Abstract
Objective
A review of all resections for recurrent or metastatic ACC was performed to identify patients who might benefit from a surgical approach, and to identify factors that might aid in prognosis among patients with metastatic disease.
Summary Background Data
Adrenocortical carcinoma (ACC) is a rare tumor, with frequent recurrences and metastases even after complete resection. Chemotherapy has limited efficacy, and surgical resection of metastatic ACC remains controversial.
Methods
A retrospective review was performed of all patients who underwent surgical intervention for metastatic ACC in a single tertiary center from 1977 to 2009. All available clinicopathologic data were analyzed to determine potential factors associated with response to treatment and survival.
Results
Fifty-seven patients underwent 116 procedures for recurrent or metastatic disease. Twenty-three resections were for liver metastases, 48 for pulmonary metastases, 22 for abdominal disease including local recurrences, and 13 were for metastases at other sites. Median and 5-year survivals from time of first metastasectomy were 2.5 years, and 41%, respectively. The median survival of patients with DFI <12 months was 1.7 years, compared to 6.6 years for patients with DFI >12 months (P = 0.015). Median survival for right versus left-sided primaries was 1.9 years versus 3.8 years (P = 0.03). Liver metastases were more common with right-sided primaries (67% vs. 41%, P = 0.05). Chemotherapy had no impact on survival.
Conclusions
Resection of recurrent or metastatic ACC is safe, and may result in prolongation of survival in selected patients with DFI greater than 1 year.
Keywords: metastatic adrenocortical carcinoma, liver resection, lung resection
INTRODUCTION
Adrenocortical carcinoma (ACC), although a rare cancer with an annual incidence of 2 per million people, is one of the most aggressive endocrine malignancies, second only to anaplastic thyroid cancer [1]. It most commonly occurs in the fourth and fifth decades, and affects women more frequently than men (1.5:1) [2]. Patients usually present with large primary tumors that have invaded adjacent organs, or with metastatic disease, giving it a generally dismal prognosis [3]. An estimated 40–60% of adult patients present with hormonal symptoms of a functional tumor, the majority of these being Cushing's syndrome. The remaining non-functional tumors may often become quite large before they are detected due to symptoms of mass effect [4]. Previously reviewed series have suggested that large tumors (>12 cm) have a negative prognosis, while other factors such as localized disease, mitotic rate less than 6 per high-powered field, absence of intratumoral hemorrhage, and complete resection have a favorable prognosis. The prognostic implications of age and functional status are less clear, although some studies have suggested that older age and cortisol production may contribute to a less favorable outcome [5]
While transient control of endocrine symptoms and modest tumor response with mitotane have been reported, overall survival is not impacted by systemic chemotherapy, and its role in the adjuvant setting remains controversial [6–8]. Radiation therapy has been used to palliate bony metastases, but ACC is generally considered radioresistant, and therefore the role of radiotherapy remains unclear [9]. In light of these findings, surgery remains the best treatment modality for ACC, and complete resection is the cornerstone of long-term survival. In the setting of metastatic and/or recurrent disease, the role of aggressive surgical therapy, especially when it requires multiple re-operations, has yet to be expounded. We undertook the present review in an attempt to identify patients who might benefit from this approach to metastatic disease, and to identify factors that might aid in prognosis among patients with metastatic disease.
METHODS
Data Collection and Clinical Assessment
A retrospective review identified all patients diagnosed with metastatic ACC who underwent resection or radiofrequency ablation (RFA) at the National Cancer Institute in Bethesda, Maryland from 1977 to 2009. Some of these patients have been included in prior publications [10,11]. Inclusion criteria were all patients with pathologically confirmed ACC from site of resection, or from a biopsy done prior to RFA. Exclusion criteria were patients who had resection of their primary only, arterial embolization, or biopsy alone. All patients were enrolled on investigational protocols and signed an institutional review board-approved consent for participation in clinical studies.
Statistical Analysis
Overall survival (OS) was calculated from the date of the first metastasectomy until the date of death or the date of the last encounter as appropriate. Disease-free survival (DFS) for those patients resected to no evidence of disease (NED), or progression-free survival (PFS) for those patients with incompletely resected or residual disease, was calculated from the date of the first metastasectomy until the date of the second recurrence or progression, or to the last follow up as appropriate. The probabilities of OS, DFS, or PFS, were calculated using the Kaplan–Meier method. The statistical significance of the difference between pairs of Kaplan–Meier curves was determined by the log-rank test for cases in which the distinguishing characteristic of the curve was known at the date of the resection. All P-values are two-tailed.
