Significance
Adenoviruses cause acute respiratory, ocular, and enteric diseases, with significant health concerns for immunocompromised individuals. Replication-deficient adenoviruses are among the most frequently used vectors for human gene therapy. However, the structural details of these large (150-MDa) and complex viral vectors remain obscure. In this study, we determined the crystal structures of all cement proteins in the context of the entire virion and, in the process, revised the existing cement protein structures and their locations. Significantly, our results revealed the structure of protein VI, for the first time to our knowledge, with its cleaved propeptide sequestered within peripentonal hexons. This permits untethering and release of the membrane-lytic segment, thereby providing the molecular basis for maturation cleavage of protein VI in adenovirus-mediated endosome disruption.
Keywords: human adenovirus, cement protein scaffold, structure–function relationships
Abstract
Adenovirus cement proteins play crucial roles in virion assembly, disassembly, cell entry, and infection. Based on a refined crystal structure of the adenovirus virion at 3.8-Å resolution, we have determined the structures of all of the cement proteins (IIIa, VI, VIII, and IX) and their organization in two distinct layers. We have significantly revised the recent cryoelectron microscopy models for proteins IIIa and IX and show that both are located on the capsid exterior. Together, the cement proteins exclusively stabilize the hexon shell, thus rendering penton vertices the weakest links of the adenovirus capsid. We describe, for the first time to our knowledge, the structure of protein VI, a key membrane-lytic molecule, and unveil its associations with VIII and core protein V, which together glue peripentonal hexons beneath the vertex region and connect them to the rest of the capsid on the interior. Following virion maturation, the cleaved N-terminal propeptide of VI is observed, reaching deep into the peripentonal hexon cavity, detached from the membrane-lytic domain, so that the latter can be released. Our results thus provide the molecular basis for the requirement of maturation cleavage of protein VI. This process is essential for untethering and release of the membrane-lytic region, which is known to mediate endosome rupture and delivery of partially disassembled virions into the host cell cytoplasm.
Human adenoviruses (HAdVs) are large (∼150 nm in diameter, 150-MDa) nonenveloped double-stranded DNA (dsDNA) viruses that cause respiratory, ocular, and enteric diseases (1). Although these diseases are self-limiting in immunocompetent individuals, they cause significant morbidity in AIDS, cancer, and organ transplant patients with compromised immune systems (2–4). Because of their broad cell tropism and ease of genome manipulation, replication-deficient or conditionally replicating HAdVs are also being evaluated in the clinic as potential vaccine and gene therapy vectors (5).
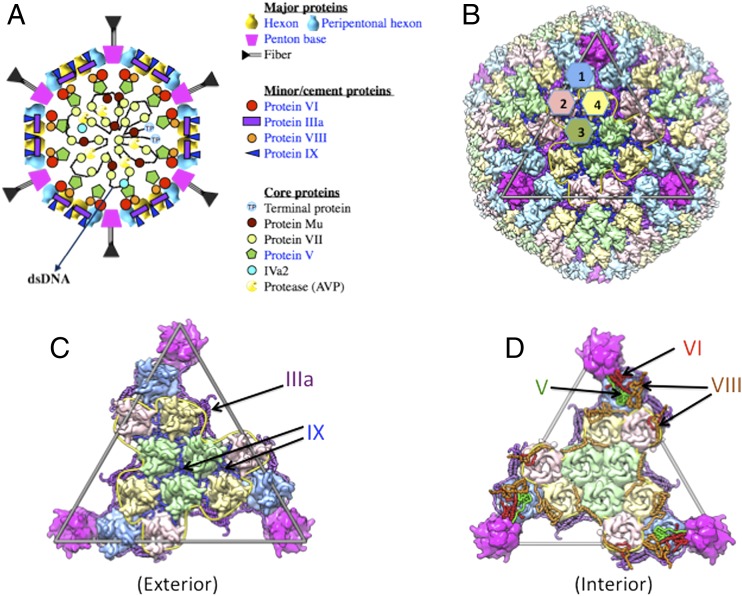
The capsid shell of an adenovirus (Ad) comprises multiple copies of three major capsid proteins (MCPs; hexon, penton base, and fiber) and four minor/cement proteins (IIIa, VI, VIII, and IX) that are organized with pseudo-T = 25 icosahedral symmetry (Fig. 1 A and B). In addition, six other proteins (V, VII, μ, IVa2, terminal protein, and adenovirus protease) are encapsidated along with the 36-kb dsDNA genome inside the capsid (Fig. 1A). The crystal structures of all three MCPs are known, and so is their organization in the capsid from prior X-ray crystallography (6–8) and cryoelectron microscopy (cryo-EM) analyses (9, 10). Recently, high-resolution structures of recombinant HAdV5 vectors have been determined using cryo-EM (11) and X-ray methods (12) that revealed the structures and organization of some of the cement proteins. Both studies agree closely on the organization of the MCPs and confirm the earlier cryo-EM observations (9, 10, 13), but neither provided significant information on the structure and location of protein VI, which serves key roles in the virus life cycle. Of note, however, the two studies differ significantly in their assignments of the cement proteins IIIa and IX. Recent cryo-EM studies reported that only protein IX molecules form “triskelion” as well as “four-helix bundle” (4-HLXB) structures and mediate the network of interactions between hexon subunits on the capsid exterior (11, 14, 15). They also suggested that the densities ascribed to α-helices beneath the vertex region belong to protein IIIa. However, based on our X-ray crystallographic data and considering the principles of quasi-equivalence (16), we earlier suggested that although the IX molecules form triskelion structures, it is rather unlikely that the C termini of IX would form 4-HLXB structures (12). Instead, we proposed that this 4-HLXB is most likely derived from a subdomain of IIIa (12).
Fig. 1.
