Summary
The adult pancreas is capable of limited regeneration after injury but has no defined stem cell population. The cell types and molecular signals that govern the production of new pancreatic tissue are not well understood. Here, we show that inactivation of the SCF-type E3 ubiquitin ligase substrate recognition component Fbw7 induces pancreatic ductal cells to reprogram into α, δ, and β cells. Loss of Fbw7 stabilized the transcription factor Ngn3, a key regulator of endocrine cell differentiation. The induced β cells resemble islet β cells in morphology and histology, express genes essential for β cell function, and release insulin after glucose challenge. Thus, loss of Fbw7 appears to reawaken an endocrine developmental differentiation program in adult pancreatic ductal cells. Our study highlights the plasticity of seemingly differentiated adult cells, identifies Fbw7 as a master regulator of cell fate decisions in the pancreas, and reveals adult pancreatic duct cells as a latent multipotent cell type.
Graphical Abstract
Highlights
-
•
Fbw7 loss induces adult pancreatic duct cell reprogramming to α, δ, and β cells
-
•
The transcription factor Ngn3 is an Fbw7 substrate
-
•
Expression of Fbw7-resistant Ngn3 is sufficient to trigger adult β cell neogenesis
-
•
Fbw7-deleted ductal cell-derived β cells show glucose-stimulated insulin secretion
The E3-ligase Fbw7 is important for maintenance of adult pancreatic ductal cell identity, and loss of Fbw7 causes transdifferentiation of ductal cells to endocrine α, δ, and β cells.
Introduction
The pancreas comprises an exocrine component (ductal and acinar cells) and an endocrine component (β cells, α cells, δ cells, pancreatic polypeptide-positive [pp] cells, and ε cells). The endocrine cells are organized in defined islet structures embedded in the acinar compartment, which function as key regulators of carbohydrate metabolism (Edlund, 2002). The autoimmune disease Type 1 diabetes irreversibly destroys insulin-secreting β cells in pancreatic islets, resulting in a lack of insulin production and hyperglycemia (Atkinson et al., 2011). Treatment is most commonly with insulin injections, but the degree of glycemic control with this approach does not compare to functional pancreatic β cells. Regenerative β cell treatments in diabetic patients could allow for the long-term restoration of normal glycemic control and thus represent a potentially curative therapy (Yi et al., 2013).
The generation of new pancreatic β cells is being pursued on several fronts in vitro, including differentiation of induced pluripotent stem cells (iPSCs) and reprogramming of other pancreatic cell types (Pagliuca and Melton, 2013). Regenerating pancreatic β cells in situ is an attractive alternative to these approaches, driven by evidence of spontaneous β cell neogenesis in the adult pancreas (Bonner-Weir et al., 2004; Dor et al., 2004; Lysy et al., 2012; Pagliuca and Melton, 2013; Teta et al., 2005). β cell regeneration during adulthood is very limited but can be achieved experimentally using pancreatic duct ligation in mice (Xu et al., 2008) and pancreatectomy in rats (Bonner-Weir et al., 2004). Inducible depletion of acinar and islet cells with diphtheria toxin showed that duct cells can give rise to both acinar and endocrine cells (Criscimanna et al., 2011). Thus, ductal cells in the adult pancreas show a latent propensity for β cell generation. Additionally, genetic approaches have converted other pancreatic cell types into β cells. Adenoviral overexpression of the three transcription factors neurogenin-3 (Ngn3), Maf1a, and Pdx1 is sufficient to convert adult acinar cells into β cells (Zhou et al., 2008), and overexpression of Pax4 converts glucagon-producing α cells into β cells (Collombat et al., 2009). However, the capacity for β cell neogenesis in the normal adult pancreas, and the regulatory events surrounding it, remain largely unknown.
Ngn3 is the earliest factor that specifically regulates the development of the endocrine compartment in the embryonic pancreas (Habener et al., 2005). Ngn3−/− mice completely lack endocrine islet development (Gradwohl et al., 2000), and transgenic overexpression of Ngn3 activates an islet differentiation program in the embryo and in cultured pancreatic ductal cell lines (Heremans et al., 2002; Schwitzgebel et al., 2000). In the adult pancreas, Ngn3 expression is very limited, but levels rise during β cell neogenesis induced by pancreatic duct ligation, where Ngn3 is required for β cell replenishment (Van de Casteele et al., 2013; Xu et al., 2008). Moreover, expansion of Ngn3+ cells bordering the ducts contributes to the β cell expansion observed when overexpressing Pax4 (Al-Hasani et al., 2013), indicating that manipulation of Ngn3 levels and/or activity may be beneficial for regeneration therapies. Ngn3 is a highly unstable protein (Roark et al., 2012), and the level and timing of its expression must be precisely controlled to ensure the correct production of β cells, but the details of its posttranslational regulation remain elusive.
Fbw7 (F-box and WD-40 domain protein 7) is the substrate recognition component of an evolutionarily conserved SCF (complex of SKP1, CUL1, and F-box protein)-type ubiquitin ligase. SCF(Fbw7) degrades proteins that function in cellular growth and division pathways, including c-Myc, cyclin E, Notch, and c-Jun (Welcker and Clurman, 2008). Emerging evidence shows that Fbw7 controls stem cell self-renewal, cell fate decisions, survival, and multipotency in numerous tissues, including the hematopoietic (Iriuchishima et al., 2011) and nervous systems (Hoeck et al., 2010; Matsumoto et al., 2011), liver (Onoyama et al., 2011), and intestine (Sancho et al., 2010). This suggests that Fbw7 has a crucial function in fundamental cell differentiation processes.
Here, we show that Fbw7 contributes to the regulation of Ngn3 stability, and loss of Fbw7 induces a direct ductal-to-β cell differentiation in the adult pancreas. Our study not only reveals a role for Fbw7 in pancreatic cell fate determination and identifies Ngn3 as a target of Fbw7 but also demonstrates that ductal cells can be induced to alter their identity in the adult pancreas in the absence of injury to the organ with a single genetic change.
Results
Fbw7 Deletion in the Pancreas Induces Scattered Duct Cells to Display Functional Mature β Cell Hallmarks
Given the role of Fbw7 in controlling cell fate decisions in other organs, we asked whether Fbw7 also functions in cell type specification in the pancreas. We deleted Fbw7 in embryonic pancreatic progenitor cells using a Cre recombinase under the control of the Pdx1 promoter (Pdx1-Cre; Fbw7f/f mice). Although pancreatic organ size and gross morphology appeared normal, histological analysis revealed increased ductal cell proliferation and an expansion of the ductal compartment (Figures S1A and S1B available online), consistent with the increase in proliferation upon Fbw7 deletion observed in other organs (Hoeck et al., 2010; Matsumoto et al., 2011; Onoyama et al., 2007, 2011; Sancho et al., 2010). Unexpectedly, scattered cells in the Pdx1-Cre; Fbw7f/f ducts showed an enlarged cytoplasm and smaller rounded nuclei when compared with surrounding ductal cells, and more closely resembled islet β cells (Figures 1A–1D). Insulin expression, which is normally restricted to islets in control mice (Figure 1E), was detected in these aberrant ductal cells (Figure 1F). The majority of insulin-positive cells in Pdx1-Cre; Fbw7f/f ducts were devoid of the ductal cell marker cytokeratin-19 (CK19), but costaining of CK19 and insulin was sometimes observed (Figure 1F), suggesting an intermediate transition state between ductal and insulin-positive cells. No insulin costaining with the acinar cell marker amylase was observed (Figures S1C and S1D). Thus, the absence of Fbw7 appears to trigger abnormal differentiation of a subset of ductal cells, biasing them toward an endocrine fate.
Figure 1.
Pdx1-Driven Deletion of Fbw7 in the Pancreas Induces Occurrence of Cells in the Ducts Displaying β Cell Hallmarks
(A–D) Hematoxylin and eosin (H&E) staining of Fbw7f/f or Pdx1-Cre; Fbw7f/f pancreas. (a) Fbw7f/f pancreatic islet; (b) Fbw7f/f duct; (c) and (d) Pdx1-Cre; Fbw7f/f cells with altered morphology in the ducts.
(E and F) Double IF for insulin (ins) and cytokeratin 19 (CK19) in Fbw7f/f (E) or Pdx1-Cre; Fbw7f/f (F) pancreas at 4 weeks.
