Abstract
Ongoing concerns over the rising cost of health care are driving large-scale changes in the way that health care is practiced and reimbursed in the United States. To effectively implement and thrive within this new health care delivery environment, academic medical institutions will need to modify financial and business models and adapt institutional cultures. In this paper, we review the expected features of the new health care environment from the perspective of academic radiology departments. Our review will include background on Accountable Care Organizations, identify challenges associated with the new managed care model, and outline key strategies—including expanding the use of existing information technology infrastructure, promoting continued medical innovation, balancing academic research with clinical care, and exploring new roles for radiologists in efficient patient management—that will ensure continued success for academic radiology.
Keywords: accountable care organization, research, informatics, quality, value
Introduction
Continued growth in health care spending with a constantly aging population has propelled concerns about the solvency of the current health care system in the United States. Health expenditure has risen dramatically over the last 50 years (17.4% of gross domestic product (GDP), compared to 11.4% for Canada in 2009), however, U.S. health performance lags behind by comparison based on indicators such as life expectancy, quality, access, efficiency, and equity (1, 2). Nonalignment of cost with performance triggered the 2010 panel discussion by the Institute of Medicine (IOM). Factors identified by the IOM as contributing to the cost-performance nonalignment included prevalence of chronic disease, lifestyle, population health demographics (such as the obesity epidemic), but also inefficient delivery of services (excess administrative costs, unnecessary services, high pricing, deficiency in preventative care, and fraud, amounting to $765 billion) (3). Furthermore, considerable variation in quality of care (as indicated by readmission rates per Medicare beneficiary) has been reported without correlation to regional costs (4, 5).
In this paper we will broadly review the landscape of the new health care delivery environment from the perspective of academic medical institutions and anticipated impact on the future of radiology. Our review will include a background on Accountable Care Organizations and challenges associated with the new managed care environment, use of technology for managing data-intensive environments, role of radiologists in medical innovation, defining new boundaries and roles for radiology in patient management, and implications of balancing academics and clinical care.
Patient Protection and Affordable Care Act
Payment reform is based on the premise that the current fee-for-service payment incentivizes physicians to increase services with consequent excess utilization. Over-utilization of sub-specialty services relative to perceived appropriate level of management in the primary care environment has resulted in the targeting of subspecialist physicians including radiologists and procedure-centric physicians such as interventional cardiologists or gastroenterologists. In an attempt to avoid overuse of imaging and subspecialist referral, a number of payment models have been put forward ranging from prospective payment for discrete episodes of care to global payment or risk-based care (6).
Direct Mechanisms for Reduced Reimbursement
Global prospective payments were a key feature of managed care programs that peaked in the 1990s. However, anticipated payment capitations were stemmed by unpopular restrictions on choice and access to services. Over a decade later, concerns regarding nonalignment of health care cost and quality have renewed the interest in global payment schemes, in part through Accountable Care Organizations (ACOs) introduced by the Patient Protection and Affordable Care Act (ACA) of 2010. The ACA also directly addresses expenditure on imaging services under the existing fee-for-service model through increases in: a) “assumed utilization rates” and b) “multiple procedure payment reduction.”
The assumed imaging utilization rate is used to determine practice expense relative value units for “expensive” imaging equipment (ostensibly encompassing computed tomography, magnetic resonance imaging, and positron emission tomography). A higher assumed utilization rate results in a lower technical component reimbursed through Medicare for each imaging study under such modalities. Since 1997, the assumed utilization rate was set at 50%, however, this has been increased to 90% beginning in 2014, as the result of the American Taxpayer Relief Act of 2012.
Multiple Procedure Payment Reduction (MPPR) is a reimbursement model designed to capture savings from efficiencies consequent to multiple services being rendered in the same session. Originally applied to surgical procedures, MPPR permitted the highest-paying surgical procedure to be reimbursed in full while additional procedures would be reimbursed at a discounted rate. In 2006, the Centers for Medicare and Medicaid Services (CMS) introduced the MPPR into imaging services by instituting a 25% reduction to the technical component of CT, CTA, MRI, MRA, and ultrasound performed on contiguous body parts within one of eleven “imaging families”. Each of these families contained billing codes for an imaging modality paired with an anatomical region (e.g., CT of the spine; MRI or MRA of the chest, abdomen, or pelvis; CT or CTA of the lower extremities). In 2011, MPPR was broadened such that the reduction in reimbursement applied when contiguous body parts were scanned regardless of the relevant code family (7). In addition, the ACA has instigated a reduction in imaging reimbursement through increasing the MPPR of the technical component of a study from 25% to 50%. In 2012, CMS also decreased imaging reimbursement by reducing the professional component of a study by 25%. CMS further intends to apply the reduced payment scheme when different physicians provide diagnostic services to the same patient in the same session, and has considered extending the MPPR to the professional and technical components of all imaging modalities (8).
In an attempt to address self-referral, the ACA requires physicians to disclose when referring patients to imaging facilities they own. However, the likelihood of such disclosure limiting self-referral may be restrained since it is not accompanied by any direct impact on imaging reimbursement.
Accountable Care Organizations
While not specifically described by the ACA in the context of medical imaging, ACOs may have the largest impact on the future practice of radiology. ACOs have been described as networks of physicians and other providers that could work together to improve the quality of health care services and reduce costs for a defined patient population. The ACO is comprised, at minimum, of primary care physicians who can serve 5,000 Medicare beneficiaries. Specialists and hospitals may be contracted. Evidence-based medicine, quality and cost control measures, and coordinated care must be demonstrated. Practitioners, including radiologists, do not have to work exclusively with an ACO.
From the outset, the ACA prescribed ACO reimbursement under a fee-for-service model, with additional shared savings revenue available in exchange for reducing expenditures below benchmarks set by the Secretary of the Department of Health and Human Services. In 2013, CMS entered the first phase of its Bundled Payments for Care Improvement initiative, which has chosen select health care organizations as partners in episodic bundling of payments. By assuming more financial risk, providers can potentially net higher reimbursement under a bundled payment model as compared to the fee-for-service model with shared savings. In the future, with expected growth of ACOs, payment models could move to partial or full capitation as providers take on full financial risk of caring for larger populations. A capitation model would theoretically reward organizations for delivering coordinated care in an effective and efficient manner. Private insurers have also experimented with bundled payments and capitation, and will likely continue to do so as the results of the various CMS payment arrangement experiments are brought to light.
Payment Structure for Providers under the ACO Model
The method of payment received by an ACO for services rendered may differ from that which it chooses to pay its providers. For example, while the ACO of the future may receive payment under a capitation model, it may pay its individual providers on a fee-for-service (FFS) basis or through direct employment. The ACR Future Trends Committee argues for preserving imaging reimbursement under a FFS payment model, or some derivative thereof, as alternative models could prove unsustainable to the ACO in the setting of high technical costs associated with unchecked overutilization. In other words, preserving the FFS model for reimbursement of imaging services within an ACO could be used to incentivize ordering providers to limit the utilization of imaging services appropriately. A more compelling approach for an ACO, where FFS is the dominant compensation model, would be to align payment with validated quality metrics that relate directly to improved patient outcomes and cost control.
