Abstract
Later-born siblings of children with autism spectrum disorder (Sibs-ASD) are at elevated risk for social impairments. Two putative predictors of later social impairment—measures of responding to joint attention and weighted triadic communication—were examined in a sample of 43 Sibs-ASD who were followed from 15 to 34 months of age. Results revealed that initial level of responding to joint attention and growth rate of weighted triadic communication predicted the degree of social impairment at the final measurement period. Additionally, both predictors were associated with later ASD diagnosis. In contrast, unweighted triadic communication, age of entry into the study, and initial language level did not predict later social impairment. The importance of considering social outcome as a continuous variable in prospective studies of Sibs-ASD is discussed.
Keywords: Risk for social impairment, Prediction, Joint attention, Younger siblings of children with ASD
Introduction
Genetic and family studies have revealed that autism is a highly heritable disorder, with some estimating heritability to be as high as 90% (Szatmari et al. 1998). The genetic risk to family members extends not only to diagnosed autism spectrum disorders (ASD), but also to milder expressions of the disorder that are below threshold for a clinical diagnosis (Bailey et al. 1998; Szatmari et al. 2000). Behaviors and traits that are conceptually similar to the core autism symptom domains but are not associated with disability or diagnosis (e.g., mild social or communication impairments) have been referred to as the “broader autism phenotype” (Piven et al. 1997; Wassink et al. 2004), and the prevalence of the broader autism phenotype in siblings of children with ASD (Sibs-ASD) has been reported to be as high as 40% (Losh et al. 2008).
Previous studies of Sibs-ASD have focused either on identifying early predictors of ASD or detecting differences between Sibs-ASD and typically developing (TD) children that may reflect genetic vulnerabilities. Studies in the latter category have found that as a group, Sibs-ASD demonstrate different patterns of social and communicative functioning compared with younger siblings of children with typical development. These differences include lower levels of smiling (Cassel et al. 2007) and eye-to-eye gaze with mothers (Merin et al. 2007), as well as more limited triadic communication skills, such as responding to joint attention, initiating joint attention, initiating requests, and using gesturing (Cassel et al. 2007; Goldberg et al. 2005; Mitchell et al. 2006; Presmanes et al. 2007; Stone et al. 2007; Yirmiya et al. 2006). Moreover, the lower levels of performance appear to characterize a substantial portion of the Sibs-ASD group (Stone et al. 2007) and remain even after the children who later receive a diagnosis of ASD are removed (Mitchell et al. 2006). It is noteworthy that many of these differences are evident in various aspects of social development.
Empirically, even the highest estimates of ASD outcomes in Sibs-ASD samples indicate that the majority of Sibs-ASD does not have clinically diagnosable social impairments (Landa and Garrett-Mayer 2006). Thus, it is important to understand the sources of the variability in social impairment within the Sibs-ASD population. Therefore, instead of focusing on mean differences between Sibs-ASD and TD groups, the present study focuses on predicting the continuum of social impairment outcomes within the Sibs-ASD group.
Traditional diagnostic conceptualizations of ASD consider the triad of symptoms that define the disorder as interrelated and co-occurring. In fact, the required presence of symptoms in all three areas (repetitive behavior/ restricted interests, social reciprocity impairment, and communicative impairment) is the logical basis for separating ASD from arguably related disorders (e.g., developmental language disorder, obsessive–compulsive disorder, pragmatic communication disorder). The over-lapping nature of symptoms is most apparent for the social and communication domains, as evidenced in the use of a combined social-communication cutoff score in the Autism Diagnostic Observation Scale (ADOS; Lord et al. 2000) diagnostic algorithm. Moreover, recent factor analysis supports the merging of social and communicative algorithms on the ADOS (Gotham et al. 2008).
Although controversial, recent conceptualizations of autism and ASD have emphasized the continuous nature of individual autism symptoms in contrast to the categorical approach used in formal diagnostic systems (Baron-Cohen 2002; Happe et al. 2006). According to this approach, the behavioral symptoms of ASD form a normal distribution across individuals with and without a diagnosis of ASD (Constantino et al. 2000, 2006). Additionally, the three core symptoms are conceptualized as separable and independent at behavioral, neurocognitive, and genetic levels (Baron-Cohen and Belmonte 2005; Happe et al. 2006). In fact, the independence of individual symptoms may be most evident in findings that family members of children with ASD rarely display the full triad of clinical symptoms (Pickles et al. 2000).
The purpose of this study was to understand the variability in the degree of later social impairments, as well as the presence of ASD, in later-born siblings of children with ASD by identifying early skills that predict individual differences in social impairment. That is, we aim to understand why some siblings are more impaired than others, not only to identify those in need of intervention services, but also to understand what goes awry in the early development of children who are at-risk for social impairment. Although inadequate for determining causality, longitudinal correlational designs have long been used to identify particularly promising factors that may influence later individual differences. In this way, a longitudinal correlational design is a good first step in understanding individual differences in particular aspects of social impairment within a group at high-risk for social impairment: Sibs-ASD.
In the present study, we take two approaches. In one approach, we follow the traditional path of predicting which Sibs-ASD are eventually diagnosed as having ASD. This approach uses conventional methodology as a means for providing practical guidelines for clinicians who seek to make longitudinal predictions of ASD outcome for at-risk children. In the second approach, social outcomes are measured using continuous variables (i.e., scores of social ability that are referenced to the mean and SD of an age-matched typically developing group). This approach is a deliberate departure from most previous studies of Sibs- ASD. Here we emphasize the continuous nature of social impairment instead of treating social impairment as a dichotomous outcome (i.e., ASD diagnosis vs. other), because (a) social impairment as a construct is an intrinsically continuous phenomenon, (b) models predicting continuous outcomes tend to be more statistically powerful than models predicting categorical outcomes (i.e., larger effect sizes for associations tend to detected) because the outcome variable contains more specific information and uses the entire range of the scale than do artificially-dichotomized outcomes, and (c) measuring the outcome as a continuous variable reminds readers that there are scientific, as well as practical, reasons for studying Sibs-ASD. Additionally, by focusing on social impairment, instead of the cluster of symptoms that define autism or ASD, we can begin to understand the sources of a particular impairment. Finally, by focusing on different aspects of social impairment (i.e., the specific skill of responding to joint attention bids [responding joint attention] and a more general measure of social skill), we can potentially identify distinct predictors for specific aspects of social impairment. Such specificity is likely to improve the basis for optimizing treatments aimed at preventing or attenuating social impairment.
