Abstract
Hexavalent chromium [Cr(VI)] is a carcinogenic genotoxin commonly found in industry and the environment. DNA damage resulting from Cr(VI) exposure triggers numerous stress responses, including activation of cell cycle checkpoints and initiation of apoptosis. Mechanisms controlling these responses, while extensively studied, have yet to be fully elucidated. Here, we demonstrate that the p38 mitogen-activated protein kinase (MAPK) is activated by Cr(VI) exposure and that inhibition of p38 function using the selective inhibitor SB203580 results in abrogation of S-phase and G2 cell cycle checkpoints in response to Cr(VI). Also, we observe that inhibition of p38 results in decreased cell survival and increased percentage of apoptotic cells following Cr(VI) treatment. Taken together, these results indicate that p38 function is critical for optimal stress response induced by Cr(VI) exposure.
Keywords: apoptosis, cell cycle checkpoints, chromium, DNA damage
Introduction
Cr(VI) is a complex genotoxin capable of inducing numerous types of genomic lesions, which if left unchecked, can result in mutation and carcinogenesis [1–3]. Similar to ultra-violet (UV) radiation exposure, Cr(VI) exposure results in replication blockage and DNA inter- and intra-strand crosslinks; however, Cr(VI) treatment also causes DNA double-strand breaks, which are a hallmark of exposure to ionizing radiation (IR) [4]. Stress responses induced by Cr(VI) include cell cycle checkpoint activation and apoptosis, and consistent with the complexities of Cr(VI) genotoxicity, mechanisms controlling Cr(VI)-induced stress responses are intricate and remain to be fully understood. Studies have shown that ataxia-telangiectasia mutated (ATM), a protein kinase critical in cellular response to IR, is activated by Cr(VI) exposure and that this activation is required for Cr(VI)-induced S-phase arrest [4]. Additionally, NF-κB and p53 are activated in response to Cr(VI) exposure, and their activation is believed to be important for mechanisms governing the initiation of apoptosis following Cr(VI) exposure [5–7].
Another protein implicated in the response to Cr(VI) is p38. This protein is a member of the MAPK family which also includes the extracellular signal-regulated kinases (ERK), c-jun N-terminal or stress-activated kinases (JNK/SAPK), and the ERK/big MAP kinase 1 (BMK1) proteins [8]. MAPKs regulate diverse signalling pathways that control cellular growth, differentiation, and proliferation. Additionally, MAPK pathways have been implicated in the control of stress response although their exact role remains in question [9–11]. For example, studies indicate a conflicting role for p38 in the initiation of apoptosis, with evidence supporting both proand anti-apoptotic properties of p38 depending on cell type and genotoxin used [12]. In addition, p38 activation is required for IR-induced G2 arrest, and p38 has been implicated in the initiation of G1, S-phase, and G2 arrest following UV exposure [13–15]. Chuang and colleagues recently demonstrated that p38 was activated in response to Cr(VI) exposure, but mechanisms by which p38 governs theCr(VI)-induced stress response remain unknown [16]. In particular, we wanted to elucidate a role for p38 in regulating cell cycle checkpoints, cell survival, and apoptosis following Cr(VI) treatment.
In this study, we used the specific p38 inhibitor SB203580 to determine the role of p38 in the Cr(VI)-induced stress response. We demonstrate that Cr(VI) induces a dose-dependent activation of S-phase and G2 checkpoints and that inhibition of p38 abrogates these checkpoint responses. Also, we reveal that treatment of cells with Cr(VI) causes a dose-dependent decrease in cell survival with an accompanying increase in percentage of apoptotic cells and that p38 inhibition further decreases cell survival and increases apoptosis in response to Cr(VI).
Materials and methods
Cell culture, Cr(VI), and SB203580 treatment
HeLa cells were purchased from the American Type Culture Collection (Manassas, VA) and were cultured at 37 °C in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in a humidified 5% CO2 atmosphere. Potassium chromate (K2CrO4) and SB203580 were obtained from Sigma (St. Louis, MO) and were dissolved in sterile PBS or DMSO, respectively.
