Abstract
The diagnostic yield of computed tomography (CT)-guided biopsies is dependent on accurate needle insertion. A laser-assisted angle selection system was custom fabricated and used during a CT-guided lung biopsy. Off-target error was measured comparing standard methods to the laser method while pointing towards the target from the skin. The difference between the planned angle and selected angle using the laser-assisted system was 2°, improved from 12° with the standard method. Although yet to be confirmed, laser-assisted angle selection systems may improve the accuracy of needle placement, which may translate into improved outcomes for certain needle based procedures.
Keywords: Biopsy, Angle selection, CT guidance, Interventional, Laser guidance
1. Introduction
Image-guided biopsies are becoming an important part of interventional radiology practice, with tissue characterization playing an increasingly vital role in drug selection, drug discovery, and prognostication in the era of personalized cancer therapies. More accurate biopsy means more accurate characterization which may be critical for heterogeneous tumors. After estimating a planned insertion angle from the preprocedural computed tomography (CT) scan, the standard method involves the physician replicating that angle using their clinical experience and visual estimation. Images are acquired again to confirm the needle angle and location, with repositioning and reimaging as necessary. Although the accuracy of angle selection can define success, it is an inexact process that is highly dependent on operator experience.
Certain laser-assisted systems improve accuracy over the conventional method [1,2]. In contrast to prior laser systems [3], this laser-assisted needle angle selection system (Laser-NASS) is integrated directly into the CT imaging software, minimizing impact upon throughput, potentially improving its clinical applicability and ease of use. In this case, Laser-NASS had markedly less error compared to the conventional method (as measured by the difference between the planned angle and actual angle).
2. Case report
A 51-year-old man with a history of chronic granulomatous disease and recurrent pneumonia presented with a low fever, persistent cough, and bilateral pulmonary infiltrates and underwent CT-guided lung biopsy. The evaluation of Laser-NASS described here was only preprocedural and was only used to select angles at the skin surface for simulated first-pass accuracy but not used for the actual needle insertion. The patient underwent written informed consent as part of an Institutional Review Board-approved clinical trial.
A standard radio-opaque skin grid and seven fiducial markers were placed on the patient, and a preprocedural CT scan was obtained. A custom electromagnetic (EM) tracking system (based upon Northern Digital Inc., Waterloo, Canada) was set up [4–6]. The fiducial markers allowed spatial registration of the CT images and measurement of the needle trajectory. The fiducials and tracking system were only used as a measurement tool. They are unrelated to laser guidance and not required for laser use. An entry point and target were then chosen in the CT image (Fig. 1), which defined a planned insertion angle and path. Initially, the conventional method for trajectory selection was performed, using only experience and intuition. Error was defined automatically by the custom software as the difference from the planned trajectory. The angle error was measured automatically by the EM tracking system in degrees, and needle trajectory error was defined as the minimum distance between the target and the actual vector.