Linking physician reimbursement to measures of quality and efficiency of service—a model known as pay-for-performance (P4P)— is increasingly being sought. Potential advantages of P4P include containment of cost, reduction of waste and inefficiency, and improvement in the quality and value of health care (9-11). Although desirable, there are significant challenges to applying P4P to radiology due to a lack of standardized radiology performance metrics and the difficulty of linking imaging with patient outcomes. Physician Quality and Reporting System (PQRS; formerly Physician Quality and Reporting Initiative or PQRI) is one of the P4P programs relevant to radiologists, providing incentives and payment adjustments for compliance with reporting and coding requirements, thereby promoting reporting of quality information. However, this program has been criticized for “rewarding physicians and practice conformance with rigid documentation, reporting, and coding requirements” rather than encouraging improved patient care. Several performance-measure goals and activities have been proposed including:
Create a set of radiology performance measures and objectively measure the quality of radiology practices;
Create outcome and process metrics that have target benchmarks for performance;
Identify metrics that emphasize the value added of radiology and are useful in continuous quality improvement within radiology practices;
Promote the widespread use of registries such as the National Radiology Data Registry;
Continue to promote the use of the Appropriateness Criteria or other forms of Decision Support in Computerized Physician Order Entry as a tool to reduce inappropriate imaging;
Develop specific performance measures as part of program accreditation (12).
Indeed, the ACA also introduced value-based payment modifiers in an attempt to move reimbursement towards P4P. These modifiers will adjust the standard Medicare physician payments to providers based on the ratio of quality of care to cost. Physicians who provide low quality care at high cost will receive lower reimbursement. The modifiers will be based on existing quality programs such as PQRS and will be phased in for all physicians by 2017. CMS has solicited help from the American Board of Radiology (ABR) and other specialty boards to develop these quality measures (13).
Providing Value for Radiology within an ACO
If the shared-savings model of the ACO experiment succeeds, referring physicians and radiologists alike will have to demonstrate their value to the system by promoting and maintaining cost-effective health care delivery. Aside from delivering a diagnostic and procedural service, there are several non-interpretive areas that provide significant opportunities for radiologists and radiology practices to add value to an ACO. The most prominent of these areas is utilization management, in which radiologists use their expertise in imaging to ensure that imaging studies are performed appropriately. Strong suggestions are being made for radiologists to align with general practitioners to guide the appropriate use of imaging and referral to subspecialists (14). One approach to effective utilization management is implementation of decision support in the context of computerized order entry (15-18). Such systems may decrease inappropriate utilization by requiring peer-to-peer consultation with a radiologist for examinations determined to be low yield and offering a means to compare utilization rates of individual providers to established benchmarks. This approach would be a natural expansion of the “reading room consultation” model and would help to counteract potential commoditization of our service by non-physician resource management groups (12).
In addition to utilization management to ensure appropriate imaging, additional non-interpretive value-added activities well-suited to radiology include managing the imaging enterprise, engaging in value-based and comparative effectiveness research, participating in hospital and medical staff governance, managing the ACO's Information Technology (IT) infrastructure, and promoting quality and safety at the departmental and institutional levels. ACO-related target activities for radiologists (non-interpretative goals) include (12):
Work with national quality groups such as the National Quality Forum to devise useful metrics for radiology;
Sponsor value-based and comparative effectiveness research;
Interact with other specialty societies to establish security for hospital-based physicians in ACOs;
Work with radiology societies to promote use of Appropriateness Criteria;
Facilitate communication among radiologists in ACOs through the creation of Accountable Care Committee and the Accountable Care Network;
Develop (or support ongoing commercial development of) a decision support tool based on the Appropriateness Criteria for utilization management;
Create management training tools for radiologists.
Promoting Quality, Safety, and Best Practices
Providing high quality, cost-effective health care is the primary goal of an ACO. Establishing comprehensive quality and safety programs to improve quality of care and patient safety, as well as reduce waste and inefficiency are necessary in order for an organization to realize this goal. Acknowledging that there are many suboptimal patient care processes within a radiology department that can lead to patient harm is the first step towards a culture of continuous quality improvement (CQI).
Application of CQI methodologies has been shown to reduce errors, improve patient outcomes, decrease costs, increase staff productivity, and improve both customer and employee satisfaction. Two business management strategies are particularly well suited for CQI in radiology departments. The first, known as “lean,” is a method of process improvement that focuses on continually identifying and eliminating waste. The second, known “Six Sigma,” focuses on minimizing the frequency of defects (19).
CQI requires a system for objective performance measurement to assess and evaluate key components of an organization by setting performance goals and trending performance over time. Fisher and colleagues described several measures of quality, including technical quality, mortality, physician satisfaction, and patient satisfaction (5). There can be translated into five major categories of performance metrics including patient safety, productivity, finance, access, and customer satisfaction (20).
Within the category of patient safety, one can define a variety of performance indicators (20):
Contrast injection extravasation rates
Contrast reaction rates
Contrast-induced nephropathy rates
Nosocomial infection rates
Medication error rates
Patient falls with harm
Compliance with critical test and results reporting
Specimen labeling errors
Compliance with universal protocol, hand hygiene
Elements of informed consent
Mislabeled examinations
Wrong site procedure, wrong procedure, wrong side procedure, and wrong patient
Major discrepancies in trainee preliminary reports
Section-specific and modality-specific performance measures can also be tracked and reported, such as CT dose reduction, sedation/analgesia documentation compliance, and correlation of imaging with surgery or pathology. Other possible methods of performance measurement include peer review, report turn-around time, participation in the American Board of Radiology maintenance of certification (MOC) program, and documentation of complication rates and diagnostic yield for procedures (21, 22).
In comparison to other medical specialties, there is currently a dearth of nationally recognized radiology-specific quality measures. Furthermore, many of the process defects and inefficiencies identified using CQI in a radiology department are specific to that local environment. In order to establish benchmark performance and share best practices, radiology must develop nationally accepted process and outcomes measures related to quality, safety, teamwork, and communication. National benchmarks proposed to assess performance and demonstrate CQI include:
Facility accreditation
MR/radiation safety programs and radiation dose index
Evaluation of service to patients and referrers
PQRS participation
MOC
AART registered technologists
ACR practice guidelines and technical standards
ACR appropriateness criteria
Finally, radiologists working under an ACO must demonstrate the value our services add to the management of patients, specifically in downstream time and resources saved. Dividing a bundled payment among involved providers may hinge upon time-and resource-saving metrics. Responsible use of imaging and the information we derive from it helps guide timely patient treatment, monitoring of therapy, and appropriate referral to subspecialists. Quantification of these savings, if responsibly performed, should work to the radiologists' advantage (23). Great transparency in quality of service through metrics may also be applied to efficiency and expenditure. Cost-transparency engines (e.g., consumer reports, CastLight Health, the in-house RBM databases, etc.) enable consumers to not only recognize but also compare what their money can buy at different institutions.