Structure and organization of human adenovirus. (A) A schematic illustration of the organization of capsid and core proteins in human adenovirus. The locations of various proteins are represented by different-colored symbols and the corresponding names are shown (Right). The indicated locations of the core proteins are approximate. Shown in blue-colored lettering are the proteins whose structures have been identified in this study. (B) Overall organization of hexon and penton base subunits exhibiting pseudo-T = 25 icosahedral symmetry. Structurally unique hexons (1–4) are color-coded in light blue, pink, green, and khaki, respectively. Penton vertices are shown in magenta. Outer cement proteins IIIa and IX are shown in purple and blue, respectively. Fiber molecules associated with the penton base are disordered. The outline of the triangular icosahedral facet is shown as a gray triangle, whereas the border of the GON hexons is indicated by yellow-colored rope. (C) An exterior view of the triangular icosahedral facet that comprises 12 hexons along with penton base vertices shown in magenta. Color representations are the same as in B. (D) An interior view of the facet in C, with three minor proteins, V (green), VI (red), and VIII (orange). It is noteworthy that a copy of V, VI, and VIII forms a ternary complex beneath the vertices, whereas VIII (orange) molecules are arranged as staples along the border (yellow-colored rope) of the GON hexons.
Here we report a revised interpretation, a paradigm shift, of the structures and locations of all of the cement proteins based on the refined crystal structure of Ad5F35 (HAdV5 vector encoding the type 35 fiber) that includes detailed models for the ordered regions of all four cement proteins (IIIa, VI, VIII, and IX). Additionally, we identified a segment of core protein V, which associates closely with protein VI. The 4-HLXB structure on the capsid exterior is a subdomain of IIIa (amino acids 101–355) that mediates interactions between group-of-nine (GON) (17) hexons (Fig. 1 C and D). The backbone path of each IX molecule is reversed from what was assigned by the cryo-EM studies (11, 14). Proteins V, VI, and VIII form a ternary complex that stabilizes the adjacent peripentonal hexons (PPHs) underneath each of the 12 vertex regions (Fig. 1D). This complex was incorrectly assigned to protein IIIa in cryo-EM studies (11, 14). Following virion maturation, the cleaved propeptide(s) of VI (pVIn; amino acids 1–33) is observed in the inner cavities of the PPHs, in agreement with recent interpretations from hydrogen–deuterium exchange mass spectrometry studies (18).
Results
Protein IIIa.
We resolved three segments of IIIa (amino acids 9–25, 48–209, and 252–355) with good certainty, aided by the use of a recently devised method for evaluating and scoring assigned sequences to features of experimental density. Unweighted best match scores (UWBMSs; 0.82 and 0.80), weighted best match scores (WBMSs; 0.64 and 0.60), and corresponding reliability indices (RIs; 3.2 and 2.1) are high for two large segments (amino acids 48–209 and 252–355) (SI Appendix, Table S4). UWBMS/WBMS values represent the fraction of residues in the polypeptide for which the electron density matches the size of the side chain. RI values indicate the significance of the best WBMS values relative to the second-best WBMS values. RI values greater than 1 (RI >> 1) indicate a higher confidence in the assigned sequences, whereas RI = 1 indicates the lack of specificity to the sequence assignment for the peptide. Details of calculating BMSs and RIs are described in SI Appendix, Materials and Methods. Even though UWBMS and WBMS values are high (0.88 and 0.76) for the short peptide (amino acids 9–25), which was built into an island of density, the corresponding RI value (1.2) is marginal.
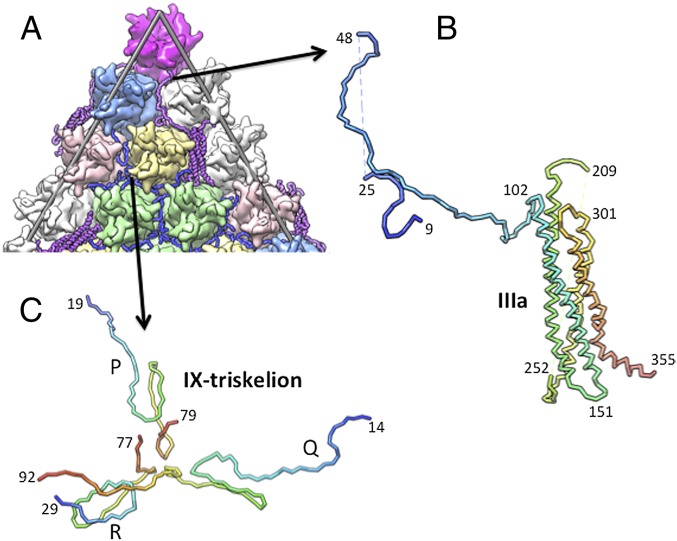
This resolved domain of IIIa mediates interactions between the hexons, including PPHs, along the group-of-twelve (GOT) hexon–GOT interfaces on the capsid exterior (Fig. 2 A and B). The characteristic signature of the IIIa structure is an antiparallel, four-helix bundle at its core and a long extended polypeptide formed by N-terminal residues (Fig. 2B and SI Appendix, Fig. S1B). The helical bundle comprises two long helix-turn-helix segments (amino acids 103–200 and 262–342) with short turns at residues 147–156 and 301–309, whereas a longer connection (amino acids 210–253) is disordered. Interestingly, the visible N-terminal segment of IIIa (amino acids 9–25 and 48–102) adopts an extended structure and mediates interactions primarily between a pair of PPHs and reaches all of the way to the penton vertices (Fig. 2A and SI Appendix, Fig. S2A). The remaining C-terminal residues (356–585) are disordered, and we surmise that they likely remain on the capsid exterior at the GON–GON interfaces near the twofold axis. In spanning ∼168 Å from the penton vertices to near the twofold axis, the ordered region of IIIa appears to function like the tape measure protein (P30) in PRD1 (19), but on the capsid exterior. The location and interactions of IIIa concur with early observations that it is surface-accessible and located near the PPHs and penton base (20, 21). Furthermore, the location of the N terminus of IIIa near the PPH and penton base also agrees with recent studies involving N-terminal labeling of IIIa (22), even though it was suggested that protein IIIa resides inside the capsid.