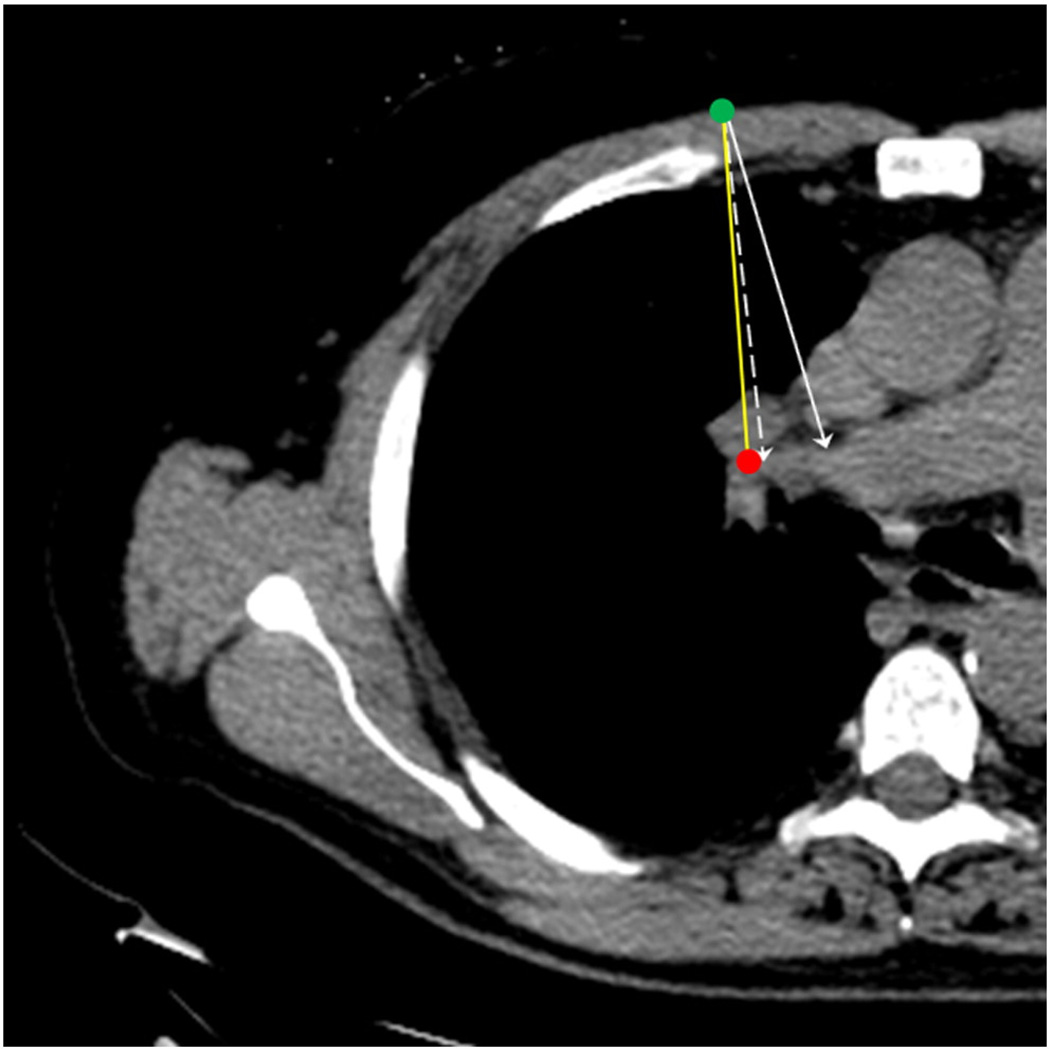
Fig. 1.
Planning CT shows planned entry point (green dot) and target (red dot). Planned path (yellow line), simulated path with Laser-NASS (dashed arrow), and simulated freehand path (solid arrow) are shown. (Paths are only illustrative as angle error may not be in the same plane.) Conventional method would have missed the target on the first needle pass, whereas the Laser-NASS would have resulted in a perfect pathway.
After the conventional method, the Laser-NASS method was followed by the same physician for comparison. Laser-NASS software semiautomatically orients the laser according to the planned angle and trajectory. The laser was Class II with four degrees of freedom (ARC Seibersdorf Research, Seibersdorf, Austria) and was mounted on a lockable passive arm with six degrees of freedom (DK Technologies, Barum, Germany) (Fig. 2). The laser produces a cross hair oriented along the planned angle parallel to the axis of the planned path. This parallel path is moved into the actual planned path when laser is moved to point at the preidentified skin entry mark without the angle changing. The cross-hair was used to orient the angle of the needle by first placing the needle tip at the skin entry mark. While holding this tip stationary in the skin, the hub was angulated until aligned into the cross hair. The angle (and thus potential path) and error for Laser-NASS were automatically recorded as 2° (vs. 12° with standard technique), and corresponding to a distance error of 10 mm (vs. 18 mm for standard technique). The actual biopsy was then completed by a second radiologist according to standard techniques.