(G–I) Double IF for Glut2 and insulin in Fbw7f/f (G) or Pdx1-Cre; Fbw7f/f (H and I) pancreas at 4 weeks. Nuclei were counterstained with DAPI.
White dashed squares in (F) and (H) represent the areas magnified in (F) and (I) respectively. n > 5 mice per genotype (representative picture shown).
See also Figure S1.
In addition to ectopic insulin-positive cells (in 17% of ducts), Pdx1-Cre; Fbw7f/f ducts also contained cells expressing the α cell marker glucagon, albeit less frequently (3% of ducts) (Figures S1E and S1F). We also observed glucagon/insulin double-positive cells in Pdx1-Cre; Fbw7f/f ducts (Figures S1G–S1I), similar to progenitor cells described in human embryonic pancreas (Piper et al., 2004). Thus, deletion of Fbw7 in the pancreas promotes the occurrence of cells coexpressing markers of different pancreatic cell lineages, a cellular phenotype that is not normally observed in the adult pancreas.
One of the features of functional β cells is the expression of the glucose transporter Glut2 (Slc2a2). In control animals, Glut2 was coexpressed with insulin in islet β cells by double insulin/glut2 immunofluorescence (IF) (Figure 1G). Glut2 was also coexpressed with insulin in the Pdx1-Cre; Fbw7f/f aberrant ductal cells (Figures 1H and 1I).
Inducible Deletion of Fbw7 in the Adult Pancreas Promotes β Cell Neogenesis
Because Pdx1-expressing progenitors give rise to all the pancreatic lineages (Oliver-Krasinski and Stoffers, 2008), the emergence of insulin-positive cells in Pdx1-Cre; Fbw7f/f ducts could be due to a developmental defect. To test whether Fbw7 deletion can induce β cell neogenesis in adult mice, and to clarify the cells that give rise to ectopic β cells, we combined inducible Fbw7 deletion using the R26-CreERT line with lineage tracing using R26-LSL-YFP. “RY” control mice express a tamoxifen-inducible form of Cre recombinase from the ubiquitous Rosa26 (R26) promoter, leading to the permanent expression of yellow fluorescent protein (YFP) in recombined cells. In the Fbw7f/f background (“RFY” line, Figure 2A), tamoxifen treatment results in recombination of the floxed Fbw7 alleles in Cre-expressing cells concomitantly with activation of YFP expression (Figure 2B).
Figure 2.
Inducible Deletion of Fbw7 in the Adult Pancreas Promotes β Cell Neogenesis
(A) Scheme of the RY (R26-CreERT; R26-LSL-YFP) and RFY (R26-CreERT; Fbw7f/f; R26-LSL-YFP) mouse models.
(B) Schematic diagram of RY pancreas before and after tamoxifen (Tam) injection.
(C) GFP immunoperoxidase staining in RY pancreas after tamoxifen injection. Section shows acinar cells, an islet, and a duct. n > 3 mice per genotype.
(D) Quantification of GFP-positive cells in the different pancreatic cell type compartments of RY (n = 3) mice 13 days postinjection. A, acinar; I, islet; D, ducts. Data are represented as mean + SEM.
(E–N) Triple IF for insulin (ins), GFP, and amylase (amy) in RFY mice 13 days after tamoxifen injection. n > 3 mice per genotype. Nuclei were counterstained with DAPI. Ducts are circled with a yellow dashed line. White dashed squares represent the area magnified in the squares shown below.
See also Figure S2.
Intraperitoneal injection of tamoxifen induced recombination in all pancreatic cell types but with different efficiencies. Almost all acinar cells showed YFP positivity (91%), while recombination occurred much less frequently in islet (10%) and ductal cells (5%) (Figures 2C and 2D). Despite the high percentage of recombination in the acinar compartment (Figure 2D), no insulin positivity was observed in cells with acinar cell morphology, and insulin/amylase double-positive cells could not be detected in the RFY pancreas (Figures 2E–2N). Likewise, direct intrapancreatic injection of 4-OH-tamoxifen into the pancreatic tail resulted in exclusive recombination in acinar cells, and here, no β cell neogenesis was observed (Figures S2A–S2D). In contrast, despite the low percentage of recombination in ducts (5%), RFY mice gave rise to insulin/green fluorescent protein (GFP) double-positive cells in ducts as early as 13 days postintraperitoneal injection of tamoxifen (Figures 2E–2N) as well as at later time points (Figures S2E and S2F). Therefore, Fbw7 deletion in ductal cells, but not in acinar cells, induces the acquisition of β cell identity.
Deletion of Fbw7 in the Pancreas Leads to Increased Ngn3 Protein Levels
Fbw7 targets many proteins involved in proliferation and differentiation for proteasomal degradation, such as N-terminally phosphorylated c-Jun (p-c-JunSer73), Notch intracellular domain 1 (NICD1), phosphorylated c-Myc, and phosphorylated Cyclin E (Welcker and Clurman, 2008). It has been shown that SCF(Fbw7)-mediated substrate degradation is tissue specific (Hoeck et al., 2010; Ishikawa et al., 2008; Nakayama and Nakayama, 2006; Onoyama et al., 2007, 2011; Sancho et al., 2010; Thompson et al., 2008; Wang et al., 2012). Western blotting of lysates from whole Pdx1-Cre; Fbw7f/f pancreas, in which Fbw7 is inactivated in all pancreatic cell types, showed increased p-c-JunSer73 and NICD1 protein levels when compared to Fbw7f/f controls, while phosphorylated c-Myc and Cyclin E levels were not substantially affected (Figure 3A; quantifications in Figures S3A and S3B). In β cells, NICD1 and p-c-JunSer73 were barely detectable, either in control RY or in Fbw7-deleted RFY pancreas (Figure S3C). In the acinar compartment, the loss of Fbw7 did not increase p-c-JunSer73 or NICD1 in RFY compared with RY mice (Figure S3D). In order to analyze Fbw7 function in ducts, we examined sections from Pdx1-Cre; Fbw7f/f animals. p-c-JunSer73 fluorescence intensity was increased in Pdx1-Cre; Fbw7f/f compared with control ducts (Figures 3B and 3C), suggesting that SCF(Fbw7) primarily acts in pancreatic ductal cells.
Figure 3.
Fbw7 Loss in the Pancreas Leads to Increased Ngn3 Protein Levels
(A–D) In (A) and (D), a western blot analysis is shown of Fbw7f/f and Pdx1-Cre; Fbw7f/f whole pancreas lysates. n = 3 mice per genotype. (B) Double IF of CK19 and p-c-JunSer73 in Fbw7f/f and Pdx1-Cre; Fbw7f/f pancreas at 4 weeks. n > 3 mice per genotype (representative picture shown). (C) Quantification of experiment in (B). p-c-Jun fluorescence intensity was measured using LSM software; each dot represents the mean intensity of a single cell. Fbw7f/f n = 471 cells (20 ducts/2 mice); Pdx1-Cre; Fbw7f/f n = 505 cells (20 ducts/3 mice).
(E) qPCR analysis of Ngn3 target genes from Fbw7f/f and Pdx1-Cre; Fbw7f/f mouse pancreas at 4 weeks. n > 3 mice per genotype. Error bars indicate SEM.
(F) IF for Insm1 in Fbw7f/f and Pdx1-Cre; Fbw7f/f pancreas at 4 weeks. Ducts are circled with a yellow dashed line.
(G) Quantification of experiment in (F). Insm1 fluorescence intensity per cell was measured as for p-c-Jun above. Fbw7f/f n = 518 cells (20 ducts/2 mice); Pdx1-Cre; Fbw7f/f n = 527 cells (20 ducts/3 mice).
Error bars in (C) and (G) represent mean ± SD. See also Figure S3 and Table S1.
