Abstract
Perfluorocarbon (PFC) emulsions can transport and release various gases based on concentration gradients. The objective of this study was to determine the possibility of carrying and delivering exogenous nitric oxide (NO) into the circulation by simply loading PFC emulsion with NO prior infusion. PFC was equilibrated with room air (PFC) or 300 ppm NO (PFC-NO) at atmospheric pressure. Isotonic saline solution was used as a volume control (Saline). PFC and PFC-NO were infused at a dose of 3.5 mL/kg in the hamster window chamber model. Blood chemistry, and systemic and microvascular hemodynamic response were measured. Infusion of PFC preloaded with NO reduced blood pressure, induced microvascular vasodilation and increased capillary perfusion; although these changes lasted less than 30 min post infusion. On the other hand, infusion of PFC (without NO) produced vasoconstriction; however, the vasoconstriction was followed by vasodilatation at 30 min post infusion. Plasma nitrite and nitrate increased 15 min after infusion of NO preloaded PFC compared to PFC, 60 min after infusion nitrite and nitrate were not different, and 90 min after infusion plasma S-nitrosothiols increased in both groups. Infusion of NO preloaded PFC resulted in acute vascular relaxation, where as infusion of PFC (without NO) produced vasoconstriction, potentially due to NO sequestration by the PFC micelles. The late effects of PFC infusion are due to NO redistribution and plasma S-nitrosothiols. Gas solubility in PFC can provide a tool to modulate plasma vasoactive NO forms availability and improve microcirculatory function and promote increased blood flow.
Keywords: Nitrite, S-nitrosothiols, endothelium, microcirculation, gas transport, vasoactive NO forms
Introduction
Perfluorocarbons (PFC) are colourless liquids, and chemically inert synthetic molecules that consist primarily of carbon and fluorine atoms.1, 2 They can dissolve gases including oxygen, carbon dioxide, nitric oxide (NO), etc.3 PFCs are hydrophobic and not miscible with water. Thus, they have to be emulsified for intravenous use by using a surface active lipid. Stable perfluorocarbon emulsions with small droplet size (0.2 μm) can be prepared with a simple technology. Their intravascular half-life depends on their molecular weight (MW), varying from hours for low MW to days for high MW.4 They are eliminated unchanged by the lungs after passing through the reticuloendothelial system.4
Under normal conditions NO is produced by various types of cells. Endothelium-derived NO plays a central role in vascular physiology, as a potent vasodilator molecule it maintains basal vasomotor tone, mediates flow dependent vasodilation, inhibits platelet aggregation and endothelium adhesion molecule expression.5 However, NO's ability to be transported by blood is limited by NO reactions with oxygen, including NO dioxygenation reaction with oxy-hemoglobin (oxy-Hb), which forms nitrate (NO3−, inert) and methemoglobin (met-Hb).6 In vitro, the reaction rate in aqueous solutions of NO with oxygen and Hb follows a second-order kinetics, thus the rate of NO disappearance is proportional to the square of NO concentration.7 At high concentrations (300 μM) in an aqueous solution, NO has been reported to have an intravascular half-life of less than 1 second.8 While at lower concentrations within the physiological range (0.01–1 μM), NO intravascular half-life has been reported to be up to 500 s.8 Despite the rapid kinetics of the NO dioxygenation reaction, the longer intravascular half-life at physiological concentrations has been explained by the compartmentalization of Hb within the erythrocyte. This compartmentalization reduces NO inactivation reaction rate by oxy-Hb by 1000-fold, thus allowing for NO to regulate vascular tone despite the presence of red blood cells (RBC) in the intravascular space.9 On the other hand, hemolysis will disrupt NO physiology signalling, as NO will be rapidly scavenged by acellular Hb producing vasoconstriction.6
NO preferential partitioning into lipid membranes is an important regulator of its physiological effects.10 NO solubility in lipid membranes is 8 times that in water, whereas for oxygen is only 3 fold that in water. Therefore, NO gas can be preloaded in PFC emulsions and intravascularly administered to deliver NO throughout the cardiovascular system. Additionally, infusion of NO loaded PFC can accelerate the formation of bioactive (nitrite and S-nitrosothiols) and bioinactive (nitrate) NO forms within the PFC micelle, as oxygen concentration in the micelle equilibrates with intravascular oxygen concentrations.11 The present study was designed to explore systemic and peripheral vascular effects of the infusion of a NO preloaded PFC emulsion. To accomplish this objective, this study compares the systemic and microvascular hemodynamic response in a hamster window chamber model to a small hypervolemic volume infusion of PFC equilibrated with NO gas (300 ppm balance nitrogen) over 90 min after infusion. NO preloaded PFC response was compared to that of room air equilibrated PFC and saline (0.9% NaCl) without NO at an identical infusion volumes.