Clinicopathological features included demographics, functional status, side of primary tumor, stage, neoadjuvant or adjuvant chemotherapy, and disease-free interval (DFI). DFI was defined as the time to first recurrence at any site after the initial operation, if the patient was originally rendered NED.
RESULTS
Patient Characteristics
Fifty-seven patients underwent 116 procedures for recurrent or metastatic disease between 1977 and 2009. Demographic data are listed in Table I. The majority of these patients presented as stage IV (44%), and had functional tumors 35 (61%). Functional tumors were defined as those that showed signs and symptoms of excess hormone production, or who had elevation of adrenal hormones on laboratory studies. At the time of first recurrence, 29 had thoracic lesions, 23 had hepatic lesions, and 17 had local recurrences. Additionally, there were 9 recurrences to other sites, such as the brain, abdominal wall, and subcutaneous tissue.
TABLE I.
Demographics of 57 Patients Who Underwent Metastasectomy or Repeat Resection for Metastatic Adrenocortical Carcinoma
| Feature | Median (range) |
|---|---|
| Age at diagnosis | 40 (6–72) |
| Sex, M/F | 21/36 |
|
| |
| No. (%) | |
|
| |
| Lee staging classification at initial presentation | |
| II | 10 (17) |
| III | 13 (23) |
| IV | 25 (44) |
| Unknown | 9 (16) |
| Hormonal status at initial presentation | |
| Non-functional | 19 (33) |
| Functional | 35 (61) |
| Unknown | 3 (5) |
| Hypercortisolism | 23 (66) |
| Hyperaldosteronism | 5 (14) |
| Virilization | 4 (11) |
| Feminization | 3 (9) |
| Side of primary tumor | |
| Right | 30 (53) |
| Left | 27 (47) |
| Right-sided primaries with 1st recurrence to liver | 16 (53) |
| Left-sided primaries with 1st recurrence to liver | 9 (33) |
| Disease status after resection of primary tumor | |
| NED | 45 (79) |
| Not NED | 10 (18) |
| Unknown | 2 (3) |
|
| |
| Sites and resections of distant metastases | |
| Total metastasectomies | 116 |
| Total liver resections | 23 |
| Total lung resections | 48 |
| Total abdominal resections (other than hepatic and primary) | 22 |
| Other sites resected | 23 |
|
| |
| Systemic therapy | Total/mitotane |
|
| |
| Neoadjuvant Chemotherapy | 6 (11%)/6 (11%) |
| Adjuvant chemotherapy | 34 (60%)/25 (44%) |
Nineteen of the 57 patients presented with synchronous disease. Of these, 5 patients presented with disease in both the liver and lung. One of these patients underwent adrenalectomy, liver resection, and a pulmonary resection at the initial operation.
Operative Findings
Nineteen patients had a local recurrence; the remaining 38 had distant metastases. Operations performed for the first recurrence consisted of 13 liver resections, 23 thoracic metastasectomies, 14 exploratory laparotomies with non-hepatic resections, and 9 other procedures (1 craniotomy and 8 RFA's; 7 to liver lesions, and 1 to a right lower lobe lesion of the lung). For all 116 metastasectomies performed, 23 were liver resections, 48 were lung or thoracic resections, 22 were exploratory laparotomies (15 of which were specifically for local recurrence), and 23 were procedures or resections at other sites. Of these, 3 interventions consisted of RFA alone, and 12 used a combination of RFA and surgical resection. The other resected sites included craniotomies (4), spine resections (3), and soft tissue resections (4). The mean number of metastasectomies on all patients was 1.8 (range: 1–7); 2 patients underwent 7 procedures.
Of the 48 thoracic resections, 22 were via right thoracotomy, 20 were via left thoracotomy, 5 were via median sternotomy, and 1 was a right video-assisted thoracoscopy. One patient underwent 7 thoracotomies for metastatic disease, and was disease free after his last thoracotomy. The survival for this patient from the time of first metastasectomy was 6.8 years.
Of the 23 liver resections, 7 patients underwent a right hepatectomy, 3 underwent a left lateral segmentectomy, 3 had a segmentectomy, 2 had a trisegmentectomy, 1 had a posterior segmentectomy, and the remaining were non-anatomical resections.