Of the known substrates increased by Fbw7 loss in the pancreas, none are thought to be involved in β cell neogenesis. While Notch has been reported to be involved in embryonic pancreatic differentiation, it has been proposed to inhibit β cell neogenesis rather than promoting it (Esni et al., 2004; Murtaugh et al., 2003). We therefore examined the possibility that Fbw7 in the pancreas may control the levels of one or more other substrates. Transcription factors involved in embryonic β cell development include Pdx1, Ngn3, Hnf3, and Hnf6 (Zaret, 2008). Pdx1, Hnf3, and Hnf6 protein levels were unaltered in Pdx1-Cre; Fbw7f/f compared with Fbw7f/f pancreas, but the protein levels of Ngn3 were strongly increased (Figure 3D; quantifications in Figures S3E and S3F). Ngn3 messenger RNA (mRNA) levels were also increased (Figure 3E), in agreement with previous reports of positive autoregulatory loops controlling Ngn3 expression (Ejarque et al., 2013; Wang et al., 2008). The increase in Ngn3 after Fbw7 loss also correlated with higher mRNA levels of the Ngn3 transcriptional target genes Insm1, HeyL, Ctgf, and Nkx2-2 (Swales et al., 2012) when analyzed by quantitative PCR (qPCR) (Figure 3E), and increased protein levels of the Ngn3 transcriptional targets NeuroD1 and Insm1 (Figures 3D, 3F, 3G, S3E, and S3F). Ngn3 is a key regulator of endocrine differentiation, making it an excellent candidate for inducing β cell neogenesis induced by Fbw7 loss.
Fbw7 Binds to, Ubiquitinates, and Induces Proteasomal Degradation of Ngn3
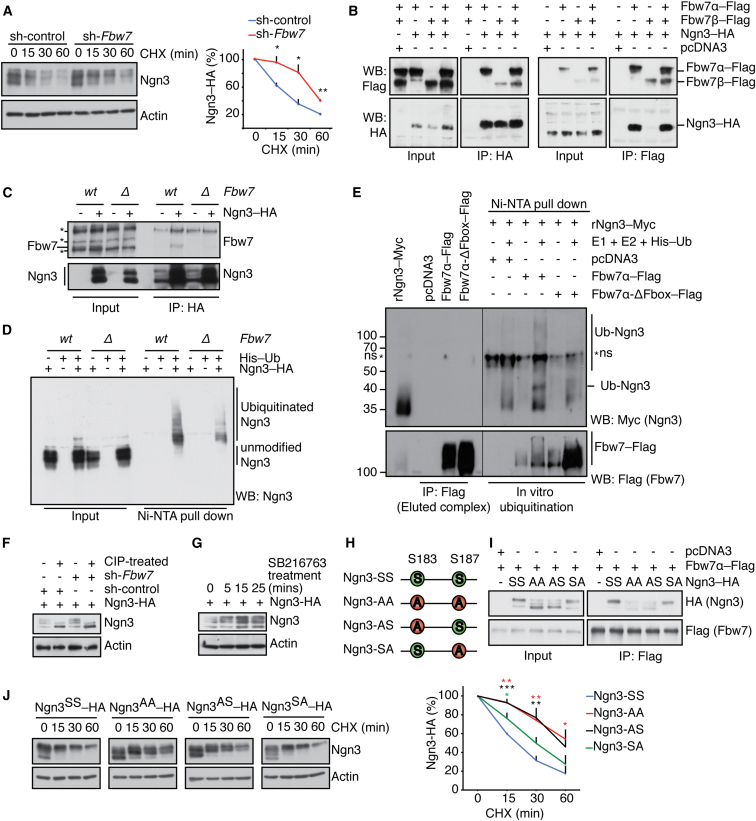
To investigate the mechanism by which Fbw7 affects Ngn3, we first analyzed the stability of Ngn3 protein using cycloheximide to inhibit protein synthesis. Ngn3 half-life was increased more than 2-fold after Fbw7 silencing, suggesting that Fbw7 acts to destabilize Ngn3 protein (Figure 4A). Ngn3-hemagglutinin (Ngn3-HA) coimmunoprecipitated Flag-tagged Fbw7 isoform-α and, to a lesser extent, isoform-β (Figure 4B, left panel; and vice versa, as shown in the right panel), and endogenous Fbw7 interacted with Ngn3-HA (Figure 4C). Ngn3 is a heavily ubiquitinated protein (Roark et al., 2012), but Ngn3 ubiquitination was strongly reduced in Fbw7Δ HCT116 cells when compared to congenic Fbw7wt cells (Figure 4D). In vitro, wild-type (WT) Fbw7–Flag protein complexes promoted efficient ubiquitination of recombinant Ngn3, but the inactive mutant Fbw7α-ΔFbox–Flag did not (Welcker et al., 2004) (Figures 4E and S4A). All together, these data suggest that Ngn3 is a substrate of the SCF(Fbw7) ubiquitin ligase.
Figure 4.
Fbw7 Binds to and Ubiquitinates Ngn3 and Induces Its Proteasomal Degradation
(A) Western blot analysis of Ngn3 protein levels during a cycloheximide (CHX) time course after silencing Fbw7. Graph shows mean Ngn3 levels normalized to actin, as a percentage of initial protein levels. n = 3 independent experiments.
(B) Ngn3–HA and Fbw7α–Flag/Fbw7β–Flag IP from cotransfected HEK293T cells. Western blots (WB) of input and IP material are shown.
(C) HA IP from HCT116-Fbw7wt and Fbw7 knockout (Fbw7Δ) cells transfected with Ngn3-HA. Anti-Fbw7 (Bethyl Laboratories) detects endogenous Fbw7.
(D) In vivo Ngn3 ubiquitination is reduced in Fbw7Δ compared with Fbw7wt cells. Ubiquitinated Ngn3 was resolved by Ni2+-NTA affinity purification and immunoblotting with anti-Ngn3 antibody.
(E) Recombinant Ngn3 (rNgn3-myc) is ubiquitinated in vitro, using Flag-IP complexes of pcDNA3, Fbw7α-Flag, or Fbw7α-ΔFbox-Flag from transfected HEK293T cells. Ubiquitinated complexes were enriched by Ni2+-NTA affinity purification before immunoblotting.
(F) Calf intestinal phosphatase (CIP) treatment of lysates from Ngn3-HA and sh-control/sh-Fbw7 cotransfected HEK293T cells.
(G) GSK3β inhibitor (SB216763) treatment of cells transfected with Ngn3-HA.
(H) Graphic scheme of Ngn3 mutant constructs generated, showing the putative GSK3β phosphorylation site in mouse Ngn3. S, serine; A, alanine.
(I) Ser183Ala mutation (AA, AS) disrupts Ngn3 interaction with Fbw7. Left: input. Right: Fbw7α-Flag IP from HEK293T cells co-transfected with Ngn3–HA or the indicated mutant construct.
(J) Ser183Ala mutation (AA, AS) increases Ngn3 stability. Ngn3 protein levels were measured after cycloheximide treatment in cells transfected with different Ngn3–HA mutants. Graph shows mean Ngn3 levels normalized to actin, as a percentage of initial protein levels.
In (B)–(I), n > 2 independent experiments. Error bars in (A) and (J) represent SEM; n = 3 independent experiments. See also Figure S4 and Table S1.
Most Fbw7 substrates contain a phosphodegron motif that serves as the recognition motif for Fbw7 interaction (Welcker and Clurman, 2008). Multiple higher molecular weight bands of Ngn3-HA detected by immunoblot collapsed after calf intestinal phosphatase (CIP) treatment, suggesting that they represent phosphorylated forms. Silencing of Fbw7 increased the levels of these higher molecular weight forms (Figure 4F). GSK3β is the kinase responsible for modifying the phosphodegron motifs of c-Myc and Notch1 (Welcker and Clurman, 2008; Welcker et al., 2004). In silico analysis revealed a GSK3β consensus site at the Ngn3 C terminus (Ser183–Ser187). GSK3β inhibitor treatment increased Ngn3 protein levels (Figure 4G), suggesting that GSK3β regulates the stability of Ngn3. We generated constructs of Ngn3 with Ser183 and/or Ser187 mutated to alanine to assess the role of the putative phosphodegron motif (Figure 4H). Mutation of the predicted GSK3β phosphorylation site Ser183 altered the electrophoretic mobility of Ngn3 protein, while mutation of Ser187 had less effect (Figure 4I, left panel). While WT Ngn3 could efficiently interact with Fbw7, the interaction was severely impaired when Ser183 was mutated (Ngn3-AA and Ngn3-AS; Figure 4I, right panel). Accordingly, while the mRNA levels from all four Ngn3 constructs were comparable, the stability of Ngn3-AA and Ngn3-AS was greatly increased (Figures S4B and S4J). These data suggest that Fbw7 directly controls Ngn3 stability by regulating its ubiquitination and proteasomal degradation and that GSK3β-mediated phosphorylation of Ser183 might regulate this process. Since Ngn3 has been shown to be involved in β cell neogenesis in the adult pancreas (Al-Hasani et al., 2013; Baeyens et al., 2006; Xu et al., 2008), accumulation of Ngn3 protein is likely to contribute to adult β cell neogenesis induced by Fbw7 inactivation.
