Abstract
Background
Variations in GABRA2 and GABRG3, genes encoding the α2 and γ3 subunits of the pentameric GABAA receptor, are associated with the risk of developing alcoholism in adults, conduct disorder at younger ages, and with differences in electroencephalographic power in the β frequency range. The SNPs associated with alcoholism did not alter the coding of these genes, and extensive DNA sequencing of GABRA2 did not find coding changes in the high-risk haplotypes. Therefore we hypothesize that the associations arise from differences in gene expression.
Methods
Here we report studies in Xenopus oocytes to examine the functional effects of altering the relative abundance of these two receptor subunits on GABA current and response to ethanol, as a model of potential effects of regulatory differences.
Results
When human α2β2γ3 subunits are co-expressed, increasing the amount of the α2 subunit mRNA increased GABA current; in contrast, increasing the amount of the γ3 subunit decreased GABA currents. Acute ethanol treatment of oocytes injected with a 1:1:1 or 2:2:1 ratio of α2:β2:γ3 subunit mRNAs resulted in significant potentiation of GABA currents, whereas ethanol inhibited GABA currents in cells injected with a 6:2:1 ratio. Overnight treatment with ethanol significantly reduced GABA currents in a manner dependent on the ratio of subunits.
Conclusions
These studies demonstrate that changes in relative expression of GABAA receptor subunits alter the response of the resulting channels to GABA and to ethanol.
Keywords: Ethanol, GABA Receptors, Voltage Clamp
Alcoholism and alcohol abuse are prevalent conditions which present a significant burden to society. Alcoholism is a complex multi-factorial disease which has a strong genetic component. Linkage and association analyses have led to the identification of a number of genes in which variations increase risk for alcoholism (for reviews, see (Dick and Foroud 2003; Edenberg and Foroud 2006; Kohnke 2008). Family-based studies demonstrated that GABRA2, encoding the α2 subunit of the GABAA receptor, is associated with alcohol dependence and with electroencephalographic differences in the β frequency range of 13–28 Hz (Edenberg et al. 2004). Alcoholics and their offspring differ from controls by having increased power in that frequency band (Bauer and Hesselbrock 1993; Costa and Bauer 1997; Rangaswamy et al. 2002; Rangaswamy et al. 2003). Bauer and Hesselbrock (Bauer and Hesselbrock 1993) reported that enhanced high frequency beta activity, originating from deep anterior regions of the frontal brain, was the best predictor of relapse in substance-dependent patients, and may also be related to initial risk for dependence. The association between GABRA2 and alcoholism has been replicated in several other populations, with the same haplotypes conferring higher risk (Covault et al. 2004; Edenberg and Foroud 2006; Enoch et al. 2006; Fehr et al. 2006; Lappalainen et al. 2005; Soyka et al. 2008). Further analyses demonstrated that at younger ages, the higher-risk alleles of GABRA2 are associated with conduct disorder (Dick et al. 2006a). GABRG3, encoding the γ3 subunit, has also been associated with alcohol dependence (Dick et al. 2004), as has GABRA1, encoding the α1 subunit (Dick et al. 2006b).
Our previous study identified multiple SNPs associated with alcoholism, none of which were within coding regions (Edenberg et al. 2004). In addition, sequencing over 40 individuals with high-risk and low-risk haplotypes revealed no nucleotide changes within the coding sequence of GABRA2. Detailed analysis of mRNA from human brain demonstrated alternative splicing and alternative promoter use in the human α2 gene (Tian et al. 2005). Promoter activity of naturally occurring haplotypes also differed (Tian et al. 2005). These observations led us to hypothesize that changes in gene regulation may underlie the alcohol-related phenotypes.
Some evidence suggests that there are innate differences in GABA receptor function between lines of rats selectively bred for alcohol preference (P and NP) (Lumeng et al. 1977). Microarray analysis has identified genes expressed differentially in the hippocampus of the P and NP lines including many genes involved in synaptic transmission (Edenberg et al. 2005). For example, the GABRB1 gene was expressed at a 1.6-fold higher level in the hippocampus of the P rat vs. the NP rat and GABRA1 gene expression was higher as well. An additional microarray study corroborated the observation that the GABRB1 gene was more highly expressed in multiple brain regions in the P vs. NP rats (Kimpel et al. 2007). Our hypothesis is that changes in expression levels of the receptor subunits alter GABA signaling.
γ-Aminobutyric acidA (GABAA) receptors are ligand-gated ion channels that mediate the majority of fast inhibitory neurotransmission in the central nervous system (Barnard et al. 1998; Mehta and Ticku 1999; Whiting et al. 1999). They are pentameric integral membrane proteins comprised of different classes of subunits. At least 6 α, 3 β, 3 γ, δ, ε, θ and π subunit genes and multiple splice variants have been identified. The majority of GABAA receptors are thought to be composed of two α, two β and one γ subunits (Chang et al. 1996; Farrar et al. 1999) with δ sometimes substituting for the γ subunit (Quirk et al. 1995). GABAA receptors are targets of many important clinical agents including benzodiazepines, barbiturates, steroids, anesthetics and anti-convulsants (Barnard et al. 1998; Korpi, Grunder, and Luddens 2002; Sieghart 2006). GABAA receptors are important in many behavioral effects of ethanol consumption including motor in-coordination, sedation, anxiolysis, tolerance, preference, hypothermia and withdrawal (Buck 1996; Grobin et al. 1998; Korpi, Grunder, and Luddens 2002). For example, ethanol mimics some of the effects of GABAA receptor modulators such as benzodiazepines, neurosteroids and barbiturates.
