Abstract
PURPOSE
The principal risks of needle biopsy are hemorrhage and implantation of tumor cells in the needle tract. This study compared hemorrhage after liver and kidney biopsy with and without radiofrequency (RF) ablation of the needle tract.
MATERIALS AND METHODS
Biopsies of liver and kidney were performed in swine through introducer needles modified to allow RF ablation with the distal 2 cm of the needle. After each biopsy, randomization determined whether the site was to undergo RF ablation during withdrawal of the introducer needle. Temperature was measured with a thermistor stylet near the needle tip, with a target temperature of 70°C–100°C with RF ablation. Blood loss was measured as grams of blood absorbed in gauze at the puncture site for 2 minutes after needle withdrawal. Selected specimens were cut for gross examination.
RESULTS
RF ablation reduced bleeding compared with absence of RF ablation in liver and kidney (P < .01), with mean blood loss reduced 63% and 97%, respectively. Mean amounts of blood loss (±SD) in the liver in the RF and no-RF groups were 2.03 g ± 4.03 (CI, 0.53–3.54 g) and 5.50 g ± 5.58 (CI, 3.33–7.66 g), respectively. Mean amounts of blood loss in the kidney in the RF and no-RF groups were 0.26 g ± 0.32 (CI, −0.01 to 0.53 g) and 8.79 g ± 7.72 (CI, 2.34–15.24 g), respectively. With RF ablation, thermal coagulation of the tissue surrounding the needle tract was observed.
CONCLUSION
RF ablation of needle biopsy tracts reduced hemorrhage after biopsy in the liver and kidney and may reduce complications of hemorrhage as well as implantation of tumor cells in the tract.
PERCUTANEOUS image-guided needle biopsy is often performed in highly vascular organs or in tumors with rich macroscopic and microscopic blood supply. The principal risks related to needle biopsy are hemorrhage and implantation of tumor cells in the needle tract. Incidences of postbiopsy hematoma in the liver have been reported in prospective studies to be 18.3% after biopsy with a 2.0-mm Tru-cut needle (1) and 23% after biopsy with a 1.6-mm Jamshidi needle (2). Patients with cirrhosis or hepatic tumors are at greater risk of hemorrhagic complications after biopsy (3), as are patients with uncorrected coagulopathy. Hemorrhage may go undetected unless the patient undergoes subsequent imaging procedures or develops alterations in hemodynamic status as a result of the blood loss. If the operator notices a large amount of blood emitting from a biopsy needle or if a particular tumor is deemed to be at high risk for hemorrhage, there are few options for the operator. Several potential approaches have been reported in the literature, including trans-needle placement of steel coils (4) and injection of fibrin (5), gelatin particles and thrombin (6), gelatin sponge pledgets (7,8), fibrinogen and thrombin (9,10), or autologous blood clot in an attempt to promote clotting and hemostasis in the needle tract. Kim et al (11) reported animal studies of radiofrequency (RF) coagulation with use of the biopsy needle itself to deliver the RF energy.
Transplantation of tumor cells along the needle tract after biopsy of a malignancy is also a risk. Incidences of needle tract implantation after biopsy of hepatocellular carcinoma have been reported in two recent series as 3.9% (12) and 5.1% (13). There are also published reports of subcutaneous seeding after fine needle biopsy of metastatic colonic adenocarcinoma (14,15) and metastatic pancreatic adenocarcinoma (16). The development of an effective, inexpensive, and technically simple method of cauterizing a coaxial needle biopsy tract could provide a solution to these difficult clinical problems.
Our objective was to design a coaxial needle biopsy system that uses RF energy to cauterize the needle tract after core biopsy to reduce the risks of hemorrhage and needle tract seeding by tumor cells. A prototype design has been tested in animals to evaluate hemorrhage at the organ surface after biopsy with cauterization of the needle tract compared with traditional needle biopsy. The hypothesis tested was that cauterization of the needle tract after coaxial needle biopsy of the liver or kidney decreases hemorrhage.