Conditional Overexpression of Ngn3-AA in the Adult Pancreatic Ducts Induces β Cell Neogenesis
To determine whether accumulation of Ngn3 protein is sufficient to induce β cell neogenesis, we generated a conditional inducible transgenic mouse line that expresses the phospho mutant, stable form of Ngn3 (Ngn3-AA) together with GFP after Cre recombination (Pdx1-Cre; Rosa26-loxSTOPlox-Ngn3-AA-IRES-GFP or Pdx1-Cre; R26-LSL-Ngn3-AA; Figure 5A). Pdx-Cre induced recombination in mouse pancreas but not in liver or tail (Figure 5B). Ngn3 protein, which is undetectable in control adult pancreas, was detected in Pdx1-Cre; R26-LSL-Ngn3-AA pancreas (Figure 5C) but not in liver. Pdx1-Cre; R26-LSL-Ngn3-AA pancreas showed increased Ngn3 and Ins2 mRNA levels when compared to unrecombined R26-LSL-Ngn3-AA mice (Figure 5D). Transgenic Ngn3-AA expression resulted in increased β cell area as analyzed by immunostaining for insulin (Figures 5E and 5F). These data suggested that the overexpression of a stable form of Ngn3 (Ngn3-AA) from embryonic pancreas development onward results in an increase in β cells. In order to test whether overexpression of Ngn3-AA in the adult pancreatic ducts was sufficient to induce β cell reprogramming, we crossed R26-LSL-Ngn3-AA mice to CK19-CreERT mice, in which the expression of tamoxifen-inducible Cre-ERT protein is driven by the promoter of the ductal marker cytokeratin 19 (Means et al., 2008) (Figure 5G). Thirteen days post-tamoxifen injection (Figure 5H), GFP expression could be detected specifically in ductal cells of CK19-CreERT; R26-LSL-Ngn3-AA mice, while it was absent in R26-LSL-Ngn3-AA ducts (Figure 5I). Furthermore, we detected a significant increase in insulin-positive ductal cells in tamoxifen-injected CK19-CreERT; R26-LSL-Ngn3-AA pancreas (Figures 5J and 5K), suggesting that Ngn3-AA overexpression in the adult pancreatic duct is sufficient to induce ductal-to-β cell conversion.
Figure 5.
Conditional Overexpression of Ngn3-AA in the Adult Pancreatic Ducts Is Sufficient to Induce β Cell Neogenesis
(A) Schematic representation of the Pdx1-Cre; R26-LSL-Ngn3-AA model.
(B) R26-LSL-Ngn3-AA recombination PCR performed in genomic DNA from tail (T), liver (L) and pancreas (P).
(C) Western blot analysis for Ngn3 in R26-LSL-Ngn3-AA or Pdx1-Cre; R26-LSL-Ngn3-AA mouse pancreas and liver.
(D) qPCR analysis of Ngn3 and Ins2 in R26-LSL-Ngn3-AA or Pdx1-Cre; R26-LSL-Ngn3-AA pancreas. n = 3 mice per genotype. Error bars indicate SEM.
(E) Insulin staining in R26-LSL-Ngn3-AA or Pdx1-Cre; R26-LSL-Ngn3-AA mouse pancreas. Scale bar, 300 μm.
(F) Quantification of insulin-positive (ins+) area in R26-LSL-Ngn3-AA (n = 14 sections; 578 islets/3 mice) or Pdx1-Cre; R26-LSL-Ngn3-AA (n = 20 sections; 900 islets/4 mice). Dots represent percentage of ins+ cells for each section. Error bars represent mean ± SEM.
(G) Scheme of the CK19-CreERT; R26-LSL-Ngn3-AA model.
(H) Schematic diagram of the CK19-CreERT; R26-LSL-Ngn3-AA pancreas before and after tamoxifen injection.
(I) GFP immunoperoxidase staining in R26-LSL-Ngn3-AA or CK19-CreERT; R26-LSL-Ngn3-AA pancreas after tamoxifen injection. Dashed square indicates the area magnified.
(J) Representative picture showing insulin-positive cells in the pancreatic duct of tamoxifen-injected CK19-CreERT; R26-LSL-Ngn3-AA mice.
In (I) and (J), n > 5 mice per genotype (representative picture shown).
(K) Quantification of insulin-positive ductal cells in tamoxifen-injected R26-LSL-Ngn3-AA (n = 5 mice/3,462 cells) and CK19-CreERT; R26-LSL-Ngn3-AA animals (n = 8 mice/5,965 cells). Data are represented as mean + SEM.
See also Table S1.
Fbw7 Deletion in the Adult Pancreatic Ducts Induces Direct Conversion of Ductal Cells into β Cells
The aforementioned data suggest that Fbw7 may control adult β cell neogenesis by regulating Ngn3 protein stability. To test whether β cells arise as a direct consequence of Fbw7 loss in ductal cells, we asked whether loss of Fbw7, specifically in the adult ductal compartment, is sufficient to achieve cell conversion. To this end, we generated CK19-CreERT; Fbw7+/+; R26-LSL-YFP (“CY”) control and CK19-CreERT; Fbw7f/f; R26-LSL-YFP (“CFY”) inducible Fbw7 deletion mice (Figures 6A and 6B). The efficiency of recombination 2 weeks after tamoxifen injection was between 40% and 50% in both CY and CFY ducts, as reported previously (Means et al., 2008). Complete recombination was confirmed by PCR analysis of genomic DNA isolated from YFP+ CFY cells (Figure 6C). qPCR analysis demonstrated that Fbw7 mRNA was highly expressed in CY ductal cells but undetectable in CFY ductal cells and mature pancreatic β-cells sorted from MIP-GFP mice (in which GFP expression is driven by the insulin promoter) (Figure 6D). These data suggest that Fbw7 mRNA is enriched in ductal cells, in agreement with substrate stabilization predominantly in this cell type after Fbw7 loss (Figure 3B).
Figure 6.
Fbw7 Deletion in the Adult Pancreatic Ducts Induces Direct Conversion of Ductal Cells into β Cells with No Intermediate Cell Division
(A) Scheme of the CY and CFY mouse model genotypes.
(B) Schematic diagram of CY pancreas before and after tamoxifen injection.
(C) PCR analysis of the Fbw7 and R26 loci on genomic DNA isolated from GFP-sorted CY and CFY cells 13 days post-tamoxifen injection. wt, Fbw7 WT allele; flox, Fbw7f unrecombined allele; Δ, Fbw7 recombined allele; R26-LSL, R26 unrecombined allele; R26-Δ, R26 recombined allele.
(D) qPCR analysis of Fbw7 mRNA in GFP+ (duct) and GFP− (endocrine and acinar) cells sorted from tamoxifen-injected CFY and CY mice (n = 6 pooled pancreas each) and GFP+ (β) cells from MIP-GFP mice (n = 3 pooled pancreas). n = 3 independent experiments.
(E and F) Quantification of number of ducts (E) or islets (F) per square millimeter in at least seven fields per mouse (representative example in adjacent picture); scale bar, 20 μm; n = 3 mice/genotype. ns, not significant.
(G) Quantification of the percentage of total ductal cells that are insulin positive in tamoxifen-injected CY (n = 10 mice/10,070 cells) and CFY mice (n = 7 mice/12,220 cells). Representative example in adjacent picture; scale bar, 20 μm.
(H–J) Triple IF for GFP, insulin (ins), and BrdU in tamoxifen-injected CY (H) or CFY (I and J) mice (5 days post-tamoxifen injection; n > 5 mice/genotype; representative picture shown). Nuclei were counterstained with DAPI. White dashed square (I) represents the area magnified (J). Error bars in (D)–(G) indicate SEM.