Fig. 2.
Structure and location of outer cement proteins. (A) A zoomed-in view of the (partial) icosahedral facet. (B) A tube representation showing the fold of protein IIIa. Rainbow coloring highlights the flow of the polypeptide chain from the N terminus (blue) to the C terminus (red). Dashed lines represent disordered residues. Selected residues are labeled. (C) Structure of one of the protein IX triskelions. Rainbow coloring blue to red shows the trail of the polypeptide chain from the N terminus to the C terminus for each of the three individual IX molecules P, Q, and R.
Association of the four-helix bundle with neighboring hexons is facilitated mostly by hydrogen bond and charge–charge interactions (SI Appendix, Table S1). Roughly two-thirds of the interactions involving the ordered regions of IIIa occur with hexon-2 and a PPH from the reference GOT/facet, and the remainder are with hexon-4′ and hexon-3′ from adjacent facets (SI Appendix, Fig. S2A). The four-helix bundle is oriented ∼35° relative to the base of the hexons, tilted down near the twofold axis and up toward the PPHs (SI Appendix, Fig. S2B). Two IIIa molecules (strict twofold-related), one from each facet, stabilize the unique edge interface between a pair of facets. A total of six IIIa molecules surround the border of each facet along three edges on the exterior (Fig. 1C and SI Appendix, Fig. S1A), three of which uniquely belong to each facet, one in each icosahedral asymmetric unit, resulting in 60 IIIa molecules that stabilize the entire virion.
The structure and location of IIIa differ from recent cryo-EM studies (11, 14), where the 4-HLXB domain was ascribed to the C termini from four molecules of protein IX. Comparison of the outer cement protein (IX) models from the cryo-EM study (11) [Protein Data Bank (PDB) ID code 3IYN] with that of the IIIa and IX triskelion structures here highlights the differences (SI Appendix, Fig. S3). Helices in the 4-HLXB from the X-ray structure are consistently longer than the corresponding helices seen in the deposited cryo-EM model (PDB ID code 3IYN) (11) (SI Appendix, Figs. S18 and S20). However, to our surprise, the density from the cryo-EM reconstruction [Electron Microscopy Data Bank (EMDB) ID code emd_5172] is consistent with the longer helices seen in IIIa (SI Appendix, Fig. S20), even though the connections between the helices are not clear. Furthermore, the cryo-EM models for protein IIIa (amino acids 7–300) were incorrectly assigned to underneath the vertex region (11) that actually belongs to a ternary complex of proteins VI and VIII and a fragment of core protein V (see below) (SI Appendix, Fig. S4).
Protein IX.
Protein IX is the most flexible molecule among the cement proteins, displaying a primarily extended structure (Fig. 2C and SI Appendix, Figs. S5–S7), perhaps dictated by the interacting hexon subunits. We traced four structurally independent molecules of IX, but at varying lengths: IX-P (amino acids 19–75), IX-Q (amino acids 12–92), IX-R (amino acids 29–77), and IX-S (amino acids 21–78) (SI Appendix, Fig. S5). The UWBMS, WBMS, and RI scores for the longest of the IX molecules (IX-Q) are 0.95, 0.9, and 3.5, respectively, which are the best match/confidence scores attained for any of the cement proteins in this study (SI Appendix, Table S4). The characteristic feature of protein IX is that it forms triskelion structure(s) consisting of three IX molecules. Two types of triskelions, termed IX-Q3 and IX-I3, occur in the Ad capsid shell. IX-Q3 is formed by three molecules of IX (IX-P, IX-Q, and IX-R) that are related by quasi (local)-threefold symmetry and primarily stabilize hexons 2, 3, and 4. Furthermore, the N terminus of IX-P clasps the nearest PPH (SI Appendix, Fig. S8). The second (IX-I3) is formed by three IX-S molecules that are related by icosahedral (strict) threefold symmetry and stabilize three hexon-3 trimers at the center of the icosahedral facet (SI Appendix, Fig. S5). Therefore, four triskelions (three IX-Q3 and one IX-I3) stabilize the hexons within a GON as well as latch onto PPHs within the facet (Fig. 1C). Hence, the Ad capsid contains 80 triskelions (240 IX molecules), 60 of which are quasi-triskelions (IX-Q3) with the remaining 20 strict triskelions (IX-I3).
Importantly, the direction of the IX polypeptide is reversed compared with previous cryo-EM reports (11, 14). Our sequence assignments agree well after reversing the chain direction, with the sole tryptophan residue (W22) in the sequence along with Y49, F79, and F81 that can be clearly identified in the difference and omit maps (Fig. 3B and SI Appendix, Fig. S6). In fact, 95% of the assigned residues matched the features of the experimental density (SI Appendix, Table S4). Also, a stretch of Ala and Ser residues occurring between amino acids 60 and 70 forms the center/core of the triskelion structure and also mediates interactions between the β-barrels of the hexons. Significantly, the revised polypeptide direction allows the C termini of all IX molecules to be surface-exposed, in agreement with previous biochemical and immunoelectron microscopy experiments (20, 21, 23). This change in chain direction also allows the organization of IX molecules in HAdVs to be consistent with the arrangement of trimeric C-terminal spikes (helical bundles) seen in nonhuman adenoviruses (24, 25), even though the corresponding trimeric spikes are disordered in human serotypes.