Twenty-two out of the 57 patients were rendered NED after the first metastasectomy (39%). This includes staged procedures; many of these resections were followed by additional operations to target other sites of disease. For example, of the 23 first recurrence operations that were performed for pulmonary disease, 8 were followed by additional pulmonary resections.
Clinical Outcomes and Prognostic Factors
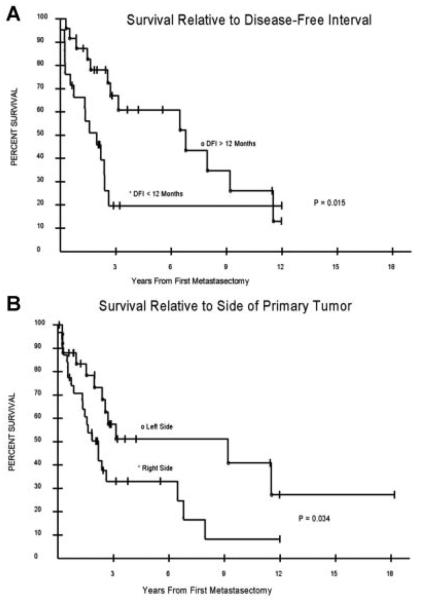
Disease-free interval was calculated for all patients who were rendered NED at their primary operation. For this population, DFI ranged between 2.8 months and greater than 12 years, with a median of 4.1 years. The median OS from the date of the first metastasectomy was 2.5 years, with a range of 2.8 months to greater than 12 years, and a 5-year survival of 41% (Fig. 1). The median potential follow up for this patient population was 8 years. Both DFI and the side of the primary lesion were significant prognostic factors. The median OS for patients with a DFI less than 12 months was 1.7 years (range: 2.8 months to >12 years), compared to 6.6 years (range: 3.6 months to >12 years) for a DFI greater than 12 months (P = 0.015). Median OS was 1.9 years (range: 3.1 months to >12 years) for right-sided primaries, compared to 3.8 years (range: 2.8 months to >18 years) for left-sided primaries (P = 0.03). Kaplan–Meier curves for these prognostic factors are shown in Figure 2A,B. The median DFS of patients rendered NED after first metastasectomy was 6 months. The median PFS of the population not rendered NED was 2.4 months. There were no 30-day post-operative deaths in this series. Liver metastases were more common (67% vs. 41%) in patients with right-sided primaries (P = 0.05).
Fig. 1.
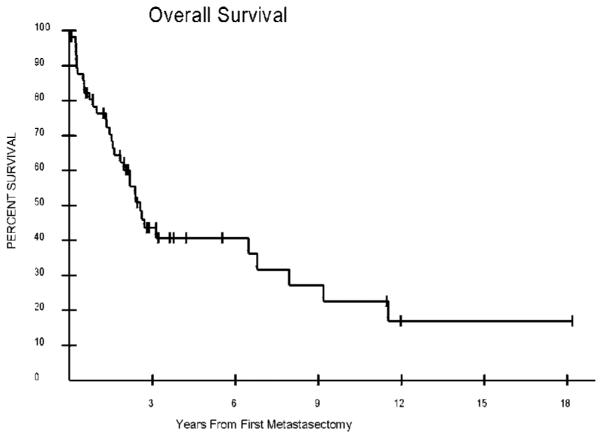
Overall survival from the time of first metastasectomy for 57 patients who all underwent at least one metastasectomy. At 5 years, the overall survival was 41%, with a median survival of 2.5 years and a range of 2.8 months to 12+ years.
Fig. 2.
Prognostic factors. A: Disease-free interval longer than 12 months was associated with longer survival (6.6 years vs. 1.7 years, P = 0.015). B: Left-sided primary was associated with a longer survival (3.8 years vs. 1.9 years, P = 0.034).
