Abstract
In Burkitt's lymphoma cells the proto-oncogene c-myc is constantly juxtaposed through chromosomal translocation to one of the immunoglobulin loci on chromosomes 14, 2 or 22. In the majority of cases the chromosomal breakpoint is localized 3' or 5' of the gene leaving the physiological c-myc transcription unit intact. As a consequence of the translocation the c-myc gene on the translocation chromosome becomes transcriptionally activated in such a manner that the c-myc promoter P1 is more active than promoter P2. In order to define elements involved in c-myc activation through t(2;8) translocation we have studied the expression of constructs consisting of c-myc and different parts of the immunoglobulin kappa locus after stable transfection into Burkitt's lymphoma cells. The c-myc gene under the control of the complete Ig kappa locus containing matrix attachment region, intron enhancer, constant kappa gene and 3' enhancer was strongly activated with predominant usage of promoter P1. Deletion analysis revealed that the intron or 3' enhancers alone activated c-myc to a much lesser extent and with normal promoter usage (P1 < P2). The cooperation of the same regulatory elements is required not only for transcriptional activation and induction of the promoter shift but also for down-regulation of promoter P1 of the translocated c-myc allele by sodium butyrate, another characteristic feature of Burkitt's lymphoma cells. This supports the notion that all elements involved in transcriptional activation and dysregulation of c-myc are contained within the myc-Ig specific minichromosome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel T. W., Mautner J., Polack A., Bornkamm G. W., Eick D. Two antisense promoters in the immunoglobulin mu-switch region drive expression of c-myc in the Burkitt's lymphoma cell line BL67. Oncogene. 1992 Jul;7(7):1267–1271. [PubMed] [Google Scholar]
- Atchison M. L., Perry R. P. Complementation between two cell lines lacking kappa enhancer activity: implications for the developmental control of immunoglobulin transcription. EMBO J. 1988 Dec 20;7(13):4213–4220. doi: 10.1002/j.1460-2075.1988.tb03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison M. L., Perry R. P. The role of the kappa enhancer and its binding factor NF-kappa B in the developmental regulation of kappa gene transcription. Cell. 1987 Jan 16;48(1):121–128. doi: 10.1016/0092-8674(87)90362-x. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Bernheim A., Berger R., Lenoir G. Cytogenetic studies on African Burkitt's lymphoma cell lines: t(8;14), t(2;8) and t(8;22) translocations. Cancer Genet Cytogenet. 1981 Jun;3(4):307–315. doi: 10.1016/0165-4608(81)90039-x. [DOI] [PubMed] [Google Scholar]