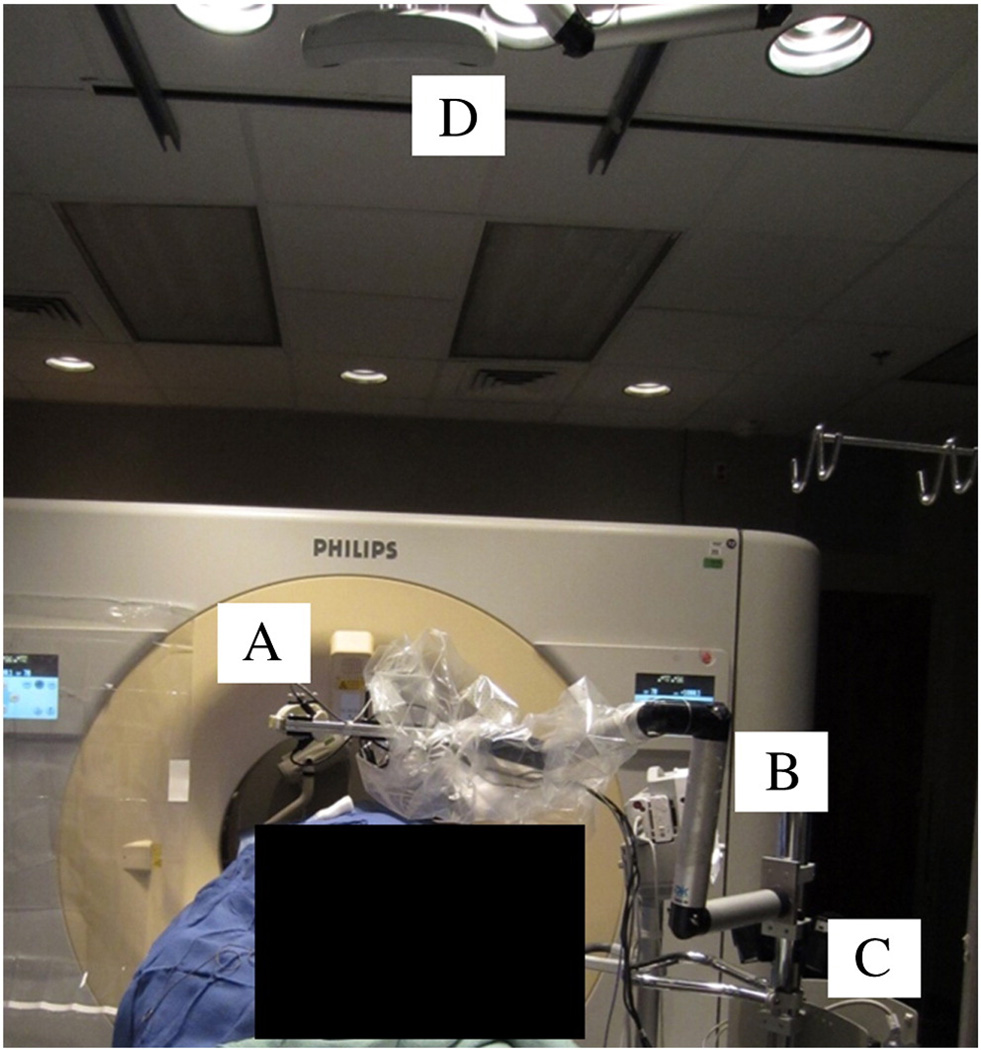
Fig. 2.
The head of the Laser-NASS (A) with the laser is mounted on the lockable passive arm (B). The Laser-NASS rests on a stable cart (C) with a height-adjustable rod. A commercial optical tracking system on the ceiling (D) is used to track the laser's motion and automatically provide a reference of the Laser-NASS to the CT, but the optical tracking is optional and only provides calibration.