We measure social impairment using both direct observation and parent report. Direct observation of social performance has the advantage of eliminating any immediate influence that parental characteristics (e.g., memory) may have on scores, but has the disadvantage of basing scores on relatively short procedures that might not represent the child’s typical performance. Parent report of social performance has the advantage of potentially drawing on a wide range of experiences with the child but the disadvantage of allowing the potential influence of characteristics of the parent to affect the accuracy of scores.
By far the majority of past studies predicting diagnosis, social development or social impairment in Sibs-ASD have relied on single-point measures to predict later social development or impairment. Although this method can provide important information, the present study employs hierarchical linear modeling (HLM) to examine growth in early social skills as predictors of social impairment. Examination of the growth rate of early social skills provides a different type of information than single-point measures and may also be useful in predicting social impairment.
Because we wish to assess whether individual differences in growth curve parameters (e.g., intercepts and slopes) predict social impairment, it is important to measure behaviors that improve (i.e., variables that increase) during the developmental period of interest (i.e., 15–34 months). In addition, because we have only four time points within which to assess growth, it is helpful to have a measure that increases in a linear fashion.
Of the many potential predictors of social impairment that are likely to grow in a somewhat linear manner during the 15–34 month period, one particularly promising predictor is responding to joint attention (RJA). RJA, also called attention following, is signaled by the child’s shifting his or her attention in response to another person’s attentional cue [e.g., point, gaze, attention-directing utterance] (McDuffie et al. 2005). RJA has been shown to increase from 9 to 18 months of age even when only one type of attentional cue (e.g., point plus gaze) is presented (Mundy et al. 2007). When a variety of attentional cues that vary systematically by salience and informational value are used, RJA has been shown to increase through 21 months of age (Deak et al. 2008). In typically developing infants, individual differences in early RJA have been associated with attention disengagement in self-regulation tasks or inhibitory control, which are in turn relate to later social competence (Morales et al. 2005; Van Hecke et al. 2007). RJA also predicts teacher reports of externalizing behavior in preschoolers with prenatal exposure to cocaine (Sheinkopf et al. 2004) and with typical development (Van Hecke et al. 2007).
Disturbances in RJA have been observed reliably in young children with autism (Mundy et al. 1986; Sigman and Ruskin 1999). Although many Sibs-ASD will not develop ASD themselves, identifying the early deficits that children with ASD show may help understand the origins of social impairment in other populations. RJA may be related to later social competence because it may: (a) mark the early development of an awareness that others have perceptions and intentions that may be indicated by their social signals; and/or (b) involve aspects of executive attention regulation, inhibitory control and self-monitoring that are critical to social development (Van Hecke et al. 2007).
A second putative predictor of later social impairment in Sibs-ASD is a new variable to the autism literature: weighted triadic communication (WTC). This type of triadic communication is child initiated, and involves the child’s use of a combination of gestures, vocalizations, gaze shifts, and sometimes symbolic means to communicate a message about an object or event to another person (Van Hecke et al. 2007). In this manuscript, we use the term child-initiated triadic communication, instead of initiating joint attention, because the latter term is sometimes used to refer to communication with a declarative function (e.g., comments) and our variable included both imperative (e.g., requests) and declarative functions. It is well accepted that unweighted triadic communication, like RJA, may be important to later social development because (a) it marks the early development of an awareness that others have perceptions and intentions that might be accessed by attending to social signals; and/or (b) it involves aspects of executive attention regulation, inhibitory control and self-monitoring that are critical to social development (Van Hecke et al. 2007). For this study, we used a weighted measure of child-initiated triadic communication to capture qualitative changes in the form of intentional communication that occur with development. By ‘weighted’, we mean that each instance of triadic communication is weighted by whether it is nonverbal (1 point), single word (2 points) or multiword (3 points). This index has been shown to grow in a linear fashion throughout the period of development that we studied (Greenwood et al. 2006).
Weighting triadic communication reflects both language use and frequency of communication. Weighted triadic communication is worth considering as a predictor of social impairment because it takes into account the developmental level of the form of the triadic communication. For example, children may begin to initiate triadic communication using nonverbal behaviors, such as eye contact. However, as language emerges, they may reduce their reliance on eye gaze and use more developmentally advanced forms of communication to engage in social exchanges about a topic of communication. Eventually, words are combined to convey meanings. Giving more weight to developmentally advanced forms of communication results in a measure that better reflects developmental level than does simple frequency of communication. Using frequency of communication as the primary unit that is weighted reflects the social nature of interaction more than vocabulary checklists or most standardized tests of language do. To determine whether WTC has any advantage over unweighted triadic communication in predicting later social impairment and ASD diagnosis, both metrics will be derived and examined. WTC has not yet been tested as a predictor of social impairment in any population.
In sum, the purpose of this study was to determine the extent to which growth in RJA and WTC predicts ASD diagnosis and the extent of social impairment in younger siblings of children with ASD. ASD diagnosis and continuous social outcomes were assessed approximately 1½ years after study entry (i.e., after 30 months of age). Social outcomes were assessed using parental report and direct observation measures. Because our goal was to predict later social impairment, social outcomes were measured about 4 months after the last period used to estimate the growth of RJA and WTC.