Fbw7 inactivation in ductal cells did not alter the number of ducts (Figure 6E) or islets (Figure 6F). However, a significant number of insulin-positive cells (almost 0.5%, i.e. ∼1% of the Fbw7 knockout cells if considering ∼50% recombination efficiency) was observed in the ducts of CFY mice, while they rarely arose in CY mice (Figure 6G). About 12% of the CFY ducts contained induced β cells, typically between one and three cells per duct. It is interesting that 3.8% of CFY ducts contained glucagon-positive α cells and 5.5% contained somatostatin-positive δ cells, while pp or amylase-positive ductal cells were never detected (Figures S4C and S4D). Thus, deletion of Fbw7 in adult pancreatic ductal cells induces conversion of some ductal cells to α or δ cells or, most frequently, to β cells.
Inactivation of Fbw7 could trigger resident ductal progenitor cell proliferation followed by redifferentiation or induce direct transdifferentiation. To distinguish between these possibilities, Fbw7 inactivation and lineage tracing was combined with long-term bromodeoxyuridine (BrdU) labeling. BrdU was incorporated in scattered cells in the pancreatic CY ducts and increased in CFY ducts (Figures 6H, 6I, and S4E). However, less than 1% of insulin-positive CFY duct cells were labeled after 2 weeks of continuous BrdU exposure, beginning directly before Fbw7 deletion (Figures 6J, S4F, and S4G). Thus Fbw7 deletion in the adult pancreatic ducts induces direct conversion of a subset of exocrine ductal cells into endocrine β cells, without a requirement for cell proliferation.
Induced β Cells in Adult Fbw7 Mutant Ducts Resemble Functional β Cells
To explore the functionality of the β cells formed after Fbw7 deletion, we performed mRNA expression profiling of GFP+ sorted cells from tamoxifen-injected CY and CFY mice and compared them to GFP+ cells from MIP-GFP mice as a positive control for β cells (Figure 7A). CFY GFP+ ductal cells showed a modest increase in expression of numerous β cell specific genes, consistent with a small subset of ductal cells undergoing β cell conversion. In agreement with the increase in Ngn3 stability in Fbw7-deleted cells, CFY GFP+ ductal cells also showed an increase in the expression of reported Ngn3 target genes (Chga, Insm1, Dll3, Syp, Chn1, HeyL, Atp2a3, and Pcsk2; Swales et al., 2012) (Figure 7B). qPCR analysis confirmed increased mRNA expression of the β cell marker genes Ins2, Gck, Pdx1, and Nkx6.1 in CFY GFP+ ductal cells compared with CY GFP+ cells (Figure 7C).
Figure 7.
Converted β Cells Induced by Fbw7 Deletion Resemble Functional Mature β Cells
(A) Schematic diagram of the comparison strategy used for the mRNA expression profile.
(B) Heat map representing expression of 15 β cell markers and 15 Ngn3 target genes in GFP+ sorted cells from tamoxifen-injected CY (n = 6), CFY (n = 6), and MIP-GFP (n = 3) mice.
(C) qPCR analysis of Ins2, Gck, Pdx1, and Nkx6.1 expression in GFP+ cells sorted from CY and CFY mice (n = 6 pooled pancreas per genotype). n = 2 independent experiments.
(D and E) Double IF of insulin (ins) together with different β cell markers or ductal markers (Sox9, Dolichos biflorus agglutinin) in ductal CFY β cells. Scale bars, 5 μm. n > 5 mice per genotype (representative picture shown).
(F) Schematic diagram of the glucose challenge experiment design and fluorescence-activated cell sorting (FACS) profile indicating the sorting gate used for the isolation of 30,000 GFP+ cells (CY, CFY: n = 6 pooled pancreas) or 5,000 GFP+ cells (MIP-GFP: n = 3 pooled pancreas).
(G and H) Secreted insulin levels measured by ELISA from 30,000 GFP+ CY, CFY (G) and 5,000 MIP-GFP (H) cells 0–60 min after the addition of 20 mM glucose. n = 3 independent experiments.
(I) Insulin secretion per β cell is equivalent in CFY duct-derived and MIP-GFP islet cells, based on 300 converted β cells per well in the experiment in (G) and 5,000 β cells per well in the experiment in (H). Error bars in (C), (G), and (I) indicate SEM.
See also Figures S5 and S6 and Table S1.
As well as showing gene expression characteristics of islet β cells, insulin-positive cells in the ducts of CFY mice costained with the functional β cell markers c-PPT, Glut2, MafA1, Nkx6.1, Pax6, PC1/3, Pdx1, Urocortin 3 (Ucn3), and Isl1, showing comparable staining to islet β cells (Figures 7D, 7E, and S5). Insulin-positive CFY ductal cells were negative for the ductal markers Sox9 and DBA, while other CFY ductal cells retained expression of these markers (Figures 7E and S5).
An important hallmark of β cell function is the ability to release insulin after glucose stimulation. To test this, we subjected GFP+ cells sorted from CY and CFY mouse pancreas to in vitro glucose challenge (Figures 7F and 7G). While CY GFP+ cells did not respond to glucose, CFY GFP+ cells showed a substantial release of insulin (Figure 7G). Each CFY well of 30,000 cells contained approximately 300 converted β cells (based on a 1% conversion frequency), which secreted 214 pg (1.07 ng/ml) of insulin, i.e., 0.71 pg per cell. By comparison, 5,000 islet β cells sorted from a MIP-GFP pancreas responded to glucose by releasing 3,378 pg (16.89 ng/ml) of insulin, i.e., 0.67 pg per cell (Figure 7H). Based on this result, the response to glucose challenge in the converted cells is comparable to that of islet β cells (Figure 7I). Thus, the β cells converted after Fbw7 loss in the adult pancreatic ducts show both the characteristic marker expression and functionality of mature β cells.
Discussion
Ectopic expression of combinations of transcription factors can induce changes of cellular fate in adult pancreatic tissue (Zhou et al., 2008); however, examples of reprogramming in vivo by loss of a single molecule are rare. In this study we show that Fbw7 maintains adult ductal cell fate, as Fbw7 inactivation results in transdifferentiation of ductal cells into α and δ cells and, predominantly, β cells. The newly formed β cells resemble islet β cells with regard to cellular morphology, marker gene expression, and insulin secretion in response to glucose challenge. Our findings indicate an unexpected plasticity of ductal cells, in which loss of a single gene (Fbw7) renders the duct cells multipotent, able to remain exocrine or transdifferentiate into endocrine α, δ, or β cell types.
Fbw7 Function in the Adult Pancreas
Fbw7 is a key regulator of stem cell function, as Fbw7 inactivation results in increased proliferation and impaired differentiation of hematopoietic, liver, intestinal, and neural progenitor cells (Hoeck et al., 2010; Iriuchishima et al., 2011; Matsumoto et al., 2011; Onoyama et al., 2011; Sancho et al., 2010, 2013). The potent tumor suppressor function of Fbw7 is likely to be a direct consequence of deregulated stem cell proliferation and differentiation (Wang et al., 2012). However, the role of Fbw7 in the pancreatic ducts is distinct from Fbw7 function in other organ systems. Ductal to endocrine cell transdifferentiation after Fbw7 loss occurs in the absence of proliferation, suggesting that the subset of cells that respond to Fbw7 deletion in this way does not behave as adult stem cells in other organ systems, requiring cell division before differentiation. Rather, Fbw7 seems to function in the adult pancreas to constantly maintain cell fate in a subset of ductal cells.
Fbw7 Loss Converts Adult Ductal Cells into Functional β Cells
We found that the expression and activity of Fbw7 in the adult pancreas is enriched in the ductal compartment. Adult pancreatic ducts have been suggested to harbor β cell progenitors, which are reactivated after challenge (Bonner-Weir et al., 2008). Pancreatic duct ligation (PDL), combined with lineage tracing of the ductal epithelium, demonstrated that a quarter of new β cells formed in response to injury were derived from ductal cells (Inada et al., 2008). In contrast, alloxan treatment was recently shown to induce transdifferentiation of acinar cells into β cells (Baeyens et al., 2014). We found that Fbw7 expression was quickly and dramatically downregulated 24 hr after PDL, but alloxan treatment induced no change in Fbw7 expression (Figures S6A and S6B). These data suggest that Fbw7 transcriptional downregulation may contribute to duct-derived β cell neogenesis in response to pancreatic injury (Figure S6C).