Methods
PFC emulsion
The PFC emulsion is 58% weight/volume (%w/v) of perfluorooctyl bromide (CF3(CF2)7Br) (Exfluor Research Corporation®, Round Rock, TX), soybean lecithin Epikuron 170, (Cargill Company®, Hamburg, Germany) as an emulsifying surfactant, (+)-α-tocopherol (Sigma Aldrich®, St. Louis, MO) as antioxidant. Dextrose 50% for osmolality equilibration, and alginic acid sodium salt solution (Sigma Aldrich®, St. Louis, MO) as viscosity agent. Briefly, PFC, soybean lecithin and the continuous phase were first homogenized (Heidolph SilentCrusher M®, Schwabach, Germany) and then emulsified (Microfluidizer M-110Y, Microfluidics Corp ®, Newton, MA) until the mean particle size was below 250 nm. The PFC emulsion was heat sterilized at 100 °C for 40 minutes and then stored at 4 °C. Some physicochemical properties of the PFC emulsion are: i) Osmolality 252 ±7 mOsm/kg H2O; ii) Mean particle size: 234±14 nm; and iii) pH: 7.34 ± 0.03
NO equilibrated PFC
PFC emulsion was degassed by bubbling pure nitrogen for 4 hours at room temperature, cold on ice for 20 min (∼0 °C), and then equilibrated with NO gas (300 ppm, 583 10-9 molar at 25°C, purified from higher oxides by passing it through a 1 M KOH solution) for 15 min. All solutions were equilibrated immediately before in vivo use.
NO and O2 partitioning Coefficient
Water (Milli-Q grade) was deoxygenated by boiling and cooling under pure nitrogen. NO equilibrated water solution was prepared in an airtight container by bubbling NO gas (300 ppm; AIRGAS, San Diego, CA), purified from higher oxides by passage through a 1 M KOH solution until the concentration reached equilibrium. NO partition coefficient for PFC was measured using the changes in NO concentrations in the NO equilibrated water (10 mL) after adding different volumes of deoxygenated PFC in the presence of nitrogen in the head space. The NO concentration was measured continuously using an ISO-NO Mark II NO electrode (World Precision Instruments, Sarasota, FL). Oxygen partition coefficient for PFC was measured by adding different volumes of deoxygenated PFC to 10 mL of water equilibrated at atmospheric O2. The O2 concentrations were measured with a 5331 oxygen electrode (YSI, Yellow Springs, OH).
Total NO in the PFC
Gaseous NO levels were measured using a Sievers 280i Chemiluminescence NO Analyzer. Briefly, 200 mL of PFC-NO were added to Milli-Q grade water (5 ml), pH was balanced using Trizma Base and Trizma HCI (Sigma-Aldrich). Solutions were continuously bubbled with pure nitrogen (200 mL/min) and gas phase was analyzed by the NO analyzer.