Methods
Participants
This study included 43 Sibs-ASD (56% male) and 24 Sibs- TD (71% male) who were recruited between 2003 and 2006 for participation in a longitudinal study of the development of social orienting. Eligibility criteria for both groups were: (1) chronological age between 12 and 23 months (inclusive) at study entry; (2) no severe sensory or motor impairments; (3) no identified metabolic or genetic disorders; (4) English as the primary language; (5) an older sibling; and (6) completion of the final evaluation at Time 5. Eligibility for the Sibs-ASD group further required: (1) that the older sibling (proband) have a diagnosis of autism, PDD-NOS, or Asperger’s disorder, which was verified by ADOS, Autism Diagnostic Interview- Revised (ADI-R; Rutter et al. 2003), and clinical diagnosis; and (2) valid data on the putative predictors for at least 3 of the 4 time points preceding the final evaluation. Four families had more than one Sibs-ASD child participating in the study; for these families, the child with the most complete data was included. When equivocal, one child from the family was selected randomly. Eligibility for the Sibs-TD group further required that the older sibling have no developmental disorders and that there be no family history of autism or mental retardation in first-degree relatives; this information was obtained through a family history interview with parents.
The Sibs-ASD were recruited from university-based autism and speech-language programs and community agencies. The Sibs-TD were recruited through a birth record database and word-of-mouth. The research protocol received approval from the Vanderbilt University Institutional Review Board, and all parents signed informed consent forms prior to participation. Compensation in the form of checks or savings bonds was provided to families following each visit.
On average, the Sibs-ASD at Time 1 were 14.9 months old (SD = 3.1), had nonverbal mental ages of 15.4 months (SD = 3.4), and cognitive standard scores of 98 (SD = 14). Probands of Sibs-ASD had the following diagnoses: autism (26), PDD-NOS (16), Asperger’s disorder (1). Most participants in both groups were Caucasian (88% of each group); twelve percent of the Sibs-TD and 7% of the Sibs-ASD were multi-racial; and the remainder was African American or Asian. The majority (56%) of Sibs-ASD had only one sibling, whereas only 38% of Sibs- TD had only one sibling. Twenty-three percent of Sibs- ASD and 38% of Sibs-TD had two siblings, and the remainder had three. There were more males than females in both Sibs-ASD (55% male) and Sibs-TD (71% male) samples. There were no significant group differences on any of these categorical variables. The Sibs-ASD and Sibs- TD groups were also comparable on child chronological age, nonverbal mental age, and maternal education level at Time 5, the age at which the Sibs-TD sample was used to reference the outcomes (see Table 1).
Table 1.
Participant description at time 5
| Variables | Sibs-ASD
|
Sibs-TD
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age in months | 33.72 | 3.48 | 34.38 | 4.04 |
| Nonverbal mental age in months | 37.65 | 8.03 | 39.58 | 9.6 |
| Maternal formal education level | 5.91 | 0.78 | 6.08 | 0.72 |
Design
This study employed a longitudinal correlational design. The two predictor variables (Responding Joint Attention, RJA; and Weighted Triadic Communication, WTC) were measured at 4 time points, each separated by approximately 4 months. ASD diagnosis and the three social outcome variables were measured at Time 5: RJA, WTC, and Social Behavior Checklist score. The Time 5 visit took place approximately 6 months after the Time 4 visit. The average age of the Sibs-ASD during this visit was 33.9 months (SD = 3.4 months). It is noteworthy that a social impairment diagnosis (i.e., ASD) tends to be more stable after 30 months of age (Turner and Stone 2007). Therefore, we are measuring diagnosis and social impairment at an age that is arguably particularly meaningful.
Because one focus of this paper is the prediction of social impairment, means and SDs from the Time 5 Sibs-TD sample were used to derive TD-referenced z-scores for the continuous outcome variables in the Sibs-ASD. Children in the Sibs-ASD group who scored below −1.25 SD (i.e., the level in SD units that corresponds to the 10th percentile) on these TD-referenced z-scores were considered to be “delayed” on the measured construct. Outcome variables for which more than 10 percent of the Sibs-ASD scored below −1.25 were considered to be sensitive measures of the type of social impairments for which Sibs-ASD children are at risk.
Procedures
Demographic Questionnaire
At the child’s first and last visits his/her parents completed a demographic questionnaire, which included information about the child’s age and the mother’s educational level. Educational level was organized into the following categories with their assigned numerical value: <7 years (1), 7–9 years (2), 10–11 years (3), high school graduation or equivalent (4), partial college (5), college graduation (6), graduate degree (7). Maternal education at Time 5 was used as a matching variable, and child age was used as amatching variable at Time 5 and a possible covariate at Time 1.
Mullen Scales of Early Learning (MSEL) (Mullen 1995)
The MSEL is a well-known and widely-used measure of cognitive function that was developed for use with children from birth through 68 months. It includes four cognitive scales: Visual Reception (nonverbal problem-solving), Fine Motor, Receptive Language, and Expressive Language. These subscales yield T-scores as well as age equivalency scores. The age equivalency scores on the Receptive Language and Expressive Language scales at Time 1 were used to quantify the developmental level of the children’s language, and were used as possible covariates.
Screening Tool for Autism in Two-Year-Olds (STAT) (Stone et al. 2000, 2004)
The STAT is an empirically-derived interactive screening instrument for autism that takes about 20 min to administer. Twelve items assessing play, communication, and imitation are presented in a non-fixed order within a play-like interaction. Though designed as an interactive screening tool, it has been used previously as a standard, semi-structured context within which children’s social-communicative behaviors can be coded (McDuffie et al. 2005) and with children under 24 months (Stone et al. 2008). In the current study the STAT was used as a context for coding unprompted triadic communication, including the form of its expression (i.e., nonverbal, single word, or multiword means), for the WTC variable.
Trained observers coded the STAT using a timed-event behavior sampling method with the aid of software (ProcoderDV; Tapp 2003) that enables computer control of digital recordings. “Triadic communication” was defined as the use of gestural, vocal, gaze, and/or symbolic communication that shows attention to the message recipient and the physical referent of communication. Communication was considered “unprompted” if it followed an adult behavior that did not provide a cue for a specific communication or occurred after the child’s own behavior. Words and word approximations were coded using the following criteria for inclusion as a “single-word” or “multi-word” acts: (a) contains at least one accurate consonant and vowel in the correct position, (b) has the correct number of syllables (exceptions made for common developmentally appropriate word derivates), (c) appears in the unabridged English language dictionary, (d) was not used imitatively, and (e) was used referentially.
