by production of IL-10; in contrast, Tregs are not critically required for H. pylori extract-mediated protection, as their depletion fails to abrogate protection.
Another cytokine known to be induced by H. pylori, IL-18 (22), also turned out to be absolutely essential for asthma prevention in the course of our studies. IL-18 is produced upon inflammasome activation by H. pylori in a variety of cell types, including DCs, and promotes Treg differentiation and H. pylori-specific tolerance in vitro and in vivo (10, 22). Whether the DC-intrinsic production of IL-18 is required for H. pylori (extract)-mediated protection against asthma remains to be addressed with suitable mouse strains.
Our data further show that two H. pylori determinants, GGT and VacA, are required for extract-mediated protection and can be administered in purified form to prevent allergic asthma. These findings are in line with earlier reports showing that both factors have a critical role in H. pylori persistence and immune modulation. Mutants lacking the ggt gene are incapable of colonizing mice persistently (19, 23), and this phenotype has been attributed to DC tolerization by GGT in vitro and in vivo (19). Similarly, a vacA gene deletion mutant fails to tolerize DCs and to induce Tregs in vivo, and is therefore effectively controlled or even cleared upon onset of an adaptive immune response (19). The fact that a mutant form of VacA lacking an amino-terminal hydrophobic region of three tandem GXXXG motifs fails to protect against asthma when administered to mice in purified form suggests that membrane insertion by VacA is required for its immunomodulatory effects. The exact mechanism and relevant target cell types of VacA in vivo remain to be elucidated in detail. Taken together, our data demonstrate, to our knowledge for the first time, that the immunomodulatory properties of a very common infectious agent in humans, H. pylori, can be exploited for therapeutic purposes in an allergy model and lend support to H. pylori-specific tolerization as a viable strategy for asthma prevention in high-risk individuals.
Materials and Methods
Animal Experimentation.
C57BL/6, BATF3−/−, IL-18R−/−, and CD11c-Cre.IL-10fl/fl mice were orally infected with H. pylori PMSS1 as described (16) or received either once-weekly oral or i.p. doses of 200 µg of extract of H. pylori wild-type PMSS1, PMSS1Δggt or PMSS1ΔvacA (19), Salmonella typhimurium, or E. coli or once-weekly i.p. doses of 25 µg of recombinant GGT, or of s1m1 type VacA (wild type or Δ6–27, ref. 20) purified from H. pylori strain 60190. Mice were sensitized by i.p. injection of 20 µg of ovalbumin (Sigma-Aldrich) emulsified in 2.25 mg of aluminum hydroxide (Alum Imject; Pierce) at 8 and 10 wk of age and challenged with 1% aerosolized ovalbumin using an ultrasonic nebulizer (NE-U17; Omron) for 20 min daily on days 31, 32, and 33 after initial sensitization. Airway resistance measurements were performed on anesthetized, intubated and mechanically ventilated mice (FinePointe Resistance and Compliance System, Buxco Electronics) in response to increasing doses of inhaled metacholine. In vivo blocking of IL-10 signaling and depletion of Tregs was achieved by two i.p. injections of 250 μg of anti-IL-10R antibody (clone 1B1.3A) and anti-CD25 antibody (clone PC-61.5, both BioXCell), respectively, during the challenge phase. CD4+CD25+ Tregs were adoptively transferred as described (9). Lungs were lavaged via the trachea with 1 mL of PBS. Broncho-alveolar lavage fluid (BALF) cells were counted using trypan blue dye exclusion. Differential cell counts of macrophages, lymphocytes, neutrophils, and eosinophils were performed on cytocentrifuged preparations stained with the Microscopy Hemacolor-Set (Merck). For lung histopathology, lungs were fixed by inflation and immersion in 10% (vol/vol) formalin and embedded in paraffin. Tissue sections were stained with H&E and periodic acid-Schiff and examined in blinded fashion on a BX40 Olympus microscope. Peribronchial inflammation was scored on a scale from 0 to 4. PAS-positive goblet cells were quantified per 1 mm of basement membrane. All animal experimentation was performed in accordance with federal, cantonal and institutional guidelines, and approved by the Zurich Cantonal Veterinary Authorities (no. 170/2009 to A.M.).
Preparation of H. pylori Extract and Purification of GGT and VacA.