Reports about the acute effects of ethanol on GABAA receptors differ. The sensitivity of GABAA receptors to ethanol may vary with subunit composition. Ethanol potently (at low mM concentrations) facilitates extrasynaptic GABAA receptors containing α4 or α6 subunits in combination with δ subunits (Sundstrom-Poromaa et al. 2002; Wallner, Hanchar, and Olsen 2003). In contrast the potency of ethanol is much lower in the more abundant synaptic GABAA receptors which contain γ subunits instead of δ subunits. However other investigators have been unable to replicate these findings and the inconsistency may be due to species differences, differences in expression levels or GABA concentrations tested, or unknown variables (Borghese and Harris 2007; Harris and Mihic 2004). In general, long-term ethanol treatment reduces GABAA receptor function by several mechanisms including changes in gene expression, posttranslational modification and subcellular localization of specific subunits (for reviews, see (Kumar, Fleming, and Morrow 2004) and (Krystal et al. 2006)). For example, chronic ethanol administration in rats reduces the level of mRNA and peptides for the α1, α2 and α3 GABAA receptor subunits in the cerebral cortex (Mhatre and Ticku 1992) but these effects differ across brain regions. Indirect evidence suggests that phosphorylation, particularly by PKC, may differentially alter GABAA receptor function in response to ethanol (Kumar, Fleming, and Morrow 2004; Kumar, Sieghart, and Morrow 2002; Morrow et al. 2004). Chronic ethanol treatment selectively increases α1 subunit protein internalization in rat cerebral cortex (Kumar et al. 2003).
In the functional studies presented here, we have mimicked changes in relative gene expression by introducing different ratios of the α2, β2 and γ3 GABAA receptor subunit mRNAs into Xenopus oocytes and have measured GABAA receptor channel function. In addition we have examined the effect of acute and chronic ethanol treatment on the electrophysiological characteristics of the channels that result from expression of different subunit ratios.
MATERIALS AND METHODS
Clones
A human GABAA subunit α2 cDNA (Hadingham et al. 1993a); GenBank accession number NM_000807) from GeneCopoeia (Germantown, MD) was subcloned into pcDNA3.1(+) (Invitrogen, Carlsbad, CA) for expression studies. The human β2 subunit cDNA ((Hadingham et al. 1993b); NM_000813) in pCDM8 was a kind gift from Peter Wingrove from Merck Laboratories. The human γ3 subunit cDNA (Hadingham et al. 1995); NM_033223) in pCMV6-XL5 was from Origene (Rockville, MD). The authenticity of all three subunits was verified by DNA sequencing before expression studies were conducted.
Xenopus oocyte expression and electrophysiology
All animal procedures were approved by the Animal Care and Use Committee at Indiana University School of Medicine. GABAA receptor proteins were expressed in Xenopus laevis oocytes after injection of in vitro transcribed mRNAs. Full-length cDNAs of each of the cloned subunits were used to generate mRNA from the T7 RNA polymerase promoter using an in vitro transcription kit (mMessage Machine, Ambion, Austin TX). RNA concentration and quality was determined by both gel electrophoresis and UV spectrophotometry. Oocytes were harvested from mature Xenopus laevis, and follicle cells were removed with 2 mg/ml collagenase type IA (Sigma, St. Louis, MO). Stage V and VI oocytes were injected with 5–30 ng of each GABAA receptor subunit mRNA as indicated in a total of 50 nL of RNAse-free H20 using a Drummond automatic microinjector. Seven ratios of GABAA subunits were tested: 1) α2β2 only in a 1:1 ratio (10 ng:10 ng); 2) α2β2γ3 in a 1:1:3 ratio (10 ng:10 ng:30 ng); 3) α2β2γ3 in a 1:1:1 ratio (10 ng:10 ng:10 ng); 4) α2β2γ3 in a 3:1:1 ratio (30 ng:10 ng:10 ng); 5) α2β2γ3 in a 2:2:1 ratio (10 ng:10 ng:5 ng); 6) α2β2γ3 in a 6:2:1 (30 ng:10 ng:5 ng); 7) α2β2γ3 in a 0.5:2:1 ratio (5 ng:20 ng:10 ng). Since the transcripts and coding regions for all 3 genes are similar in length the molarities will also be similar when 1:1:1 quantities of RNA are injected. Because all 3 genes have optimal Kozak sequences, the ratios of injected subunit mRNAs should be reflected in the amount of specific subunit protein expressed. Oocytes were stored in Oocyte Ringer solution (50% Leibovitz’s L15 medium, 15 mM HEPES, 0.8 mM L-glutamine, 0.04 mg/mL gentamycin) at 17° C.
