Abstract
Background
A von Frey filament (vFF) is a type of aesthesiometer usually made of nylon perpendicularly held in a base. It can be used in paw withdrawal pain threshold assessment, one of the most popular tests for pain evaluation using animal models. For this test, a set of filaments, each able to exert a different force, is applied to the animal paw, from the weakest to the strongest, until the paw is withdrawn.
New Method
We made 20 low cost vFF using nylon filaments of different lengths and constant diameter glued perpendicularly to the ends of popsicle sticks. They were calibrated using a laboratory balance scale. Building and calibrating took around four hours and confirmed the theoretical prediction that the force exerted is inversely proportional to the length and directly proportional to the width of the filament.
Results
The calibration showed that they were precise and accurate. We analyzed the paw withdrawal threshold assessed with the set of home-made vFF and with a high quality commercial set of 5 monofilaments vFF (Stoelting, Wood Dale, USA) in two groups (n=5) of healthy mice.
Comparison with existing methods
The home-made vFF precisely and accurately measured the hind paw withdrawal threshold (20.3±0.9 g). The commercial vFF have different diameters while our set has the same diameter avoiding the problem of lower sensitivity to larger diameter filaments.
Conclusion
Building a set of vFF is easy, cost effective and, depending on the kind of tests, can increase precision and accuracy of animal nociception evaluation.
Keywords: von Frey filament, pain evaluation, home-made von Frey filament, validation
1. INTRODUCTION
Pain is one of the most researched areas of medicine. It is a consequence of the increasing life expectancy of the world population, the high costs and the side effects of analgesics (Chau et al., 2008). Notwithstanding, in times of world recession and financial crises one of the first sectors to feel the scarcity of funding is experimental science investigations. Moreover, there are an increasing number of new laboratories, the majority in developing countries, particularly in the BRICS group (Brazil, Russia, India, China, and South Africa). Nevertheless funding for these laboratories is restricted and the building of homemade equipment is usual. One of the most frequently used devices for pain evaluation is the von Frey filament (vFF). A set of commercial vFF costs approximately 500 USD, however, in this study we will show how someone can easily handcraft a very precise and accurate set of vFF for less than 5 USD.
1.1. Relevance of pain tests using von Frey filaments
Animal models are very important to test new medications or procedures, especially for pain management. However, pain cannot be directly monitored - it can only be estimated by examining the response to a specific stimulus like mechanical stimulation with vFF (Pitcher et al., 1999, Le Bars et al., 2001). To perform von Frey nociception tests the filament is applied at the “end point” of response. In animals, these tests are usually performed at the hind paw, and paw withdrawal, sustained retraction, licking and/or flinching are considered as a response to the stimulus (Lambert et al., 2009, Mogil et al., 2001).
The vFF have a broad scope of application such as pain evaluation in conscious animals with neuropathic lesions (Luo et al., 1999, Martinez-Caro and Laird, 2000), anesthetized animals (Krauspe et al., 1992), headache studies performed either with humans (Cooke et al., 2007) or with rats (Oshinsky and Gomonchareonsiri, 2007, Oshinsky et al., 2012) and also to evaluate nociception after central nervous system modulation (Burstein et al., 1998, Bartsch et al., 2004).
Trauma and surgery frequently induce nerve injury, resulting in permanent or temporary disturbances in pain perception. To identify nerve injury, to grade its severity, to monitor its recovery, to facilitate the transfer of information between medical units and to prepare medico-legal reports, it is useful to have tests suitable for daily clinical practice and able to be universally carried out (Poort et al., 2009, Robinson et al., 1992). These tests can be performed with home-made vFF as they are low cost and they can be easily crafted and calibrated in medical units. In addition, a superficial area of anesthesia (or hypo/hyperesthesia) can be mapped by applying the von Frey filament stimulus within the lesion and the surrounding areas in small steps until a sensation is felt (Stubhaug et al., 1997).
1.2. Pain evaluation with von Frey Filaments
The most used series of commercial von Frey filaments was developed by Semmes et al. (Semmes et al., 1960) for mapping tactile sensation in patients with brain damage. There are several methodologies of pain assessment with vFF, although, there is no clear evidence that one is superior. Some methods are based on nociceptive scales (Ho Kim and Mo Chung, 1992, Takasaki et al., 2001): a set of 3 or 4 different filaments representing the type of stimuli (e.g., touch, pain, strong pain) is applied to the animal, and the percentage of responses to 5 to 10 applications of each stimulus is tabulated. This methodology is important for mapping an area affected by neurological damage, surgery or peripheral neuropathy.