H. pylori was cultured in Brucella broth supplemented with 10% FCS, pelleted by centrifugation, and washed once with PBS. Bacteria were subjected to three freeze/thaw cycles and disrupted by three passes through a French pressure cell press (Stansted Fluid Power, Cell Pressure Homogenizer) at 30,000 bar. Cell debris was removed by centrifugation and the supernatant filtered through a 2-μm filter. Protein concentrations were determined by BCA Protein Kit (R&D Systems). H. pylori VacA was purified using published procedures (24, 25), with the following slight modifications. H. pylori strain 60190 was cultured in sulfite-free Brucella broth containing either cholesterol or 0.5% charcoal. After centrifugation of the culture, supernatant proteins were precipitated with a 50% saturated solution of ammonium sulfate. The oligomeric form of VacA was isolated by gel filtration chromatography with a Superose 6 HR 16/50 column in PBS containing 0.02% sodium azide and 1 mM EDTA. H. pylori GGT was purified as described (19). Protocols for lung single cell preparation, flow cytometry, cytokine ELISAs, and preparation of murine and human DCs and DC/T-cell cocultures can be found in SI Materials and Methods.
Statistics.
All statistical analysis was performed using Graph Pad prism 5.0 software. The Mann–Whitney test was used throughout. P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Hermelijn Smits and Maria Yazdanbakhsh (Leiden University Medical Center) for their help with culturing human DCs. This study was funded by Swiss National Science Foundation Project Grant 310030_143608/1 (to A.M.), the National Institutes of Health Grants AI039657 and CA116087 (to T.L.C.), and Department of Veterans Affairs (T.L.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410579111/-/DCSupplemental.
References
- 1.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7(12):887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amberbir A, et al. Effects of Helicobacter pylori, geohelminth infection and selected commensal bacteria on the risk of allergic disease and sensitization in 3-year-old Ethiopian children. Clin Exp Allergy. 2011;41(10):1422–1430. doi: 10.1111/j.1365-2222.2011.03831.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167(8):821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198(4):553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reibman J, et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS ONE. 2008;3(12):e4060. doi: 10.1371/journal.pone.0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbarth O, et al. Helicobacter pylori colonisation and eczema. J Epidemiol Community Health. 2007;61(7):638–640. doi: 10.1136/jech.2006.046706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold IC, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121(8):3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oertli M, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122(3):1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salama NR, Hartung ML, Müller A. Life in the human stomach: Persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11(6):385–399. doi: 10.1038/nrmicro3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 13.Parsonnet J, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 14.Rad R, et al. Extracellular and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology. 2009;136(7):2247–2257. doi: 10.1053/j.gastro.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 15.Sayi A, et al. TLR-2-activated B cells suppress Helicobacter-induced preneoplastic gastric immunopathology by inducing T regulatory-1 cells. J Immunol. 2011;186(2):878–890. doi: 10.4049/jimmunol.1002269. [DOI] [PubMed] [Google Scholar]
- 16.Arnold IC, et al. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140(1):199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ismail HF, Fick P, Zhang J, Lynch RG, Berg DJ. Depletion of neutrophils in IL-10(-/-) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J Immunol. 2003;170(7):3782–3789. doi: 10.4049/jimmunol.170.7.3782. [DOI] [PubMed] [Google Scholar]
- 18.Edelson BT, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207(4):823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oertli M, et al. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci USA. 2013;110(8):3047–3052. doi: 10.1073/pnas.1211248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinion-Dubiel AD, et al. A dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J Biol Chem. 1999;274(53):37736–37742. doi: 10.1074/jbc.274.53.37736. [DOI] [PubMed] [Google Scholar]
- 21.Arnold B, Schüler T, Hämmerling GJ. Control of peripheral T-lymphocyte tolerance in neonates and adults. Trends Immunol. 2005;26(8):406–411. doi: 10.1016/j.it.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Hitzler I, et al. Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1β and IL-18. J Immunol. 2012;188(8):3594–3602. doi: 10.4049/jimmunol.1103212. [DOI] [PubMed] [Google Scholar]
- 23.Chevalier C, Thiberge JM, Ferrero RL, Labigne A. Essential role of Helicobacter pylori gamma-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Mol Microbiol. 1999;31(5):1359–1372. doi: 10.1046/j.1365-2958.1999.01271.x. [DOI] [PubMed] [Google Scholar]
- 24.Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267(15):10570–10575. [PubMed] [Google Scholar]
- 25.Cover TL, Hanson PI, Heuser JE. Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating cytotoxin, reveals its pattern of assembly. J Cell Biol. 1997;138(4):759–769. doi: 10.1083/jcb.138.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.