Two-electrode voltage experiments were conducted three days after RNA injection. Oocytes were voltage-clamped at −70 mV with two glass electrodes filled with 3 M KCl and having a resistance of 0.5–1.5 MΩ using an OC-725 Oocyte Clamp (Warner Instrument Corporation, Hamden, CT). GABA-induced chloride currents were digitized with an Axon Instruments Digidata 1322A (Sunnyvale, CA) and recorded on a computer using pCLAMP software. Oocytes were rapidly superfused (at 5 ml/min) with ND96 (96 mM NaCl, 1.8 mM CaCl2, 2 mM KCl, 1 mM MgCl2 and 5 mM HEPES, pH 7.6) or ND96 containing the indicated concentrations of GABA, ethanol or both GABA and ethanol.
In some experiments, oocytes were exposed to GABA (1 or 10 μM) only once for 30 seconds before drug washout (see Figures 1, 2A and 4). In GABA concentration-response curve experiments (Figure 2B and C), each oocyte served as its own control, i.e., multiple concentrations of GABA (300 nM–30 μM) where tested on each cell in a random order with a 5 minute wash-out between GABA applications. In acute ethanol experiments, each oocyte served as its own control and the response to 1 μM GABA before and after 44 mM ethanol pretreatment was compared after a 5 minute wash-out. Specifically, GABA responses were tested before ethanol perfusion and after a 60 second pretreatment with ethanol. Immediately after the ethanol pretreatment 1 μM GABA was again superfused for 30 seconds in the continued presence of 44 mM ethanol. In chronic ethanol experiments, half of the oocytes from an injection group were incubated with ethanol (44 mM or 100 mM) in Oocyte Ringer solution for 16 hours immediately before electrophysiological recording (approximately 2 days after RNA injection).
Fig. 1.
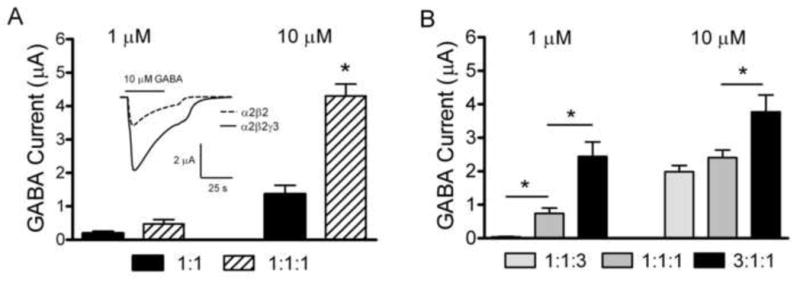
GABA currents as a function of the ratio of α2, β2 and γ3 subunit mRNAs A. Peak current amplitudes in response to 1 or 10 μM GABA in oocytes injected with a 1:1 ratio of α2:β2 subunit mRNAs or a 1:1:1 ratio of α2:β2:γ3 subunit mRNAs. Inset depicts representative inward GABA currents in response to 10 μM GABA. The solid line above the traces indicates GABA application. B. Peak current amplitudes in response to 1 or 10 μM GABA in oocytes injected with either 1:1:3 or 1:1:1 or 3:1:1 ratios of α2:β2:γ3 subunit mRNAs. The data are shown as the mean ± SEM. * indicates a significant difference between groups (p < 0.05).
Fig. 2.
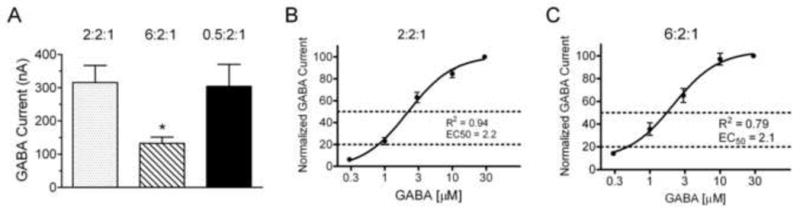
GABA currents as a function of the ratio of α2, β2 and γ3 subunit mRNAs. A. Peak current amplitudes in response to 10 μM GABA in oocytes injected with either 2:2:1 or 6:2:1 or 0.5:2:1 ratios of α2:β2:γ3 subunit mRNAs. B–C. GABA concentration-response curves in oocytes injected with either 2:2:1 (B) or 6:2:1 (C). Peak currents at each GABA concentration were normalized to the current at 30 μM GABA for each oocyte and averaged within groups. Data are presented as mean ± SE. The solid line through the data indicates the non-linear regression fit with the correlation coefficient (R2) and calculated EC50 values below. Dotted lines indicate the location of EC50 and EC20 on each graph.
Fig. 4.
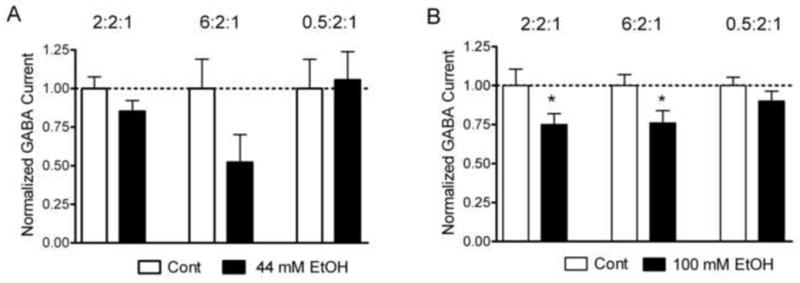
Effects of chronic ethanol treatment on GABA current amplitude. A. Peak current amplitudes in response to 10 μM GABA in oocytes treated for 16 hours with 44 mM ethanol after injection with 2:2:1 or 6:2:1 or 0.5:2:1 ratios of α2:β2:γ3 subunit mRNAs. B. Peak current amplitudes in response to 10 μM GABA in oocytes treated for 16 hours with 100 mM ethanol after injection with 2:2:1 or 6:2:1 or 0.5:2:1 ratios of α2:β2:γ3 subunit mRNAs. Data are normalized to peak current amplitudes of control oocytes; symbols as in Figure 1.