3. Discussion
An integrated laser-guided angle selection system was used during a CT-guided biopsy as part of an ongoing clinical trial and showed improved accuracy over freehand angle selection in this patient. Limitations of the system include additional set-up time, cost, and an additional operator at the Laser-NASS workstation, although the latter may not be required for clinical use. Laser-NASS would not require EM tracking for validation in the clinical setting, nor is optical tracking (which automates calibration) absolutely required. Laser-NASS is integrated with the CT software and does not require time-consuming calibration to the table. In contrast to one currently available commercial platform, Laser-NASS does not require the operator to align the laser or manually enter angle numbers, although it still requires the conventional method of skin entry point selection [7].
More patients are needed to fully assess the accuracy of the system and the reproducibility of the results, but the anecdotal data in this patient are interesting. Improved accuracy using laser assistance for needle orientation was shown in this patient. Such a tool could also improve standardization for the inexperienced user. Although purely speculative, such a system could potentially offer advantages and should be further studied to assess for outcomes of diagnostic yield, needle repositioning, intraoperative risk, radiation exposure, and procedure time [8–10].
Acknowledgments
Thanks to Ankur Kapoor, PhD who assisted in software and hardware design and custom integration. This study was supported in part by the Intramural Research Program of the National Institutes of Health (NIH) and the Center for Interventional Oncology. The opinions expressed herein do not necessarily represent the opinions of the U.S. Government or the NIH. This research was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Leona M. and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at http://www.fnih.org/work/programs-development/medical-research-scholars-program. NIH and Philips Healthcare have a cooperative research and development agreement.
References
- 1.Pereles FS, Baker M, Baldwin R, Krupinski E, Unger EC. Accuracy of CT biopsy: laser guidance versus conventional freehand techniques. Acad Radiol. 1998;5(11):766–770. doi: 10.1016/s1076-6332(98)80260-2. [DOI] [PubMed] [Google Scholar]
- 2.Jacobi V, Thalhammer A, Kirchner J. Value of a laser guidance system for CT interventions: a phantom study. Eur Radiol. 1999;9(1):137–140. doi: 10.1007/s003300050644. [DOI] [PubMed] [Google Scholar]
- 3.Miaux Y, Guermazi A, Gossot D, Bourrier P, Angoulvant D, Khairoune A, et al. Laser guidance system for CT-guided procedures. Radiology. 1995;194(1):282–284. doi: 10.1148/radiology.194.1.7997570. [DOI] [PubMed] [Google Scholar]
- 4.Solomon SB, Dickfeld T, Calkins H. Real-time cardiac catheter navigation on three-dimensional CT images. J Interv Card Electrophysiol. 2003;8(1):27–36. doi: 10.1023/a:1022379612437. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SB, White P, Wiener CM, Orens JB, Wang KP. Three-dimensional CT-guided bronchoscopy with a real-time electromagnetic position sensor: a comparison of two image registration methods. Chest. 2000;118(6):1783–1787. doi: 10.1378/chest.118.6.1783. [DOI] [PubMed] [Google Scholar]
- 6.Frantz DD, Wiles AD, Leis SE, Kirsch SR. Accuracy assessment protocols for electromagnetic tracking systems. Phys Med Biol. 2003;48(14):2241–2251. doi: 10.1088/0031-9155/48/14/314. [DOI] [PubMed] [Google Scholar]
- 7.Varro Z, Locklin JK, Wood BJ. Laser navigation for radiofrequency ablation. Cardiovasc Intervent Radiol. 2004;27(5):512–515. doi: 10.1007/s00270-003-4033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereles FS, Ozgur HT, Lund PJ, Unger EC. Potentials of a new laser guidance system for percutaneous musculoskeletal procedures. Skeletal Radiol. 1998;27(1):18–21. doi: 10.1007/s002560050328. [DOI] [PubMed] [Google Scholar]
- 9.Reyes GD. A guidance device for CT-guided procedures. Radiology. 1990;176(3):863–864. doi: 10.1148/radiology.176.3.2389049. [DOI] [PubMed] [Google Scholar]
- 10.Ozdoba C, Voigt K, Nüsslin F. New device for CT-targeted percutaneous punctures. Radiology. 1991;180(2):576–578. doi: 10.1148/radiology.180.2.2068333. [DOI] [PubMed] [Google Scholar]