Anticipated shared savings in the ACO model would likely demonstrate dwindling variation in year-to-year savings, and eventually there would be an expected reduction in imaging (and other sub-specialty) utilization. Given the loss in reimbursement in a fee-for-service model that would result from declining imaging volume, salaried compensation with additional compensation for management of resources or quality and safety programs may be the best option for radiologists under a shared savings model. Consideration should also be given to the number of radiologists being trained, since the number of available positions may be reduced.
Health Information Technology: A Key to Data Management
The past 20 years have seen a transformation of medicine toward an increasingly complex and data-driven model of care. These rapid changes are the result of several distinct but intersecting trends including scientific advances in medical imaging, genomics, laboratory and pathological diagnostics, updated health care information technology infrastructure, and cultural shifts in medicine toward evidence-based decision-making. The proliferation of data has transformed the clinical practice of medicine. Breast cancer is now identified with respect to its subtypes defined by genetics, tissue type, and extent of spread, with equally many variations in therapy. In this new paradigm, patient information is patient management.
Technologies to manage health information, such as Electronic Medical Records (EMR), Radiology Information Systems (RIS), and Picture Archiving and Communication Systems (PACS), have been critical in the transformation of modern health care (24, 25). Improvement in IT infrastructure has been accelerated by the Health Information Technology for Economic and Clinical Health (HITECH) Act of 2009 (26) through incentives for the implementation and adoption of electronic health records to encourage health information exchange (27).
Development of technologies related to acquisition, storage, retrieval, display, and distribution of medical imaging have not been specifically addressed under the umbrella of “Meaningful Use” (MU), a collective of government-sponsored initiatives designed to encourage providers to use health care information technology solutions to improve the quality of care while lowering costs. The Centers for Medicare and Medicaid Services (CMS) have not precisely defined the term “meaningful,” but instead apply MU to any application of technology that achieves defined patient and provider functionality, divided in different stages and through the incorporation of core objectives (https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/downloads/MU_Stage1_ReqOverview.pdf).
Data-Intensive Environment
Radiology has always been at forefront of health IT, particularly in the management of large data sets generated by imaging. In order to keep up with the widespread adoption of multi-detector CT and MRI, radiologists led the incorporation of PACS into clinical practice, first in large institutions, followed by smaller practices and organizations (28, 29). Combined with emerging web technology and other ancillary systems, PACS has become a central component of most radiology departments, supporting a more efficient workflow, reducing operating costs, and improving the communication between radiologists, technologists, referring physicians, and patients (30, 31).
RIS is a computerized database used by radiology departments to store, manipulate and distribute patient radiological data. It commonly consists of patient identification and exam history, patient scheduling and tracking, and result reporting, as part of the Hospital Information System (HIS) or EMR. RIS serves the bridge between EMR and PACS. In recent years, continued enhancements in RIS and PACS have resulted in more sophisticated, logical, and effective systems which take advantage of Web technology to efficiently distribute images to non-radiology departments (32).
Connectivity, Interoperability, and Image Transfer
Efficient and reliable EMR/RIS/PACS systems require industry accepted communication protocols and standards such as DICOM and Health Level (HL7). Initiatives—such as “Integrating the Health Care Enterprise” (IHE), sponsored by the Hospital Information Management Systems Society and the Radiological Society of North America, are focused on improved end-user access to clinical information across all systems within the health care delivery network (32). In addition, there has been growing interest in the implementation of novel web-based patient engagement technologies (e.g. “MyChart”), enabling patients to access their own health records (though often abridged). Such tools will invariably have downstream ramifications such as language modification to use “patient-friendly” terminology in order to avoid patient confusion and anxiety. Patient, or customer, access to electronic medical reports is here but the extension of such means of communication necessitates collaboration between patient care teams.
Although most vendors have cooperated vis-à-vis the goal of systems integration, full integration continues to be hampered by business and political interests (32). Such challenges in the medical IT infrastructure exist not only within individual institutions, but are protracted at the inter-institutional level, hampering consolidation of patient records and, limiting data universal accessibility (27).
To put inter-institutional connectivity into context, an important topic to be discussed is re-imaging in transfer patients. In the United States alone, there are an estimated 2.2 million patient transfers between hospitals each year. These are commonly critically ill and extensively imaged patients, initially evaluated at community hospitals and regional medical centers, subsequently transported to tertiary care hospitals, where CT repeat rates of up to 58% are observed. Unnecessary re-imaging in these patients adds cost, delays care, and results in higher levels of radiation exposure and contrast administration (33-35). Improved methods of transferring imaging data and reports to receiving institutions should increase efficiency, reduce errors and the need for repeat imaging (33). Not only transfer of medical data, but also consistency in imaging quality, and requirement of secondary opinions on outside studies are important considerations from the perspective of workflow, and reimbursement.
Research and Innovation in Radiology
Continued emphasis on innovation and progress in medical imaging will be essential to maintaining and augmenting radiology's value in the health care environment of the future. Affirmation of sustained innovation in radiology can occur through areas such as ongoing technical advancements in diagnostic modalities, development of novel imaging techniques and minimally invasive, image-guided therapeutic procedures (36-49). However, declining resources to fund development of new imaging technologies, and an increasingly cost-conscious health care system will likely restrict not only research development but also clinical implementation of radiology innovation on a fiscal basis. In a system with diminishing resources, the need for continued imaging research requires strong justification. Demonstration of the clinical value of new imaging techniques requires maintaining a focus on conducting rigorous comparative effectiveness research to establish the true benefits of these innovations.
Beyond economic challenges, additional hurdles to modern radiologic innovation include the lack of extensive research training among radiology residency training programs; lack of recruitment of research experts to the specialty, especially engineers, biochemists, biophysicists, geneticists, computer scientists and physicists; lack of appropriate departmental resources to support modern radiologic research, including insufficient time for research, inadequate mentoring or support, and insufficient funding for young investigators; lack of immediate fiscal value in an environment that emphasizes maximal clinical productivity; and lack of promotion of and participation in interdisciplinary research (50-55). These barriers, while significant, can serve as a cautionary roadmap for radiology research in the complex health care environment of the future.
Walking the academic-clinical tight rope
The challenge facing many academic departments in the new health care delivery climate is a question of priority. What kind of department do we want to be? How can we integrate research into our department and training programs? Given the constant encroachment of other sub-specialties on medical imaging, how do we envision radiology in the future? What important clinical questions can and will be answered with imaging? How can we continue to be an integral part of patient care? No doubt, the decisions we make regarding radiology research today will influence who we are tomorrow.
Over the last decade, academic medical centers have shifted emphasis from research to service, recognizing that the ivory tower existence is at risk of extinction without the balance of excellence in service. Competition from private practice for health insurance contracts and physician referrals, continued cuts in reimbursement, and economic pressures to increase RVUs have all resulted in increased clinical focus with less time for research, teaching and mentoring. The very nature of the academic environment is at odds with high volume turnover of clinical studies because only extra-mural funding and publication of peer reviewed manuscripts are often measures of merit (57), and training of residents and fellows is a both mandatory and a necessity. Such challenges could be in part be addressed by the establishment of the “expert clinician” for a “clinical” rather than “research” track. While clinical tracks do exist within the academic environment, they are typically associated with less prestige and considered less desirable. Within the ACO model, the expert clinician may be a means to bridge the clinical and academic mission, and extend services into the medical community.