Data analysis
For each experiment, all treatment and injection groups were completed and replicated in at least 3 individual batches of oocytes from different donors to confirm the reproducibility of the findings. Peak current amplitudes were measured in individual oocytes after GABA treatment and averaged within treatment and injection groups. Data are displayed as the average ± SEM in bar graphs with the number of oocytes tested indicated in the results and/or figure legend. For GABA concentration response experiments, peak current amplitudes for each GABA concentration were normalized to the peak current amplitude from the 30 μM GABA response for each oocyte and averaged within groups. Non-linear regression analysis was used to fit the data and determine GABA EC50 values. For acute ethanol treatment experiments where oocytes served as their own controls the data were normalized to peak current amplitude before ethanol and averaged within groups. In chronic ethanol treatment experiments, individual GABA currents were normalized to the average current amplitude of control groups with no ethanol treatment within the same experiment and the mean ratios ± SEM are displayed in bar graphs. Paired t-tests or ANOVA with Tukey’s posthoc analysis was used to compare groups.
RESULTS
Oocytes were injected with a total of 20–50 ng of mRNA, which is less than the translational capacity of oocytes (Moar et al. 1971). We focused on the α2 and γ3 subunits as their genes are associated with alcohol related phenotypes. The β2 subunit was utilized throughout these studies as it is one of the most abundant of GABAA β subunits in the brain (Laurie, Wisden, and Seeburg 1992).
To assess the role of the α2, β2 and γ3 subunits in forming functional GABAA receptors we compared the amplitudes and characteristics of GABA induced currents produced in oocytes injected with a 1:1 ratio of α2:β2 mRNAs to oocytes injected with a 1:1:1 ratio of α2:β2:γ3 mRNAs. We routinely tested uninjected oocytes for endogenous GABA currents and no currents were detected under these recording conditions. Injecting α2 mRNA alone (up to 30 ng per oocyte) did not produce detectable currents when tested with up to 100 μM GABA. Functional GABAA receptors were produced by injection of mRNAs for two subunits, α2 and β2, however injecting mRNAs for all three subunits produced significantly greater currents at 10 μM GABA (1.38 ± 0.26 μA (n = 35) vs. 4.30 ± 0.35 μA (n = 31, p < 0.01) (Figure 1A). Currents induced by 1 μM GABA were slightly larger but not significantly so when all 3 subunits were expressed compared to 2 subunits (0.19 ± 0.06 μA (n = 6) vs. 0.47 ± 0.13 μA (n = 8). No differences were evident between injection groups in the rates or degree of desensitization in the GABA-induced inward currents (see inset in Figure 1A).
To test the hypothesis that changing the relative abundance of α2, β2, and γ3 subunit mRNAs would alter the characteristics of the expressed channels, we increased the amount of α2 or γ3 mRNA injected relative to the other subunits. Oocytes were injected with a 1:1:3 ratio or a 1:1:1 ratio or a 3:1:1 ratio of α2, β2, and γ3 subunit mRNAs. The peak current amplitudes elicited by 1 μM GABA (Figure 1B) were significantly greater when subunits were injected in a 3:1:1 ratio (2.44 ± 0.43 μA, n = 17) than when they were injected in a 1:1:1 ratio (0.74 ± 0.16 μA, n = 20). The peak current amplitudes were significantly smaller when subunits were injected in a 1:1:3 ratio (0.06 ± 0.01 μA, n = 18) than when they were injected in a 1:1:1 ratio. Likewise greater currents were induced by 10 μM GABA (Figure 1B) in oocytes injected with the 3:1:1 ratio of subunits (3.77 ± 0.50 μA, n = 48) compared to the 1:1:1 ratio group (2.41 ± 0.22 μA, n = 66). At this higher level of GABA, there was little difference between the 1:1:3 ratio group (1.98 ± 0.19 μA, n = 79) and the 1:1:1 group.
To more accurately reflect the most common native structure of GABAA receptors (two α, two β and one γ subunit), we examined the effects of increasing or decreasing the relative abundance of the α2 subunit compared to a baseline ratio of 2:2:1. As seen in Figure 2A, the 2:2:1 ratio group (316 ± 51 nA, n = 52) produced significantly greater currents in response to 10 μM GABA than the 6:2:1 ratio group (133 ± 19 nA, n = 36). In these experiments, the 0.5:2:1 group (304 ± 66 nA, n = 24) did not significantly differ from the baseline 2:2:1 group.