Although it is conceivable that β cells produced elsewhere could migrate to the ducts, the location of induced β cells embedded within the ducts suggests that they originated in this compartment. Several lines of evidence support this interpretation: first, “transition” cells coexpressing ductal cell (CK19) and β cell (ins) markers are observed after Fbw7 loss; second, genetic models in which recombination is inefficient in ducts (such as RFY mice injected intrapancreatically with OH-tamoxifen) do not induce β cell neogenesis; and third, duct-specific Fbw7 loss combined with lineage tracing (our CFY model) induces the occurrence of ductal YFP-labeled insulin-positive cells.
Insulin-positive cells are also observed in ducts of normal unchallenged mice, albeit rarely (Teta et al., 2005), a finding we confirmed in this study. It is not known whether this spontaneous transdifferentiation process is similar to the reprogramming induced by Fbw7 inactivation. However, bihormonal insulin/glucagon double-positive cells—presumptive α and β cell precursors during embryonic pancreas development (De Krijger et al., 1992) that we also observed in Fbw7 deleted ducts—have not been described in unchallenged adult WT pancreas. Similarly, CK19/insulin transdifferentiation intermediates have not been reported in normal mice. Thus, β cell reprogramming induced by Fbw7 inactivation appears to be a distinct process from the spontaneous appearance of insulin-positive cells in WT pancreatic ducts and may represent a reawakening of a multipotent state.
A frequent stumbling block in previous models inducing cell reprogramming has been the functionality of the newly formed β cells. It is important to note that Fbw7-mutant induced β cells secrete comparable amounts of insulin after glucose challenge as bona fide β cells isolated from MIP-GFP mice. Therefore, Fbw7 loss appears to trigger the conversion of adult pancreatic ductal cells into apparently functional β cells.
The Fbw7-Ngn3 Axis as a Mechanism Regulating Adult β Cell Neogenesis
The activity of Fbw7 toward different substrates is tissue specific, and our results show that, in the pancreas, c-Jun, Notch, and Ngn3 levels are increased after Fbw7 deletion. While c-Jun has no reported function in pancreatic cell fate decisions, the Notch signaling pathway is thought to inhibit pancreatic endocrine development (Apelqvist et al., 1999; Fujikura et al., 2006; Jensen et al., 2000; Oka et al., 1995). In contrast, we find that endocrine differentiation in the ducts after Fbw7 deletion is accompanied by an increase in NICD1 levels. This could imply that Notch has different roles in embryonic and adult pancreatic β cell differentiation, but it is also possible that increased Notch signaling is not required for β cell neogenesis after Fbw7 loss, and β cell neogenesis is induced despite an overall increase in Notch levels.
The stabilization of Ngn3 after Fbw7 loss is consistent with a strong proendocrine signal. Ngn3 has been previously reported to be required for PDL- and Pax4 overexpression-induced β cell neogenesis (Al-Hasani et al., 2013; Xu et al., 2008), and our data show that Ngn3 stabilization in the ducts is sufficient to induce β cell neogenesis (Figure 5). Despite its essential role in endocrine differentiation, and the reported Ngn3 instability at the protein level, the regulatory mechanisms that control the abundance of Ngn3 are not fully understood. In this study, we show that Ngn3 is a substrate for SCF(Fbw7). Ngn3 behaves as a canonical Fbw7 substrate, containing a GSK3 consensus phosphorylation site that, when mutated, increases the stability of the protein. Our data indicate that Ngn3 stabilization after Fbw7 loss contributes to a transdifferentiation program, inducing ductal cells to differentiate into β cells. Induction of adult β cell neogenesis is desirable for diabetes treatment, and modulation of the Fbw7-Ngn3 axis could potentially be exploited as a therapeutic approach toward generation of new β cells for cell replacement therapies.
Experimental Procedures
Mouse Lines
The Pdx1-Cre (Hingorani et al., 2003), R26-LSL-YFP (Srinivas et al., 2001), CK19-CreERT (Means et al., 2008), R26-CreERT (Ventura et al., 2007), MIP-GFP (Hara et al., 2003), and Fbw7f/f (Jandke et al., 2011) mouse lines have been previously described. The R26-LSL-Ngn3-AA mouse was generated using mouse Ngn3-AA complementary DNA (cDNA) to create a conditional Rosa26-Ngn3-AA-IRES-eGFP-pA+ targeting vector as described elsewhere (Nyabi et al., 2009), followed by selection of embryonic stem cell clones targeted with linearized vector and generation of chimeric Swiss diploid embryos. All animal experiments were approved by the CRUK London Research Institute Animal Ethics Committee and conformed with UK Home Office regulations under the Animals (Scientific Procedures) Act 1986 including Amendment Regulations 2012.
Cell Lines and Plasmids
HCT116-Fbw7wt and HCT116-Fbw7Δ cells, and Fbw7α–Flag and Fbw7β–Flag constructs, have been described elsewhere (Grim et al., 2008). Full-length Ngn3 cDNA from mouse embryonic pancreas was obtained by PCR and cloned into pcDNA3 to generate the pcDNA3-Ngn3 plasmid. Mutation of Ngn3 Ser183/Ser187 to alanine was achieved by conventional PCR site-directed mutagenesis. p-RS-sh-control and p-RS-sh-Fbw7 constructs were generated by cloning short hairpin-containing oligos into the pRS vector (Addgene).
Genetic Labeling Experiments
For all experiments, adult (6–9 weeks except where indicated) age- and strain-matched animals were used. Mice were either injected intraperitoneally with 100 μg/g body weight of tamoxifen dissolved in peanut oil (at least three mice per genotype) or intrapancreatically injected with 20 μl–50 μM 4-OH-tamoxifen (two mice per genotype) as indicated. Analyses were performed 5/13 days (short term) or 60/82 days (long term) postinjection. Where indicated, BrdU (0.8 mg/ml) was given in drinking water 1 day before the first tamoxifen injection and kept until the end of the experiment. The pancreas was excised, processed, and stained as described in the Supplemental Experimental Procedures.
IF and Immunohistochemistry Staining
Rhodamine-DBA (Sigma) was used to detect ductal cells by confocal microscopy. IF and immunohistochemistry staining was performed as described elsewhere (Sancho et al., 2010). Antibodies are listed in the Supplemental Experimental Procedures. Quantification of the insulin-positive area in Ngn3 conditional transgenic mouse pancreas was performed on NanoZoomer 2.0-HT (HAMAMATSU) scanned slides using AdobePS-CS5.
Western Blot Analysis
Pancreas lysates were homogenized in RIPA lysis buffer supplemented with protease inhibitor (Sigma). 293T cells were lysed in NP-40 lysis buffer. Immunoblots were carried out as described elsewhere (Nateri et al., 2005). Antibodies are listed in the Supplemental Experimental Procedures.
Immunoprecipitations
Human embryonic kidney 293T (HEK293T) cells coexpressing HA-Ngn3 and Fbw7-Flag were treated for 5 hr with proteasome inhibitor MG-132 (25 μM; Calbiochem), lysed with 0.2% NP40 buffer, and incubated with anti-Flag or anti-HA agarose beads (Sigma). For the endogenous Fbw7-Ngn3 interaction assay, Ngn3 was immunoprecipitated from HCT116-Fbw7wt and HCT116-Fbw7Δ cells transfected with Ngn3-AA. Endogenous Fbw7 in inputs and immunoprecipitation (IP) samples was detected using anti-Fbw7 antibody (Bethyl Laboratories).
Ubiquitination Assays
For in vivo ubiquitination assays, His-Ub was affinity purified with nickel-nitrilotriacetic acid (NTA)-agarose beads, as described elsewhere (Davies et al., 2010). In vitro ubiquitination assays with Fbw7 and Fbw7α-ΔFbox-Flag immunoprecipitated complexes were performed as described elsewhere (Popov et al., 2007).
Fluorescence-Activated Cell Sorting Analysis
Single pancreatic cell suspensions were obtained by 30 min digestion in 1.6 mg/ml collagenase type IV (Whorttington), followed by filtration on a 70 μm nylon mesh. Cells from six age-matched (6- to 8-week-old) mice per genotype were sorted for GFP expression for each independent experiment.
