Abstract
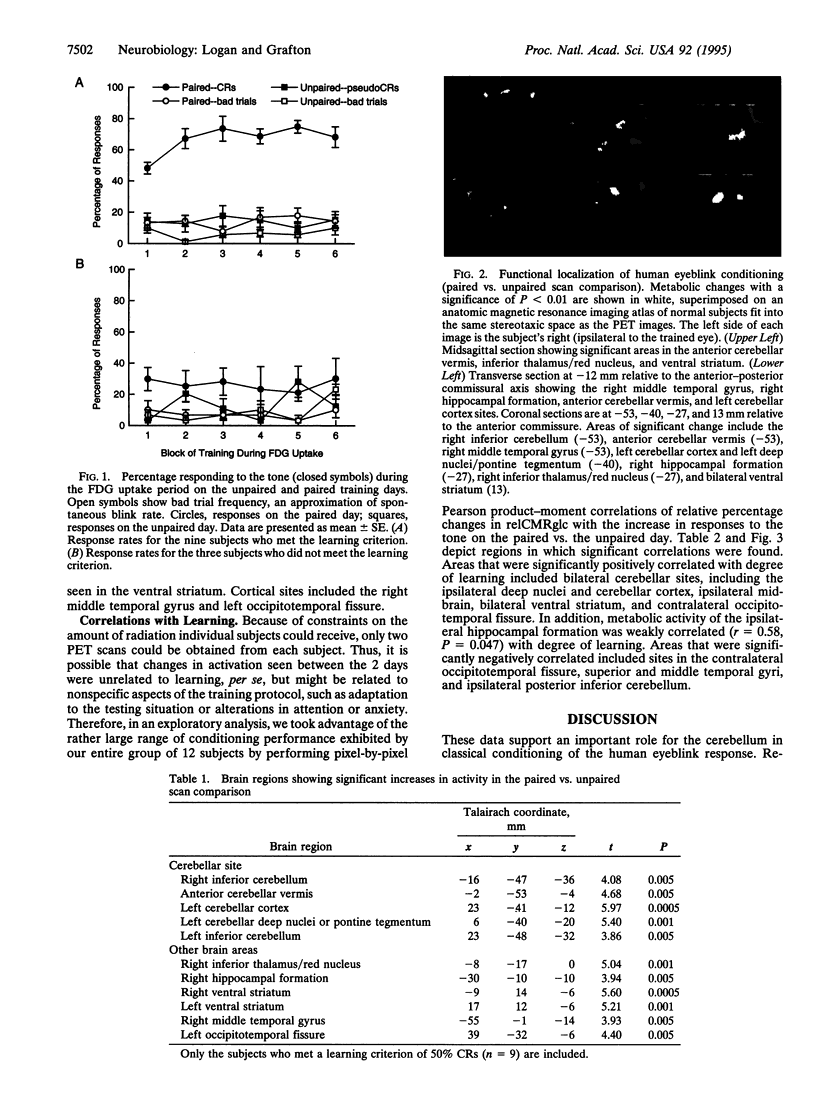
Relative cerebral glucose metabolism was examined with positron-emission tomography (PET) as a measure of neuronal activation during performance of the classically conditioned eyeblink response in 12 young adult subjects. Each subject received three sessions: (i) a control session with PET scan in which unpaired presentations of the tone conditioned stimulus and corneal airpuff unconditioned stimulus were administered, (ii) a paired training session to allow associative learning to occur, and (iii) a paired test session with PET scan. Brain regions exhibiting learning-related activation were identified as those areas that showed significant differences in glucose metabolism between the unpaired control condition and well-trained state in the 9 subjects who met the learning criterion. Areas showing significant activation included bilateral sites in the inferior cerebellar cortex/deep nuclei, anterior cerebellar vermis, contralateral cerebellar cortex and pontine tegmentum, ipsilateral inferior thalamus/red nucleus, ipsilateral hippocampal formation, ipsilateral lateral temporal cortex, and bilateral ventral striatum. Among all subjects, including those who did not meet the learning criterion, metabolic changes in ipsilateral cerebellar nuclei, bilateral cerebellar cortex, anterior vermis, contralateral pontine tegmentum, ipsilateral hippocampal formation, and bilateral striatum correlated with degree of learning. The localization to cerebellum and its associated brainstem circuitry is consistent with neurobiological studies in the rabbit model of eyeblink classical conditioning and neuropsychological studies in brain-damaged humans. In addition, these data support a role for the hippocampus in conditioning and suggest that the ventral striatum may also be involved.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett E. J., Brodie J. D., Wolf A. P., Christman D. R., Laska E., Meissner M. Reproducibility of cerebral glucose metabolic measurements in resting human subjects. J Cereb Blood Flow Metab. 1988 Aug;8(4):502–512. doi: 10.1038/jcbfm.1988.91. [DOI] [PubMed] [Google Scholar]
- Berger T. W., Alger B., Thompson R. F. Neuronal substrate of classical conditioning in the hippocampus. Science. 1976 Apr 30;192(4238):483–485. doi: 10.1126/science.1257783. [DOI] [PubMed] [Google Scholar]
- Berger T. W., Orr W. B. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behav Brain Res. 1983 Apr;8(1):49–68. doi: 10.1016/0166-4328(83)90171-7. [DOI] [PubMed] [Google Scholar]
- Berger T. W., Thompson R. F. Neuronal plasticity in the limbic system during classical conditioning of the rabbit nictitating membrane response. II: Septum and mammillary bodies. Brain Res. 1978 Nov 10;156(2):293–314. doi: 10.1016/0006-8993(78)90510-3. [DOI] [PubMed] [Google Scholar]
- Berry S. D., Thompson R. F. Medial septal lesions retard classical conditioning of the nicitating membrane response in rabbits. Science. 1979 Jul 13;205(4402):209–211. doi: 10.1126/science.451592. [DOI] [PubMed] [Google Scholar]
- Clark G. A., McCormick D. A., Lavond D. G., Thompson R. F. Effects of lesions of cerebellar nuclei on conditioned behavioral and hippocampal neuronal responses. Brain Res. 1984 Jan 16;291(1):125–136. doi: 10.1016/0006-8993(84)90658-9. [DOI] [PubMed] [Google Scholar]