GABA concentration response curves were determined to compare GABA current characteristics in cells expressing differing subunit ratios (Figure 2B and 2C). EC50 values for the GABA response did not differ between oocytes expressing 2:2:1 (95% confidence interval = 1.65–2.93 μM, n = 9) and 6:2:1 (95% confidence interval = 1.37–3.36 μM, n = 12) ratios of subunits. The GABA concentration of 30 μM was saturating; 100 μM GABA elicited responses equal to or less than the 30 μM concentration (data not shown).
We examined the effect of acute ethanol exposure on the GABA response in oocytes injected with 1:1:1, 2:2:1 or 6:2:1 ratios of α2, β2, and γ3 subunit mRNAs (Figure 3). Each oocyte was challenged with 1 μM GABA while monitoring current size under voltage-clamp conditions before and after ethanol pretreatment. This GABA concentration (1 μM) approximates the EC20 for GABA as determined above (see Figure 2B and 2C) and should allow detection of either potentiation or inhibition of GABA response by ethanol. GABA currents were normalized to control currents before ethanol treatment for each oocyte. Ethanol significantly potentiated the amplitudes of GABA-induced currents by 93% in oocytes injected with a 1:1:1 ratio of α2, β2, and γ3 subunit mRNAs (p < 0.01, n = 16). Ethanol also potentiated the amplitudes of GABA-induced currents by 24% in oocytes injected with a 2:2:1 ratio of α2, β2, and γ3 subunit mRNAs (p < 0.01, n = 15). In contrast, ethanol inhibited the amplitudes of GABA-induced currents by 19% in oocytes injected with a 6:2:1 ratio of α2, β2, and γ3 subunit mRNAs (p < 0.01, n = 19).
Fig. 3.
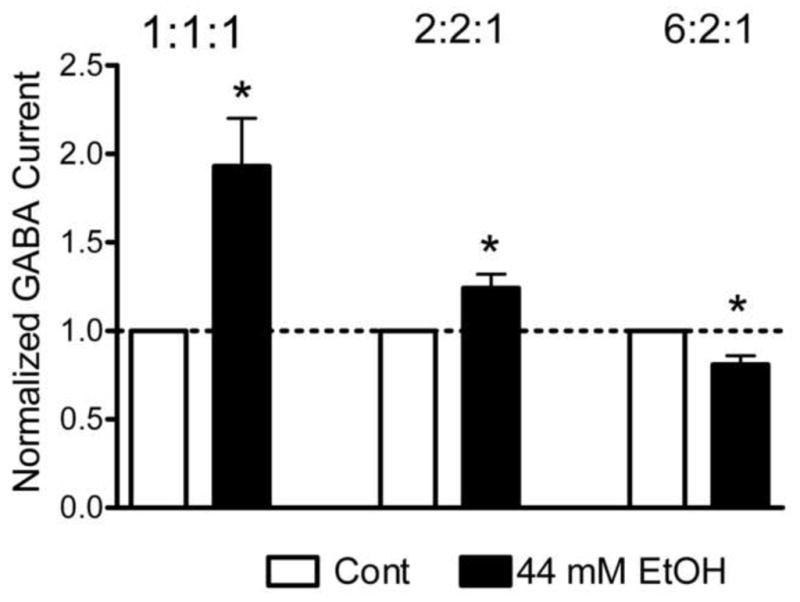
Effects of acute ethanol treatment on GABA current amplitude. A. Peak current amplitudes in response to 1 μM GABA before and after 44 mM ethanol pretreatment in oocytes expressing either 1:1:1 or 2:2:1 or 6:2:1 ratios of α2:β2:γ3 subunit mRNAs. Data are normalized to peak current amplitudes in response to GABA from each oocyte before ethanol treatment. Ethanol was applied for 60 seconds before and concurrently with GABA administration. * indicates a significant difference between groups (p < 0.01) using paired t-tests.
Because chronic ethanol exposure affects GABA responses, we determined the effect of prolonged ethanol exposure on GABAA receptors composed of different ratios of subunits. For chronic treatment studies, half of the oocytes from each injection group were incubated in Oocyte Ringer containing ethanol for 16 hours immediately prior to voltage-clamp recording, while the control cells were incubated in Oocyte Ringer alone. Ethanol (44 or 100 mM as indicated) did not alter pH but slightly decreased osmolarity of the Oocyte Ringer solution. Resting membrane potentials and leakage current under initial voltage clamp conditions did not differ between the ethanol-treated groups and controls (data not shown) which suggests that the overall health of the oocytes is similar in controls and ethanol-treated groups. As shown in Figure 4A, chronic ethanol (44 mM) had no significant effect on GABA currents (1.0 ± 0.07, n = 20 vs. 0.85 ± 0.07, n = 18; p = 0.16) in oocytes injected with the presumed native 2:2:1 ratio of α2β2γ3 subunits. Likewise, chronic ethanol (44 mM) did not significantly change, although a trend is apparent, GABA currents in oocytes injected with a 6:2:1 ratio of subunits (1.00 ± 0.19, n = 14 vs. 0.52 ± 0.18, n = 8; p = 0.11), and had no effect on oocytes injected with a 0.5:2:1 ratio (1.00 ± 0.19, n = 12 vs. 1.06 ± 0.18, n = 17; p=0.84). In contrast, 100 mM ethanol inhibited GABA currents by 25% (1.0 ± 0.11, n = 23 vs. 0.75 ± 0.0.07, n = 28, p < 0.05) in oocytes injected with the presumed native 2:2:1 ratio of α2β2γ3 subunits (Figure 4B). Chronic ethanol also reduced GABA currents in oocytes injected with a 6:2:1 ratio of subunits (1.00 ± 0.07, n = 38 vs. 0.76 ± 0.08, n = 43, p < 0.05), but had no effect on oocytes injected with a 0.5:2:1 ratio (1.00 ± 0.06, n = 23 vs. 0.92 ± 0.08, n = 28; p = 0.42).