Animal Preparation
In vivo experiments were performed using golden Syrian hamsters. Animal handling and care were provided following the procedures outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). The study was approved by the local Animal Committee. The hamster window chamber model is widely used for microvascular studies, complete surgical technique is described in detail elsewhere.12 Two days after window chamber implantation, the animals were anesthetized and implanted with arterial (carotid artery) and venous (jugular vein) catheters (PE-50) for monitoring and infusion of test solutions. The experiments were performed one or two days after catheter implantation without the use of anaesthetics.
Inclusion Criteria
Animals were considered suitable for the experiments if: 1) systemic parameters were within normal range, namely, heart rate (HR) > 340 beat/min, mean arterial blood pressure (MAP) > 80 mmHg, systemic hematocrit (Hct) > 45%, and arterial oxygen partial pressure (PaO2) > 50 mmHg13; and, 2) microscopic examination of the tissue in the window chamber observed under ×650 magnification did not reveal signs of edema or bleeding.
Systemic Parameters and Blood Chemistry
MAP and heart rate (HR) were recorded continuously (MP 150, Biopac System; Santa Barbara, CA). Hematocrit (Hct) was measured by centrifugation. Hemoglobin (Hb) was determined spectrophotometrically (B-Hemoglobin, Hemocue, Stockholm, Sweden). Met-Hb level was measured via the cyanomethemoglobin method.14 Arterial blood gases were measured using blood chemistry analyzer (248, Bayer, Norwood, MA).
Microhemodynamics
Arteriolar and venular blood flow velocities were measured on-line using the photodiode cross-correlation method (Photo Diode/Velocity Tracker Model 102B, Vista Electronics, San Diego, CA). The measured centerline velocity (V) was corrected according to vessel size to obtain the mean RBC velocity.15 A video image-shearing method was used to measure vessel diameter (D). Blood flow (Q) was calculated from the measured values as Q = π × V (D/2)2. This calculation assumes a parabolic velocity profile and has been found to be applicable to tubes of 15 - 80 μm internal diameters and for Hcts in the range of 6 - 60%.15
Functional Capillary Density (FCD)
Functional capillaries are defined as those capillary segments with RBC transit of at least a single cell in a 45s period. Each field had between two and five capillary segments with RBC flow. FCD (cm-1), i.e., was estimated by measuring and adding the length of capillaries with RBC transit in the field of view, and dividing it by the area of the observed field. The relative change in FCD from baseline levels after each intervention is an indicative of the extent of capillary perfusion.
Plasma nitrite/nitrate
Blood samples were collected from the carotid artery catheter and centrifuged to separate RBCs and plasma. Plasma proteins were removed by adding equivolume of methanol, and centrifuging at 15000 rpm for 10 min at 4°C. Concentration of nitrite and nitrate in the supernatant were measured with a NO× analyzer (ENO-20; Eicom, Kyoto, Japan). This analyzer combines the Griess method and high-performance liquid chromatography.
Chemiluminescent plasma S-nitrosothiols
Gas phase NO release from S-nitrosothiols was measured using a Sievers 280i Chemiluminescence NO Analyzer (GE Healthcare, Boulder, CO). Calibration was performed with air passed through a Sievers NO zero filter and 25 ppm NO (balance N2) gas. Arterial blood samples were centrifuged to separate RBCs and plasma. A Copper(I) and cysteine (Cu(I)/Cys) solution (1 mM l-cysteine; 100 μM cuprous chloride in 400 ml distilled water), prepared the day of the experiment and adjusted to pH 6.5 with sodium hydroxide, was used to chelate S-nitroso groups.16 The solution was placed in a water-jacketed purge vessel and maintained at 50°C using a circulating water bath. Then, 200 μL of plasma were injected sparged with 50 mL/min of nitrogen. Additional, pure nitrogen was supplied to the reaction vessel to match the instrument collection rate (200 mL/min). Plasma S-nitrosothiols were only measured at the end of the experimental protocol.