Redefining Radiology Boundaries
For decades, image interpretation was the exclusive domain of radiologists due to the confines imposed by the immobility of early imaging equipment and the requirement of hard copy images. However, technologic advances resulted in an eventual leveling of the medical imaging playing field: many imaging devices became portable and images became instantly transmissible via PACS. As imaging evolved into the key tool in diagnosis and management, other specialties became engaged in providing and interpreting imaging examinations (58). These changes have led to conflicts between radiology and non-radiology departments in some areas. Although it can be argued that imaging interpretations generated by a radiology department provide a higher quality of care at a lower price compared to imaging performed by non-radiology specialists (59, 60), competition between closely associated disciplines has blurred the boundaries between specialties and will likely only increase in the future. Therefore, radiologists may be best advised to focus less on actually winning the turf war and more on how to better negotiate these boundaries.
Radiology is at an important crossroads: successful negotiation will maintain radiology's central role in patient care and technologic innovation. Failed negotiation may result in further commoditization and fragmentation of medical imaging; in this setting, the radiologist will be quickly marginalized and supplanted by eager non-radiology specialists. However, a properly trained and cognizant radiologist has the potential to wield major advantages over other medical specialists (61). Therefore, successful negotiation of radiology's future will likely require a multi-faceted approach spanning categories such as service, quality control, and organization.
Service
Consistent high-level service is critical for radiologists to maintain a strong referral base from their clinical colleagues. The foundation of high-level service begins with education; radiology training programs and their guiding organizations must continue to modify requirements to accommodate the needs of the evolving health care environment so that graduates provide clinically relevant information more accurately and efficiently than their non-radiology colleagues. One way that this is happening currently is through revision of the residency curriculum by the American Board of Radiology, and the Accreditation Council for Graduate Medical Education to allow a higher level of sub-specialization (62). As radiologic knowledge continues to grow in breadth and depth, encouraging radiology residents to specialize will help to ensure that their clinical input will meet the expectations of clinical specialists and outpace other non-radiology specialists who also perform and interpret imaging examinations. In the future, standardization of fellowship curricula and national accreditation of all programs may serve to further enhance the contribution made by their graduates. Additionally, as the guiding and certifying bodies in radiology, continue to develop more specialized, clinically relevant maintenance of certification, this process can be used to substantiate the radiologist's role and to fulfill criteria set forth by government reimbursement programs such as ACOs (63).
Quality Control
In addition to mastery of image interpretation, radiologists can better negotiate their role in the health care system further developing expertise in the technology of the image acquisition process. Radiology residents and attending physicians alike should embrace their radiologic physics education with the same enthusiasm as their clinical radiology education. Recently, revision of the physics examination to include more clinically relevant material is a step by the American Board of Radiology to emphasize the need for re-dedication to the physics curriculum throughout residency (64). This revision has prompted some residency programs to create a more interactive, image-rich longitudinal physics curriculum (65). An understanding of image acquisition, display, and trouble shooting will position the radiologist as a central figure in the decision making process with hospital administration and policy makers. This deeper understanding of the imaging process can lead to improved clinical service through efficient creation of post-processed images and reports customized for the referring clinician. Finally, the combination of clinical and technical expertise parlay into quality control and safety. Since quality control and patient safety programs are entities highly valued by hospital administrations and government agencies, radiology's commitment to these programs is an avenue to demonstrate value and retain control of imaging equipment, protocols and policies.
Organization
As utilization control and cost containment continue to influence health care policy, the specialty of radiology can better negotiate its future by aligning itself with principles of the ACA and patient centered ACOs (66). For example, since the anticipated health care savings in the ACO model is partly based on decreasing unnecessary referrals and diagnostic testing. Radiologists are already well equipped to offer expertise with respect to the appropriateness of a diagnostic imaging test. By embracing this role within the ACO, radiologists can act as imaging gatekeepers within their own hospital system, as often occurs in commonwealth countries. This trend toward more patient-centric care will require radiologists to embrace more clinical interaction. In doing so, radiologists can assume a greater influence in patient care by more actively triaging and referring patients to other specialists (56).
In addition, the application of decision support systems and ACR developed appropriateness criteria, will enable radiologist to assume a pivotal role at the local and national level in the revision of diagnostic imaging utilization and reimbursement (67). Ultimately, radiology's success will hinge on its ability to function as part of a multidisciplinary team to curtail overutilization of imaging (68). In addition to radiology's role at local and national levels, increasing the degree of organization within radiology departments and groups can lead to additional benefits. Consolidation of all imaging services may serve to standardize management and simplify the process for referring physicians (55). The consolidation may incorporate appropriateness criteria, specific agreements with the referring clinicians, and 24 hour access to imaging subspecialists (55). The streamlining of imaging services, more efficient management of the EMR and assurance of quality and safety initiatives can be embraced by hospital managers, insurance representatives and clinicians alike.
Finally, the specialty of radiology must increase its organized efforts to address self-referral by non-radiologists. National radiology organizations can continue to highlight the fact that imaging self-referral by non-radiologists leads to increased costs, increased utilization and decreased quality (69, 70).
A Broader Picture of Patient Care and Information Management
In the new model of information-directed patient management, imaging represents a major source of patient health information across the entire spectrum of care provided and by a wide range of providers. As such, the specialty of radiology is uniquely poised to effectively guide multispecialty patient care across care boundaries. This strength of radiology demands consideration of novel, radiology-based approaches to patient management that recognize developing trends in the new model of health care delivery while maintaining sensitivity to issues of cost.
Radiologists as Consultants
One straightforward adaptation to enhance the yield and efficient utilization of radiology services is to replace imaging orders with consultation or a broader “umbrella” request category, rather than orders for specific imaging protocol, or specific interventional procedure. The advantage of radiology input at the order-entry level rather than a purely clinician-driven ordering is likely to improve the quality of clinical information available to the radiologist. Having access to robust clinical information has been shown to significantly improve the accuracy of radiologic interpretation (71-73), and is likely to increase the accuracy and specificity of radiologic interpretation, thereby enhancing the diagnostic yield of each study and reducing waste in a cost-conscious medical system. Early efforts to address this problem revolved around the implementation of decision support systems in the setting of computerized physician order entry (16, 74-78). With greater availability and sophistication of electronic medical record systems, access to pertinent clinical information should become integral to radiology workflow. Such an approach would allow the radiologist to assume greater responsibility for selecting and protocoling appropriate radiologic examinations (79). This consultative service would contribute to the non-interpretive value provided by the radiologist and help to reduce the burden on primary clinical providers to remain up-to-date on advances in imaging, and indeed, pilot studies making use of this approach have been received favorably (80). Moreover, packaging the multiple services of study selection, protocoling, and interpretation within the umbrella of a single consultation request would create an organizational schema more compatible with the reimbursement structure championed within the ACO model of care. Part of the consultation process could be instituted through electronic order entry permitting appropriate correction of requests by the radiologist according to accepted standards of care, and evidence-based medicine.