DNA Isolation and Allele Recombination PCR
Genomic DNA from GFP+/GFP− cells sorted from six tamoxifen-injected CFY and CY mice was isolated by digestion in DirectPCR Lysis Reagent (Viagen). PCR primers used to detect the efficiency of recombination of Fbw7 and R26 alleles are given in the Supplemental Experimental Procedures.
Microarray Analysis and qPCR
RNA was isolated from sorted GFP+ cells from tamoxifen-injected CY and CFY mice (six pooled pancreas per genotype) or MIP-GFP mice (three pooled pancreas) using a RNeasy Micro Kit (QIAGEN). RNA microarray hybridizations were performed by the Cancer Research UK Manchester Institute Microarray Service using the GeneChip Mouse Gene 1.0 ST array (Affymetrix) after genome amplification of the RNA.
For qPCR analysis of sorted cells, RNA was isolated as described for the microarray, and cDNA amplification was performed using the Quantitect whole transcriptome amplification kit (QIAGEN). For qPCR analysis in Pdx1-Cre; Fbw7f/f mice, RNA was isolated using the RNeasy Mini Kit (QIAGEN), and cDNA was generated using the Transcriptor First Strand cDNA Synthesis Kit (Roche). Diluted cDNAs were used for qPCR SYBR-Green detection of target genes, using primer sequences given in the Supplemental Experimental Procedures.
Glucose Challenge In Vitro
Determination of insulin release after glucose challenge was performed as described elsewhere (Banga et al., 2012), with minor modifications. Briefly, 30,000 GFP+ sorted CY or CFY cells (from six pooled pancreas per genotype) or 5,000 GFP+ cells from MIP-GFP pancreas were plated per well in Dulbecco’s modified Eagle’s medium without serum/glucose/phenol red. Cells were starved for 2 hr, the medium was changed, and 20 mM glucose was added (final volume, 200 μl). Insulin concentration was determined in supernatants using the Mouse Insulin ELISA Kit (Crystal Chem).
Statistics
Statistical evaluation was performed using the Student’s unpaired t test. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001 were considered statistically significant. See Table S1 for exact p values.
Acknowledgments
We are grateful to the London Research Institute Animal Unit, Equipment Park, FACS facility; Bradley Spencer-Dene and Emma Nye in the Experimental Histopathology lab; and Richard Mitter in the Bioinformatics facility for technical help. We thank Hamamatsu for allowing us to perform the full slide scans using their NanoZoomer 2.0-HT. We thank D. Tuveson for Pdx1-Cre mice; J.J. Haigh for providing the pRosa26-DV1 vector used for generating the Ngn3-AA conditional transgenic mouse; and Nikita Popov and Bruce Clurman for providing reagents. We thank Stephen Pollard, Taija Makinen, and Ilaria Malanchi for critical reading of the manuscript. R.S. was funded by Marie Curie (MEIF-CT-2006-041119), MRC (G0901677), and ERC (281661) grants. R.G. was supported by an EMBO long-term fellowship. A.B. and R.S. are listed as inventors on an International Patent Application based on this work. The London Research Institute is funded by Cancer Research UK.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Accession Numbers
The Gene Expression Omnibus accession number for the microarray data reported in Figure 7B is GSE58969.
Supplemental Information
References
- Al-Hasani K., Pfeifer A., Courtney M., Ben-Othman N., Gjernes E., Vieira A., Druelle N., Avolio F., Ravassard P., Leuckx G. Adult duct-lining cells can reprogram into β-like cells able to counter repeated cycles of toxin-induced diabetes. Dev. Cell. 2013;26:86–100. doi: 10.1016/j.devcel.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Apelqvist A., Li H., Sommer L., Beatus P., Anderson D.J., Honjo T., Hrabe de Angelis M., Lendahl U., Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Atkinson M.A., Bluestone J.A., Eisenbarth G.S., Hebrok M., Herold K.C., Accili D., Pietropaolo M., Arvan P.R., Von Herrath M., Markel D.S., Rhodes C.J. How does type 1 diabetes develop?: the notion of homicide or β-cell suicide revisited. Diabetes. 2011;60:1370–1379. doi: 10.2337/db10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens L., Bonné S., German M.S., Ravassard P., Heimberg H., Bouwens L. Ngn3 expression during postnatal in vitro beta cell neogenesis induced by the JAK/STAT pathway. Cell Death Differ. 2006;13:1892–1899. doi: 10.1038/sj.cdd.4401883. [DOI] [PubMed] [Google Scholar]
- Baeyens L., Lemper M., Leuckx G., De Groef S., Bonfanti P., Stangé G., Shemer R., Nord C., Scheel D.W., Pan F.C. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat. Biotechnol. 2014;32:76–83. doi: 10.1038/nbt.2747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Banga A., Akinci E., Greder L.V., Dutton J.R., Slack J.M. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc. Natl. Acad. Sci. USA. 2012;109:15336–15341. doi: 10.1073/pnas.1201701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S., Toschi E., Inada A., Reitz P., Fonseca S.Y., Aye T., Sharma A. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr. Diabetes. 2004;5(Suppl 2):16–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Inada A., Yatoh S., Li W.-C., Aye T., Toschi E., Sharma A. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem. Soc. Trans. 2008;36:353–356. doi: 10.1042/BST0360353. [DOI] [PubMed] [Google Scholar]
- Collombat P., Xu X., Ravassard P., Sosa-Pineda B., Dussaud S., Billestrup N., Madsen O.D., Serup P., Heimberg H., Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimanna A., Speicher J.A., Houshmand G., Shiota C., Prasadan K., Ji B., Logsdon C.D., Gittes G.K., Esni F. Duct cells contribute to regeneration of endocrine and acinar cells following pancreatic damage in adult mice. Gastroenterology. 2011;141:1451–1462. doi: 10.1053/j.gastro.2011.07.003. 1462.e1–1462.e1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C.C., Chakraborty A., Cipriani F., Haigh K., Haigh J.J., Behrens A. Identification of a co-activator that links growth factor signalling to c-Jun/AP-1 activation. Nat. Cell Biol. 2010;12:963–972. doi: 10.1038/ncb2098. [DOI] [PubMed] [Google Scholar]
- De Krijger R.R., Aanstoot H.J., Kranenburg G., Reinhard M., Visser W.J., Bruining G.J. The midgestational human fetal pancreas contains cells coexpressing islet hormones. Dev. Biol. 1992;153:368–375. doi: 10.1016/0012-1606(92)90121-v. [DOI] [PubMed] [Google Scholar]
- Dor Y., Brown J., Martinez O.I., Melton D.A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Edlund H. Pancreatic organogenesis—developmental mechanisms and implications for therapy. Nat. Rev. Genet. 2002;3:524–532. doi: 10.1038/nrg841. [DOI] [PubMed] [Google Scholar]
- Ejarque M., Cervantes S., Pujadas G., Tutusaus A., Sanchez L., Gasa R. Neurogenin3 cooperates with Foxa2 to autoactivate its own expression. J. Biol. Chem. 2013;288:11705–11717. doi: 10.1074/jbc.M112.388173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esni F., Ghosh B., Biankin A.V., Lin J.W., Albert M.A., Yu X., MacDonald R.J., Civin C.I., Real F.X., Pack M.A. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- Fujikura J., Hosoda K., Iwakura H., Tomita T., Noguchi M., Masuzaki H., Tanigaki K., Yabe D., Honjo T., Nakao K. Notch/Rbp-j signaling prevents premature endocrine and ductal cell differentiation in the pancreas. Cell Metab. 2006;3:59–65. doi: 10.1016/j.cmet.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Gradwohl G., Dierich A., LeMeur M., Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim J.E., Gustafson M.P., Hirata R.K., Hagar A.C., Swanger J., Welcker M., Hwang H.C., Ericsson J., Russell D.W., Clurman B.E. Isoform- and cell cycle-dependent substrate degradation by the Fbw7 ubiquitin ligase. J. Cell Biol. 2008;181:913–920. doi: 10.1083/jcb.200802076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J.F., Kemp D.M., Thomas M.K. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–1034. doi: 10.1210/en.2004-1576. [DOI] [PubMed] [Google Scholar]