Most of the researchers use the vFF to estimate the pain threshold in animals. Various methodologies are available and, in the simplest one, the test begins with the application of the weakest filament of the series to the animal body region. If the animal does not remove the body part from the stimulus a stronger filament is used in a sequence. The pain threshold is considered the force of the filament which causes the withdrawal (Tal and Bennett, 1994). Another approach uses the up-down method, starting the test with the strongest filament, and going down with the forces successively, until the animal does not react accordingly, and the corresponding filament force determines the pain threshold (Bennett, 1993).
A third methodology begins with a middle force filament. Whenever a positive response to a stimulus occurs, the next weaker von Frey filament is applied, and whenever a negative response occurs, the next stronger force is applied. The pain threshold is determined by the first change in response (Chaplan et al., 1994). A fourth methodology starts similarly, but the testing continues for five additional stimuli after the first change in response occurs (Li et al., 2000). The pattern of responses is converted to a 50% von Frey threshold using the technique described by Dixon (Dixon, 1980).
1.3. Von Frey filaments
The physiologist Maximilian von Frey was a pioneer in the study of pain in the late 19th century. To develop these studies he designed a set of filaments each with a different stiffness to evaluate pain sensitivity to touch in humans (Schmidt et al., 1997). These original filaments were made with animal hairs like pig bristles or wispy hairs from a squirrel’s tail. With the evolution of the technique, the animal fibers were replaced with manufactured filaments, giving rise to better accuracy and reproducibility. These devices are based on Euler’s buckling law, a law of physics which states that an elastic fiber with constant diameter, with one of its ends pressed vertically to a surface such as the skin, and the other end fixed, exerts a constant force. Before the filament starts to bend, the force can be increased, but after bending, the vertical force is constant. The force is directly proportional to the stiffness, directly related to the thickness of the filament and inversely proportional to its squared length (Fruhstorfer et al., 2001, Mogil et al., 2001).
An important parameter of the commercial filaments is the grade or number (M), which is very often confused with the applied force (F). The set of filament numbers create a logarithmic scale (Levin et al., 1978), which is given by:
This parameter is important not only for performing the experiment but also to calibrate and compare equivalent sets of filaments. The vFF can be easily calibrated with a laboratory balance to determine the applied force and consequently the filament number (Levin et al., 1978, Lambert et al., 2009). In this article we will refer to the force in gram-force (gf), which is a unit of force numerically equal to the weight in grams. The SI derived unit for force is the newton (N). Therefore, 1 gf is approximately 9.8 mN.
We aimed to demonstrate that homemade von Frey filaments (HmvFF) can be customized to allow a broad range of applications since their bending forces can be precise and accurately controlled. As they are low-cost and easily crafted, HmvFF are adequate for laboratories with low budget or for ones that are not specialized in pain assessment. Clinicians can also benefit from HmvFF, since they can allow quantification of the progression of a nerve injury as well as its treatment, using a cost effective device.
Hence, the objective of this study was to describe how to build, test and validate a set of HmvFF, comparing the results with a commercial set. We show that this equipment produces reliable results and consequently can be used for nociception assessment in animal models. The tests chosen to make the comparison with an animal model were the paw withdrawal threshold with HmvFF and the nociception score both with a set of high quality commercial vFF and with the HmvFF set developed here.
2. MATERIAL AND METHODS
2.1. Animals
All animal procedures were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital (protocol # 2010N000202) and met the guidelines of the National Institutes of Health. Adult male BALB/c mice (weight 20–24 g; Charles River Laboratories, Wilmington, MA) were used in this study. The animals were housed at five mice per cage and were maintained on a 12-hour light – 12-hour dark cycle with access to food and water ad libitum.
2.2. Construction of HmvFF and commercial vFF
The HmvFF construction was carried out with low-cost materials that are easy to find in common suppliers. Therefore, we used nylon monofilament fishing line (0.35 mm diameter, Scientific Anglers™ Full Sinking Fly Fishing Line) to make the tips of the HmvFF. The monofilament line was cut with scissors in different lengths. The supports for the filaments were made by popsicle sticks (CreateArt) with dimensions of 1.0 X 11.2 X 0.3 cm. Nylon filaments and popsicles sticks were glued using Loctite® 495™ Super Bonder® Instant Adhesive.