Direct Patient Communication
A second, critical component of reform is direct communication with patients. Though there has been much interest in direct radiologist-to-patient communication in recent years (81-86), such practice is uncommon outside of mammography. Research has shown that while patients remain confused or unaware of the important role that radiologists play in their care, they do appreciate expert consultation in this arena (85). Direct communication emphasizes the expert role of imaging specialists and allows patients the rare opportunity to discuss their imaging with the physician most qualified to respond to their concerns. Besides reinforcing the professional identities of radiologists as highly trained and subspecialized physicians, these high-fidelity channels of communication are likely to reduce the number of “forgotten findings,” simultaneously ensuring timely patient care and reducing the radiologists' medicolegal liability (87).
Radiologist Management of Imaging Follow up
Radiologists should also assume a greater role in actively managing radiology follow up. For the purpose of illustration, consider the familiar example of an incidentally detected pulmonary nodule. Traditionally, patients with incidental nodules are referred back to their primary care providers with the suggestion to reimage at a pre-specified time interval. The primary provider, though continuing to serve a vital role in the management of the patient's other conditions, adds little to the management of the pulmonary nodule other than to enter an order into the system for a repeat imaging study at a later date. In fact, the current arrangement creates many opportunities for mismanagement or noncompliance with follow up recommendations (88) and unnecessarily burdens the primary physician with administrative tasks. In contrast, there is much appeal in a more efficient alternative scenario in which the radiologist manages the work up of the nodule, first by informing the patient of the finding, and then by performing serial imaging according to established guidelines. Further, it requires virtually no additional effort on the part of the radiologist, since scheduling of exams is an established function of every radiology practice. In a larger sense, the proposal that radiologists manage imaging follow up may be more accurately characterized as a proposal to shift away from performing specific tasks (“acquire chest CT”) to answering specific diagnostic questions (“characterize the pulmonary nodule, with imaging as necessary”). Such a shift does not need to be limited to serial imaging, but may also extend to cases in which multimodality imaging is required to arrive at a specific diagnosis. For instance, imaging to evaluate abdominal pain may commence with a CT study, but if a gynecological abnormality is suspected based on the radiologist's interpretation of the CT, ultrasound or MRI could be performed at the radiologist's discretion. As noted previously, these multiple services could be bundled under the header of a single, well-defined consultation request, facilitating reimbursement of these services under the ACO model.
Imaging as the Basis of Subspecialist Referral
The final component of the radiologist's revised role in patient management is findings-based subspecialty referral. Radiologists today practice in an highly inefficient spoke-and-wheel referral model in which patients are referred by a provider for radiologic evaluation, imaging results are relayed back to the requesting provider along with management recommendations, the primary provider refers the patient for specialist care, and the specialists again communicate with the radiologist for results of the study (Figure 1). This nonlinear arrangement is highly redundant and introduces needless inefficiencies in communication. A better, more streamlined alternative would be for the radiologist to communicate results and refer the patient to an appropriate specialist (Figure 2). Such a paradigm would simply formalize and optimize what is already done at many U.S. hospitals. Many specialist consultants will not see a patient without appropriate imaging being available at the time of consultation. Moreover, referring providers frequently request guidance on “what to do next” based on imaging findings. Based on his/her extensive knowledge of imaging and their familiarity with the preferences of various medical and surgical teams based on daily interactions, the radiologist is in a unique position to make such recommendations, guide referral of patients when appropriate, and ensure closure of the communication loop between multiple teams of providers. Besides increasing the quality and yield of specialty referral, radiologist-managed guidance of patient referral also supports the larger trend toward care delivery by primary physicians who may be relatively less familiar with obscure diseases and injuries, but may nonetheless be able to effectively manage patient care when their knowledge is supplemented with that of a subspecialized radiologist (12, 89-92).
Figure 1.
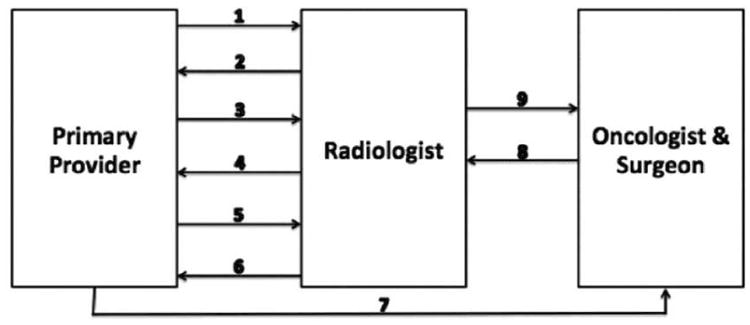
Current model for evaluation of a patient with a pulmonary nodule. The patient is seen by a primary provider, who identifies a history of smoking and refers the patient for radiological evaluation (1). The primary provider is notified of the abnormal result of the screening examination (2) and manages subsequent surveillance imaging (3). The primary provider is then notified by the radiologist that the nodule exhibits suspicious features and warrants biopsy (4) and responds by referring the patient back to the radiologist for biopsy (5). The results of the biopsy are sent to the primary physician (6), who refers the patient to oncology and surgical specialists (7), who in turn meet with the radiologist (8) to obtain his or her input about the extent of disease (9).
Figure 2.

Streamlined model for evaluation of a patient with a pulmonary nodule. A patient is first seen by a primary provider, who identifies a history of smoking. The primary physician then refers the patient to a radiologist who selects the most appropriate screening study (1). If the screening study identifies an abnormality, the radiologist enrolls the patient in a standardized surveillance protocol (2). If surveillance imaging reveals suspicious behavior of the nodule, a chest biopsy is performed (3). If the biopsy reveals malignancy, the radiologist refers the patient onward for subspecialist care (4).
Conclusion
As a specialty looking into the future we must recognize the need to redefine our field. It is imperative for our profession, starting at the trainee level, to understand the changing tide of the new health care environment and the challenges that have brought us here. Radiologists must continue to bring innovation to the diagnosis and treatment of disease, but must go further to extend the boundaries of our field to involve broader aspects of patient care. We hope that this review will provide guidance to those seeking to take the first bold steps into the future.
Acknowledgments
Grant Support: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aliya Qayyum, University of Texas MD Anderson Cancer Center, Department of Radiology, Division of Diagnostic Imaging, 1515 Holcombe, Unit 1473, Houston, Texas 77030-4009.
John-Paul J. Yu, University of California, San Francisco, Department of Radiology and Biomedical Imaging, 505 Parnassus Avenue, M-391, San Francisco, CA 94143-0628.
Akash P. Kansagra, University of California, San Francisco, Department of Radiology and Biomedical Imaging, 505 Parnassus Avenue, M-391, San Francisco, CA 94143-0628.
Nathaniel von Fischer, University of Cincinnati, Department of Radiology, 234 Goodman Street, PO Box 670761, Cincinnati, OH 45267-0761.
Daniel Costa, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX 75390-9061.
Matthew Heller, University of Pittsburgh Medical Center, Department of Radiology, 3950 Presby South Tower, 200 Lothrop Street, Pittsburgh, PA 15213.
Stamatis Kantartzis, University of Pittsburgh Medical Center, Department of Radiology, 3950 Presby South Tower, 200 Lothrop Street, Pittsburgh, PA 15213.