- Blasquez V. C., Hale M. A., Trevorrow K. W., Garrard W. T. Immunoglobulin kappa gene enhancers synergistically activate gene expression but independently determine chromatin structure. J Biol Chem. 1992 Nov 25;267(33):23888–23893. [PubMed] [Google Scholar]
- Blasquez V. C., Xu M., Moses S. C., Garrard W. T. Immunoglobulin kappa gene expression after stable integration. I. Role of the intronic MAR and enhancer in plasmacytoma cells. J Biol Chem. 1989 Dec 15;264(35):21183–21189. [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M., Senear A. W., Warren R., Palmiter R. D. Somatic expression of herpes thymidine kinase in mice following injection of a fusion gene into eggs. Cell. 1981 Nov;27(1 Pt 2):223–231. doi: 10.1016/0092-8674(81)90376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E., Dalla-Favera R., Bentley D., Groudine M. Mutations in the first exon are associated with altered transcription of c-myc in Burkitt lymphoma. Science. 1987 Nov 27;238(4831):1272–1275. doi: 10.1126/science.3685977. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Croce C. M. Role of chromosome translocations in human neoplasia. Cell. 1987 Apr 24;49(2):155–156. doi: 10.1016/0092-8674(87)90552-6. [DOI] [PubMed] [Google Scholar]
- Eick D., Bornkamm G. W. Expression of normal and translocated c-myc alleles in Burkitt's lymphoma cells: evidence for different regulation. EMBO J. 1989 Jul;8(7):1965–1972. doi: 10.1002/j.1460-2075.1989.tb03602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D., Bornkamm G. W. Transcriptional arrest within the first exon is a fast control mechanism in c-myc gene expression. Nucleic Acids Res. 1986 Nov 11;14(21):8331–8346. doi: 10.1093/nar/14.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D., Piechaczyk M., Henglein B., Blanchard J. M., Traub B., Kofler E., Wiest S., Lenoir G. M., Bornkamm G. W. Aberrant c-myc RNAs of Burkitt's lymphoma cells have longer half-lives. EMBO J. 1985 Dec 30;4(13B):3717–3725. doi: 10.1002/j.1460-2075.1985.tb04140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D., Polack A., Kofler E., Bornkamm G. W. The block of elongation in c-myc exon 1 is abolished in Burkitt's lymphoma cell lines with variant translocation. Oncogene. 1988 Oct;3(4):397–403. [PubMed] [Google Scholar]