- Daum I., Channon S., Canavan A. G. Classical conditioning in patients with severe memory problems. J Neurol Neurosurg Psychiatry. 1989 Jan;52(1):47–51. doi: 10.1136/jnnp.52.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum I., Channon S., Polkey C. E., Gray J. A. Classical conditioning after temporal lobe lesions in man: impairment in conditional discrimination. Behav Neurosci. 1991 Jun;105(3):396–408. doi: 10.1037//0735-7044.105.3.396. [DOI] [PubMed] [Google Scholar]
- Daum I., Schugens M. M., Ackermann H., Lutzenberger W., Dichgans J., Birbaumer N. Classical conditioning after cerebellar lesions in humans. Behav Neurosci. 1993 Oct;107(5):748–756. doi: 10.1037//0735-7044.107.5.748. [DOI] [PubMed] [Google Scholar]
- Durkin M., Prescott L., Furchtgott E., Cantor J., Powell D. A. Concomitant eyeblink and heart rate classical conditioning in young, middle-aged, and elderly human subjects. Psychol Aging. 1993 Dec;8(4):571–581. doi: 10.1037//0882-7974.8.4.571. [DOI] [PubMed] [Google Scholar]
- Fox P. C., Eichenbaum H., Butter C. M. The role of frontal cortex-reticular interactions in performance and extinction of the classically conditioned nictitating membrane response in the rabbit. Behav Brain Res. 1982 Jun;5(2):143–156. doi: 10.1016/0166-4328(82)90049-3. [DOI] [PubMed] [Google Scholar]
- Gould T. J., Sears L. L., Steinmetz J. E. Possible CS and US pathways for rabbit classical eyelid conditioning: electrophysiological evidence for projections from the pontine nuclei and inferior olive to cerebellar cortex and nuclei. Behav Neural Biol. 1993 Sep;60(2):172–185. doi: 10.1016/0163-1047(93)90285-p. [DOI] [PubMed] [Google Scholar]
- Heindel W. C., Salmon D. P., Shults C. W., Walicke P. A., Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer's, Huntington's, and Parkinson's disease patients. J Neurosci. 1989 Feb;9(2):582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton B. J., Thompson R. F. Conditioning using a cerebral cortical conditioned stimulus is dependent on the cerebellum and brain stem circuitry. Behav Neurosci. 1992 Jun;106(3):509–517. doi: 10.1037//0735-7044.106.3.509. [DOI] [PubMed] [Google Scholar]
- Krupa D. J., Thompson J. K., Thompson R. F. Localization of a memory trace in the mammalian brain. Science. 1993 May 14;260(5110):989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Mazziotta J. C., Phelps M. E., Meadors A. K., Ricci A., Winter K., Bentson J. R. Anatomical localization schemes for use in positron computed tomography using a specially designed headholder. J Comput Assist Tomogr. 1982 Aug;6(4):848–853. doi: 10.1097/00004728-198208000-00043. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Thompson R. F. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984 Jan 20;223(4633):296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Thompson R. F. Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid response. J Neurosci. 1984 Nov;4(11):2811–2822. doi: 10.1523/JNEUROSCI.04-11-02811.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan S. E., Sunderland T., McIntosh A. R., Herscovitch P., Schreurs B. G. A functional anatomical study of associative learning in humans. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8122–8126. doi: 10.1073/pnas.91.17.