Microvascular Experimental Setup
The unanesthetized animal was placed in a restraining tube with a longitudinal slit from which the window chamber protruded, then fixed to the microscopic stage of a transillumination intravital microscope (BX51WI, Olympus, New Hyde Park, NY). The animals were given 20 min to adjust to the tube environment before any measurement was performed. The tissue image was projected onto a CCD camera (COHU 4815) connected to a video recorder and viewed on a monitor. Measurements were carried out using a 40× (LUMPFL-WIR, numerical aperture 0.8, Olympus) water immersion objective. Microhemodynamic measurements were compared to baseline levels obtained before the experimental procedure. The same vessels and functional capillary fields were followed throughout the experiment so that direct comparisons to their baseline levels could be performed allowing for more robust statistics for small sample populations.
Experimental groups
Animals were randomly divided into three experimental groups. Groups were labelled based on the test material: PFC-NO (PFC equilibrated with 300 ppm of NO gas balanced nitrogen), PFC (PFC equilibrated with room air), and Saline (0.9% NaCl, equilibrated with room air).
Experimental protocol
Animals were characterized at baseline. Then, they were infused a volume of 3 mL/kg (equivalent to 5% of the animal's blood volume) of test solution (defined in the experimental groups) via the jugular vein catheter at a rate of 0.1 mL/min. Animals were observed for 90 min after infusion of test solution. At the end of this observation time, blood was collected for viscosity and plasma S-nitrosthiols measurements.
Experimental protocol during NO synthase inhibition
NO synthase inhibition was induced by continuous i.v infusion of 20mg/kg per hour of L-NAME (L-NG-Nitroarginine methyl ester, Cayman Chemical, Ann Arbor, MI) dissolved in saline. L-NAME infusion was maintained over the entire protocol. After 20 min from the beginning of L-NAME infusion and when stable blood pressure effect was obtained, animals were infused with PFC-NO (PFC equilibrated with 300 ppm of NO gas balanced nitrogen), PFC (PFC equilibrated with room air) via the jugular vein at a rate of 0.1 mL/min. L-NAME infusion was only stopped during test solution injection.
Data analysis
Results are presented as mean ± standard deviation. Data within each group were analyzed using analysis of variance for repeated measurements (ANOVA, Kruskal-Wallis test). When appropriate, post hoc analyses were performed with the Dunns multiple comparison test. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, Inc., San Diego, CA). Changes were considered statistically significant if P < 0.05.
Results
PFC emulsion partition coefficient
The NO partition coefficient for PFCs was calculated by the change in NO concentration in the aqueous phase (563 ± 22 nM) after addition of 0.1, 0.5 and 1 mL of deoxygenated PFC (558 ± 24; 487 ± 19; 454 ± 11 nM) or 0.1, 0.5 and 1 mL of deoxygenated water (548 ± 10; 553 ± 17; 533 ± 8 nM). PFC NO partition coefficient was calculated as the ratio of the equilibrium concentrations between the aqueous and PFC phases. PFC NO partition coefficient indicates that NO concentration in the PFC micelle is 2.4 ± 0.3 times higher than in an aqueous solution. Oxygen partition coefficient for PFCs was calculated, based on the changes in concentration in the aqueous phase (224 ± 14 μM) after addition of 0.1, 0.5 and 1 mL of deoxygenated PFC (223 ± 6; 210 ± 9; 189 ± 5 μM) or 0.1, 0.5 and 1 mL of deoxygenated water (222 ± 4; 213 ± 7; 206 ± 8 μM). PFC oxygen partition coefficient is 1.6 ± 0.2 times higher than in an aqueous solution. NO solubility in PFC was 11.6 times the solubility of oxygen in PFC.