- Graham M., Adams J. M. Chromosome 8 breakpoint far 3' of the c-myc oncogene in a Burkitt's lymphoma 2;8 variant translocation is equivalent to the murine pvt-1 locus. EMBO J. 1986 Nov;5(11):2845–2851. doi: 10.1002/j.1460-2075.1986.tb04578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F., Lombardi L., Inghirami G., Sternas L., Cechova K., Dalla-Favera R. Negative autoregulation of c-myc gene expression is inactivated in transformed cells. EMBO J. 1990 Dec;9(12):3913–3922. doi: 10.1002/j.1460-2075.1990.tb07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl P., Lipp M. Generation of a variant t(2;8) translocation of Burkitt's lymphoma by site-specific recombination via the kappa light-chain joining signals. Mol Cell Biol. 1987 Jun;7(6):2037–2045. doi: 10.1128/mcb.7.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A. C., Gillies S. D., Saito H., Wood C., Wiman K., Hayward W. S., Tonegawa S. Activation of a translocated human c-myc gene by an enhancer in the immunoglobulin heavy-chain locus. 1984 Jan 26-Feb 1Nature. 307(5949):334–340. doi: 10.1038/307334a0. [DOI] [PubMed] [Google Scholar]
- Henglein B., Synovzik H., Groitl P., Bornkamm G. W., Hartl P., Lipp M. Three breakpoints of variant t(2;8) translocations in Burkitt's lymphoma cells fall within a region 140 kilobases distal from c-myc. Mol Cell Biol. 1989 May;9(5):2105–2113. doi: 10.1128/mcb.9.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis G. F., Mitchell K. F., Battey J., Potter H., Taub R., Lenoir G. M., Leder P. A variant translocation places the lambda immunoglobulin genes 3' to the c-myc oncogene in Burkitt's lymphoma. Nature. 1984 Feb 23;307(5953):752–755. doi: 10.1038/307752a0. [DOI] [PubMed] [Google Scholar]
- Joos S., Haluska F. G., Falk M. H., Henglein B., Hameister H., Croce C. M., Bornkamm G. W. Mapping chromosomal breakpoints of Burkitt's t(8;14) translocations far upstream of c-myc. Cancer Res. 1992 Dec 1;52(23):6547–6552. [PubMed] [Google Scholar]
- Judde J. G., Max E. E. Characterization of the human immunoglobulin kappa gene 3' enhancer: functional importance of three motifs that demonstrate B-cell-specific in vivo footprints. Mol Cell Biol. 1992 Nov;12(11):5206–5216. doi: 10.1128/mcb.12.11.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klehr D., Schlake T., Maass K., Bode J. Scaffold-attached regions (SAR elements) mediate transcriptional effects due to butyrate. Biochemistry. 1992 Mar 31;31(12):3222–3229. doi: 10.1021/bi00127a025. [DOI] [PubMed] [Google Scholar]