The variable derived from this procedure was the weighted frequency of unprompted triadic communication, obtained by multiplying each unprompted triadic communication act by the weight assigned to the behavior used to communicate (nonverbal = 1, single word = 2, multiword = 3). The growth curve for the weighted frequency of triadic communication from Times 1–4 was analyzed as a putative predictor and a TD-referenced z-score transformation for the Time 5 score was analyzed as an outcome variable. Interobserver reliability (intra-class correlation coefficients, ICC) on weighted triadic communication was based on independent codings of an average of 22% (SD = 5%) of the sessions, randomly selected from the entire data set at each time period. The average ICC was .97 (SD = .02).
Responding to Joint Attention (RJA) (Deak et al. 2008; Presmanes et al. 2007)
This procedure is described in greater detail in the Presmanes et al. paper. Children were seated facing the stimulus display at a small table with age-appropriate toys. The experimenter sat on either the child’s right or left side (counterbalanced) and delivered attentional cues while the child was visually engaged with toys. Eight novel target stimuli were displayed on individual plexiglass shelves in a 3 × 3 matrix across one wall, with a camera placed in the middle of the bottom row. Children’s gaze toward the stimuli was recorded by three cameras (one in front, and one at the child’s far left and far right) positioned to capture the child’s head and upper body movements.
Ten different types of attention-eliciting prompts were used, each containing a different combination of physical and verbal directives (i.e., head and gaze shifts, pointing gestures, and eliciting and directing verbalizations). Each RJA prompt type was repeated twice, once with the experimenter on the child’s left and once on the right. Each trial lasted 10 s, during which time the experimenter held the physical position and facial expression constant. The accuracy with which children located the target was evaluated for each trial that the child fixated on a location on the stimulus wall by coders unaware of the correct target location.
Trained observers blind to group status coded the RJA task from media files with the aid of ProcoderDV software (Tapp 2003). Coders indicated which of the eight stimuli the child looked at (if any) in the 10-s period following each cue. “Hits” were scored by comparing the coded gaze location with the actual target location in that trial. If the code matched the target location, a score of 1 was given. If the coded location was vertically adjacent to the target location, a score of 0.5 was given. If the coded location was not vertically adjacent to the target or no gaze location was coded, a score of 0 was given. Growth on the accuracy raw scores from Time 1 to Time 4 was used a putative predictor and the TD referenced z-score transformation of the accuracy scores from Time 5 was used an outcome variable. Interobserver reliability (ICC) on RJA raw scores was based on independent codings of an average of 28% (SD = 3%) of the sessions, which were randomly selected from each time period. The average ICC was .96 (SD = .02).
Social Behavior Checklist (SBC)
The SBC is a broad-based parental report measure of normative social behaviors that represents an expanded version of the Preschool Social Behavior Checklist (Stone and Lemanek 1990). The 30 developmentally-ordered items on the SBC represent a wide range of social behaviors occurring in infancy through the preschool years that reflect common deficit areas for young children with ASD (e.g., expression of affect, imitation, toy play, peer interactions). The specific items selected for inclusion on the SBC are independent of a child’s level of language development, which renders this measure especially well-suited to young children with autism. Items are presented as behavioral descriptions (e.g., “shows interest in other children”) and parents rate how true each description is on a 3-point Likert-like scale. Psychometric properties in the present sample revealed good internal consistency (standardized alpha = .95 for Sibs-ASD and .86 for Sibs-TD). The TD-referenced z-score transformation of the Time 5 SBC score was used as an outcome measure of overall social behavior from the parents’ perspective.
Diagnostic Measures
The Autism Diagnostic Observation Scale (ADOS) (Lord et al. 2000), Autism Diagnostic Interview-Revised (ADI-R) (Rutter et al. 2003), and clinical diagnosis from a licensed psychologist with autism experience were used to establish Time 5 diagnostic outcomes of the Sibs-ASD to and verify ASD diagnoses of Sibs-ASD probands. The ADI-R is an investigator-based interview for parents/caregivers that provides explicit scoring criteria and cutoff scores in the domains of social reciprocity, language and communication, and restricted and repetitive activities. Scores are used to determine whether the individual’s diagnostic classification is consistent with an autism spectrum disorder. Clinical diagnosis was assigned by a licensed psychologist, based on results of the ADOS and ADI-R, with reference to DSM-IV-TR diagnostic criteria (APA 2000). The classifications of Autism and PDD-NOS were based on the child’s clinical diagnosis. This Time 5 dichotomous variable was dummy-coded (1 = ASD, 0 = typical) and analyzed as an outcome.
Results
Diagnosis
Six of the children (15%) were diagnosed with ASD (autism = 3; PDD/NOS = 3). The remaining 85% were not diagnosed as having ASD (or other formal diagnoses). However, eight of the children (20%) who were not diagnosed as having ASD presented behaviors that led the clinician to have significant concerns about the social development of the children and seven of the nine children scored above the ASD threshold on the Reciprocal Social Interaction algorithm scale of the ADOS.
Social Impairment Outcomes
As mentioned earlier, the performance of the Sibs-TD sample was used to provide age-expected benchmarks for the continuous social outcomes. The means and SDs obtained by the Sibs-TD were used to transform the raw scores for the Sibs-ASD sample into “TD-referenced z-scores.” Table 2 indicates the means and SD for the two groups. The TD-referenced SBC (M = −.99; SD = 1.95) and TD-referenced RJA (M = −.68; SD = 1.61) were significantly below zero (i.e., the mean for the TD sample), t(40) = −3.6; p = .001; d = .51, −2.58, p = .124, d = .42, respectively. In contrast, the TD-referenced WTC was not different from zero, M = −.03, SD = .9, (39) = 1.5, p = .13; t(41) = .06; p = .95, respectively. Additionally, the proportion of Sibs-ASD who scored under 1.25 (i.e., below the 10th percentile with reference to the TD sample) for the putative Time 5 outcomes was .37, .34, and .11 on the TD-referenced RJA, SBC, and WTC, respectively. The proportion obtained for the TD-referenced WTC was about what would be expected for a typically developing sample; consequently, the WTC was not considered an outcome that was sensitive to social impairment at Time 5 for the Sibs-ASD sample. Two Time 5 variables were thus included as social impairment outcomes in this study: TD-referenced RJA scores, and TD-referenced SBC scores.