R. Scooter Plowman, University of Kansas, School of Medicine, 3901 Rainbow Boulevard, Kansas City, KS 66160.
Jason Itri, University of Pittsburgh Medical Center, Department of Radiology, 3950 Presby South Tower, 200 Lothrop Street, Street Pittsburgh, PA 15213.
References
- 1.Fineberg HV. Shattuck Lecture. A successful and sustainable health system--how to get there from here. N Engl J Med. 2012;366(11):1020–7. doi: 10.1056/NEJMsa1114777. [DOI] [PubMed] [Google Scholar]
- 2.Davis KSC, Stremikis K. Mirror, mirror on the wall: how the performance of the US health care system compares internationally. New York: Commonwealth Fund; 2010. [Google Scholar]
- 3.Yong PL, Saunders RS, Olsen LA, editors. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington (DC): 2010. [PubMed] [Google Scholar]
- 4.McCarthy DHS, Schoen C, Cantor JC, Belloff D. Aiming higher: results from a state scorecard on health system performance. 2009 [Google Scholar]
- 5.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138(4):273–87. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 6.Landon BE. Keeping score under a global payment system. N Engl J Med. 2012;366(5):393–5. doi: 10.1056/NEJMp1112637. [DOI] [PubMed] [Google Scholar]
- 7.Payment Policies Under the Physician Fee Schedule, Five-Year Review of Work Relative Value Units, Clinical Laboratory Fee Schedule: Signature on Requisition, and Other Revisions to Part B for CY 2012. Federal Register. 76(288) Final Rule. [PubMed] [Google Scholar]
- 8.Revisions to Payment Policies Under the Physician Fee Schedule, DME Face-to-Face Encounters, Elimination of the Requirement for Termination of Non-Random Prepayment Complex Medical Review and Other Revisions to Part B for CY 2013. Federal Register. 77(222) [PubMed] [Google Scholar]
- 9.Seidel RL, Baumgarten DA. Pay for performance: survey of diagnostic radiology faculty and trainees. J Am Coll Radiol. 2007;4(6):411–5. doi: 10.1016/j.jacr.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Moser JW, Wilcox PA, Bjork SS, et al. Pay for performance in radiology: ACR white paper. J Am Coll Radiol. 2006;3(9):650–64. doi: 10.1016/j.jacr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.McVey LR. Pay-for-performance radiology: a new concept. Radiol Manage. 1999;21(3):18–21. [PubMed] [Google Scholar]
- 12.Allen B, Jr, Levin DC, Brant-Zawadzki M, et al. ACR white paper: Strategies for radiologists in the era of health care reform and accountable care organizations: a report from the ACR Future Trends Committee. J Am Coll Radiol. 2011;8(5):309–17. doi: 10.1016/j.jacr.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Becker GJ, Bosma JL, Burleson J, et al. Introduction to value-based payment modifiers. J Am Coll Radiol. 2012;9(10):718–24. doi: 10.1016/j.jacr.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Enzmann DR. Radiology's value chain. Radiology. 2012;263(1):243–52. doi: 10.1148/radiol.12110227. [DOI] [PubMed] [Google Scholar]
- 15.Kahn CE., Jr Artificial intelligence in radiology: decision support systems. Radiographics. 1994;14(4):849–61. doi: 10.1148/radiographics.14.4.7938772. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal DI, Weilburg JB, Schultz T, et al. Radiology order entry with decision support: initial clinical experience. J Am Coll Radiol. 2006;3(10):799–806. doi: 10.1016/j.jacr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan B. Evaluating informatics applications--clinical decision support systems literature review. Int J Med Inform. 2001;64(1):15–37. doi: 10.1016/s1386-5056(01)00183-6. [DOI] [PubMed] [Google Scholar]
- 18.Miller RA. Medical diagnostic decision support systems--past, present, and future: a threaded bibliography and brief commentary. J Am Med Inform Assoc. 1994;1(1):8–27. doi: 10.1136/jamia.1994.95236141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruskal JB, Reedy A, Pascal L, et al. Quality initiatives: lean approach to improving performance and efficiency in a radiology department. Radiographics. 2012;32(2):573–87. doi: 10.1148/rg.322115128. [DOI] [PubMed] [Google Scholar]
- 20.Ondategui-Parra S, Bhagwat JG, Gill IE, et al. Essential practice performance measurement. J Am Coll Radiol. 2004;1(8):559–66. doi: 10.1016/j.jacr.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Johnson CD, Krecke KN, Miranda R, et al. Quality initiatives: developing a radiology quality and safety program: a primer. Radiographics. 2009;29(4):951–9. doi: 10.1148/rg.294095006. [DOI] [PubMed] [Google Scholar]
- 22.Mendiratta-Lala M, Eisenberg RL, Steele JR, et al. Quality initiatives: measuring and managing the procedural competency of radiologists. Radiographics. 2011;31(5):1477–88. doi: 10.1148/rg.315105242. [DOI] [PubMed] [Google Scholar]
- 23.Lee CI, Enzmann DR. Measuring radiology's value in time saved. J Am Coll Radiol. 2012;9(10):713–7. doi: 10.1016/j.jacr.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Thompson TG, Brailer DJ. The decade of health information technology: delivering consumer-centric and information-rich health care. Washington, DC: US Department of Health and Human Services; 2004. [Google Scholar]
- 25.Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–52. doi: 10.7326/0003-4819-144-10-200605160-00125. [DOI] [PubMed] [Google Scholar]
- 26.Blumenthal D. Stimulating the adoption of health information technology. N Engl J Med. 2009;360(15):1477–9. doi: 10.1056/NEJMp0901592. [DOI] [PubMed] [Google Scholar]
- 27.Flanagan PT, Relyea-Chew A, Gross JA, et al. Using the Internet for Image Transfer in a Regional Trauma Network: Effect on CT Repeat Rate, Cost, and Radiation Exposure. J Am Coll Radiol. 2012;9(9):648–56. doi: 10.1016/j.jacr.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Buntin MB, Burke MF, Hoaglin MC, et al. The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Affairs. 2011;30(3):464–71. doi: 10.1377/hlthaff.2011.0178. [DOI] [PubMed] [Google Scholar]
- 29.Channin DS, Bowers G, Nagy P. Should radiology IT be owned by the chief information officer? J Digit Imaging. 2009;22(3):218–21. doi: 10.1007/s10278-009-9196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arenson RL. PACS: current status and cost-effectiveness. Eur Radiol. 2000;10(Suppl 3):S354–6. doi: 10.1007/pl00014092. [DOI] [PubMed] [Google Scholar]