G2 checkpoint assay
HeLa cells were treated with SB203580 at a 10µM dose for 1 h prior to Cr(VI) treatment. SB203580-treated and untreated cells were then exposed to indicated doses of Cr(VI) for 4 h. Cells were then harvested using trypsin, washed in PBS, and fixed in 70% ethanol. Cells were incubated with primary anti-phospho-histone H3 antibody at 1:100 dilution at room temperature for 3 h, and then with FITC-conjugated goat anti-rabbit secondary antibody at 1:30 dilution for 30 min at room temperature. DNA was then stained using propidium iodide and cellular florescence was determined using a FACS calibur flow cytometer.
S-phase checkpoint assay
Activation of the S-phase checkpoint was determined as previously reported [4]. Briefly, cells were pre-labelled for ~24 h by culture in complete growth media containing 10 nCi/ml [14C]-thymidine. Medium was then replaced with normal DMEM, and the cells were allowed to recover for 6 h. The cells were then treated with 10µM concentrations of SB203580 or left untreated. The experimental groups were then exposed to indicated doses of Cr(VI), followed by pulse-labelling with 2.5µCi/ml [3H]-thymidine for 15 min. Following pulse-labelling, cells were harvested, washed twice with PBS, and fixed in cold (−4 °C) 70% methanol. Afterwards, the cells were applied to Whatman GF/A filters, which were then rinsed sequentially in 70 and 95% methanol, air-dried and then placed in glass vials containing 3ml of scintillation fluid. The filters were subsequently analysed on a Beckman scintillation counter with windows set to record both 14C dpm and 3H dpm. The measure of DNA synthesis was derived from resulting ratios of 3H cpm to 14C cpm and corrected for counts resulting from channel cross-over.
MTT assay
HeLa cells were plated in 96-well plates at a density of 1 × 104 and allowed to grow overnight. These cells were then either treated with SB203580 at 10µM or left untreated. Cells were then exposed to the stated concentrations of Cr(VI) for 24 h, following which 20µl of MTT reagent (Sigma) (5 mg/ml dissolved in PBS) was added to each well for a period of 5 h. The medium was then removed and 100µl of DMSO was added to each well. After gentle mixing, the plates were incubated at 37 °C for 5 min. Absorbance at 550 nm was then determined using a BioRad m680 microplate reader.
TUNEL assay