Table 2.
Means and SDs for the time 5 social outcomes
| Variable | Raw scores
|
TD-referenced scores
|
||||
|---|---|---|---|---|---|---|
| Sibs-TD
|
Sibs-ASD
|
Sibs-ASD
|
||||
| Mean | SD | Mean | SD | Mean | SD | |
| RJA | 7.9 | 2.2 | 10.5 | 5.0 | −.68 | 1.61 |
| SBC | 53.8 | 5.4 | 48.47 | 10.5 | −.99 | 1.95 |
| WTC | 32.5 | 26.2 | 31.8 | 23.7 | −.03 | 0.90 |
Intercorrelation of the Time 5 Measures of Social Impairment and Diagnosis
The two continuous measures of social impairment (i.e., TD-referenced RJA and TD-referenced SBC) were not related, r = .03, ns. However, ASD diagnosis was moderately related to TD-referenced RJA, r = .4, p = .01, and TD-referenced SBC, r = −.39, p = .02. The predictors of each outcome were examined separately.
Descriptive Statistics for the Putative Predictors
The means and SDs for the putative predictors (i.e., RJA and WTC) at Times 1–4 are in Table 3. In addition, the means for unweighted triadic communication have been included for comparison. As expected, means for RJA and WTC demonstrate growth over time, whereas this was not true for the unweighted triadic communication variable. This information is included to provide a context for interpreting the results of the study.
Table 3.
Means and SDs for putative predictors and unweighted triadic communication in Sibs-ASD at times 1–4
| Variables | Time 1
|
Time 2
|
Time 3
|
Time 4
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| RJA | 3.4 | 2.7 | 4.6 | 3.0 | 5.2 | 3.1 | 5.3 | 3.4 |
| WTC | 14.4 | 10.2 | 19.9 | 14.3 | 19.3 | 14.0 | 22.1 | 17.9 |
| Unweighted triadic comm. | 13.1 | 6.7 | 15.6 | 10.1 | 13.4 | 7.6 | 11.7 | 6.5 |
Time 1 Age and Language Level as Potential Covariates of Social Impairment and Diagnosis
Because the ages of children in the Sibs-ASD group varied at the Time 1 evaluation, it was important to determine whether age alone could account for variance in outcomes. Age at Time 1 did not correlate with the Time 5 TD-referenced RJA scores, r = .05, p = .76, the TD-referenced SBC scores, r = .02, p = .89, or ASD diagnosis, Wald statistic = 1.2, p = .26.
A similar analysis was performed for the MSEL language subscales. Because age equivalency scores for the two language subscales were highly intercorrelated, r = .64, p<.001, the average of the two age equivalency scores was used as the putative predictor of social impairment outcomes. Language level at Time 1 was not related to Time 5 TD-referenced RJA scores or to TD-referenced SBC scores, r = .11, 23, ns, respectively. Therefore, neither language level nor age was covaried when testing predictors of continuous social impairment outcomes. However, language level at Time 1 was predictive of Time 5 ASD diagnosis, Wald statistic (1) = 4.44, p = .04, sensitivity = .17, specificity = .97, positive predictive value = .5. Therefore, the growth curves for RJA and WTC were assessed along with language level in predicting ASD diagnosis.
Growth Curves for RJA and WTC
Hierarchical Linear Modeling (HLM) software was used to estimate the growth curves of the RJA and WTC variables. Age of evaluation was centered at the average age of Sibs- ASD at the Time 1 evaluation (i.e., 14.88 months). Centering age at the average Time 1 age allowed the intercept to be interpreted as the estimated level of RJA or WTC at the average age of Time 1 evaluation (i.e., estimated initial level of RJA or WTC). Using the difference in the deviance statistics for nested models to determine the best fitting growth curve, RJA growth was best estimated with a fixed (significantly different from zero) and random (significant individual differences) intercept and a fixed (significantly different from zero) slope, difference χ2(1) = 7.7.; p = .006. In contrast, WTC growth was best estimated with a fixed (significantly different from zero) intercept and a fixed (significantly different from zero) and random (significant individual differences) slope, difference χ2(1) = 12.25; p = .001. Because only four measurement periods were used, the most complex growth model we could consider was quadratic. However, the accelerator parameter was not needed. In general, growth curve modeling indicated that simple linear models (i.e., a straight line) best fit the growth of WTC and RJA.
Relation of Predictors with Time 5 Social Impairment and Diagnosis
ASD diagnosis at Time 5 varied as a function of WTC slope, Wald statistic (1) = 5.3, p = .02, sensitivity = .5, specificity = .97, positive predictive value = .75, and RJA at Time 1, Wald statistic (1) = 4.2, p = .04, sensitivity = .17, specificity = .94, positive predictive value = .33. Neither WTC slope or RJA at Time 1 had unique predictive value after controlling for Time 1 language age, although each p value was<.10.
The aspects of the growth curves that can vary as a function of the outcome variables are those showing significant individual differences (i.e., random effects). In the present study, these parameters are the intercept for RJA (i.e., initial level of RJA) and the slope for WTC (i.e., rate of growth of WTC). Associations between individual differences in these random parameters and outcomes can be tested using HLM by treating the social impairment outcomes as Level 2 variables. Separate HLM models were conducted for each Time 5 social impairment variable (TD-referenced RJA, and TD-referenced SBC) on each random effect (intercept of RJA and slope of WTC).