DISCUSSION
Variations in genes encoding the α and γ subunits of the GABAA receptor are associated with risk for alcoholism (Dick et al. 2004; Edenberg et al. 2004); the association of GABRA2 has been replicated in several populations (Covault et al. 2004; Edenberg and Foroud 2006; Enoch et al. 2006; Fehr et al. 2006; Lappalainen et al. 2005; Soyka et al. 2008). Variations in GABRA2 have also been associated with power in the beta frequency band (13–28 Hz) of the EEG (Edenberg et al. 2004). Yet the associated variations did not affect the amino acid sequences, suggesting that expression differences might be involved (Edenberg et al. 2004). Alternative splicing and alternative promoter use have been demonstrated in the human GABRA2 gene (Tian et al. 2005). To test the hypothesis that variations in the ratios of GABAA receptor subunits would affect receptor function, we manipulated the subunit ratios in the Xenopus oocyte expression system and measured the resulting GABA currents. We modeled changes in gene expression by injecting Xenopus oocytes with different ratios of α2, β2 and γ3 subunits and then compared GABA currents.
Initial studies of newly cloned GABAA receptors in heterologous expression studies indicated that some combinations of α and β subunits can form functional receptors (Schofield et al. 1987; Ymer et al. 1989) albeit lacking some characteristics of native channels (Pritchett et al. 1989). We found that functional GABAA channels can be formed from α2 and β2 subunits alone, although the currents are smaller than those produced by all 3 subunits (Figure 1A). To test our hypothesis that changing the relative abundance of individual GABAA receptor subunits could alter functional responses, we modified the amounts of either the α2 or γ3 subunit, keeping the concentration of the β2 subunit constant. As previous recombinant GABAA receptor studies had utilized 1:1:1 ratios we initially compared the 1:1:1 ratio with three-fold increases in either α2 (3:1:1) or γ3 subunits (1:1:3). GABAA receptors formed from different ratios of subunits had different responses to GABA. Increasing the amount of α2 mRNA (3:1:1 ratio of α:β:γ) produced significantly greater GABA currents than a 1:1:1 ratio of subunits (Figure 1B). Increasing the amount of γ3 subunit mRNA (1:1:3 ratio) reduced the GABA currents compared to either the 1:1:1 or 3:1:1 ratio of subunits.
As the majority of native GABAA receptors are believed to be composed of two α, two β and single γ or δ subunits (Chang et al. 1996; Farrar et al. 1999; Quirk et al. 1995) we did additional comparisons in cells expressing 2:2:1 ratios and increased or decreased the α2 abundance However, increasing the ratio of α2 mRNA above the baseline of a 2:2:1 ratio led to decreased GABA currents (Figure 2A). Increasing the α2 or γ3 subunits may alter the formation of functional channels bi-directionally by changing receptor assembly or membrane trafficking (Kittler, McAinsh, and Moss 2002). Overall our study indicates that small changes in the relative abundance of subunit mRNA injected affect GABAA receptor current amplitude.
While GABAA receptor gene variants are believed to modulate the predisposition to alcoholism, the effects of ethanol on GABAA receptor function are not well-understood. The effects of acute ethanol treatment on GABAA receptor function vary widely in vivo and in vitro. A number of reports suggest that low concentrations of ethanol (<5 mM) potentiate GABA responses in some cell types or with specific subunit combinations, notably α4/6 and δ (Borghese and Harris 2007; Sundstrom-Poromaa et al. 2002; Wallner, Hanchar, and Olsen 2003; Wallner, Hanchar, and Olsen 2006). In our study, higher but still pharmacologically relevant concentrations of ethanol (44–100 mM) were required to see effects with “less-sensitive” GABAA subunit combinations. Acute ethanol treatment significantly potentiated GABA induced currents in cells injected with a 1:1:1 and 2:2:1 ratios of human α2β2γ3 subunits, whereas ethanol inhibited GABA responses in cells injected with a 6:2:1 ratio. Our results are notable for two reasons as we describe significant ethanol potentiation in GABAA receptors lacking α4/6 and/or δ subunits and we observed potentiation or inhibition by ethanol which was dependent on subunit ratio. The specificity of the response, i.e. potentiation vs. inhibition, is of interest because it demonstrates that changes in subunit abundance can alter response to ethanol.