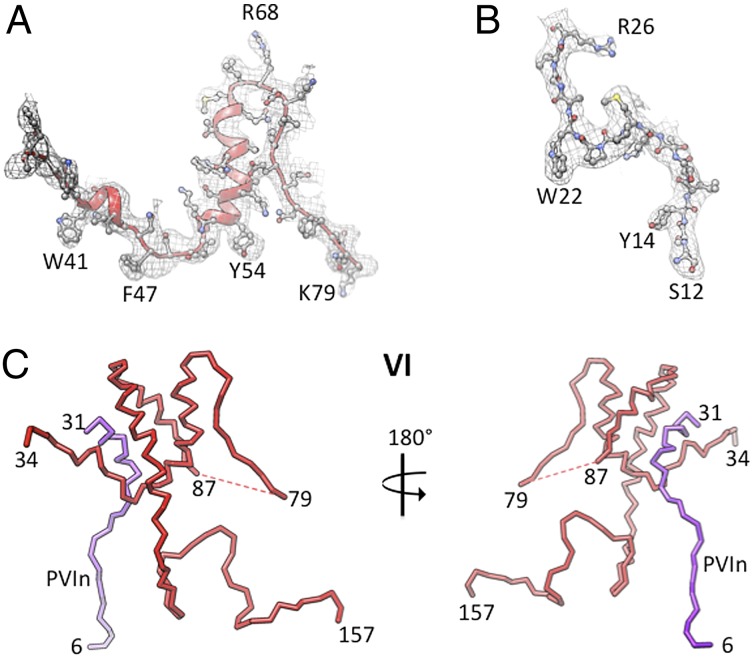
Fig. 3.
Representative electron densities and the structure of protein VI. (A) NCS-averaged 3Fo − 2Fc electron density map (contoured at 1σ) corresponding to the membrane-lytic peptide of VI. The equivalent Fo − Fc map, calculated by omitting protein VI, is shown in SI Appendix, Fig. S14. (B) Similar NCS-averaged electron density for the N-terminal part of the IX-Q molecule. (C) Stick diagram showing the fold of protein VI. The cleaved propeptide (pVIn) (amino acids 6–31) is shown in purple color.
Protein VI.
Here we report to our knowledge the first structure of protein VI, which plays multiple roles in the adenovirus life cycle—it functions as a cofactor for the adenovirus protease (AVP) and as a chaperone for nuclear transport, and is essential for virus assembly and endosome lysis (26–30). One copy of protein VI is found intimately associated with the base of each PPH on the capsid interior. We were able to resolve residues 6–31, 34–79, and 87–157 of protein VI, which interact primarily with the bases of PPHs; the remaining C-terminal residues (158–239) were disordered. High BMS values and reliability indices of 2.4, 1.9, and 1.4, respectively, for these peptides indicate greater confidence in the sequence assignments (SI Appendix, Table S4). A representative [noncrystallographic symmetry (NCS)-averaged] electron density for the membrane-lytic region of VI is shown in Fig. 3A. Protein VI has a helical core and unstructured N and C termini (Fig. 3C). The fold of protein VI appears to be distinct and has no known structural homologs. The presence of a helical core is in agreement with observations from modeling and CD spectroscopy studies (29, 31). After proteolysis by the AVP, the ends of the newly formed fragments (6–31 and 34–157) are separated by ∼24 Å, perhaps due to rearrangement following the cleavage (Fig. 3C and SI Appendix, Fig. S9A).
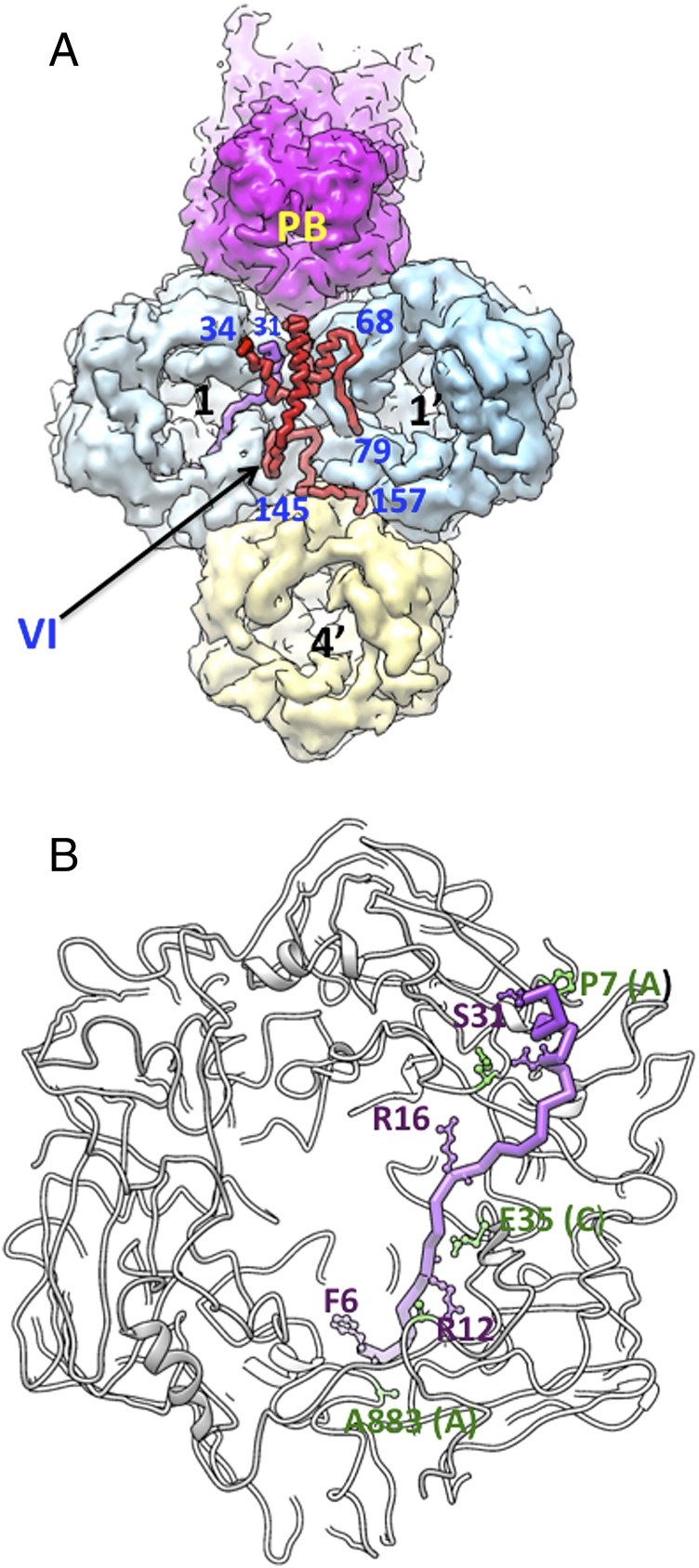
It appears that the majority of residues (amino acids 1–21) in the cleaved 33-residue N-terminal propeptide are buried in the cavities of the PPHs and that the remaining ordered residues (amino acids 22–31) interact with the N terminus (amino acids 5–17) of the A subunit (Fig. 4 A and B and SI Appendix, Fig. S10 and Table S2 detail the interactions between VI and the PPHs). The propeptide interactions with the base of the hexons concur with recent hydrogen–deuterium exchange mass spectrometry studies (18) as well as with affinity measurements between VI and the hexon (32). Moreover, although it was not reported by Liu et al. (11), electron density attributable to pVIn can be seen associated with PPHs in their high-resolution cryo-EM map deposited in the EMDB (ID code emd_5172) (SI Appendix, Fig. S20). Owing to steric constraints, it is unlikely that more than one copy of VI would bind to each hexon trimer in the assembled virion. In addition, hexon-4 might not accommodate even one VI molecule without causing severe steric clashes with icosahedral symmetry-related molecules.