MATERIALS AND METHODS
Device
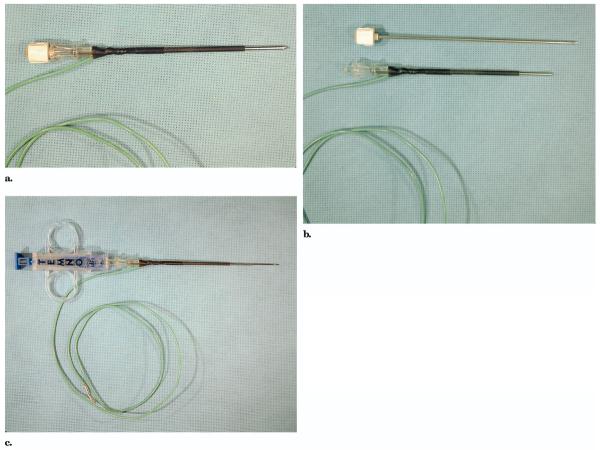
A 14-gauge, 10-cm coaxial introducer needle (Temno; Allegiance Healthcare, McGaw Park, IL) with a removable pencil-point stylet was modified to serve as an RF ablation electrode after core biopsy (Fig 1). The proximal 8 cm of the metal shaft was electrically insulated by nonconducting heat-shrinkable plastic (SPC Technology, Chicago, IL) around the circumference. Electrical connection with the needle was established by encasing the bare end of an 18-gauge wire within the plastic sheath in direct contact with the needle. The distal 2 cm of the shaft at the tip remained exposed to act as a monopolar electrode for deposition of alternating current in the RF range. The outer diameter of the insulated portion of the needle was 3.0 mm, equivalent to approximately 11 gauge.
Figure 1.
Biopsy introducer needle with the obturator in place (a), with the obturator removed (b), and with the biopsy needle in place (c). All but the distal 2 cm of the needle shaft are insulated with shrink-wrapped plastic. The needle is electrically connected to the RF generator by a wire with one end enclosed in the shrink-wrapped plastic.
Experimental Protocol
The needle was tested in seven large crossbred domestic swine (141 kg ± 76) following protocol development in one pig under an animal use protocol approved by the institutional animal care and use committee. Anesthesia was induced with a mixture of tiletamine HCL and zolazepam HCL (Telazol; Fort Dodge Animal Health, Overland Park, KS) and xylazine (Phoenix Pharmaceutical, St. Joseph, MO) injected intramuscularly, followed by an intravenous injection of an anesthetic combination of tiletamine HCL and zolazepam HCL, xylazine, atropine sulfate (AmVet, Lexington, KY), ketamine (Phoenix Pharmaceuticals), and butorphanol tartrate (Torbugesic; Wyeth, Madison, NJ), as described by Wray-Cahen et al (17). Pigs were intubated and mechanically ventilated. General anesthesia was maintained with 1%–3% isoflurane (Abbott Laboratories, North Chicago, IL). The pigs were placed in a left lateral decubitus position and a combined laparotomy and thoracotomy was performed to expose the liver in six animals. The kidneys were exposed in four animals. Four disposable single-foil ground pads were placed on each pig, one on each shoulder and one on each thigh. The modified introducer needle and all grounding pads were connected to a 200-W, 480-kHz RF generator (CC1 Cosman coagulator system; Radionics, Burlington, MA).
Multiple biopsies were performed on each animal in the study. For each biopsy, the modified introducer needle was inserted into the liver or kidney without imaging guidance. For placement into the kidney, the modified introducer needle was inserted into the kidney perpendicular to the surface along the greater curvature. To monitor temperature near the tip of the introducer needle, a 22-gauge SMK pole needle (Radionics) was placed in tandem, parallel to the shaft of the introducer needle with the tips of the needles at the same depth within 1 cm of each other. The position of the needle was confirmed by ultrasonography, and a 25-gauge thermistor stylet connected to a digital thermometry monitor (TCA-2; Radionics) was inserted into the SMK pole needle for temperature measurements. A biopsy was performed through the introducer needle with a standard unmodified disposable 16-gauge biopsy gun with a 20-mm notch (Temno) deployed through the modified introducer needle. Randomization to the RF ablation group or the no–RF ablation group occurred after biopsy and withdrawal of the biopsy needle. In both groups, the SMK pole needle, thermistor, and introducer needle were simultaneously withdrawn. In the RF ablation group, the RF current was applied to the introducer needle as the thermistor and two needles were slowly withdrawn from the liver, with a goal of maintaining the measured temperature between 70°C and 100°C. The temperature range was chosen based on the feasibility study in which (i) bleeding was not well-controlled at temperatures lower than 70°C and (ii) tissue adhesion to the needle occurred at temperatures substantially higher than 100°C. Tissue adhesion also occurred if the needle was allowed to remain stationary for an extended period of time, in which case the tissue sheared on removal, potentially increasing hemorrhage. After the liver biopsies in the fifth animal (pig E), the needles were withdrawn more slowly for longer ablation of the last 2 cm of the needle tract. For both treatment groups, blood loss at the organ surface was measured by absorbing the blood with preweighed gauze pads for exactly 2 minutes and recording the differences in measured weight. The collected blood included hemorrhage from both needle puncture sites. The biopsy sites were marked at the capsule with suture for specimen collection after animal sacrifice by exsanguination. Specimens were fixed in 10% buffered formalin. Selected specimens were cut for gross examination.