Apoptosis was determined using the terminal deoxynucleotidyltransferase dUTP nick end labelling (TUNEL) assay as described in the Apo-Direct Kit obtained from BD Biosciences. Briefly, HeLa cells were plated at a density of 1.0 × 106 in T25 flasks and allowed to grow overnight. Cells were then exposed to SB203580 as described earlier or left untreated. The experimental groups were then treated with Cr(VI) at indicated doses for 24 h, following which the cells were harvested, fixed, and stained according to the manufacturer’s protocol. Cellular florescence was determined using a FACS calibur flow cytometer (BD Biosciences).
Results
Inhibition of p38 abrogates Cr(VI)-induced G2 arrest
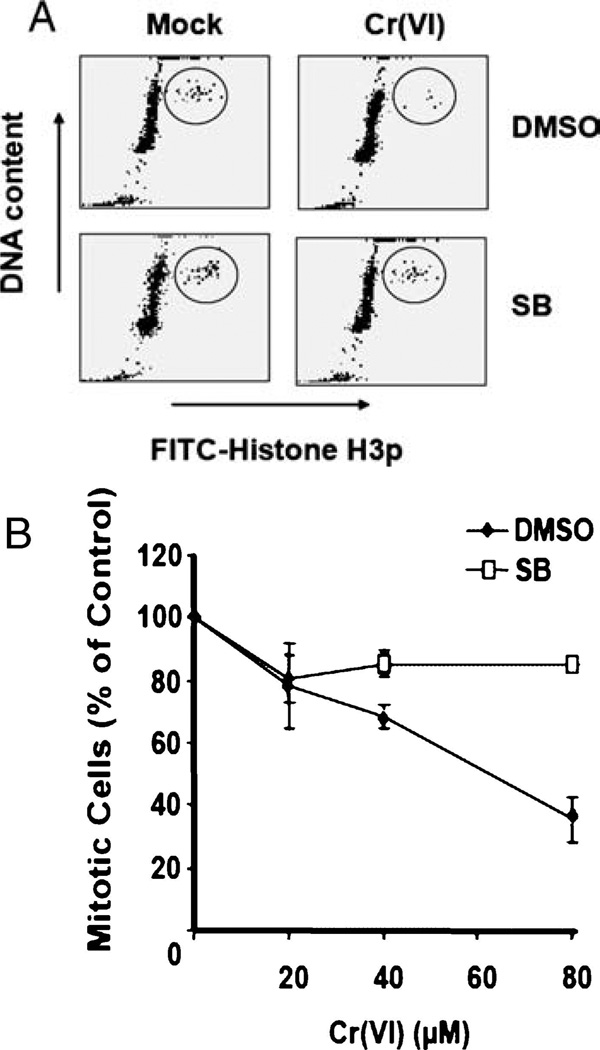
Initiation of the G2 checkpoint occurs in response to DNA damage following replication and serves to prevent cells with potentially deleterious mutations from dividing and passing those mutations on to daughter cells [17]. p38 has been implicated in G2 checkpoint activation following UV and IR treatment [15]. Since Cr(VI) treatment results in damage similar to that induced by UV and IR, we decided to test if p38 function was required for the Cr(VI)-induced G2 checkpoint. In order to examine p38 function, we treated the cells with SB203580, a well-characterized inhibitor of p38α and p38β, prior to Cr(VI) exposure [18, 19]. Following SB203580 treatment, we exposed HeLa cells to 20, 40, or 80µM doses of Cr(VI) for 4 h, after which cells were harvested and fixed. Cellular DNA content was determined by staining with propidium iodide (PI), and mitotic cells were identified by staining with an antibody specific for phosphorylated histone H3 (H3p). Since histone H3 is specifically phosphorylated at the onset of mitosis, observation of both H3p and PI staining allows us to differentiate between cells in G2 and mitosis [20]. Flow cytometric analysis revealed a significant reduction in mitotic cells following Cr(VI) treatment, indicative of activation of the G2/M checkpoint. G2/M checkpoint activation is a dose- and time-dependent occurrence that requires functional ATM (data not shown). Cells treated with SB203580 exhibited no significant reduction in mitotic cells in the presence of Cr(VI) (Fig. 1A). Cr(VI)-induced G2 arrest was dose-dependent and was abrogated by inhibition of p38 (Fig. 1B), suggesting a role of p38 in activation of the G2/M checkpoint in response to Cr(VI) exposure.
Fig. 1.
p38 function is required for Cr(VI)-induced G2 arrest. HeLa cells were treated with indicated doses of Cr(VI) for 4 h in the presence or absence of SB203580. (A) A dot plot of H3p vs. DNA content illustrates mitotic cells before and after Cr(VI) treatment. (B) Quantitative analysis of mitotic percentage of SB203580-treated or untreated cells exposed to increasing Cr(VI) doses. Error bars = ±1 S.D. are representative of three independent experiments.