Fig. 4.
Interactions between protein VI and the PPHs. (A) A hybrid (surface and tube) representation illustrating associations mediated by one copy of VI (red tube) gluing the adjacent PPHs (1,1′) and connecting them to hexon-4′ arising from the neighboring GON tile. The cleaved propeptide of VI (purple tube) remains associated with PPH-1 inside the hexon cavity. Certain residues of VI are identified with blue-colored labels. The penton base (PB) is shown in magenta. (B) A close-up view of the propeptide (purple tube) interactions with PPH-1, shown as a gray ribbon. A few residues that are involved in propeptide (purple) and hexon (green) interactions are labeled. Names of hexon subunits (A and C) are shown in parentheses.
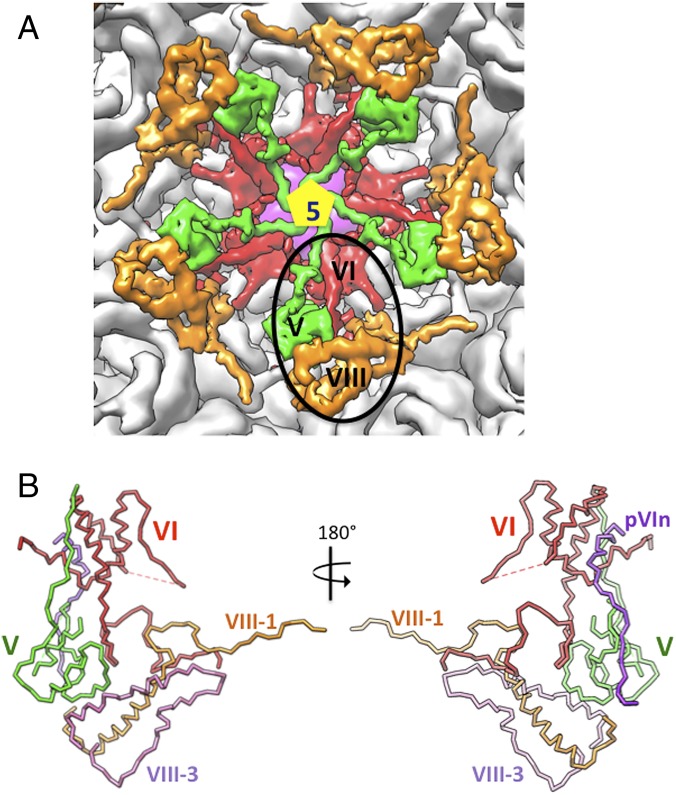
Of note, most of the residues (34–50) in the membrane-lytic region (amino acids 34–54) of VI that were predicted to form an amphipathic helix (29) do not conform to the helical structure in the context of the virion (Fig. 3C and SI Appendix, Fig. S9A). However, like other amphipathic helices (33), they may in fact adopt a helical conformation after they come in contact with the endosomal membrane, following their release from the partially disassembled virion(s). Also, a key leucine (L40) that has been shown to impact endosome rupture (31) may influence the above helix formation (SI Appendix, Fig. S9A). In addition to its role in cell entry, the ordered portion of the mature VI molecule (amino acids 34–157) stabilizes the capsid by gluing two PPHs together and joining them with hexon-4 of an adjacent GON hexon (Fig. 4A). The residues following the membrane-lytic region (amino acids 68–79) closely interact with the neighboring PPH (Fig. 4A). The first helix of the helix-turn-helix (amino acids 87–101) wedges in between two PPHs near the vertex region, thus acting as “molecular glue.” In addition, residues 139–145 of the unstructured region mediate interactions at the opposite end of this PPH–PPH interface. The C-terminal residues (amino acids 145–157) join the PPHs to the adjacent GON (Fig. 4A). A set of five such glue-like interactions joins five PPHs surrounding the penton vertices to the five GON tiles (Fig. 5A). Notably, one VI molecule together with one V and one VIII-A form a close ternary complex (Fig. 5B) that bolsters interactions between the PPHs and the neighboring GONs.
Fig. 5.
Organization of cement proteins underneath the vertex region. (A) Five copies of the ternary complex formed by proteins V (green), VI (red), and VIII (orange) glue the peripentonal hexons (gray) to each other and connect them to the adjacent GONs. One of the copies is highlighted by a black oval. (B) A stick diagram showing the structure of the ternary complex. Color assignments of the proteins are as in A, with the exception that the propeptide of VI (pVIn) is shown in purple and the C-terminal fragment of VIII (VIII-3) is shown in orchid, whereas the N-terminal fragment of VIII (VIII-1) is shown in orange.