Long-term ethanol consumption results in the development of tolerance to many of the GABA-mediated effects of ethanol and this adaptation is usually associated with diminished GABA receptor function (reviewed by Kumar, Fleming, and Morrow 2004). Changes in GABA function after chronic ethanol treatment may be due to changes in gene regulation, assembly and trafficking or posttranslational modification. The oocyte expression system as used here does not allow us to examine changes in gene regulation as the regulatory sequences are lacking in the injected cRNA and some modulatory proteins aren’t present. However oocytes do contain many common signaling proteins, including various isoforms of PKC, which are hypothesized to regulate GABA function after ethanol treatment, so some aspects of chronic ethanol treatment on specific subunit combinations may be initially studied on oocytes. As seen in Figure 4, ethanol treatment for 16 hours significantly reduced GABA responses in oocytes injected with a 2:2:1 ratio and a 6:2:1 ratio of subunits, while not affecting current amplitude of oocytes injected with 0.5:2:1 ratios. This result suggests that the effect is specific to particular subunit ratios and not due to non-specific effects of ethanol on the membrane or viability of the cells. In oocytes, either trafficking or posttranslational modifications may be occurring and may be mechanistically related. For example, it has been previously shown that GABAA receptors are internalized in a subunit-specific manner in oocytes after stimulation with PMA, a protein kinase C activator (Chapell et al. 1998; Filippova et al. 2000). Although there is no direct evidence that GABAA subunits are phosphorylated in vivo after ethanol treatment, it has been hypothesized that chronic ethanol may increase phosphorylation of GABAA subunits and alter expression, function or trafficking (Kumar, Fleming, and Morrow 2004).
It is unclear whether GABAA receptors composed of the α2β2γ3 subunit combination exist in vivo in areas likely to be involved in alcohol-related phenotypes or predisposition to alcoholism. However based on in situ hybridization and immunocytochemistry studies it is likely that these subunit proteins co-localize in several brain regions which may be important in observed alcohol related phenotypes such as cortex and mesolimbic areas (reward pathways). The α2 subunit is found in 35% of GABAA receptors and in high concentrations in cortex, striatum, nucleus accumbens, septum, dentate gyrus and amygdala and hypothalamus. The γ3 subunit is less abundant but enriched in some regions which could contribute to risk for alcoholism including cortex, basal nuclei and hippocampus. The β2 subunit has not been linked to alcoholism but is the most abundant β isoform in mature brain. It is also important to point out that although our study has demonstrated differences in response to ethanol based on this subunit combination all 3 subunits may not be required to see similar functional changes.
This report demonstrates that altering the relative abundance of GABAA receptor subunits results in functional changes in response to both GABA and ethanol. Since GABAA receptor activity is critical for synaptic inhibition throughout the brain, alterations in GABA responsiveness may have wide-reaching impacts on the general state of central nervous system excitability. This study suggests that variations in the relative expression of GABAA receptor subunit genes may result in differences in response to both GABA and ethanol, and thereby such expression differences may modulate the propensity towards alcoholism.
Acknowledgments
This research was supported by NIH grant U10AA08403 (the Collaborative Study on the Genetics of Alcoholism).
The Collaborative Study on the Genetics of Alcoholism (COGA), Co-Principal Investigators B. Porjesz, V. Hesselbrock, H. Edenberg, L. Bierut, includes nine different centers where data collection, analysis, and storage take place.
The nine sites and Principal Investigators and Co-Investigators are:
University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., T. Foroud); University of Iowa (S. Kuperman, R. Crowe); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, A. Goate, J. Rice); University of California at San Diego (M. Schuckit); Howard University (R. Taylor); Rutgers University (J. Tischfield); Southwest Foundation (L. Almasy). Zhaoxia Ren serves as the NIAAA Staff Collaborator. This national collaborative study is supported by the NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
In memory of Henri Begleiter and Theodore Reich, Principal and Co-Principal Investigators of COGA since its inception; we are indebted to their leadership in the establishment and nurturing of COGA, and acknowledge with great admiration their seminal scientific contributions to the field.