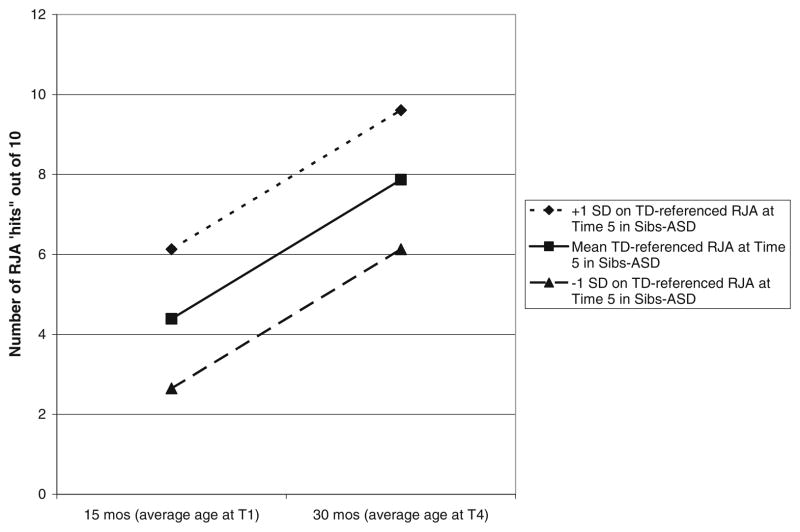
As before, the difference in deviance statistics was used to determine whether adding the social impairment variable at Level 2 improved the model. Results revealed that TD-referenced RJA at Time 5 varied as a function of RJA intercept, difference χ2(1) = 26.2; p<.001. Figure 1 illustrates this association. The pseudo R square change for this association was .67. Using the observed Time 1 RJA score as the predictor of Time 5 TD-referenced RJA, the association was .43; p = .007. Other associations with TD-referenced RJA were nonsignificant and of trivial size.
Fig. 1.
Growth in estimated RJA ‘hits’ as a function of degree of impairment in RJA at Time 5
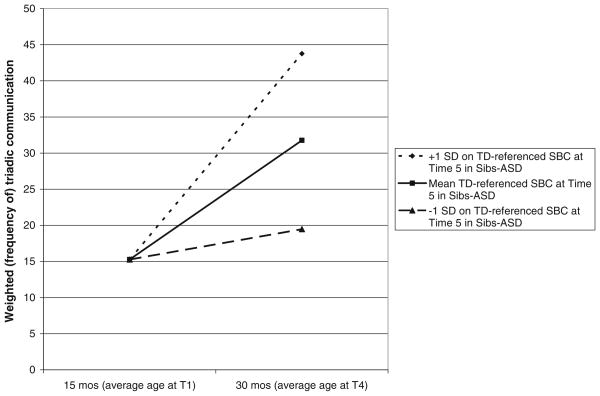
TD-referenced SBC at Time 5 varied as a function of WTC slope, difference χ2(1) = 5.6; p<.02. This association is illustrated in Fig. 2. The pseudo R square change for this association was .12. Using the ordinary least squares estimate of each participant’s growth curve for WTC as a predictor of TD-referenced SBC at Time 5, the association .46; p = .004. Other associations with TD-referenced SBC were nonsignificant and of trivial effect size.
Fig. 2.
Growth in estimated WTC as a function of degree of impairment in SBC at Time 5
Secondary Analysis of Unweighted Triadic Communication as a Potential Predictor of Social Impairment or Diagnosis
In Table 3, we see that the means for the Unweighted Triadic Communication variable does not increase within the Sibs-ASD sample with time, as expected. Additionally, the data did not meet an important assumption of growth curve modeling (i.e., homogeneity of Level 1 residuals); no model for growth of unweighted triadic communication could be fit to the data. For example, adding a fixed or random slope parameter to the model did not improve the fit of linear growth models to the data over and above using only the Time 1 unweighted triadic communication variable alone. Finally, the observed Time 1 unweighted triadic communication was not significantly related to either Time 5 ASD diagnosis, Wald statistic(1) = .15, p = .7, TD-referenced RJA, r = −.11, or TD-referenced SBC, r = .10.
Discussion
This study evaluated whether initial value and growth rate of two early social skills (i.e., responding to joint attention [RJA] and weighted triadic communication [WTC]) predicted social impairment in a group of younger siblings of children with ASD (Sibs-ASD). Impairment in important social outcomes was measured at the age at which individual differences in ASD diagnosis are relatively stable (i.e., after 30 months; Turner and Stone 2007). An age-matched group of younger siblings of children with typical development was used to provide benchmarks used to quantify the degree of impairment or delay in Time 5 social outcome for Sibs-ASD. Two continuous social outcome measures, representing observational (RJA) and parent report (SBC) data, were used: the TD-referenced RJA score, and the TD-referenced Social Behavior Checklist (SBC) score. ASD diagnosis at Time 5 was determined by experienced clinical psychologists on the basis of clinical judgment, ADOS and ADI-R data.
As a group, Sibs-ASD were delayed in both RJA and more general social skills (as measured by the SBC). However, only about 35% (i.e., n = 15) of the sample scored under the 10th percentile of performance as defined by the typically developing group. Additionally, only 15% (i.e., n = 6) received an ASD diagnosis at Time 5. Finally, there was a large degree of variation on the two social impairment measures within the Sibs-ASD group. Growth curve modeling indicated that simple linear models (i.e., a straight line) best fit the growth of WTC and RJA predictors. There was significant variance for only the growth rate (i.e., slope) of the WTC and for only the estimated initial value (i.e., intercept) of RJA. Therefore, only these parameters from the growth curves could be used to predict later social impairment. Results revealed that initial RJA level strongly predicted later degree of impairment in directly-observed RJA, and growth rate of WTC moderately predicted later degree of impairment in parental reports of social development. Age at entry, initial language level and unweighted triadic communication did not predict later social impairment. However, initial language age equivalence scores did predict ASD diagnosis.