Residues 135–157 of a second molecule of VI-B, along with another molecule of VIII-B, associate with hexon-2, whereas the rest of VI-B, including the N-terminal propeptide, is disordered (SI Appendix, Fig. S11B). This binary complex between VI-B and VIII-B mediates interactions between the same adjacent GON (facet) that are stabilized by the above (V–VI–VIII) ternary complex (SI Appendix, Fig. S11A). This accounts for 120 of ∼350 molecules of VI believed to be present in the adenovirus virion based on biochemical (34) and mass spectrometry proteomics studies (35). However, another 60 molecules of VI could sterically be accommodated associating with 60 copies of hexon-3 (i.e., one VI per hexon), thereby increasing the number of VI molecules interacting with the hexons to 180. The remaining (∼170) molecules of protein VI, if indeed present, might be associated with the nucleoprotein core of HAdV.
Protein VIII.
Based on biochemical and structural studies it was shown that two structurally distinct copies of protein VIII (designated A and B) interact with the bases of the hexons on the capsid interior (13, 14, 20, 21). Protein VIII is processed by AVP at two locations (amino acids 111 and 157), resulting in three fragments (amino acids 1–111, 112–157, and 158–227). We could trace residues 33–90 of fragment 1; 163–215 in fragment 3 in VIII-A; and residues 31–86 of fragment 1 in VIII-B (SI Appendix, Figs. S9C and S11B). Reliability indices for fragments 1 and 3 of VIII-A are 1.9 and 1.5, respectively, and along with high UWBMS values indicate that ∼90% of assigned residues match the experimental density (SI Appendix, Table S4). No density was observed corresponding to fragment 2 in either VIII-A or VIII-B, suggesting that it might have been released from the virion after the processing by AVP. The structure of fragment 1 mostly agrees with that seen in the high-resolution cryo-EM reconstruction (11), but the fragment 3 structure differs completely from that reported by cryo-EM (SI Appendix, Fig. S12).
As described above, VIII-A forms a ternary complex with proteins VI and V that interacts with PPHs, whereas a second molecule of VIII (VIII-B) forms a binary complex with a short fragment of VI-B that closely associates with hexon-2 in the reference GON. Both unique copies of VIII (A and B) line the border of the GONs and mediate interactions between hexons from adjacent GONs and PPHs (Fig. 1D and SI Appendix, Fig. S11). A total of 120 copies of VIII glue the PPHs and GONs together on the interior of the Ad capsid.
Protein V.
Even though protein V is considered a core protein and along with protein VII is known to interact with the Ad genome, it also interacts with protein VI based on cross-linking experiments (21, 36). We could trace 72 (amino acids 208–219 and 236–295) of the 368 residues of protein V (SI Appendix, Fig. S9B). The ordered region of V includes two short helices (amino acids 208–219 and 259–271), with the rest forming an extended structure. Even though the BMS values (0.9 and 0.85) are high, the reliability indices (1.0 and 1.3) are marginal for the respective peptides, particularly for the short segment of 12 residues (amino acids 208–219) (SI Appendix, Table S4). This short peptide, which forms a helix, was built into an island of density found close to the larger segment (amino acids 236–295). Significantly, 85% of sequence assignments for the larger segment of 60 residues agree well with the protein V sequence, which is more than any other cement protein sequence on the capsid interior. The visible C terminus of V (amino acids 289–295) interacts with VI (amino acids 103–115) as the former reaches underneath the vertex region (Fig. 5A). Based on this organization, the disordered and highly basic N-terminal part of V likely interacts with the genome, which is also disordered in the HAdV crystal structure. The structurally ordered region of protein V lies beneath protein VI (closer to the center of the particle) and does not directly interact with the hexon subunits but likely mediates interactions between the (disordered) nucleoprotein core and the capsid shell through its interactions with protein VI. Protein V also interacts with the C-terminal fragment 3 of VIII-A in forming the V–VI–VIII ternary complex that stabilizes the PPHs and links them with adjacent GONs (Fig. 5 and SI Appendix, Fig. S11A).
Discussion
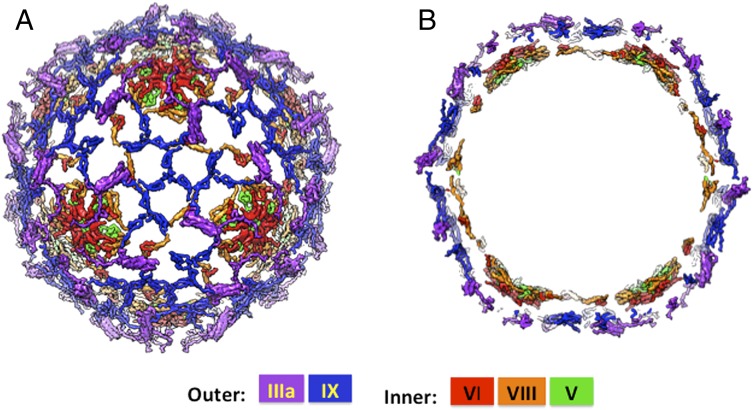
The refined crystallographic model of HAdV, one of the largest biomolecules determined by X-ray crystallography, reveals the structures and organization of all of the cement proteins and the partially ordered core protein V, which together stabilize the Ad capsid shell. The cement proteins are organized in two distinct layers (Fig. 6), which exclusively stabilize the hexon shell including PPHs, thus rendering the penton vertices the weakest links of the HAdV capsid and enabling their release into the low-pH endosome. The outer layer is formed by proteins IX and IIIa, which mediate interactions within and between GON/GOT hexons, respectively. Notably, the N terminus of IIIa tethers the PPHs, connecting them with GONs on the capsid exterior. These interactions are consistent with experimental observations that protein IIIa is surface-accessible and is released along with the peripentonal hexons (21). In addition, reversal of the chain direction of protein IX molecules allows the surface exposure of their C termini, in agreement with immunoelectron microscopy results (23). Moreover, protein IX triskelion structures in all likelihood are enforced by the adjoining hexon subunits, as there are hardly any interactions between individual IX molecules. Together, IIIa and IX form a nearly contiguous hexagonal lattice (SI Appendix, Fig. S13A), which surrounds the hexons within each GON. The inner cement protein layer (SI Appendix, Fig. S13B), although not as contiguous as the outer layer, is formed by proteins V, VI, and VIII, where VI and VIII closely associate with the bases of the hexons by stapling them along the GON–PPH and GON–GON interfaces. However, the ordered segments of protein V seen in the crystal structure do not directly contact the hexon shell. The locations of the “hot spots” of cement protein interactions that surround the hexon subunits are complementary in the upper and lower layers. Interestingly, some of the distinct features observed in the X-ray structure compared with the deposited cryo-EM model (PDB ID code 3IYN) (e.g., longer helices in IIIa and the sequestered pVIn peptide inside the PPH) are supported by the cryo-EM density (SI Appendix, Fig. S20), but were not previously considered (11).