The commercial vFF (Stoelting, Wood Dale, USA) series is a standardized set of filaments, all of constant length but varying in diameter. Each filament is individually calibrated by the manufacturer to deliver its targeted force within a 5% standard deviation. The support is similar to a plastic pencil. The filaments are retractile and made of flexible plastic. Therefore, they are durable and biocompatible.
2.3. Designing and calibrating von Frey filaments
We easily handcrafted 20 low cost vFF with nylon tips glued perpendicularly to the endpoint of popsicle sticks (figure 1). In order to obtain a range of forces we made the vFF with different lengths, but the same diameter. Some HmvFF have two (or three) nylon filaments glued together only by the endpoint to duplicate (or triplicate) the force. We were careful to make the end point with only one nylon filament to ensure the same area was always touching the animal. The forces exerted by HmvFF were calibrated with a weighing scale (Andrews, 1993). The average forces ± standard deviation of 10 measurements are shown in figure 1.
Figure 1.
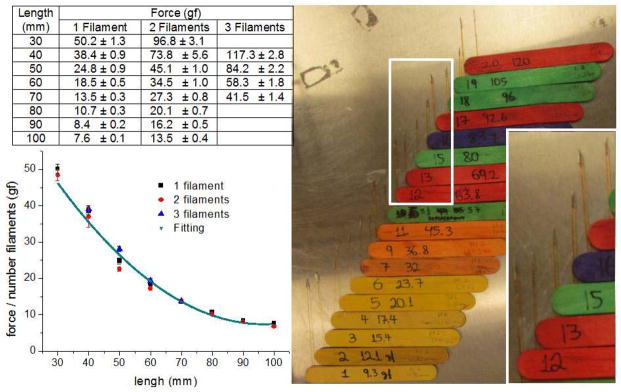
Picture of the HmvFF, with enlarged detail for the tips. Table showing average ± SD of bending force of HmvFF with one, two or three associated filaments and lengths from 30 to 100 mm. Graph of force divided by number of associated filaments Vs. length when fitted by a second order polynomial function (R2 = 0.975).
According to Euler’s equation, the bending force of the filament is inversely proportional to the squared length (L) and is directly proportional to the number of associated filaments. The HmvFF forces divided by the number of associated filaments were fitted by a second order polynomial function as predicted by Euler’s equation (figure 1). The whole process of handcrafting and calibrating took around four hours and the material used was less expensive than five dollars.
2.4. Assessment of pain sensation using von Frey filaments
The mice were placed individually in a cage (10 X 20 X 15 cm) whose floor was constructed with meshed metal wire (squares of 5 X 5 mm). Noxious stimulus was obtained when a commercial vFF of bending forces of 10, 15, 26, 60, 100 gf, or a HmvFF of bending forces raging from 7.6 to 27.3 gf (table 1) were pressed perpendicularly upward, through the cage floor, against the plantar hind paw skin and held for approximately 3 s until it slightly buckled.
Stimuli with the same filament were applied five times to the hind paw at intervals of several seconds. The responses to these stimuli were ranked as follows: 0, no response; 1, move paw away from vFF; and 2, immediate flinching or licking of the hind paw. A nociceptive score, based on Takasaki et al., 2001 (Takasaki et al., 2001), of each animal was calculated as the sum of the responses to the five stimuli. This gives rise to a scale that ranges from 0 (no response for all noxious stimuli) to 10 (high response for all noxious stimuli). The nociceptive score of the whole group was the average of the individual scores.
The assessment of pain threshold is more direct: the animals were placed and noxiously stimulated in the same way as described above. However, for this test, the mice were successively stimulated starting with the weakest to the strongest filaments. The sequence of stimuli was stopped when the mouse reacted with immediate flinching or licking of the hind paw. The force of the last used filament was considered the pain threshold. The group pain threshold was the average of individual thresholds.
2.5. Statistical analyses
The mean hind paw withdrawal thresholds and nociceptive scores were analyzed using one-way ANOVA and Tukey’s test. The mean ± standard deviation (SD) withdrawal threshold and nociceptive score values between different groups or within the same group at different time points were considered significantly different when P < 0.05.
3. Results
In this section we compare the mice behavior during the evaluation of pain with commercial and homemade von Frey Filaments.