Inhibition of p38 blocks S-phase arrest induced by Cr(VI)
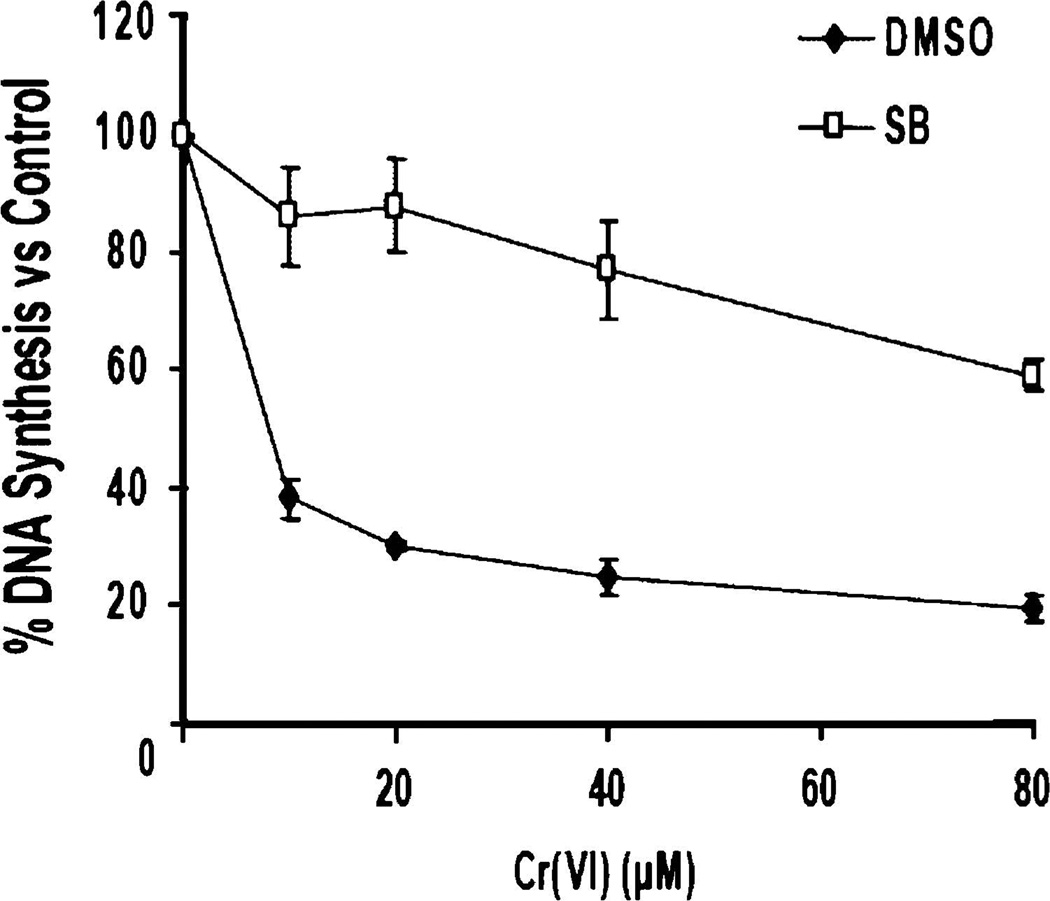
The S-phase cell cycle checkpoint occurs in response toDNA damage incurred during DNA replication. Initiation of this checkpoint serves to limit mutation by halting replication so that the damage can be assessed and either repaired or apoptotic processes can be initiated [17]. Activation of the S-phase checkpoint can be determined using an assay that measures [3H]-thymidine incorporation into nuclear DNA following DNA damage. MAPKAP, a downstream target of p38, has been shown to be required for UV-induced S-phase arrest [14]. Since p38 is activated in HeLa cells by Cr(VI) exposure (data not shown), we decided to determine if p38was required for Cr(VI)-induced S-phase arrest. HeLa cells were exposed to 10, 20, 40, or 80µM doses of Cr(VI) for 4 h. After Cr(VI) exposure, the S-phase checkpoint assay was performed to determine DNA synthesis in the cell populations. Figure 2 illustrates that there is a dose-dependent decrease after Cr(VI) exposure, indicative of S-phase arrest. This arrest is abrogated by treatment of cells with SB203580, indicating that this arrest is dependent upon p38 function.
Fig. 2.
Cr(VI)-induced S-phase arrest is dependent on p38 function. HeLa cells treated with increasing doses of Cr(VI) for 4 h in the presence or absence of SB203580 were subjected to S-phase checkpoint analysis. A reduction in DNA synthesis following Cr(VI) exposure is indicative of checkpoint activation. Error bars = ±1 S.D., graphed is the mean of three independent experiments.