Other prognostic factors that were evaluated, but not found to be statistically significant, included OS by sex, stage, functional status, NED at initial operation, and by administration of chemotherapy (Table II). Median OS was 2.7 years versus 1.7 years for women and men, respectively. For stage II patients at initial presentation, median survival was 1.9 years versus 2.3 and 2.4 years for stages III and IV (P = 0.86). Median survival for patients with non-functional tumors was 5.9 years versus 1.8 years for those who presented with Cushing's syndrome. Median survival for all patients with any functional tumor was 2.2 years. The difference in survival between functional and non-functional tumors did not reach statistical significance (P = 0.12). Forty-five patients were rendered NED at the initial operation; their median survival was 2.6 years versus 1.6 years in the patients who were not rendered NED at initial operation (P = 0.34). Of the 6 patients who received neoadjuvant chemotherapy, median survival was 3.9 years versus 2.4 years for those who did not. For those receiving adjuvant chemotherapy, the median survival was 2.4 years versus 2.6 years for those who did not (P = 0.8). Of the 34 patients receiving adjuvant therapy, 25 patients received mitotane, either alone or with other drugs. Eleven received only mitotane in the adjuvant setting. The other commonly used adjuvant drugs were cisplatin, doxorubicin, and etoposide; each was administered in 8 patients, alone or in combination with other drugs. Gemcitabine was used in 2 patients.
TABLE II.
Selected Prognostic Factors
| P-value | |
|---|---|
| DFI >12 months | 0.015 |
| Left-sided primary tumor | 0.034 |
| Neoadjuvant chemotherapy | 0.46 |
| NED at initial operation | 0.34 |
| Female gender | 0.29 |
| Nonfunctional tumor | 0.22 |
| First recurrence to site other than liver | 0.39 |
DISCUSSION
Complete surgical resection is the established approach for the treatment of primary ACC, and previous reports have demonstrated that the effectiveness of other modalities is limited at best [6,12]. Repeat surgical resections for metastatic or recurrent disease, while commonly practiced at large cancer centers, is not as well established in the literature. We therefore reviewed our experience with this disease at a single institution, in order to describe the outcome of patients who have undergone metastasectomies for ACC. We found that DFI greater than 1 year, and left-sided primaries were associated with improved survival.
The first statistically significant prognosticator in this series was DFI; those with a DFI over 1 year had a 5 years improved median survival. As there are not many other studies that specifically evaluate patient outcome after metastasectomy (single or multiple) for ACC, there is not a substantial amount of previously reported data on the influence of DFI in determining survival after repeat resections. While not well established in ACC due to the limited number of cases, a direct relationship of DFI to survival has been well documented in surgical oncology literature [11,13,14]. This observation, along with our findings, suggests that the evaluation of patients for repeat resections should include the length of DFI, with a longer interval portending a better survival outcome.
In our series, 53% of patients initially had a right-sided primary; interestingly, these patients had a statistically significant decreased survival compared to those who had a left-sided primary. When further investigated, the patients with right-sided primaries were more likely to have their first metastasis to the liver than those with left-sided primaries (53% vs. 33%). This may have contributed to the difference in survival observed in this population, although there was not a statistically significant difference in the survival of patients who had their first recurrence to the liver versus those who did not. Based on the fact that liver metastases that portray worse prognosis in ACC were more common in right-sided primaries, we theoretically could set the null hypothesis as following: Right-sided primaries have worse prognosis. Accepting this null hypothesis, as we have done in our study, might be erroneous, that is, accepting the null hypothesis incorrectly or type-II error. This is usually the case when the sample size is too small. We do not see any anatomical or physiological basis for this observation and thus conclude that it is a type-II statistical error.
The majority (79%) of patients were rendered NED at their initial resection, indicating a strong propensity for ACC to recur, even after an oncologically sound operation. Most reviews do note a significantly improved survival associated with complete or curative resection of the primary [8,12,15,16]. Our population also had a 1-year improvement in median survival in patients rendered NED at the primary operation, but this difference was not statistically significant. There was no difference in survival for our patients who were rendered NED at the first metastasectomy compared to those who were not (2.4 years vs. 2.39 years). This finding is most likely confounded by the fact that many of these procedures were staged, and these patients went on to subsequent resections, which may have rendered them NED. The median survival after complete resections ranges from 28 to 74 months in other studies [2,6,17]. This is consistent with our observed median survival of 2.6 years.
All patients reviewed here had at least one procedure for recurrence or metastatic disease, and about half (49%) had at least two procedures for recurrences. As our group reviewed specifically those patients who underwent metastasectomies, 53% of our population initially presented with stage IV disease. Previous reviews of ACC have reported that metastatic disease on initial presentation ranges between 18% and 39% [2,5,17]. The most common repeat resection performed in this series was for recurrence in the lung, representing 46% of all repeat metastasectomies. Similarly, Schulick's review noted that 55% of resected metastases were in the lung. This observation underscores that ACC has significant metastatic potential, in addition to being locally recurrent.