References
- Barnard EA, et al. International Union of Pharmacology. XV Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50 (2):291–313. [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. EEG, autonomic and subjective correlates of the risk for alcoholism. J Stud Alcohol. 1993;54 (5):577–589. doi: 10.15288/jsa.1993.54.577. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41 (3):155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck KJ. New insight into the mechanisms of ethanol effects on GABAA receptor function and expression, and their relevance to behavior. Alcohol Clin Exp Res. 1996;20 (8 Suppl):198A–202A. doi: 10.1111/j.1530-0277.1996.tb01776.x. [DOI] [PubMed] [Google Scholar]
- Chang Y, et al. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16 (17):5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapell R, et al. Activation of protein kinase C induces gamma-aminobutyric acid type A receptor internalization in Xenopus oocytes. J Biol Chem. 1998;273 (49):32595–32601. doi: 10.1074/jbc.273.49.32595. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L. Quantitative electroencephalographic differences associated with alcohol, cocaine, heroin and dual-substance dependence. Drug Alcohol Depend. 1997;46 (1–2):87–93. doi: 10.1016/s0376-8716(97)00058-6. [DOI] [PubMed] [Google Scholar]
- Covault J, et al. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129 (1):104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Dick DM, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006a;36 (4):577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dick DM, et al. Association of GABRG3 with alcohol dependence. Alcohol Clin Exp Res. 2004;28 (1):4–9. doi: 10.1097/01.ALC.0000108645.54345.98. [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T. Candidate genes for alcohol dependence: a review of genetic evidence from human studies. Alcohol Clin Exp Res. 2003;27 (5):868–879. doi: 10.1097/01.ALC.0000065436.24221.63. [DOI] [PubMed] [Google Scholar]
- Dick DM, et al. Association between GABRA1 and drinking behaviors in the collaborative study on the genetics of alcoholism sample. Alcohol Clin Exp Res. 2006b;30 (7):1101–1110. doi: 10.1111/j.1530-0277.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74 (4):705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T. The genetics of alcoholism: identifying specific genes through family studies. Addict Biol. 2006;11 (3–4):386–396. doi: 10.1111/j.1369-1600.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, et al. Gene expression in the hippocampus of inbred alcohol-preferring and -nonpreferring rats. Genes Brain Behav. 2005;4 (1):20–30. doi: 10.1111/j.1601-183X.2004.00091.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA, et al. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006;141 (6):599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar SJ, et al. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J Biol Chem. 1999;274 (15):10100–10104. doi: 10.1074/jbc.274.15.10100. [DOI] [PubMed] [Google Scholar]
- Fehr C, et al. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16 (1):9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Filippova N, et al. Regulation of recombinant gamma-aminobutyric acid (GABA)(A) and GABA(C) receptors by protein kinase C. Mol Pharmacol. 2000;57 (5):847–856. [PubMed] [Google Scholar]
- Grobin AC, et al. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139 (1–2):2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, et al. Expression and pharmacology of human GABAA receptors containing gamma 3 subunits. Eur J Pharmacol. 1995;291 (3):301–309. doi: 10.1016/0922-4106(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, et al. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol Pharmacol. 1993a;43 (6):970–975. [PubMed] [Google Scholar]
- Hadingham KL, et al. Role of the beta subunit in determining the pharmacology of human gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1993b;44 (6):1211–1218. [PubMed] [Google Scholar]
- Harris RA, Mihic SJ. Alcohol and inhibitory receptors: unexpected specificity from a nonspecific drug. Proc Natl Acad Sci U S A. 2004;101 (1):2–3. doi: 10.1073/pnas.0307281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpel MW, et al. Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol. 2007;41 (2):95–132. doi: 10.1016/j.alcohol.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol. 2002;26 (2–3):251–268. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- Kohnke MD. Approach to the genetics of alcoholism: a review based on pathophysiology. Biochem Pharmacol. 2008;75 (1):160–177. doi: 10.1016/j.bcp.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Grunder G, Luddens H. Drug interactions at GABA(A) receptors. Prog Neurobiol. 2002;67 (2):113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, et al. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63 (9):957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101 (3):211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kumar S, et al. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86 (3):700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Sieghart W, Morrow AL. Association of protein kinase C with GABA(A) receptors containing alpha1 and alpha4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J Neurochem. 2002;82 (1):110–117. doi: 10.1046/j.1471-4159.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, et al. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29 (4):493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III Embryonic and postnatal development. J Neurosci. 1992;12 (11):4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng L, Hawkens DT, Li T-K. New strains of rats with alcohol preference and nonpreference. In: Thurman RG, et al., editors. Alcohol and Aldehyde Metabolizing Systems. Academic Press; New York: 1977. pp. 537–544. [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999;29 (2–3):196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Ticku MK. Chronic ethanol administration alters gamma-aminobutyric acidA receptor gene expression. Mol Pharmacol. 1992;42 (3):415–422. [PubMed] [Google Scholar]
- Moar VA, et al. Translational capacity of living frog eggs and oocytes, as judged by messenger RNA injection. J Mol Biol. 1971;61 (1):93–103. doi: 10.1016/0022-2836(71)90208-7. [DOI] [PubMed] [Google Scholar]
- Morrow AL, et al. Ethanol effects on cell signaling mechanisms. Alcohol Clin Exp Res. 2004;28 (2):217–227. doi: 10.1097/01.alc.0000113439.97498.ac. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, et al. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338 (6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Quirk K, et al. Characterisation of delta-subunit containing GABAA receptors from rat brain. Eur J Pharmacol. 1995;290 (3):175–181. doi: 10.1016/0922-4106(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, et al. Theta power in the EEG of alcoholics. Alcohol Clin Exp Res. 2003;27 (4):607–615. doi: 10.1097/01.ALC.0000060523.95470.8F. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, et al. Beta power in the EEG of alcoholics. Biol Psychiatry. 2002;52 (8):831–842. doi: 10.1016/s0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- Schofield PR, et al. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987;328 (6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol. 2006;54:231–263. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- Soyka M, et al. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2008;42 (3):184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, et al. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5 (8):721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, et al. Alternative splicing and promoter use in the human GABRA2 gene. Brain Res Mol Brain Res. 2005;137 (1–2):174–183. doi: 10.1016/j.molbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100 (25):15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose acute alcohol effects on GABA A receptor subtypes. Pharmacol Ther. 2006;112 (2):513–528. doi: 10.1016/j.pharmthera.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, et al. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Ymer S, et al. GABAA receptor beta subunit heterogeneity: functional expression of cloned cDNAs. EMBO J. 1989;8 (6):1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