Different measures of social impairment had different predictors. The fact that early use of RJA predicts the extent to which Sibs-ASD were delayed in their RJA 19 months later is especially important. Establishing joint attention about other’s referents of interest is a primary way children learn about the environment and respond to others in social interaction. The measurement context we used for the RJA variables utilized adult attention-directing cues that varied systematically along a continuum of saliency. Some of these cues were particularly difficult (e.g., gaze to object alone) and some were quite easy (e.g., call child’s name, point and gaze to object); even at the final measurement some children were far below the ceiling on this measure. Using attention-directing cues that vary in saliency, redundancy, and information allowed us to measure individual differences in RJA that may be more predictive of later delay in RJA skills than more commonly used single-cue methods of measuring RJA. Additionally, learning to respond to others’ attentional cues early in development may be important for preventing delays in later RJA competence, which may in turn affect children’s learning about their environment.
Growth rate on Weighted Triadic Communication predicted individual differences on parent reports of their children’s social skills. The SBC is a more comprehensive assessment of social interaction than the RJA because of the breadth of items it covers. Examples of these are “Attempts to gain adult’s attention” and “Imitates others” at the low end of the scale and “Uses pretend play” and “Plays table games” at the high end of the scale. More advanced items require knowledge of social conventions. Most items require attention to a shared focus and another person. Weighted frequency of triadic communication gives higher weight to triadic communication that uses a socially-defined code (i.e., language) to communicate about a shared focus of attention. It was growth rate of WTC (i.e., the extent to which children grew in the frequency and conventionality of their triadic communication) that predicted later social impairment. Because WTC is influenced by both frequency of child-initiated triadic communication and language use, it is useful to note that mere frequency of triadic communication (i.e., unweighted triadic communication) at any time period did not predict later social impairment; neither did initial language level as assessed by the Mullen. The Mullen assesses use and understanding of language under demand conditions, but may not assess communicative use of language. This is the first study showing the value of WTC in the autism literature and constitutes one of the contributions of the current study.
ASD diagnosis was predicted by language age, rate of growth of WTC, and initial RJA. When considered alone, the positive predictive power for later ASD diagnosis was greatest for rate of growth of WTC. However, after controlling for the other two predictors, none of the predictors of later diagnosis accounted for unique variance in diagnosis. Thus, despite its predictive utility for general social impairment, measuring rate of growth of WTC would not appear to be a cost-effective screening strategy for identifying children at risk for a later ASD diagnosis.
An important methodological consideration is whether observers coding the predictor variables were blind to social impairment or ASD diagnostic status. The short answer is ‘mostly’, although exact numbers and thus proportion of data that could be affected is unavailable. The more complete answer is (a) most of the coding was done by people who were not aware of time 5 outcome scores, (b) a small minority of the children’s Time 1–Time 4 tapes were coded after the Time 5 procedures were administered, (c) an even smaller minority of these children at Time 5 were ‘unusual’ and observers may have noticed this, and (d) two of the primary coders for the predictor variables also administered some of the Time 5 procedures. Our conclusion is that while it is possible that correlated measurement error for a minority of participants could have influenced the effect size of the reported associations, it is highly unlikely that correlated measurement error could explain the results.
Although longitudinal correlational designs provide two of the three criteria for establishing causality between predictor and criterion variables (i.e., a statistically reliable association, the putative cause occurs prior to the putative affected variable; Beakley and Ludlow 1992), the current study does not provide unequivocal evidence that low scores on the predictors cause the social impairment. Like all nonexperimental research designs, unmeasured third variables may be related to both the predictor and the criterion variables. If future studies using experimental designs support the hypothesis that RJA and WTC are causally related to later social development in this population, then the current findings add to the evidence that RJA and WTC are appropriate treatment goals for Sibs- ASD who are deficient in these skills. Replication of these findings is needed.
Acknowledgments
We thank the families that participated in this study and the following research staff, graduate students, and clinical staff that made this research possible: Linda Ashford, Eric Esters, Jennifer Foss-Feig, James Kretzer, Justin Lane, Evon Lee, Laura McLean, Caitlin McMahon, Melissa Moss, Joanna Mussey, Ayesha Nasmyth, Anne Osberger, Alison Presmanes, Stacie Pozdol, Amy Swanson, Meredith Townes, Holly Turbeville, Lauren Turner, Teresa Ulman, and Kelly Wendel. This research was supported by grant number R01 HD043292, with additional support from grant numbers P30 HD15052, T32 HD 07226, and the Vanderbilt Kennedy Center Marino Autism Research Institute (MARI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies or the National Institutes of Health.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders-iv-text revision. 4. Washington, DC: American Psychiatric Association; 2000. rev. [Google Scholar]
- Bailey A, Palferman S, Heavey L, Le Couteur A. Autism: The phenotype in relatives. Journal of Autism and Developmental Disorders. 1998;28:369–392. doi: 10.1023/A:1026048320785. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. Trends in Cognitive Sciences. 2002;6:248–254. doi: 10.1016/S1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Belmonte MK. Autism: A window onto the development of the social and the analytic brain. Annual Review of Neuroscience. 2005;28:109–126. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- Beakley B, Ludlow P. The philosophy of the mind: Classical problems/contemporary issues. Cambridge, MA: The MIT Press; 1992. [Google Scholar]
- Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, et al. A case-control family history study of autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Cassel TD, Messinger DS, Ibanez LV, Haltigan JD, Acosta SI, Buchman AC. Early social and emotional communication in the infant siblings of children with autism spectrum disorders: An examination of the broad phenotype. Journal of Autism and Developmental Disorders. 2007;37:122–132. doi: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, et al. Autistic social impairment in the siblings of children with pervasive developmental disorders. The American Journal of Psychiatry. 2006;163:294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Pryzbeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. Journal of Developmental and Behavioral Pediatrics. 2000;21:2–11. doi: 10.1097/00004703-200002000-00001. [DOI] [PubMed] [Google Scholar]