- Klobeck H. G., Combriato G., Zachau H. G. N segment insertion and region-directed somatic hypermutation in a kappa gene of a t(2;8) chromosomal translocation. Nucleic Acids Res. 1987 Jun 25;15(12):4877–4888. doi: 10.1093/nar/15.12.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumm A., Meulia T., Brunvand M., Groudine M. The block to transcriptional elongation within the human c-myc gene is determined in the promoter-proximal region. Genes Dev. 1992 Nov;6(11):2201–2213. doi: 10.1101/gad.6.11.2201. [DOI] [PubMed] [Google Scholar]
- Lee D. Y., Hayes J. J., Pruss D., Wolffe A. P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993 Jan 15;72(1):73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- Mengle-Gaw L., Rabbitts T. H. A human chromosome 8 region with abnormalities in B cell, HTLV-I+ T cell and c-myc amplified tumours. EMBO J. 1987 Jul;6(7):1959–1965. doi: 10.1002/j.1460-2075.1987.tb02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulia T., Krumm A., Spencer C., Groudine M. Sequences in the human c-myc P2 promoter affect the elongation and premature termination of transcripts initiated from the upstream P1 promoter. Mol Cell Biol. 1992 Oct;12(10):4590–4600. doi: 10.1128/mcb.12.10.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. B., Neuberger M. S. The immunoglobulin kappa locus contains a second, stronger B-cell-specific enhancer which is located downstream of the constant region. EMBO J. 1989 Jul;8(7):1959–1964. doi: 10.1002/j.1460-2075.1989.tb03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B., Stappert H., Reth M. A physical map and analysis of the murine C kappa-RS region show the presence of a conserved element. Eur J Immunol. 1990 Jun;20(6):1409–1411. doi: 10.1002/eji.1830200631. [DOI] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. The mechanism of inactivation of the normal c-myc gene locus in human Burkitt lymphoma cells. Oncogene. 1988 May;2(5):493–498. [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Transgenic mice. Cell. 1985 Jun;41(2):343–345. doi: 10.1016/s0092-8674(85)80004-0. [DOI] [PubMed] [Google Scholar]
- Pettersson S., Cook G. P., Brüggemann M., Williams G. T., Neuberger M. S. A second B cell-specific enhancer 3' of the immunoglobulin heavy-chain locus. Nature. 1990 Mar 8;344(6262):165–168. doi: 10.1038/344165a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Polack A., Eick D., Koch E., Bornkamm G. W. Truncation does not abrogate transcriptional downregulation of the c-myc gene by sodium butyrate in Burkitt's lymphoma cells. EMBO J. 1987 Oct;6(10):2959–2964. doi: 10.1002/j.1460-2075.1987.tb02601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