p38 promotes cell survival following Cr(VI) exposure
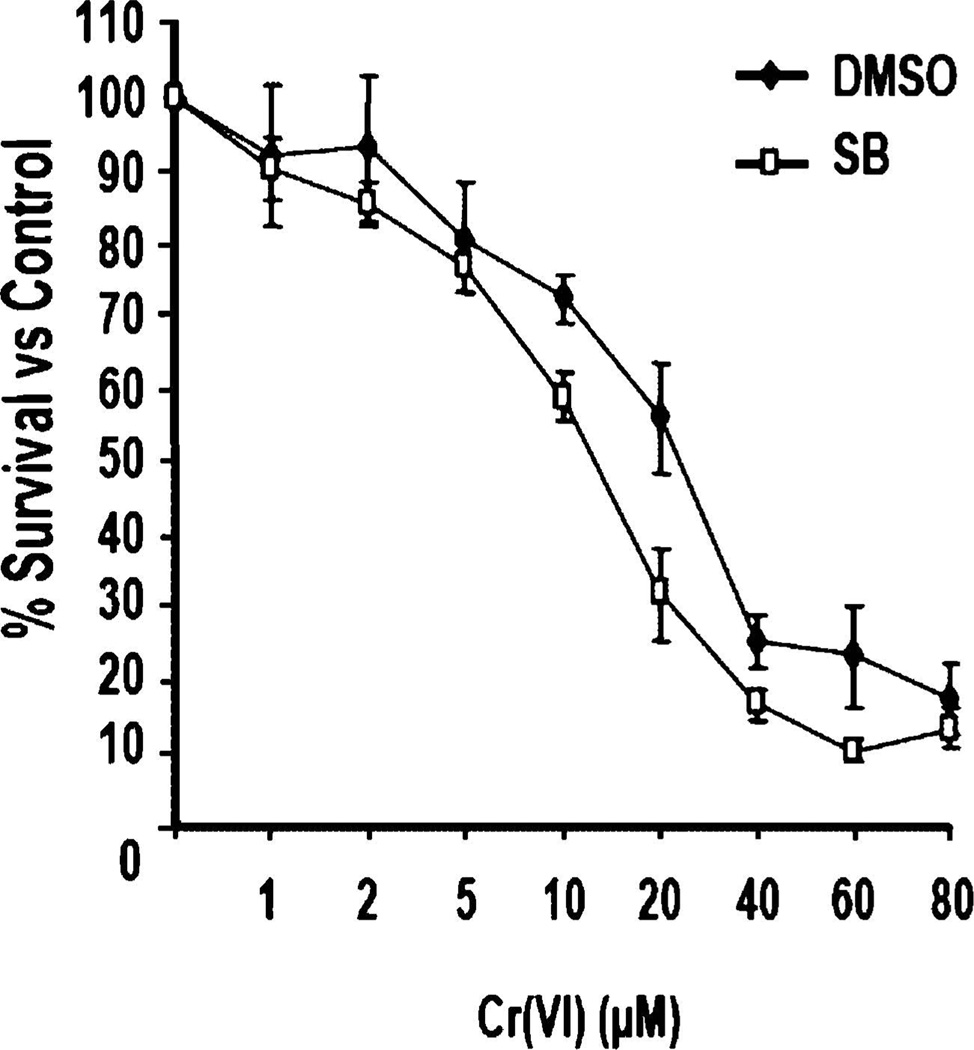
There is some conflict over the role that p38 plays in cell survival following DNA damage. Some studies indicate that p38 promotes cell survival [21, 22], while it has also been demonstrated that p38 has no effect on cell survival [16]. To further clarify the role of p38 in cell survival following Cr(VI) exposure, HeLa cells were treated with or without SB203580 and then exposed to doses of Cr(VI) ranging from 1 to 80µM for 24 h. MTT analysis was then performed to determine the percentage of surviving cells compared to untreated. Both experimental groups exhibited a dose-dependent decrease in cell survival following Cr(VI) exposure; however, cells exposed to SB203580 showed a reduction in cell survival between 10 and 60µM doses (Fig. 3). Although the observed differences in survival are not profound, they are statistically significant, suggesting that p38 plays a role in promoting cell survival in response to Cr(VI).