Interestingly, stage at presentation did not predict survival. For stage II patients at initial presentation (T2N0M0), median survival was 1.9 years versus 2.3 and 2.4 years for stages III and IV (P = 0.86). How is it possible that stage II patients fared worse than those of higher stages? One potential explanation could be selection bias. While it is true that when one looks at large population, stage II at presentation should fare better than later stages, it is known that certain early stage cancers have bad prognosis, that is, the outliers for given stage. In the NIH, we see mostly patients who fail several treatments before coming to the NIH. A stage IV patient that makes it to the NIH, and makes it long enough to get at least two resections, clearly has a different stage IV tumor biology, potentially more indolent than the average stage IV. Whereas a patient who presents as stage II and then progresses fast to metastatic or recurrent disease arguably has potentially a more aggressive biology.
Consistent with observed demographics in the literature, our population was predominantly female (63%), and patients most commonly presented in the 5th–6th decade of life [2,3,6]. The lung and liver were common sites of first metastasis (39% and 28%, respectively) in our population; in 9 patients metastases to both the lung and liver were detected at the same time. Prior reviews have indicated the liver as the most frequent site of metastatic disease, with the lung listed as another common site [2,6,7,17].
Most series reported either an equal prevalence of right-sided ACC versus left-sided ACC, or a slight predominance of left-sided lesions. A 1993 analysis from Wooten and King [18] that reviewed a total of 1,891 patients found a left-sided incidence of 52.8%. Schulick and Brennan's [6] review contained 56 patients with right-sided tumors and 57 presenting with tumors on the left. Bilimora et al.'s [2] publication of 3,982 patients from the National Cancer Database observed 49.6% presented with tumors on the left, compared to 41.3% on the right, the remainder presenting bilaterally or not specified. An Italian retrospective series of 129 cases saw no difference in the side of presentation [17].
As reflected in our review, a wide range of chemotherapeutic drugs are implemented to medically treat ACC, with mitotane being the most commonly used. While some studies have shown partial tumor regression and transient control of endocrine symptoms, most fail to show an improvement in survival [15,18–20]. In a retrospective analysis from Grubbs et al. [3], adjuvant mitotane did not significantly affect OS, but did improve DFS from 12 to 30 months. A review of 55 patients from Holland reported a survival advantage in unresectable or recurrent ACC treated with mitotane, when the serum drug levels were kept greater than 14 mg/L [12]. Of note, significant toxicities are reported with mitotane, including lethargy, depression, nausea, anorexia, as well as adrenal insufficiency, due to the drug's-specific targeting of the adrenal cortex and the resulting toxicity to normal adrenal tissue. An isomer of the pesticide DDT, mitotane has a narrow therapeutic window, and its toxicity is often dose limiting [11]. In our review, the number of patients receiving neoadjuvant therapy was small [5], making it difficult to draw conclusions from this group. Sixty percent of our patient population received adjuvant chemotherapy; 74% of these regimens included or solely consisted of mitotane. The comparable median survival between these two groups is consistent with other reports in the literature. This emphasizes the importance of complete surgical resection, given the poor efficacy of systemic drugs.
While complete resection of ACC remains the standard of care, a multidisciplinary approach including RFA can be important for this rare and aggressive disease. The use of RFA in this series is predominantly in addition to a surgical resection; only 3 procedures consisted of RFA alone. The integration of this modality into our series demonstrates the safety of this procedure at a specialized institution, and its usefulness when combined with surgery to treat lesions that might otherwise be considered unresectable. Previous reports indicate a palliative role for RFA as well [10].
Given the retrospective nature of this review, it is difficult to draw concrete conclusions from the associations observed. However, this is a large series for this particular disease, and the patient demographics are similar to those observed in other studies. The prognostic indicators associated with survival also reflect those observed by other authors, with the exception of the side of the primary tumor [11]. Our observation that right-sided primaries have diminished survival may be explained by the propensity of these tumors to invade or metastasize to the liver. However, the selection bias inherent in this type of review precludes a definitive conclusion on the significance of a right primary tumor versus left primary tumor.
From this and other studies, recurrent and metastatic ACC carries a poor prognosis and has no effective systemic therapy. Previously established is the improved survival with complete resections, and our data indicate that the longer the patient is recurrence-free, the better outcome they are likely to have. Furthermore, diminished survival and a likelihood of hepatic metastases were observed in patients with right-sided primaries. Of note, there were no 30-day mortalities in the patients undergoing surgery for resection of metastatic or recurrent disease, indicating the relative safety of this approach. Therefore, the decision to surgically treat metastatic and recurrent disease should involve DFI from the last resection as well as technical feasibility.