- Hara M., Wang X., Kawamura T., Bindokas V.P., Dizon R.F., Alcoser S.Y., Magnuson M.A., Bell G.I. Transgenic mice with green fluorescent protein-labeled pancreatic beta -cells. Am. J. Physiol. Endocrinol. Metab. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- Heremans Y., Van De Casteele M., in’t Veld P., Gradwohl G., Serup P., Madsen O., Pipeleers D., Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J. Cell Biol. 2002;159:303–312. doi: 10.1083/jcb.200203074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani S.R., Petricoin E.F., Maitra A., Rajapakse V., King C., Jacobetz M.A., Ross S., Conrads T.P., Veenstra T.D., Hitt B.A. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Hoeck J.D., Jandke A., Blake S.M., Nye E., Spencer-Dene B., Brandner S., Behrens A. Fbw7 controls neural stem cell differentiation and progenitor apoptosis via Notch and c-Jun. Nat. Neurosci. 2010;13:1365–1372. doi: 10.1038/nn.2644. [DOI] [PubMed] [Google Scholar]
- Inada A., Nienaber C., Katsuta H., Fujitani Y., Levine J., Morita R., Sharma A., Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. USA. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriuchishima H., Takubo K., Matsuoka S., Onoyama I., Nakayama K.I., Nojima Y., Suda T. Ex vivo maintenance of hematopoietic stem cells by quiescence induction through Fbxw7 overexpression. Blood. 2011;117:2373–2377. doi: 10.1182/blood-2010-07-294801. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y., Onoyama I., Nakayama K.I., Nakayama K. Notch-dependent cell cycle arrest and apoptosis in mouse embryonic fibroblasts lacking Fbxw7. Oncogene. 2008;27:6164–6174. doi: 10.1038/onc.2008.216. [DOI] [PubMed] [Google Scholar]
- Jandke A., Da Costa C., Sancho R., Nye E., Spencer-Dene B., Behrens A. The F-box protein Fbw7 is required for cerebellar development. Dev. Biol. 2011;358:201–212. doi: 10.1016/j.ydbio.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Jensen J., Pedersen E.E., Galante P., Hald J., Heller R.S., Ishibashi M., Kageyama R., Guillemot F., Serup P., Madsen O.D. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Lysy P.A., Weir G.C., Bonner-Weir S. Concise review: pancreas regeneration: recent advances and perspectives. Stem Cells Transl. Med. 2012;1:150–159. doi: 10.5966/sctm.2011-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Onoyama I., Sunabori T., Kageyama R., Okano H., Nakayama K.I. Fbxw7-dependent degradation of Notch is required for control of “stemness” and neuronal-glial differentiation in neural stem cells. J. Biol. Chem. 2011;286:13754–13764. doi: 10.1074/jbc.M110.194936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A.L., Xu Y., Ray K.C., Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46:318–323. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh L.C., Stanger B.Z., Kwan K.M., Melton D.A. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K.I., Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat. Rev. Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- Nateri A.S., Spencer-Dene B., Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- Nyabi O., Naessens M., Haigh K., Gembarska A., Goossens S., Maetens M., De Clercq S., Drogat B., Haenebalcke L., Bartunkova S. Efficient mouse transgenesis using Gateway-compatible ROSA26 locus targeting vectors and F1 hybrid ES cells. Nucleic Acids Res. 2009;37:e55. doi: 10.1093/nar/gkp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka C., Nakano T., Wakeham A., de la Pompa J.L., Mori C., Sakai T., Okazaki S., Kawaichi M., Shiota K., Mak T.W., Honjo T. Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development. 1995;121:3291–3301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- Oliver-Krasinski J.M., Stoffers D.A. On the origin of the beta cell. Genes Dev. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoyama I., Tsunematsu R., Matsumoto A., Kimura T., de Alborán I.M., Nakayama K., Nakayama K.I. Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis. J. Exp. Med. 2007;204:2875–2888. doi: 10.1084/jem.20062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoyama I., Suzuki A., Matsumoto A., Tomita K., Katagiri H., Oike Y., Nakayama K., Nakayama K.I. Fbxw7 regulates lipid metabolism and cell fate decisions in the mouse liver. J. Clin. Invest. 2011;121:342–354. doi: 10.1172/JCI40725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca F.W., Melton D.A. How to make a functional β-cell. Development. 2013;140:2472–2483. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper K., Brickwood S., Turnpenny L.W., Cameron I.T., Ball S.G., Wilson D.I., Hanley N.A. Beta cell differentiation during early human pancreas development. J. Endocrinol. 2004;181:11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- Popov N., Wanzel M., Madiredjo M., Zhang D., Beijersbergen R., Bernards R., Moll R., Elledge S.J., Eilers M. The ubiquitin-specific protease USP28 is required for MYC stability. Nat. Cell Biol. 2007;9:765–774. doi: 10.1038/ncb1601. [DOI] [PubMed] [Google Scholar]
- Roark R., Itzhaki L., Philpott A. Complex regulation controls Neurogenin3 proteolysis. Biol. Open. 2012;1:1264–1272. doi: 10.1242/bio.20121750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho R., Jandke A., Davis H., Diefenbacher M.E., Tomlinson I., Behrens A. F-box and WD repeat domain-containing 7 regulates intestinal cell lineage commitment and is a haploinsufficient tumor suppressor. Gastroenterology. 2010;139:929–941. doi: 10.1053/j.gastro.2010.05.078. [DOI] [PubMed] [Google Scholar]
- Sancho R., Blake S.M., Tendeng C., Clurman B.E., Lewis J., Behrens A. Fbw7 repression by hes5 creates a feedback loop that modulates Notch-mediated intestinal and neural stem cell fate decisions. PLoS Biol. 2013;11:e1001586. doi: 10.1371/journal.pbio.1001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitzgebel V.M., Scheel D.W., Conners J.R., Kalamaras J., Lee J.E., Anderson D.J., Sussel L., Johnson J.D., German M.S. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swales N., Martens G.A., Bonné S., Heremans Y., Borup R., Van de Casteele M., Ling Z., Pipeleers D., Ravassard P., Nielsen F. Plasticity of adult human pancreatic duct cells by neurogenin3-mediated reprogramming. PLoS ONE. 2012;7:e37055. doi: 10.1371/journal.pone.0037055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teta M., Long S.Y., Wartschow L.M., Rankin M.M., Kushner J.A. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- Thompson B.J., Jankovic V., Gao J., Buonamici S., Vest A., Lee J.M., Zavadil J., Nimer S.D., Aifantis I. Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J. Exp. Med. 2008;205:1395–1408. doi: 10.1084/jem.20080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Casteele M., Leuckx G., Baeyens L., Cai Y., Yuchi Y., Coppens V., De Groef S., Eriksson M., Svensson C., Ahlgren U. Neurogenin 3+ cells contribute to β-cell neogenesis and proliferation in injured adult mouse pancreas. Cell Death Dis. 2013;4:e523. doi: 10.1038/cddis.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A., Kirsch D.G., McLaughlin M.E., Tuveson D.A., Grimm J., Lintault L., Newman J., Reczek E.E., Weissleder R., Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- Wang S., Hecksher-Sorensen J., Xu Y., Zhao A., Dor Y., Rosenberg L., Serup P., Gu G. Myt1 and Ngn3 form a feed-forward expression loop to promote endocrine islet cell differentiation. Dev. Biol. 2008;317:531–540. doi: 10.1016/j.ydbio.2008.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Inuzuka H., Fukushima H., Wan L., Gao D., Shaik S., Sarkar F.H., Wei W. Emerging roles of the FBW7 tumour suppressor in stem cell differentiation. EMBO Rep. 2012;13:36–43. doi: 10.1038/embor.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker M., Clurman B.E. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat. Rev. Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- Welcker M., Orian A., Jin J., Grim J.E., Harper J.W., Eisenman R.N., Clurman B.E. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl. Acad. Sci. USA. 2004;101:9085–9090. doi: 10.1073/pnas.0402770101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., D’Hoker J., Stangé G., Bonné S., De Leu N., Xiao X., Van de Casteele M., Mellitzer G., Ling Z., Pipeleers D. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Yi P., Park J.S., Melton D.A. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell. 2013;153:747–758. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zaret K.S. Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat. Rev. Genet. 2008;9:329–340. doi: 10.1038/nrg2318. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.