- Deak GO, Walden T, Kaiser MG, Lewis A. Driven from distraction: How infants respond to parents’ attempts to elicit and re-direct their attention. Infant Behavior and Development. 2008;31:34–50. doi: 10.1016/j.infbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Goldberg WA, Jarvis KL, Osann K, Laulhere TM, Straub C, Thomas E, et al. Brief report: Early social communication behaviors in the younger siblings of children with autism. Journal of Autism and Developmental Disorders. 2005;35:657–664. doi: 10.1007/s10803-005-0009-6. [DOI] [PubMed] [Google Scholar]
- Gotham K, Risi S, Dawson G, Tager-Flusberg H, Joseph R, Carter A, et al. A replication of the autism diagnostic observation schedule (ADOS) revised algorithms. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:642–651. doi: 10.1097/CHI.0b013e31816bffb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood CR, Carta JJ, Walker D, Hughes K, Weathers M. Preliminary investigations of the application of the early communication indicator for infants and toddlers. Journal of Early Intervention. 2006;28:178–196. doi: 10.1177/105381510602800306. [DOI] [Google Scholar]
- Happe F, Ronald A, Plomin R. Time to give up on a single explanation of autism. Nature Neuroscience. 2006;9:1218– 1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule—generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2008;147:424–433. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Yoder PJ, Stone WL. Prelinguistic predictors of vocabulary in young children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research: JSLHR. 2005;48:1080–1097. doi: 10.1044/1092-4388(2005/075). [DOI] [PubMed] [Google Scholar]
- Merin N, Young GS, Ozonoff S, Rogers SJ. Visual fixation patterns during reciprocal social interaction distinguish a subgroup of 6-month-old infants at-risk for autism from comparison infants. Journal of Autism and Developmental Disorders. 2007;37:108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Brian J, Zwaigenbaum L, et al. Early language and communication development of infants later diagnosed with autism spectrum disorder. Journal of Developmental and Behavioral Pediatrics. 2006;27:69–78. doi: 10.1097/00004703-200604002-00004. [DOI] [PubMed] [Google Scholar]
- Morales M, Mundy P, Crowson M, Neal AR, Delgado CEF. Individual differences in infant attention skills, joint attention, and emotion regulation behavior. International Journal of Behavioral Development. 2005;29:373–377. [Google Scholar]
- Mullen EM. Mullen scales of early learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Mundy P, Block J, Van Hecke A, Delgado CEF, Parlade M, Parmares Y. Individual differences and the development of joint attention in infancy. Child Development. 2007;78:705– 722. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits in autism: The contribution of non-verbal communication measures. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1986;27:657–669. doi: 10.1111/j.1469-610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Pickles A, Starr E, Kazak S, Bolton P, Papanikolaou K, Bailey A, et al. Variable expression of the autism broader phenotype: Findings from extended pedigrees. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41:491–502. doi: 10.1111/1469-7610.00634. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. The American Journal of Psychiatry. 1997;154:185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- Presmanes A, Walden T, Stone W, Yoder P. Effects of different attentional cues on responding to joint attention in younger siblings of children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:133–144. doi: 10.1007/s10803-006-0338-0. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. ADI-R: The autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Sheinkopf SJ, Mundy P, Claussen A, Willoughby J. Infant joint attention skill and preschool behavioral outcomes in at-risk children. Development and Psychopathology. 2004;16:273– 291. doi: 10.1017/S0954579404044517. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ruskin E. Continuity and change in the social competence of children with autism, Down syndrome, and developmental delays. Monographs of the Society for Research in Child Development. 1999;64(1):v–v114. doi: 10.1111/1540-5834.00002. [DOI] [PubMed] [Google Scholar]
- Stone WL, Coonrod EE, Ousley OY. Brief report: Screening tool for autism in two-year-olds (STAT): Development and preliminary data. Journal of Autism and Developmental Disorders. 2000;30:607–612. doi: 10.1023/A:1005647629002. [DOI] [PubMed] [Google Scholar]
- Stone WL, Coonrod EE, Turner LM, Pozdol S. Psychometric properties of the STAT for early autism screening. Journal of Autism and Developmental Disorders. 2004;34:691–701. doi: 10.1007/s10803-004-5289-8. [DOI] [PubMed] [Google Scholar]
- Stone WL, Lemanek KL. Parental report of social behaviors in autistic preschoolers. Journal of Autism and Developmental Disorders. 1990;20:513–522. doi: 10.1007/BF02216056. [DOI] [PubMed] [Google Scholar]
- Stone WL, McMahon C, Henderson LM. Use of the screening tool for autism in two-year-olds (STAT) for children under 24 months: An exploratory study. Autism. 2008;12:573–589. doi: 10.1177/1362361308096403. [DOI] [PubMed] [Google Scholar]
- Stone WL, McMahon CR, Yoder PJ, Walden TA. Early social-communicative and cognitive development of younger siblings of children with autism spectrum disorders. Archives of Pediatrics and Adolescent Medicine. 2007;161:384–390. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Jones MB, Zwaigenbaum L, MacLean JE. Genetics of autism: Overview and new directions. Journal of Autism and Developmental Disorders. 1998;28:351–368. doi: 10.1023/A:1026096203946. [DOI] [PubMed] [Google Scholar]
- Szatmari P, MacLean JE, Jones MB, Bryson SE, Zwaigenbaum L, Bartolucci G, et al. The familial aggregation of the lesser variant in biological and non-biological relatives of PDD probands: A family history study. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41:579–586. doi: 10.1111/1469-7610.00644. [DOI] [PubMed] [Google Scholar]
- Tapp J. ProcoderDV. Nashville, TN: Vanderbilt Kennedy Center; 2003. [Google Scholar]
- Turner LM, Stone WL. Variability in outcome for children with an ASD diagnosis at age 2. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48:793–802. doi: 10.1111/j.1469-7610.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- Van Hecke A, Mundy P, Acra CF, Block J, Delgado CEF, Parlade M, et al. Infant joint attention, temperament, and social competence in preschool children. Child Development. 2007;78:53–69. doi: 10.1111/j.1467-8624.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink TH, Brzustowicz LM, Bartlett CW, Szatmari P. The search for autism disease genes. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:272–283. doi: 10.1002/mrdd.20041. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: Social engagement, communication, and cognition. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47:511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]