Fig. 3.
p38 promotes cell survival following Cr(VI) exposure. HeLa cells were exposed to increasing doses of Cr(VI) for 24 h in the presence or absence of SB203580.MTTanalysiswas used to determine the percentage of surviving cells. Error bars = ±1 S.D., graphed is the mean of six independent experiments.
p38 protects cells from Cr(VI)-induced apoptosis
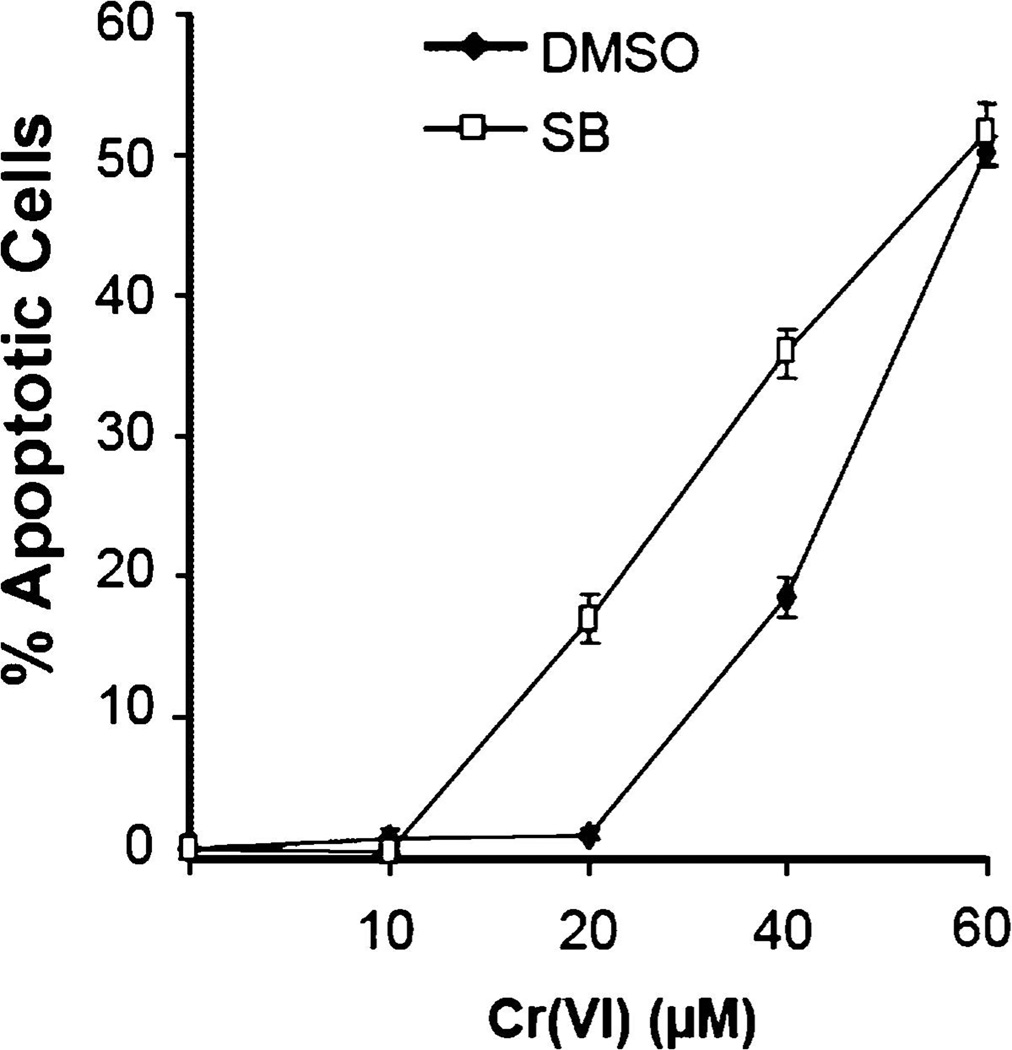
p38 pathways regulating apoptosis in response to extracellular signals have been extensively studied, and evidence exists to support both pro- and anti-apoptotic functions depending upon cell type and conditions [12]. However, there has been little focus placed on studying the role of p38 in apoptosis induced by DNA damage. In order to study the role of p38 in Cr(VI)-induced apoptosis, we exposed HeLa cells treated with or without SB203580 to 10, 20, 40, or 60µM doses of Cr(VI) for 24 h. After Cr(VI) exposure, apoptotic cells were identified by TUNEL assay. Cells with inhibited p38 function exhibited a significant increase in percentage of apoptotic cells at 20 and 40µM Cr(VI) concentrations compared to cells with normal p38 function (Fig. 4), suggesting a role for p38 in limiting Cr(VI)-induced apoptosis.