Statistical Evaluation
For the liver biopsies, at least seven and no more than 12 biopsies were performed on the liver of six animals in the study. For the kidney biopsies, at least three and no more than five biopsies were performed on the kidneys of four animals. The treatments (RF ablation or no RF ablation) were randomly assigned within each animal for each biopsy site. Randomization was blinded. A two-tailed Student t test (unpaired) was used to determine if the mean blood loss after biopsy was significantly different between the treatment groups. One liver data point was removed as an outliner from the no-RF treatment group because it was more than five SDs greater than the mean of all the individual weights in that treatment group. This data point numerically increased the difference between the two treatment groups, but it also increased the treatment variation three fold. Results are presented as means ± SD along with the 95% confidence intervals. Probability values of < 0.05 were considered statistically significant.
RESULTS
Biopsy with ablation with RF energy during withdrawal of the introducer needle resulted in thermal coagulation of the needle tract compared with simple biopsy without RF ablation (Fig 2). The unstained cross-sections show that tissue slightly remote from the needle was uninjured.
Figure 2.
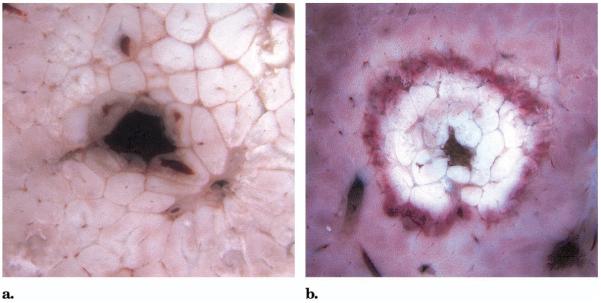
Gross cross-sections through the biopsy introducer needle tract in liver without RF ablation (a) and with RF ablation (b) during withdrawal of the needle.
Blood loss for each liver biopsy in the RF ablation and no–RF ablation groups within each animal is presented in Table 1. The biopsies in the RF group had significantly reduced bleeding compared with those in the no-RF group (P < .01). The mean blood loss for biopsies in the RF group was 63% lower than in the no-RF group.
Table 1.
Grams of Blood Lost after Liver Biopsy at Each Biopsy Site with and without RF Ablation*
| No RF (n = 28)† |
RF (n = 30)‡ |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pig | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 |
| A | 2.8 | 8.4 | 0.9 | 1.0 | 1.3 | – | 0.2 | 0.8 | 0.4 | 0.0 | 0.1 | – |
| B | 5.5 | 4.3 | 13.8 | – | – | – | 1.4 | 1.5 | 0.1 | 0.4 | – | – |
| C | 3.1 | 1.0 | 0.6 | 1.2 | 4.6 | 8.8 | 0.0 | 0.0 | 0.2 | 9.1 | 0.1 | 2.3 |
| D | 8.3 | 0.8 | 2.7 | 9.7 | 0.9 | – | 1.5 | 2.8 | 0.0 | 0.1 | – | – |
| E | 9.8 | 5.7 | 13.2 | 1.3 | 70.0§ | – | 0.1 | 0.2 | 0.1 | 2.0 | 14.5 | 0.2 |
| F | 15.4 | 3.2 | 0.2 | 22.9 | 2.5 | – | 0.0 | 0.2 | 8.2 | 0.0 | 14.5 | – |
P = .0086.
Mean blood loss ± SD, 5.50 mL ± 5.58; 95% CI, 3.33–7.66 mL.
Mean blood loss ± SD, 2.03 mL ± 4.03; 95% CI, 0.53–3.54 mL.
This value was not included in mean, SD, or statistical comparison because it was more than 5 SDs greater than the mean of all the individual weights in that treatment group.
Blood loss for each renal biopsy in the RF and no-RF groups within each animal are presented in Table 2. The biopsies in the RF group had greatly reduced bleeding compared with those in the no-RF group (P < .01). The mean blood loss for biopsies in the RF group was 97% lower than in the no-RF group.