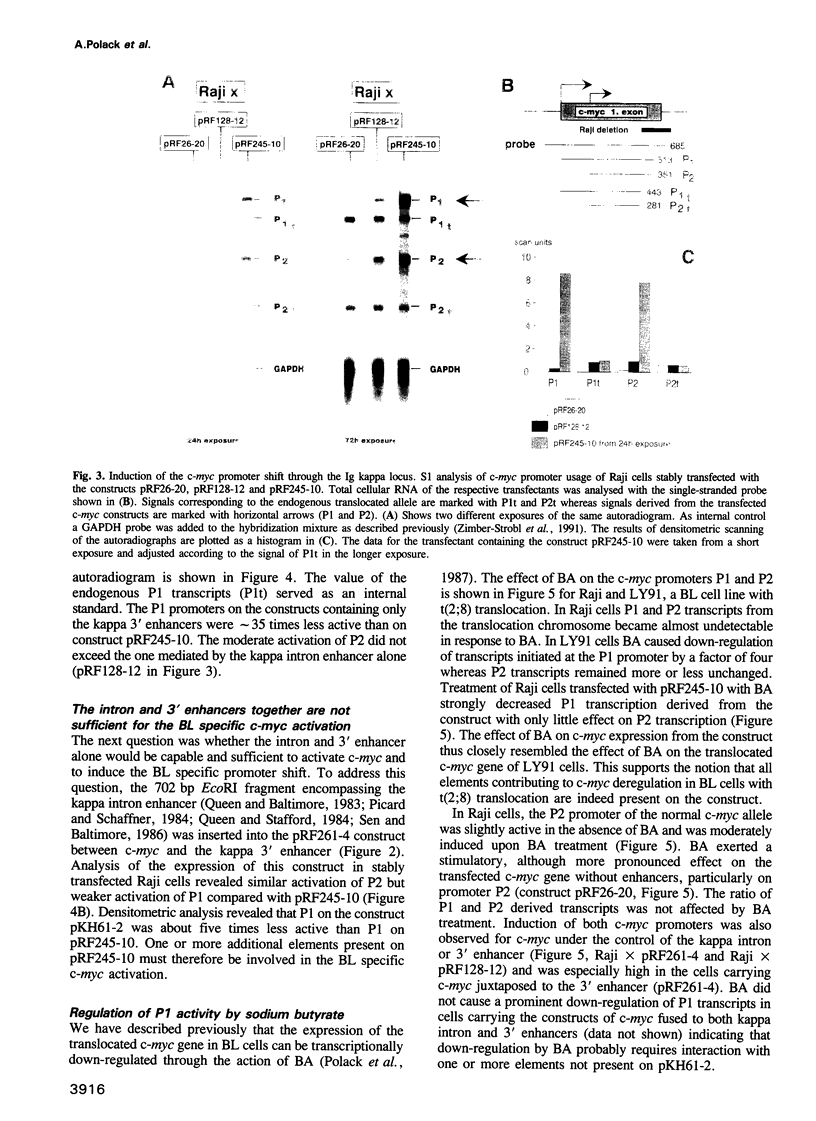
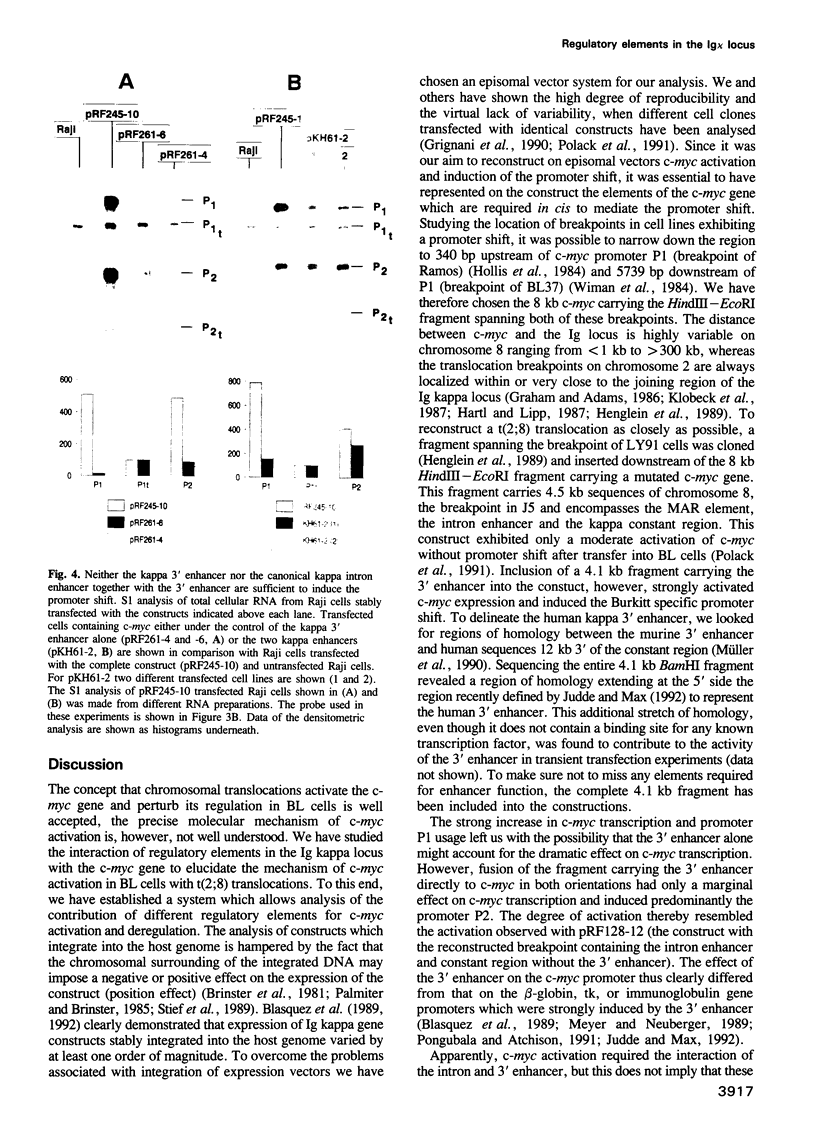
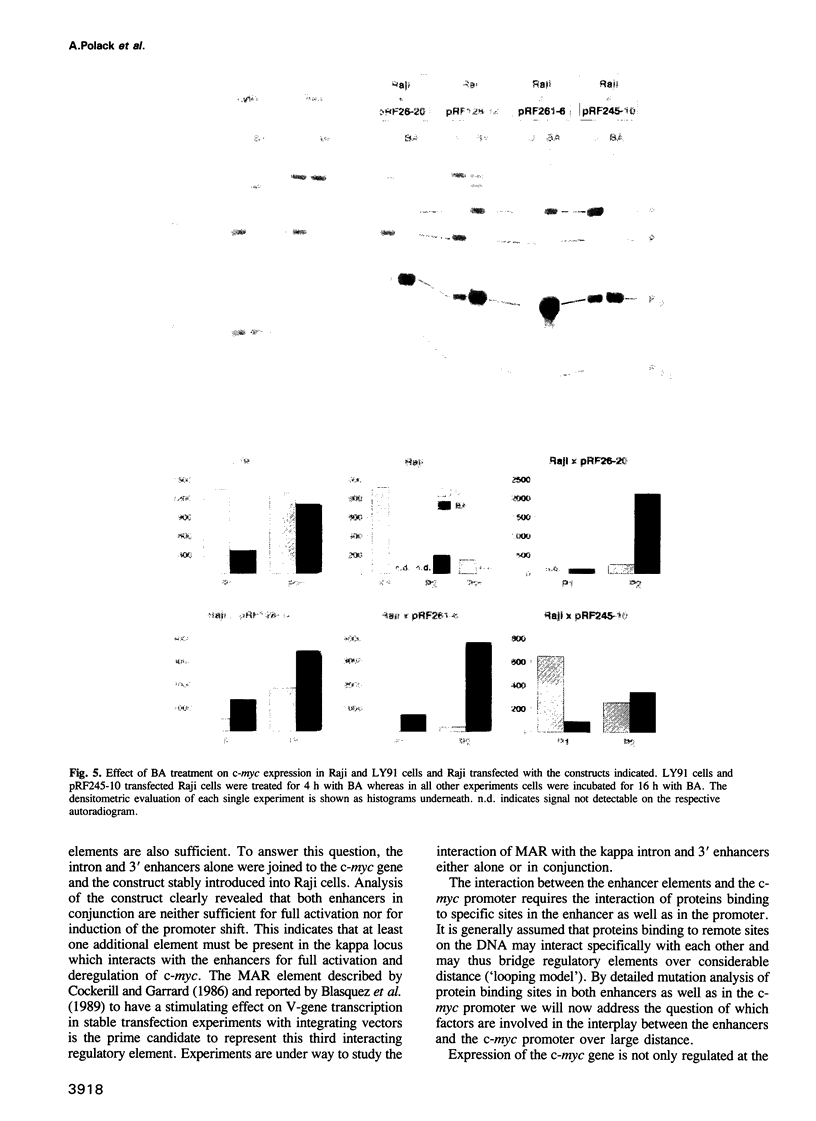
- Polack A., Strobl L., Feederle R., Schweizer M., Koch E., Eick D., Wiegand H., Bornkamm G. W. The intron enhancer of the immunoglobulin kappa gene activates c-myc but does not induce the Burkitt-specific promoter shift. Oncogene. 1991 Nov;6(11):2033–2040. [PubMed] [Google Scholar]
- Pongubala J. M., Atchison M. L. Functional characterization of the developmentally controlled immunoglobulin kappa 3' enhancer: regulation by Id, a repressor of helix-loop-helix transcription factors. Mol Cell Biol. 1991 Feb;11(2):1040–1047. doi: 10.1128/mcb.11.2.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongubala J. M., Nagulapalli S., Klemsz M. J., McKercher S. R., Maki R. A., Atchison M. L. PU.1 recruits a second nuclear factor to a site important for immunoglobulin kappa 3' enhancer activity. Mol Cell Biol. 1992 Jan;12(1):368–378. doi: 10.1128/mcb.12.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Queen C., Stafford J. Fine mapping of an immunoglobulin gene activator. Mol Cell Biol. 1984 Jun;4(6):1042–1049. doi: 10.1128/mcb.4.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Forster A., Baer R., Hamlyn P. H. Transcription enhancer identified near the human C mu immunoglobulin heavy chain gene is unavailable to the translocated c-myc gene in a Burkitt lymphoma. Nature. 1983 Dec 22;306(5945):806–809. doi: 10.1038/306806a0. [DOI] [PubMed] [Google Scholar]
- Richman A., Hayday A. Normal expression of a rearranged and mutated c-myc oncogene after transfection into fibroblasts. Science. 1989 Oct 27;246(4929):494–497. doi: 10.1126/science.2683072. [DOI] [PubMed] [Google Scholar]
- Richman A., Hayday A. Serum-inducible expression of transfected human c-myc genes. Mol Cell Biol. 1989 Nov;9(11):4962–4969. doi: 10.1128/mcb.9.11.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., LeStrange R. C., Novak U., Hayward W. S., Groudine M. The block to transcription elongation is promoter dependent in normal and Burkitt's lymphoma c-myc alleles. Genes Dev. 1990 Jan;4(1):75–88. doi: 10.1101/gad.4.1.75. [DOI] [PubMed] [Google Scholar]
- Stief A., Winter D. M., Strätling W. H., Sippel A. E. A nuclear DNA attachment element mediates elevated and position-independent gene activity. Nature. 1989 Sep 28;341(6240):343–345. doi: 10.1038/341343a0. [DOI] [PubMed] [Google Scholar]