Fig. 4.
p38 protects cells from Cr(VI)-induced apoptosis. HeLa cells were exposed to 10, 20, 40, or 60µM doses of Cr(VI) for 24 h in the presence or absence of SB203580. TUNEL analysis was performed to determine apoptotic percentages in the experimental populations. Error bars = ±1 S.D., graphed is the mean of three independent experiments.
Discussion
Numerous proteins play important roles in the cellular response to Cr(VI), but due to the complexities of Cr(VI)-induced genotoxicity the exact mechanisms regulating that response remain poorly understood. Of particular importance are the pathways controlling the initiation of cell cycle checkpoints and apoptosis because these mechanisms are crucial for limiting mutation and carcinogenesis. In this study, we have clarified a role for p38 in the Cr(VI)-induced stress response by demonstrating that it is required for cell cycle checkpoint initiation, cell survival, and limiting apoptosis.
Previous studies have demonstrated that p38 is required for IR-induced G2 checkpoint and have implicated p38 in UV-induced G1, S, and G2 checkpoints. Here we have shown that p38 is required for Cr(VI)-induced S-phase and G2 checkpoints, indicating a crucial role for p38 in the Cr(VI)-induced cell cycle checkpoints. Although the results of this study shed light on p38’s role in Cr(VI)-induced cell cycle checkpoints, the upstream mechanisms regulating p38 function are unclear. It has been observed that ATM regulates p38 activation after IR and that this ATM-dependent activation is required for G2 checkpoint activation. Additionally, observations indicate requirements for both ATM and ATR in Cr(VI)-induced cell cycle checkpoints. Since Cr(VI) induces DNA damage known to trigger activation of both ATM and ATR, it will be interesting to examine whether these proteins regulate p38-dependent checkpoint activation after Cr(VI) exposure.
The role of p38 in cell survival and apoptosis has been extensively examined, but the results of these studies are inconsistent. Data indicate that p38-dependent pathways promote apoptosis and cell death, but it has also been reported that p38 signalling promotes cell survival and growth. These findings indicate a dual role for p38 in the cellular stress response that appears to be specific to the type of cell and apoptotic stimulus studied. This study focuses on the role of p38 in cell survival and apoptosis in response to Cr(VI) exposure. Our findings indicate that cells with functional p38 exhibited a reduction in cell death and apoptosis following Cr(VI) exposure compared to cells treated with p38 inhibitor. This indicates that p38 functions to promote cell survival in the face of Cr(VI)-induced stress. Because p38 is also required for cell cycle checkpoint activation following Cr(VI) treatment, it will be interesting to examine the role of upstream regulators of cell cycle checkpoint activation, such as ATM and ATR, in influencing p38-mediated cell survival.
In conclusion, the results of our study demonstrate that p38 is required for cell cycle checkpoint activation and cell survival in the face of Cr(VI)-induced stress. These findings provide valuable insight into mechanisms important in the cellular response to Cr(VI) and hint at new avenues of study into the mechanisms regulating the function of p38 in the DNA damage response.
Acknowledgments
The authors would like to thank all members in the Xu laboratory for their helpful comments in preparing the manuscript. This work was supported by NIH Grant ES013301-01 to B. Xu and a NIOSH Student Training Grant and a Cancer Association of Greater New Orleans Student Training Grant to T.P. Wakeman.