- 31.Roberson GH, Shieh YY. Radiology information systems, picture archiving and communication systems, teleradiology--overview and design criteria. J Digit Imaging. 1998;11(4 Suppl 2):2–7. doi: 10.1007/BF03168169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boochever SS. HIS/RIS/PACS integration: getting to the gold standard. Radiol Manage. 2004;26(3):16–24. quiz 5-7. [PubMed] [Google Scholar]
- 33.Sung JC, Sodickson A, Ledbetter S. Outside CT imaging among emergency department transfer patients. J Am Coll Radiol. 2009;6(9):626–32. doi: 10.1016/j.jacr.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Gupta R, Greer SE, Martin ED. Inefficiencies in a rural trauma system: the burden of repeat imaging in interfacility transfers. J Trauma. 2010;69(2):253–5. doi: 10.1097/TA.0b013e3181e4d579. [DOI] [PubMed] [Google Scholar]
- 35.Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008;(7):1–38. [PubMed] [Google Scholar]
- 36.Pinthus JH, Farrokhyar F, Hassouna MM, et al. Single-session primary high-intensity focused ultrasonography treatment for localized prostate cancer: biochemical outcomes using third generation-based technology. Br J Urol Int. 2012;110(8):1142–8. doi: 10.1111/j.1464-410X.2012.10945.x. [DOI] [PubMed] [Google Scholar]
- 37.Rouviere O, Glas L, Girouin N, et al. Prostate Cancer Ablation with Transrectal High-Intensity Focused Ultrasound: Assessment of Tissue Destruction with Contrast-enhanced US. Radiology. 2011;259(2):583–91. doi: 10.1148/radiol.11101489. [DOI] [PubMed] [Google Scholar]
- 38.Kim HS, Baik JH, Pham LD, et al. MR-guided High-intensity Focused Ultrasound Treatment for Symptomatic Uterine Leiomyomata: Long-term Outcomes. Acad Radiol. 2011;18:970–6. doi: 10.1016/j.acra.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YS, Kim JH, Rhim H, et al. Volumetric MR-guided High-Intensity Focused Ultrasound Ablation with a One-Layer Strategy to Treat Large Uterine Fibroids: Initial Clinical Outcomes. Radiology. 2012;263(2):600–9. doi: 10.1148/radiol.12111707. [DOI] [PubMed] [Google Scholar]
- 40.Cho ZH, Kang CK, Han JY, et al. Observation of Lenticulostriate Arteries in the Human Brain In Vivo Using 7.0T MR Angiography. Stroke. 2008;39:1604–6. doi: 10.1161/STROKEAHA.107.508002. [DOI] [PubMed] [Google Scholar]
- 41.Conijn MMA, Geerlings MI, Biessels GJ, et al. Cerebral Microbleeds on MR Imaging: Comparison between 1.5 and 7T. Am J Neuroradiol. 2011;32:1043–9. doi: 10.3174/ajnr.A2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manglaviti G, Tresoldi S, Guerrer CS, et al. In Vivo Evaluation of the Chemical Composition of Urinary Stones Using Dual-Energy CT. AJR Am J Roentgenol. 2011;197:W76–W83. doi: 10.2214/AJR.10.5217. [DOI] [PubMed] [Google Scholar]
- 43.Song KD, Kim CK, Park BK, et al. Utility of Iodine Overlay Technique and Virtual Unenhanced Images for Characterization of Renal Masses by Dual-Energy CT. AJR Am J Roentgenol. 2011;197:W1076–W82. doi: 10.2214/AJR.11.6922. [DOI] [PubMed] [Google Scholar]
- 44.Neville AM, Gupta RT, Miller CM, et al. Detection of Renal Lesion Enhancement with Dual-Energy Multidetector CT. Radiology. 2011;259:173–83. doi: 10.1148/radiol.10101170. [DOI] [PubMed] [Google Scholar]
- 45.Massoud TF. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes & Development. 2003;17(5):545–80. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 46.Contag CH. In Vivo Pathology: Seeing with Molecular Specificity and Cellular Resolution in the Living Body. Annu Rev Pathol Mech Dis. 2007;2(1):277–305. doi: 10.1146/annurev.pathol.2.010506.091930. [DOI] [PubMed] [Google Scholar]
- 47.Miller JC, Thrall JH. Clinical molecular imaging. J Am Coll Radiol. 2004;1(1):4–23. doi: 10.1016/S1546-1440(03)00025-5. [DOI] [PubMed] [Google Scholar]
- 48.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219(2):316–33. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 49.Margulis AR. Molecular imaging: love it or lose it. Radiology. 2012;264(1):5. doi: 10.1148/radiol.12120339. [DOI] [PubMed] [Google Scholar]
- 50.Nathan DG, Varmus HE. The National Institutes of Health and clinical research: a progress report. Nat Med. 2000;6(11):1201–4. doi: 10.1038/81282. [DOI] [PubMed] [Google Scholar]
- 51.Lee CI, Forman HP. What we can and cannot see coming. Radiology. 2010;257(2):313–4. doi: 10.1148/radiol.10101437. [DOI] [PubMed] [Google Scholar]
- 52.Budoff MJ, Hamirani YS, Gao YL, et al. Measurement of thoracic bone mineral density with quantitative CT. Radiology. 2010;257(2):434–40. doi: 10.1148/radiol.10100132. [DOI] [PubMed] [Google Scholar]
- 53.Shemesh J, Henschke CI, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257(2):541–8. doi: 10.1148/radiol.10100383. [DOI] [PubMed] [Google Scholar]
- 54.Gondrie MJ, Mali WP, Jacobs PC, et al. Cardiovascular disease: prediction with ancillary aortic findings on chest CT scans in routine practice. Radiology. 2010;257(2):549–59. doi: 10.1148/radiol.10100054. [DOI] [PubMed] [Google Scholar]
- 55.Krestin GP. Maintaining identity in a changing environment: the professional and organizational future of radiology. Radiology. 2009;250(3):612–7. doi: 10.1148/radiol.2503081791. [DOI] [PubMed] [Google Scholar]
- 56.Arenson R, Dunnick NR. Training a better radiologist. J Am Coll Radiol. 2006;3(6):389–93. doi: 10.1016/j.jacr.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Campbell EG, Weissman JS, Blumenthal D. Relationship between market competition and the activities and attitudes of medical school faculty. JAMA. 1997;278(3):222–6. [PubMed] [Google Scholar]
- 58.Enzmann DR, Schomer DF. Analysis of Radiology Business Models. J Am Coll Radiol. 2012 doi: 10.1016/j.jacr.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Levin DC, Rao VM. Turf wars in radiology: introduction. J Am Coll Radiol. 2004;1(1):23–5. doi: 10.1016/S1546-1440(03)00013-9. [DOI] [PubMed] [Google Scholar]
- 60.Levin DC, Rao VM. Turf wars in radiology: the overutilization of imaging resulting from self-referral. J Am Coll Radiol. 2004;1(3):169–72. doi: 10.1016/j.jacr.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Hendee WR. An opportunity for radiology. Radiology. 2006;238(2):389–94. doi: 10.1148/radiol.2382051177. [DOI] [PubMed] [Google Scholar]
- 62.Amis ES, Jr, Dunnick NR. Improvement in radiology education: joint efforts of the American Board of Radiology and the Diagnostic Radiology Residency Review Committee. J Am Coll Radiol. 2009;6(2):103–5. doi: 10.1016/j.jacr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Breslau J, Lexa FJ. A radiologist's primer on accountable care organizations. J Am Coll Radiol. 2011;8(3):164–8. doi: 10.1016/j.jacr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Nachiappan AC, Wynne DM, Katz DP, et al. A proposed medical physics curriculum: preparing for the 2013 ABR examination. J Am Coll Radiol. 2011;8(1):53–7. doi: 10.1016/j.jacr.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 65.Nachiappan AC, Lee SR, Willis MH, et al. Clinically oriented three-year medical physics curriculum: a new design for the future. AJR Am J Roentgenol. 2012;199(3):635–43. doi: 10.2214/AJR.11.7356. [DOI] [PubMed] [Google Scholar]