Table 2.
Grams of Blood Lost after Kidney Biopsy at Each Biopsy Site with and without RF Ablation*
| No RF† (n = 8) |
RF‡ (n = 8) |
||||
|---|---|---|---|---|---|
| Pig | Site 1 | Site 2 | Site 3 | Site 1 | Site 2 |
| B | 1.8 | – | – | 0.0 | 0.2 |
| E | 2.9 | 8.3 | – | 0.0 | 0.2 |
| F | 6.9 | 2.6 | – | 0.1 | 0.9 |
| G | 10.6 | 25.5 | 11.7 | 0.6 | 0.1 |
P = .0075.
Mean blood loss ± SD, 8.79 mL ± 7.72; 95% CI, 2.34–15.24 mL.
Mean blood loss ± SD, 0.26 mL ± 0.32; 95% CI, -0.01 to 0.53 mL.
DISCUSSION
Hemorrhagic complications after percutaneous biopsy are not an uncommon clinical problem (1,2). In addition, liver biopsy in the setting of cirrhosis, hepatic malignancy, or uncorrected coagulopathy presents a higher risk (3). Multiple or large-diameter biopsies, like sampling for DNA analysis, may also pose an increased risk of bleeding. Postbiopsy imaging is not routinely performed unless there is hemodynamic compromise or symptoms or other secondary evidence of ongoing bleeding.
RF ablation of the introducer needle tract after biopsy reduced bleeding at the organ surface by 63% in the liver and by 97% in the kidney. This application of RF ablation may offer a method for prevention of hemorrhage after biopsy, particularly in clinical settings in which the patient may be at greater risk. RF ablation of the needle tract offers advantages over alternative embolic or prothrombotic approaches to treat hemorrhage described in the literature in that it eliminates the risk of vascular embolization and avoids placement of implants (4–10). Cauterization of the needle tract may also reduce the incidence of seeding by malignant cells.
Kim et al (11) reported a method of electrocautery of the tract with use of the biopsy needle itself as the electrode. Although there was no gross or histologic evidence of thermal injury to the specimens at 25 W, there was thermal injury evident in portions of the specimens at 125 W. The method presented here eliminated the risk of specimen injury because the RF ablation is conducted with the introducer needle. Also, in the setting of multiple biopsies, only the introducer needle tract would be subjected to the RF ablation, rather than each individual biopsy tract.
RF ablation of needle biopsy tracts may improve the safety of percutaneous needle biopsy of the liver and kidney, offering advantages over embolic or prothrombotic methods of hemostasis. RF ablation to arrest postbiopsy hemorrhage in patients with coagulopathy or in patients with especially vascular tumors may present less risk of vascular thrombosis or embolization compared with the injection of particulates such as collagen pledgets or thrombin/collagen preparations. Thermal ablation may also reduce the risk of implantation of tumor cells as a result of the heating of the needle surface and needle tract. In the future, integration of a thermistor at the tip of the introducer needle could improve the safety and effectiveness of this device. A similar design for cauterizing catheters may be useful to establish hemostasis on catheter removal from organs or vessels. The safety of minimally invasive outpatient procedures could be improved with the additional tool of percutaneous cauterization.
Acknowledgments
The authors thank Isaac Chang, PhD, a contributing investigator, for his preparation of the introducer needle prototype, and acknowledge the animal care and technical support of Autumn Ashby and the animal care support of Richard Cullison, DVM, Samuel Howard, Mark Henderson, and Mark McDonald.
The mention of commercial products, their source, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the US Food and Drug Administration, the National Institutes of Health, the Department of Health and Human Services, or the Public Health Service. This work was sponsored by the Diagnostic Radiology Department, Warren Grant Magnuson Clinical Center, National Institutes of Health, Bethesda, MD. A patent application on the device described in this article is pending on behalf of B.J.W. and W.F.P.