REFERENCES
- 1.Schteingart DE, Doherty GM, Gauger PG, et al. Management of patients with adrenal cancer: Recommendations of an International Consensus Conference. Endocr Relat Cancer. 2005;12:667–680. doi: 10.1677/erc.1.01029. [DOI] [PubMed] [Google Scholar]
- 2.Bilimora KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: Treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 3.Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: Surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol. 2010;17:263–270. doi: 10.1245/s10434-009-0716-x. [DOI] [PubMed] [Google Scholar]
- 4.Wandoloski M, Bussey KJ, Demeure MJ. Adrenocortical cancer. Surg Clin North Am. 2009;89:1255–1267. doi: 10.1016/j.suc.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Weiss LM, Medeiros LJ, Vickery AL. Pathological features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol. 1988;13:202–206. doi: 10.1097/00000478-198903000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6:719–726. doi: 10.1007/s10434-999-0719-7. [DOI] [PubMed] [Google Scholar]
- 7.Di Carlo I, Toro A, Sparatore F, et al. Liver resection for hepatic metastases from adrenocortical F carcinoma. HPB. 2006;8:106–109. doi: 10.1080/13651820500471848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Icard P, Chapuis Y, Adnreeassian B, et al. Adrenocortical carcinoma in surgically treated patients: A retrospective study on 156 cases by the French Association of Endocrine Surgeons. Surgery. 1992;112:972–980. [PubMed] [Google Scholar]
- 9.Polat B, Fassnacht M, Pfreundner L, et al. Radiotherapy in adrenocortical carcinoma. Cancer. 2009;115:2816–2823. doi: 10.1002/cncr.24331. [DOI] [PubMed] [Google Scholar]
- 10.Wood BJ, Abraham J, Hvizda JL, et al. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer. 2003;97:554–560. doi: 10.1002/cncr.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ripley RT, Kemp CD, Davis JL, et al. Liver Resection and ablation for metastatic adrenocortical carcinoma. Ann Surg Oncol. 2011;18:1972–1979. doi: 10.1245/s10434-011-1564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haak HR, Hermans J, van de Velde CJ, et al. Optimal treatment of adrenocortical carcinoma with mitotane: Results in a consecutive series of 96 patients. Br J Cancer. 1994;69:947–951. doi: 10.1038/bjc.1994.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng YC, Ueno NT. Improvement of survival and prospect of cure in patients with metastatic breast cancer. Breast Cancer. 2011 May 13; doi: 10.1007/s12282-011-0276-3. [Epub ahead of print]. PMID 21567170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leong SP, Nakakura EK, Pollock R, et al. Unique patterns of metastases in common and rare types of malignancy. J Surg Oncol. 2011;103:607–614. doi: 10.1002/jso.21841. [DOI] [PubMed] [Google Scholar]
- 15.Soreide JA, Braband K, Thoresen SO. Adrenal cortical carcinoma in Norway, 1970–1984. World J Surg. 1992;16:663–668. doi: 10.1007/BF02067349. [DOI] [PubMed] [Google Scholar]
- 16.Zografos GC, Driscoll DL, Karakousis CP, et al. Adrenal adenocarcinoma: A review of 53 cases. J Surg Oncol. 1994;55:160–164. doi: 10.1002/jso.2930550306. [DOI] [PubMed] [Google Scholar]
- 17.Crucitti F, Bellantone R, Ferrante A, et al. The Italian Registry for Adrenal Cortical Carcinoma: Analysis of a multiinstitutional series of 129 patients. Surgery. 1996;119:161–170. doi: 10.1016/s0039-6060(96)80164-4. [DOI] [PubMed] [Google Scholar]
- 18.Wooten MD, King DK. Adrenal cortical carcinoma. Cancer. 1993;72:3145–3355. doi: 10.1002/1097-0142(19931201)72:11<3145::aid-cncr2820721105>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Pommier MF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;122:963–971. [PubMed] [Google Scholar]
- 20.Luton J, Cerdas S, Line B, et al. Clincal features of adrenocortical carcinoma, prognostic factors, the effect of mitotane therapy. NEJM. 1990;322:1195–1201. doi: 10.1056/NEJM199004263221705. [DOI] [PubMed] [Google Scholar]