- Strobl L. J., Eick D. Hold back of RNA polymerase II at the transcription start site mediates down-regulation of c-myc in vivo. EMBO J. 1992 Sep;11(9):3307–3314. doi: 10.1002/j.1460-2075.1992.tb05409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl L. J., Kohlhuber F., Mautner J., Polack A., Eick D. Absence of a paused transcription complex from the c-myc P2 promoter of the translocation chromosome in Burkitt's lymphoma cells: implication for the c-myc P1/P2 promoter shift. Oncogene. 1993 Jun;8(6):1437–1447. [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R., Kelly K., Battey J., Latt S., Lenoir G. M., Tantravahi U., Tu Z., Leder P. A novel alteration in the structure of an activated c-myc gene in a variant t(2;8) Burkitt lymphoma. Cell. 1984 Jun;37(2):511–520. doi: 10.1016/0092-8674(84)90381-7. [DOI] [PubMed] [Google Scholar]
- Taub R., Moulding C., Battey J., Murphy W., Vasicek T., Lenoir G. M., Leder P. Activation and somatic mutation of the translocated c-myc gene in burkitt lymphoma cells. Cell. 1984 Feb;36(2):339–348. doi: 10.1016/0092-8674(84)90227-7. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Hayday A. C., Keating A. Electric field-mediated DNA transfer: transient and stable gene expression in human and mouse lymphoid cells. Mol Cell Biol. 1986 Feb;6(2):703–706. doi: 10.1128/mcb.6.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman K. G., Clarkson B., Hayday A. C., Saito H., Tonegawa S., Hayward W. S. Activation of a translocated c-myc gene: role of structural alterations in the upstream region. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6798–6802. doi: 10.1073/pnas.81.21.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S., Mirels L. F., Calayag M. C., Bishop J. M. Premature termination of transcription from the P1 promoter of the mouse c-myc gene. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11383–11387. doi: 10.1073/pnas.88.24.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. Q., Bauer S. R., Mushinski J. F., Marcu K. B. Chromosome translocations clustered 5' of the murine c-myc gene qualitatively affect promoter usage: implications for the site of normal c-myc regulation. EMBO J. 1985 Jun;4(6):1441–1447. doi: 10.1002/j.1460-2075.1985.tb03800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimber-Strobl U., Suentzenich K. O., Laux G., Eick D., Cordier M., Calender A., Billaud M., Lenoir G. M., Bornkamm G. W. Epstein-Barr virus nuclear antigen 2 activates transcription of the terminal protein gene. J Virol. 1991 Jan;65(1):415–423. doi: 10.1128/jvi.65.1.415-423.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]