- 66.Shortell SM, Casalino LP, Fisher ES. How the center for Medicare and Medicaid innovation should test accountable care organizations. Health Aff (Millwood) 2010;29(7):1293–8. doi: 10.1377/hlthaff.2010.0453. [DOI] [PubMed] [Google Scholar]
- 67.Bernardy M, Ullrich CG, Rawson JV, et al. Strategies for managing imaging utilization. J Am Coll Radiol. 2009;6(12):844–50. doi: 10.1016/j.jacr.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Hillman BJ, Goldsmith JC. The uncritical use of high-tech medical imaging. N Engl J Med. 2010;363(1):4–6. doi: 10.1056/NEJMp1003173. [DOI] [PubMed] [Google Scholar]
- 69.Levin DC, Rao VM. Turf wars in radiology: updated evidence on the relationship between self-referral and the overutilization of imaging. J Am Coll Radiol. 2008;5(7):806–10. doi: 10.1016/j.jacr.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 70.Hillman BJ, Joseph CA, Mabry MR, et al. Frequency and costs of diagnostic imaging in office practice--a comparison of self-referring and radiologist-referring physicians. N Engl J Med. 1990;323(23):1604–8. doi: 10.1056/NEJM199012063232306. [DOI] [PubMed] [Google Scholar]
- 71.Berbaum KS, Franken EA, Jr, el-Khoury GY. Impact of clinical history on radiographic detection of fractures: a comparison of radiologists and orthopedists. AJR Am J Roentgenol. 1989;153(6):1221–4. doi: 10.2214/ajr.153.6.1221. [DOI] [PubMed] [Google Scholar]
- 72.Loy CT, Irwig L. Accuracy of diagnostic tests read with and without clinical information: a systematic review. JAMA. 2004;292(13):1602–9. doi: 10.1001/jama.292.13.1602. [DOI] [PubMed] [Google Scholar]
- 73.Mullins ME, Lev MH, Schellingerhout D, et al. Influence of availability of clinical history on detection of early stroke using unenhanced CT and diffusion-weighted MR imaging. AJR Am J Roentgenol. 2002;179(1):223–8. doi: 10.2214/ajr.179.1.1790223. [DOI] [PubMed] [Google Scholar]
- 74.Chiunda AB, Mohammed TL. Knowledge of ACR thoracic imaging Appropriateness Criteria(R) among trainees: one institution's experience. Acad Radiol. 2012;19(5):635–9. doi: 10.1016/j.acra.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 75.Bautista AB, Burgos A, Nickel BJ, et al. Do Clinicians Use the American College of Radiology Appropriateness Criteria in the Management of Their Patients? AJR Am J Roentgenol. 2009;192(6):1581–5. doi: 10.2214/AJR.08.1622. [DOI] [PubMed] [Google Scholar]
- 76.Blackmore CC, Mecklenburg RS, Kaplan GS. Effectiveness of clinical decision support in controlling inappropriate imaging. J Am Coll Radiol. 2011;8(1):19–25. doi: 10.1016/j.jacr.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Georgiou A, Prgomet M, Markewycz A, et al. The impact of computerized provider order entry systems on medical-imaging services: a systematic review. J Am Med Inform Assoc. 2011;18(3):335–40. doi: 10.1136/amiajnl-2010-000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanders DL, Miller RA. The effects on clinician ordering patterns of a computerized decision support system for neuroradiology imaging studies. Proc AMIA Symp. 2001:583–7. [PMC free article] [PubMed] [Google Scholar]
- 79.Cascade PN. The American College of Radiology. ACR Appropriateness Criteria project. Radiology. 2000;214(Suppl):3–46. doi: 10.1148/radiology.214.1.r00ja493. [DOI] [PubMed] [Google Scholar]
- 80.Khorasani R, Silverman SG, Meyer JE, et al. Design and implementation of a new radiology consultation service in a teaching hospital. AJR Am J Roentgenol. 1994;163(2):457–9. doi: 10.2214/ajr.163.2.8037049. [DOI] [PubMed] [Google Scholar]
- 81.Basu PA, Ruiz-Wibbelsmann JA, Spielman SB, et al. Creating a Patient-Centered Imaging Service: Determining What Patients Want. AJR Am J Roentgenol. 2011;196(3):605–10. doi: 10.2214/AJR.10.5333. [DOI] [PubMed] [Google Scholar]
- 82.Berlin L. Communicating results of all radiologic examinations directly to patients: Has the time come? AJR Am J Roentgenol. 2007;189(6):1275–82. doi: 10.2214/AJR.07.2740. [DOI] [PubMed] [Google Scholar]
- 83.Berlin L. Communicating Results of All Outpatient Radiologic Examinations Directly to Patients: The Time Has Come. AJR Am J Roentgenol. 2009;192(3):571–3. doi: 10.2214/AJR.08.1954. [DOI] [PubMed] [Google Scholar]
- 84.Brandt-Zawadski M, Kerlan RK. Patient-centered Radiology: Use It or Lose It! Acad Radiol. 2009;16(5):521–3. doi: 10.1016/j.acra.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 85.Kuhlman M, Meyer M, Krupinski EA. Direct Reporting of Results to Patients: The Future of Radiology? Acad Radiol. 2012;19(6):646–50. doi: 10.1016/j.acra.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 86.Margulis AR, Sostman HD. Radiologist-patient contact during the performance of cross-sectional examinations. J Am Coll Radiol. 2004;1(3):162–3. doi: 10.1016/j.jacr.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 87.Berlin L. Failure of radiologic communication: an increasing cause of malpractice litigation and harm to patients. App Radiol. 2010;39:17–23. [Google Scholar]
- 88.Yetisgen-Yildiz M, Gunn ML, Xia F, et al. Automatic identification of critical follow-up recommendation sentences in radiology reports. AMIA Annu Symp Proc. 2011;2011:1593–602. [PMC free article] [PubMed] [Google Scholar]
- 89.Radiology and primary care in Europe. Insights Imaging. 2010;1(2):46–52. doi: 10.1007/s13244-010-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Apthorp LA, Daly CA, Morrison ID, et al. Direct access MRI for general practitioners--influence on patient management. Clin Radiol. 1998;53(1):58–60. doi: 10.1016/s0009-9260(98)80036-6. [DOI] [PubMed] [Google Scholar]
- 91.Knechtges PM, Carlos RC. The evolving role of radiologists within the health care system. J Am Coll Radiol. 2007;4(9):626–35. doi: 10.1016/j.jacr.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Royal College of General Practioners. Clinical radiology and the patients of general practitioners: Joint statement of the Royal College of General Practioners and Royal College of Radiology. 2004 [Google Scholar]