3.1. Assessment of pain in healthy mice
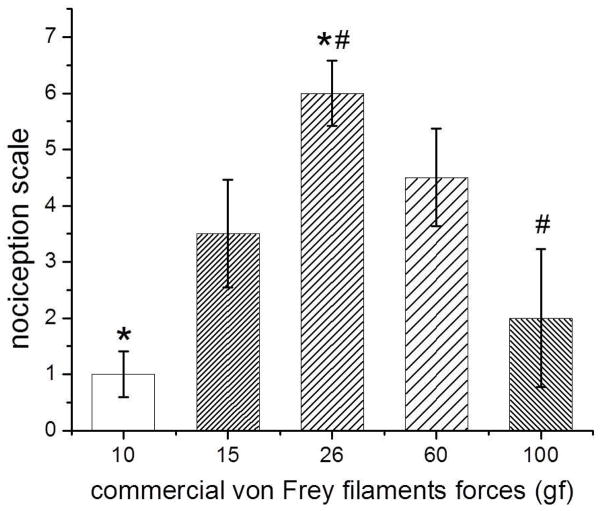
One group of mice (n = 5) was tested with commercial vFF and other (n = 5) with the set of HmvFF. Preliminary tests, with commercial vFF of bending forces of 10, 15, 26, 60, 100 gf, were made and we observed that the animals’ response increased from 10 to 26 gf and after that it started to decrease until the 100 gf. This was a non-expected behavior which happened due to the increase in the contact area of the filament. Therefore we chose 10 gf and 26 gf vFF to produce the painful stimulus since the former had the lowest sensitivity and the latter the highest (figure 2).
Figure 2.
Healthy mice sensitivity to commercial vFF with different bending forces and diameters. * and # means statistical difference of 26 gf filament against 10 gf and 100 gf filaments, respectively, (p < 0.05).
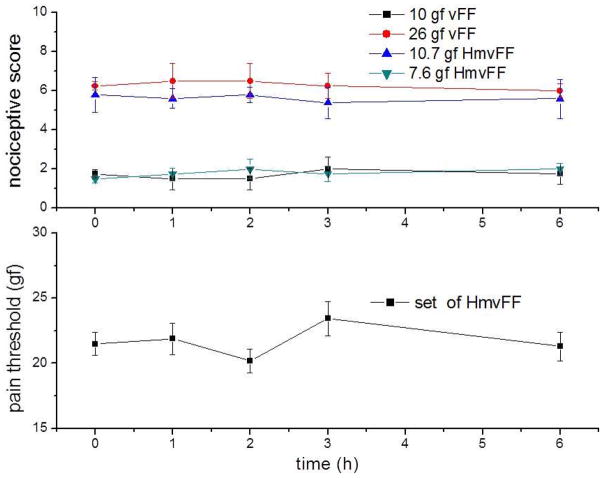
There was no statistically significant variation in the nociceptive scores between the five evaluations during the six hours of evaluation, for both sets of filaments (figure 3).The nociceptive scores for mice tested with 10 and 26 gf commercial vFF and with 7.6 and 10.7 gf HmvFF were 1.5 ± 0.6, 6.3 ± 0.2, 1.75 ± 0.4 and 5.4 ± 0.8, respectively. The sensitivity of the animals to the 10.7 gf HmvFF is as high as to the 26 gf commercial vFF. This can be explained when the corresponding pressures (force/area) are compared instead of forces. The pressures applied by 10 and 26 gf commercial vFF or 7.6 and 10.7 gf HmvFF, are 8.2, 12.0, 8.5 and 12.0 mN/mm2, respectively. Therefore, filaments which apply approximately the same pressure evoke the same level of response no matter if they are commercial or homemade von Frey Filaments.
Figure 3.
Average ± SD of nociceptive score and pain threshold of mice. Nociceptive scores were equivalent for 26 gf vFF and 12 gf HmvFF since they apply similar pressure (higher than 10 gf vFF). There was no statistical difference for five tests over six hours (p < 0.05).
For mice tested with the set of HmvFF the minimal pain threshold was 20.2 ± 2.9 gf. Similarly to the nociceptive score test, there was no statistically significant variation of the pain threshold during the six hours of evaluation (figure 3). Both behaviors are important evidences for the reproducibility and constancy of the animal response to the HmvFF. Pain threshold does not decrease after successive applications, so we can infer that HmvFF stimuli are not harmful, otherwise a hyperalgesic state could lead to a decrease in pain threshold. It did not increase either, showing that the animal did not become used to the test. In addition, we repeated this test five times a day during seven consecutive days and there was no statistical difference in the pain threshold for all these tests.