References
- 1.Ding M, Shi XL. Molecular mechanisms of Cr(VI)-induced carcinogenesis. Mol Cell Biochem. 2002;234–235:293–300. [PubMed] [Google Scholar]
- 2.Gaggelli E, Berti F, D’Amelio N, Gaggelli N, Valensin G, Bovalini L, Paffetti A, Trabalzini L. Metabolic pathways of carcinogenic chromium. Environ Health Perspect. 2002;110(Suppl 5):733–738. doi: 10.1289/ehp.02110s5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugden KD, Martin BD. Guanine and 7,8-dihydro-8-oxo-guanine-specific oxidation in DNA by chromium[V] Environ Health Perspect. 2002;110(Suppl 5):725–728. doi: 10.1289/ehp.02110s5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakeman TP, Kim WJ, Callens S, Chiu A, Brown KD, Xu B. TheATM-SMC1 pathway is essential for activation of the chromium (VI)-induced S-phase checkpoint. Mutat Res. 2004;554:241–251. doi: 10.1016/j.mrfmmm.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Shumilla JA, Broderick RJ, Wang Y, Barchowsky A. Chromium (VI) in-hibits the transcriptional activity of nuclear factor-kappaB by decreasing the interaction of p65 with cAMP-responsive element-binding protein-binding protein. J Biol Chem. 1999;274:36207–36212. doi: 10.1074/jbc.274.51.36207. [DOI] [PubMed] [Google Scholar]
- 6.Shi X, Ding M, Ye J, Wang S, Leonard SS, Zang L, Castranova V, Vallyathan V, Chiu A, Dalal N, Liu K. Cr(IV) causes activation of nuclear transcription factor-kappa B, DNA strand breaks and dG hydroxylation via free radical reactions. J Inorg Biochem. 1999;75:37–44. doi: 10.1016/S0162-0134(99)00030-6. [DOI] [PubMed] [Google Scholar]
- 7.Ha L, Ceryak S, Patierno SR. Chromium (VI) activates ataxia telangiectasia mutated (ATM) protein. Requirement of ATM for both apoptosis and recovery from terminal growth arrest. J Biol Chem. 2003;278:17885–17894. doi: 10.1074/jbc.M210560200. [DOI] [PubMed] [Google Scholar]
- 8.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 9.Seger R, Krebs EG. The MAPK signaling cascade. FASEBJ. 1995;9:726–735. [PubMed] [Google Scholar]
- 10.Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 11.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: timing is everything. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 12.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, McGowan CH, Zhao M, He L, Downey JS, Fearns C, Wang Y, Huang S, Han J. Involvement of the MKK6-p38gamma cascade in gamma-radiation-induced cell cycle arrest. Mol Cell Biol. 2000;20:454–4552. doi: 10.1128/mcb.20.13.4543-4552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manke IA, Nguyen A, Lim D, Stewart MQ, Elia AE, Yaffe MB. MAP-KAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol Cell. 2005;17:37–48. doi: 10.1016/j.molcel.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O, Appella E, Fornace AJ., Jr Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature. 2001;411:102–107. doi: 10.1038/35075107. [DOI] [PubMed] [Google Scholar]
- 16.Chuang SM, Liou GY, Yang JL. Activation of JNK, p38 and ERK mitogen-activated protein kinases by chromium(VI) is mediated through oxidative stress but does not affect cytotoxicity. Carcinogenesis. 2000;21:1491–1500. [PubMed] [Google Scholar]
- 17.Elledge SJ. Cell cycle checkpoints: Preventing and identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 18.Salmon RA, Foltz IN, Young PR, Schrader JW. The p38 mitogen-activated protein kinase is activated by ligation of the T or B lymphocyte antigen receptors, Fas or CD40, but suppression of kinase activity does not inhibit apoptosis induced by antigen receptors. J Immunol. 1997;159:5309–5317. [PubMed] [Google Scholar]
- 19.Zhang J, Gao JX, Salojin K, Shao Q, Grattan M, Meagher C, Laird DW, Delovitch TL. Regulation of fas ligand expression during activation-induced cell death in T cells by p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase. J Exp Med. 2000;191:1017–1030. doi: 10.1084/jem.191.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu B, Kim ST, Kastan MB. Involvement of Brca1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol Cell Biol. 2001;21:3445–3450. doi: 10.1128/MCB.21.10.3445-3450.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Fang M, Lu Y, Lu Y, Mills GB, Fan Z. Involvement of JNK-mediated pathway in EGF-mediated protection against paclitaxel-induced apoptosis in SiHa human cervical cancer cells. Br J Cancer. 2001;85:303–311. doi: 10.1054/bjoc.2001.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JG, Yuk Y, Rhim H, Yi SY, Yoo YS. Role of p38 MAPK in the regulation of apoptosis signaling induced by TNF-alpha in differentiated PC12 cells. J Biochem Mol Biol. 2002;35:267–272. doi: 10.5483/bmbrep.2002.35.3.267. [DOI] [PubMed] [Google Scholar]