Abbreviation
- RF
radiofrequency
References
- 1.Sugano S, Sumino Y, Hatori T, Mizugami H, Kawafune T, Abei T. Incidence of ultrasound-detected intrahepatic hematomas due to Tru-cut needle liver biopsy. Dig Dis Sci. 1991;36:1229–1233. doi: 10.1007/BF01307514. [DOI] [PubMed] [Google Scholar]
- 2.Minuk GY, Sutherland LR, Wiseman DA, MacDonald FR, Ding DL. Prospective study of the incidence of ultrasound-detected intrahepatic and subcapsular hematomas in patients randomized to 6 or 24 hours of bed rest after percutaneous liver biopsy. Gastroenterology. 1987;92:290–293. doi: 10.1016/0016-5085(87)90119-3. [DOI] [PubMed] [Google Scholar]
- 3.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy: a multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–173. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 4.Allison DJ, Adam A. Percutaneous liver biopsy and track embolization with steel coils. Radiology. 1988;169:261–263. doi: 10.1148/radiology.169.1.3420270. [DOI] [PubMed] [Google Scholar]
- 5.Chisholm RA, Jones SN, Lees WR. Fibrin sealant as a plug for the post liver biopsy needle track. Clin Radiol. 1989;40:627–628. doi: 10.1016/s0009-9260(89)80326-5. [DOI] [PubMed] [Google Scholar]
- 6.Zins M, Vilgrain V, Gayno S, et al. US-guided percutaneous liver biopsy with plugging of the needle track: a prospective study in 72 high-risk patients. Radiology. 1992;184:841–843. doi: 10.1148/radiology.184.3.1509076. [DOI] [PubMed] [Google Scholar]
- 7.Smith TP, McDermott VG, Ayoub DM, Suhocki PV, Stackhouse DJ. Percutaneous transhepatic liver biopsy with tract embolization. Radiology. 1996;198:769–774. doi: 10.1148/radiology.198.3.8628869. [DOI] [PubMed] [Google Scholar]
- 8.Fandrich CA, Davies RP, Hall PM. Small gauge Gelfoam plug liver biopsy in high risk patients: safety and diagnostic value. Australas Radiol. 1996;40:230–234. doi: 10.1111/j.1440-1673.1996.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 9.Falstrom JK, Moore MM, Caldwell SH, Matsumoto AH, Abbott RD, Spotnitz WD. Use of fibrin sealant to reduce bleeding after needle liver biopsy in an anticoagulated canine model: work in progress. J Vasc Interv Radiol. 1999;10:457–462. doi: 10.1016/s1051-0443(99)70065-5. [DOI] [PubMed] [Google Scholar]
- 10.Paulson EK, Stephenson GR, Neal MC, Rossin V, Lawson JH. Use of fibrin sealant as a hemostatic agent after liver biopsy in swine. J Vasc Interv Radiol. 2000;11:905–911. doi: 10.1016/s1051-0443(07)61810-7. [DOI] [PubMed] [Google Scholar]
- 11.Kim EH, Kopecky KK, Cummings OW, Dreesen RG, Pound DC. Electrocautery of the tract after needle biopsy of the liver to reduce blood loss. Experience in the canine model. Invest Radiol. 1993;28:228–230. doi: 10.1097/00004424-199303000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Lim HK, Lee WJ, Cho JM, Jang HJ. Needle-tract implantation in hepatocellular carcinoma: frequency and CT findings after biopsy with a 19.5-gauge automated biopsy gun. Abdom Imaging. 2000;25:246–250. doi: 10.1007/s002610000025. [DOI] [PubMed] [Google Scholar]
- 13.Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary? Liver Transpl. 2000;6:67–72. doi: 10.1002/lt.500060103. [DOI] [PubMed] [Google Scholar]
- 14.McGrath FP, Gibney RG, Rowley VA, Scudamore CH. Cutaneous seeding following fine needle biopsy of colonic liver metastases. Clin Radiol. 1991;43:130–131. doi: 10.1016/s0009-9260(05)81594-6. [DOI] [PubMed] [Google Scholar]
- 15.Goletti O, Chiarugi M, Buccianti P, Macchiarini P. Subcutaneous implantation of liver metastasis after fine needle biopsy. Eur J Surg Oncol. 1992;18:636–637. [PubMed] [Google Scholar]
- 16.de Sio I, Castellano L, Calandra M, Del Vecchio-Blanco C. Subcutaneous needle-tract seeding after fine needle aspiration biopsy of pancreatic liver metastasis. Eur J Ultrasound. 2002;15:65–68. doi: 10.1016/s0929-8266(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 17.Wray-Cahen D, Vossoughi J, Karanian JW. Large animal models in pre-clinical trials. In: Vossoughi J, Kipshidze N, Karanian JW, editors. Stent graft update. Medical and Engineering Publishing; Washington, DC: 2000. pp. 201–214. [Google Scholar]