The response to the commercial vFF was constant too, thus confirming that both commercial and home-made vFF give the same results regarding to the constancy of the animal behavior. It is worth noting that the standard deviations obtained for the pain thresholds (HmvFF) are small values (forces around 2 or 3 gf), showing the good sensitivity of the set of filaments we built, and the possibility of obtaining a fine evaluation to distinguish pain conditions.
4. Discussion
The purpose of this study was to suggest an easy way of building a set of HmvFF, as an alternative to commercial ones. The projected HmvFF consists of wood sticks with nylon filaments firmly glued to them. The difference in bending force was obtained due to the different lengths of the nylon filaments. We showed how to easily handcraft these low-cost HmvFF. The filament calibration can be done using a weighing scale, a common laboratory device. The set of HmvFF is well suited to test mice paw withdrawal response in normal conditions. Furthermore, the reproducibility of the test with HmvFF was very good: the results did not change during the first six hours of tests or even after 35 tests over seven consecutive days of evaluation.
Direct comparisons of nociceptive score obtained by stimuli of HmvFF and commercial vFF which apply the same pressure were similar. Therefore, HmvFF can reproduce the results of commercial one. The coefficient of variation (standard deviation divided by the mean), is similar for pain threshold, measured using HmvFF, and the nociceptive scale, measured using commercial vFF. Therefore, both can produce results with the same relative precision. However, HmvFF can be tailored in a broader range of forces to determine a different interval of pain thresholds, and with smaller force increments between successive filaments. Therefore they are useful for pain evaluation of mice and other laboratory animals in normal or hypo/hyperesthesia conditions. The data suggest that HmvFF can improve the paw withdrawal reflex measurement since they can be customized to have slightly different bending forces.
5. Conclusion
Sets of HmvFF are accurate, precise, and cost-effective and can be easily handcrafted for specific applications. They are versatile either for different kinds of tests, since they can reproduce the tests made with commercial vFF or for the distinct conditions of the subjects, since the animal can be in a normal or hypo/hyperesthesia state.
Highlights.
Building a set of low-cost von Frey Filaments for pain assessment.
The calibration showed home-made von Frey Filaments are precise and accurate.
Animal response to Home-made and commercial von Frey filaments are similar.
Acknowledgments
The authors are grateful to the Brazilian agencies CAPES (12957-12-7) and CNPq (140216/2011-1) for financial support. Research in the Hamblin laboratory is supported by US NIH R01AI050875
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ANDREWS K. The effect of changes in temperature and humidity on the accuracy of von Frey hairs. Journal of neuroscience methods. 1993;50:91–93. doi: 10.1016/0165-0270(93)90059-z. [DOI] [PubMed] [Google Scholar]
- BARTSCH T, KNIGHT YE, GOADSBY PJ. Activation of 5-HT1B/1D receptor in the periaqueductal gray inhibits nociception. Annals of neurology. 2004;56:371–381. doi: 10.1002/ana.20193. [DOI] [PubMed] [Google Scholar]
- BENNETT GJ. An animal model of neuropathic pain: A review. Muscle & Nerve. 1993;16:1040–1048. doi: 10.1002/mus.880161007. [DOI] [PubMed] [Google Scholar]
- BURSTEIN R, YAMAMURA H, MALICK A, STRASSMAN AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. Journal of neurophysiology. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- CHAPLAN S, BACH F, POGREL J, CHUNG J, YAKSH T. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- CHAU DL, WALKER V, PAI L, CHO LM. Opiates and elderly: use and side effects. Clinical interventions in aging. 2008;3:273. doi: 10.2147/cia.s1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOKE L, ELIASZIW M, BECKER WJ. Cutaneous allodynia in transformed migraine patients. Headache: The Journal of Head and Face Pain. 2007;47:531–539. doi: 10.1111/j.1526-4610.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- DIXON WJ. Efficient analysis of experimental observations. Annual review of pharmacology and toxicology. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- FRUHSTORFER H, GROSS W, SELBMANN O. von Frey hairs: new materials for a new design. European Journal of Pain. 2001;5:341–342. doi: 10.1053/eujp.2001.0250. [DOI] [PubMed] [Google Scholar]