Fig. 6.
Organization of cement proteins in human adenovirus. (A) Organization of the five different minor/cement proteins IIIa (purple), IX (blue), V (green), VI (red), and VIII (orange) that are present in multiple copies. Color assignments of inner and outer cement proteins are indicated (Lower). (B) Cross-section showing the double-layered organization of the cement proteins. The outer layer is composed of proteins IIIa and IX and the inner layer is formed by proteins V, VI, and VIII. The encapsidated DNA is not ordered in the averaged electron density maps, computed from diffraction data in the resolution range 20–3.8 Å.
Most importantly, the structural models for VI and V provide insights into the need for proteolytic processing of VI so it can be released, in turn leading to partial exposure of the DNA. Proteolytic processing of VI is necessary for untethering and release of the membrane-lytic region, which is implicated in endosome lysis (29, 30). Consistent with this cleavage requirement, abrogation of such processing of VI by the viral protease, as in the case of Ad-ts1 mutant viruses, makes them noninfectious (37). Furthermore, the close apposition of five copies of protein VI near the icosahedral vertices allows cross-linking of lysine residues K45 and K70 from neighboring molecules (∼4.5 Å apart), and the close proximity of proteins V and VI would allow the cross-linking between V and the dimer of VI (Fig. 5 and SI Appendix, Fig. S15) (21, 36). The release of the mature VI molecules would occur concomitant with the loss of peripentonal hexons, followed by the release of partially disassembled virions into the cytoplasm.
Materials and Methods
A form of recombinant human adenovirus serotype 5 vector displaying a short and flexible fiber from HAdV serotype 35, termed Ad5F35 (also known as Ad35F), was purified and crystallized as described previously (38). Diffraction data were collected at beamline 23-ID-D, General Medical Sciences and Cancer Institute's structural biology facility (GM/CA), Advanced Photon Source. The crystal structure was determined using molecular replacement methods using Phaser (39). Notably, high-resolution features of the electron density maps were significantly improved by omitting data at resolutions lower than 20 Å, which is akin to B-factor sharpening of electron density maps. All of the initial cement protein backbone traces were built into difference (Fo − Fc) and/or omit (5Fo − 4Fc) maps. Only polyalanine traces of the cement proteins were used to calculate electron density maps, to avoid any model bias. We devised a method for evaluating the reliability of assigned amino acid sequences to the experimental electron density (see SI Appendix, Materials and Methods for details). The reliability scores for most of the sequence assignments of the cement proteins are high. The X-ray model was refined using the program X-PLOR (40). Further details of experimental procedures are described in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to Drs. Jack Johnson, Ian Wilson, and Timothy Baker for their comments on the manuscript, as well as to Dr. Crystal Moyer for careful reading of it; to Tina-Marie Mullen for her assistance with vector production; and to Drs. Kundhavai Natchiar and Nhung Huynh for their support with data collection. We acknowledge the cryo-EM maps and models received from Drs. Phoebe Stewart and Guy Schoehn. We also thank Dr. Bob Fischetti and colleagues at the GM/CA beamline at the Advanced Photon Source (APS), Chicago, for technical support with synchrotron data collection. We also thank Dr. Tom Goddard for his expert advice on using special features of the Chimera program. We acknowledge support from National Institutes of Health (NIH) Grants AI070771 and AI103692 (to V.S.R.) and HL054352 (to G.R.N.). GM/CA has been funded with federal funds from the NIH (Y1-CO-1020, Y1-GM-11040), and the APS is supported by the US Department of Energy under Contract DE-AC02-06CH11357.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The coordinates and structure factors of the refined Ad5F35 structure reported in this paper have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4CWU).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408462111/-/DCSupplemental.