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer J. R., Jr, Deyo R. A., Disterhoft J. F. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990 Apr;104(2):243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Packard M. G., McGaugh J. L. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci. 1992 Jun;106(3):439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Penick S., Solomon P. R. Hippocampus, context, and conditioning. Behav Neurosci. 1991 Oct;105(5):611–617. doi: 10.1037//0735-7044.105.5.611. [DOI] [PubMed] [Google Scholar]
- Perrett S. P., Ruiz B. P., Mauk M. D. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993 Apr;13(4):1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. A., Mankowski D., Buchanan S. Concomitant heart rate and corneoretinal potential conditioning in the rabbit (Oryctolagus cuniculus): effects of caudate lesions. Physiol Behav. 1978 Feb;20(2):143–150. doi: 10.1016/0031-9384(78)90066-5. [DOI] [PubMed] [Google Scholar]
- Richardson R. T., DeLong M. R. A reappraisal of the functions of the nucleus basalis of Meynert. Trends Neurosci. 1988 Jun;11(6):264–267. doi: 10.1016/0166-2236(88)90107-5. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr J. A., Taylor A. E., Lang A. E. Procedural learning and neostriatal dysfunction in man. Brain. 1988 Aug;111(Pt 4):941–959. doi: 10.1093/brain/111.4.941. [DOI] [PubMed] [Google Scholar]
- Solomon P. R., Solomon S. D., Schaaf E. V., Perry H. E. Altered activity in the hippocampus is more detrimental to classical conditioning than removing the structure. Science. 1983 Apr 15;220(4594):329–331. doi: 10.1126/science.6836277. [DOI] [PubMed] [Google Scholar]
- Solomon P. R., Stowe G. T., Pendlbeury W. W. Disrupted eyelid conditioning in a patient with damage to cerebellar afferents. Behav Neurosci. 1989 Aug;103(4):898–902. doi: 10.1037//0735-7044.103.4.898. [DOI] [PubMed] [Google Scholar]
- Steinmetz J. E., Lavond D. G., Thompson R. F. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3(3):225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Thompson R. F., Krupa D. J. Organization of memory traces in the mammalian brain. Annu Rev Neurosci. 1994;17:519–549. doi: 10.1146/annurev.ne.17.030194.002511. [DOI] [PubMed] [Google Scholar]
- Topka H., Valls-Solé J., Massaquoi S. G., Hallett M. Deficit in classical conditioning in patients with cerebellar degeneration. Brain. 1993 Aug;116(Pt 4):961–969. doi: 10.1093/brain/116.4.961. [DOI] [PubMed] [Google Scholar]
- White I. M., Miller D. P., White W., Dike G. L., Rebec G. V., Steinmetz J. E. Neuronal activity in rabbit neostriatum during classical eyelid conditioning. Exp Brain Res. 1994;99(2):179–190. doi: 10.1007/BF00239585. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak D. S., Thompson R. F. Classical conditioning of the eyeblink response in the delay paradigm in adults aged 18-83 years. Psychol Aging. 1988 Sep;3(3):219–229. doi: 10.1037//0882-7974.3.3.219. [DOI] [PubMed] [Google Scholar]
- Woods R. P., Cherry S. R., Mazziotta J. C. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992 Jul-Aug;16(4):620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Worsley K. J., Evans A. C., Marrett S., Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992 Nov;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]