- HO KIM S, MO CHUNG J. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- KRAUSPE R, SCHMIDT M, SCHAIBLE HG. Sensory innervation of the anterior cruciate ligament. An electrophysiological study of the response properties of single identified mechanoreceptors in the cat. The Journal of bone and joint surgery. 1992;74:390. American volume. [PubMed] [Google Scholar]
- LAMBERT GA, MALLOS G, ZAGAMI AS. Von Frey’s hairs–a review of their technology and use–a novel automated von Frey device for improved testing for hyperalgesia. Journal of neuroscience methods. 2009;177:420–426. doi: 10.1016/j.jneumeth.2008.10.033. [DOI] [PubMed] [Google Scholar]
- LE BARS D, GOZARIU M, CADDEN SW. Animal models of nociception. Pharmacological reviews. 2001;53:597–652. [PubMed] [Google Scholar]
- LEVIN S, PEARSALL G, RUDERMAN RJ. Von Frey’s method of measuring pressure sensibility in the hand: an engineering analysis of the Weinstein-Semmes pressure aesthesiometer. The Journal of hand surgery. 1978;3:211–216. doi: 10.1016/s0363-5023(78)80084-7. [DOI] [PubMed] [Google Scholar]
- LI Y, DORSI MJ, MEYER RA, BELZBERG AJ. Mechanical hyperalgesia after an L5 spinal nerve lesion in the rat is not dependent on input from injured nerve fibers. Pain. 2000;85:493–502. doi: 10.1016/S0304-3959(00)00250-5. [DOI] [PubMed] [Google Scholar]
- LUO ZD, CHAPLAN S, SCOTT B, CIZKOVA D, CALCUTT N, YAKSH T. Neuronal nitric oxide synthase mRNA upregulation in rat sensory neurons after spinal nerve ligation: lack of a role in allodynia development. The Journal of neuroscience. 1999;19:9201–9208. doi: 10.1523/JNEUROSCI.19-21-09201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINEZ-CARO L, LAIRD JM. Allodynia and hyperalgesia evoked by sciatic mononeuropathy in NK1 receptor knockout mice. Neuroreport. 2000;11:1213–1217. doi: 10.1097/00001756-200004270-00014. [DOI] [PubMed] [Google Scholar]
- MOGIL JS, WILSON SG, WAN Y. Assessing nociception in murine subjects. Methods in pain research. 2001:11–39. [Google Scholar]
- OSHINSKY ML, GOMONCHAREONSIRI S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache: The Journal of Head and Face Pain. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSHINSKY ML, SANGHVI MM, MAXWELL CR, GONZALEZ D, SPANGENBERG RJ, COOPER M, SILBERSTEIN SD. Spontaneous trigeminal allodynia in rats: a model of primary headache. Headache: The Journal of Head and Face Pain. 2012;52:1336–1349. doi: 10.1111/j.1526-4610.2012.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITCHER GM, RITCHIE J, HENRY JL. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. Journal of neuroscience methods. 1999;87:185–193. doi: 10.1016/s0165-0270(99)00004-7. [DOI] [PubMed] [Google Scholar]
- POORT LJ, VAN NECK JW, VAN DER WAL KGH. Sensory Testing of Inferior Alveolar Nerve Injuries: A Review of Methods Used in Prospective Studies. Journal of Oral and Maxillofacial Surgery. 2009;67:292–300. doi: 10.1016/j.joms.2008.06.076. [DOI] [PubMed] [Google Scholar]
- ROBINSON PP, SMITH KG, JOHNSON FP, COPPINS DA. Equipment and methods for simple sensory testing. British Journal of Oral and Maxillofacial Surgery. 1992;30:387–389. doi: 10.1016/0266-4356(92)90206-x. [DOI] [PubMed] [Google Scholar]
- SCHMIDT R, SCHMELZ M, RINGKAMP M, HANDWERKER HO, TOREBJÖRK HE. Innervation territories of mechanically activated C nociceptor units in human skin. Journal of neurophysiology. 1997;78:2641–2648. doi: 10.1152/jn.1997.78.5.2641. [DOI] [PubMed] [Google Scholar]
- SEMMES J, WEINSTEIN S, GHENT L, TEUBER H-L. Somatosensory changes after penetrating brain wounds in man 1960 [Google Scholar]
- STUBHAUG A, BREIVIK H, EIDE P, KREUNEN M, FOSS A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiologica Scandinavica. 1997;41:1124–1132. doi: 10.1111/j.1399-6576.1997.tb04854.x. [DOI] [PubMed] [Google Scholar]
- TAKASAKI I, ANDOH T, NOJIMA H, SHIRAKI K, KURAISHI Y. Gabapentin antinociception in mice with acute herpetic pain induced by herpes simplex virus infection. Journal of Pharmacology and Experimental Therapeutics. 2001;296:270–275. [PubMed] [Google Scholar]
- TAL M, BENNETT GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57:375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]