References
- 1.Wold WSM, Horwitz MS. Adenoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5th Ed. Vol 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2395–2436. [Google Scholar]
- 2.Hierholzer JC. Adenoviruses in the immunocompromised host. Clin Microbiol Rev. 1992;5(3):262–274. doi: 10.1128/cmr.5.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman JA. Adenoviral disease in pediatric solid organ transplant recipients. Pediatr Transplant. 2006;10(1):17–25. doi: 10.1111/j.1399-3046.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 4.Lenaerts L, De Clercq E, Naesens L. Clinical features and treatment of adenovirus infections. Rev Med Virol. 2008;18(6):357–374. doi: 10.1002/rmv.589. [DOI] [PubMed] [Google Scholar]
- 5.Campos SK, Barry MA. Current advances and future challenges in adenoviral vector biology and targeting. Curr Gene Ther. 2007;7(3):189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rux JJ, Burnett RM. Type-specific epitope locations revealed by X-ray crystallographic study of adenovirus type 5 hexon. Mol Ther. 2000;1(1):18–30. doi: 10.1006/mthe.1999.0001. [DOI] [PubMed] [Google Scholar]
- 7.Zubieta C, Schoehn G, Chroboczek J, Cusack S. The structure of the human adenovirus 2 penton. Mol Cell. 2005;17(1):121–135. doi: 10.1016/j.molcel.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 8.van Raaij MJ, Mitraki A, Lavigne G, Cusack S. A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature. 1999;401(6756):935–938. doi: 10.1038/44880. [DOI] [PubMed] [Google Scholar]
- 9.Stewart PL, Burnett RM, Cyrklaff M, Fuller SD. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991;67(1):145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- 10.Stewart PL, Fuller SD, Burnett RM. Difference imaging of adenovirus: Bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 1993;12(7):2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, et al. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science. 2010;329(5995):1038–1043. doi: 10.1126/science.1187433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy VS, Natchiar SK, Stewart PL, Nemerow GR. Crystal structure of human adenovirus at 3.5 Å resolution. Science. 2010;329(5995):1071–1075. doi: 10.1126/science.1187292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabry CM, et al. A quasi-atomic model of human adenovirus type 5 capsid. EMBO J. 2005;24(9):1645–1654. doi: 10.1038/sj.emboj.7600653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saban SD, Silvestry M, Nemerow GR, Stewart PL. Visualization of alpha-helices in a 6-Ångstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. J Virol. 2006;80(24):12049–12059. doi: 10.1128/JVI.01652-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabry CM, et al. The C-terminal domains of adenovirus serotype 5 protein IX assemble into an antiparallel structure on the facets of the capsid. J Virol. 2009;83(2):1135–1139. doi: 10.1128/JVI.01808-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caspar DLD, Klug A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- 17.Smith KO, Gehle WD, Trousdale MD. Architecture of the adenovirus capsid. J Bacteriol. 1965;90(1):254–261. doi: 10.1128/jb.90.1.254-261.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snijder J, et al. The cleaved N-terminus of pVI binds peripentonal hexons in mature adenovirus. J Mol Biol. 2014;426(9):1971–1979. doi: 10.1016/j.jmb.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrescia NG, et al. Insights into assembly from structural analysis of bacteriophage PRD1. Nature. 2004;432(7013):68–74. doi: 10.1038/nature03056. [DOI] [PubMed] [Google Scholar]
- 20.Everitt E, Sundquist B, Pettersson U, Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- 21.Everitt E, Lutter L, Philipson L. Structural proteins of adenoviruses. XII. Location and neighbor relationship among proteins of adenovirion type 2 as revealed by enzymatic iodination, immunoprecipitation and chemical cross-linking. Virology. 1975;67(1):197–208. doi: 10.1016/0042-6822(75)90417-1. [DOI] [PubMed] [Google Scholar]
- 22.San Martín C, et al. Localization of the N-terminus of minor coat protein IIIa in the adenovirus capsid. J Mol Biol. 2008;383(4):923–934. doi: 10.1016/j.jmb.2008.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akalu A, Liebermann H, Bauer U, Granzow H, Seidel W. The subgenus-specific C-terminal region of protein IX is located on the surface of the adenovirus capsid. J Virol. 1999;73(7):6182–6187. doi: 10.1128/jvi.73.7.6182-6187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoehn G, et al. Three-dimensional structure of canine adenovirus serotype 2 capsid. J Virol. 2008;82(7):3192–3203. doi: 10.1128/JVI.02393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng L, et al. Cryo-EM structures of two bovine adenovirus type 3 intermediates. Virology. 2014;450-451:174–181. doi: 10.1016/j.virol.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Mangel WF, McGrath WJ, Toledo DL, Anderson CW. Viral DNA and a viral peptide can act as cofactors of adenovirus virion proteinase activity. Nature. 1993;361(6409):274–275. doi: 10.1038/361274a0. [DOI] [PubMed] [Google Scholar]
- 27.Wodrich H, et al. Switch from capsid protein import to adenovirus assembly by cleavage of nuclear transport signals. EMBO J. 2003;22(23):6245–6255. doi: 10.1093/emboj/cdg614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75(3):477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 29.Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J Virol. 2005;79(4):1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maier O, Galan DL, Wodrich H, Wiethoff CM. An N-terminal domain of adenovirus protein VI fragments membranes by inducing positive membrane curvature. Virology. 2010;402(1):11–19. doi: 10.1016/j.virol.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moyer CL, Wiethoff CM, Maier O, Smith JG, Nemerow GR. Functional genetic and biophysical analyses of membrane disruption by human adenovirus. J Virol. 2011;85(6):2631–2641. doi: 10.1128/JVI.02321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graziano V, et al. Regulation of a viral proteinase by a peptide and DNA in one-dimensional space: II. Adenovirus proteinase is activated in an unusual one-dimensional biochemical reaction. J Biol Chem. 2013;288(3):2068–2080. doi: 10.1074/jbc.M112.407312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drin G, Antonny B. Amphipathic helices and membrane curvature. FEBS Lett. 2010;584(9):1840–1847. doi: 10.1016/j.febslet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 34.van Oostrum J, Burnett RM. Molecular composition of the adenovirus type 2 virion. J Virol. 1985;56(2):439–448. doi: 10.1128/jvi.56.2.439-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benevento M, et al. Adenovirus composition, proteolysis, and disassembly studied by in-depth qualitative and quantitative proteomics. J Biol Chem. 2014;289(16):11421–11430. doi: 10.1074/jbc.M113.537498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterjee PK, Vayda ME, Flint SJ. Interactions among the three adenovirus core proteins. J Virol. 1985;55(2):379–386. doi: 10.1128/jvi.55.2.379-386.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy VS, et al. Crystallization and preliminary X-ray diffraction analysis of human adenovirus. Virology. 2010;402(1):209–214. doi: 10.1016/j.virol.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCoy AJ, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunger AT. X-PLOR, Version 3.1: A System for X-Ray Crystallography and NMR. New Haven, CT: Yale Univ Press; 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.