In vivo results
All animals tolerated the hypervolemic infusion and entire protocol without visible signs of discomfort. The animals were randomly assigned to the experimental groups as follows: Saline (n = 5; 68 ± 5 g); PFC (n = 5; 66 ± 7 g); PFC-NO (n = 5; 63 ± 6 g). Six additional animals (66 ± 5 g) were used to obtain reference values for plasma NO forms (nitrite, nitrate, S-nitrosothiols), plasma COP and blood rheology.
Systemic Parameters
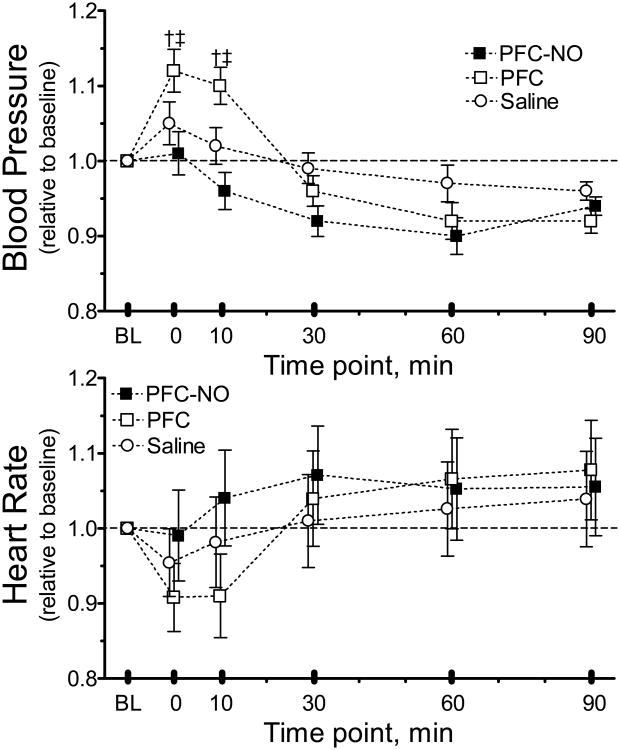
The infusion of any solution did not significantly reduce Hct or Hb. All systemic parameters remained in the physiological ranges. MAP was statistically significantly increased from baseline 10 min after the infusion for the PFC group, whereas infusion of PFC-NO decreased MAP (Figure 1.). Systemic parameters before and after infusion are presented in Table 1.
Figure 1. Changes in mean arterial blood pressure and heart rate.
PFC-NO, PFC preloaded with NO; (solid square); PFC, PFC without NO (hollow square); and Saline, volume control without NO (hollow circle). †, P<0.05 compared to saline. ‡, P<0.05 compared to PFC.
Table 1.
Systemic hemodynamics and blood chemistry.
| Control (n = 6) | Baseline (n = 15) | 1h After infusion | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Saline (n = 5) | PFC-NO (n = 5) | PFC (n = 5) | ||||
| Systemic Hemodynamics | ||||||
| MAP | mmHg | 104 ± 7 | 105 ± 5 | 103 ± 5 | 98 ± 6 | 95 ± 5 |
| HR | bpm | 424 ± 30 | 428 ± 22 | 438 ± 34 | 428 ± 26 | 416 ± 28 |
|
| ||||||
| Laboratory Parameters | ||||||
| Arterial pH | 7.36 ± 0.02 | 7.36 ± 0.01 | 7.35 ± 0.03 | 7.37 ± 0.02 | 7.36 ± 0.03 ' | |
| PaO2 | mmHg | 55.8 ± 5.3 | 57.6 ± 4.8 | 56.3 ± 4.6 | 59.3 ± 5.4 | 58.8 ± 4.7 |
| PaCO2 | mmHg | 49.4 ± 5.0 | 50.2 ± 4.8 | 51.4 ± 5.0 | 51.9 ± 4.9 | 53.9 ± 4.6 |
| Hct | % | 49.3 ± 1.8 | 48.8 ± 0.8 | 49.0 ± 1.3 | 48.1 ± 1.4 | 48.2 ± 1.5 |
| Hb | g/dL | 14.7 ± 0.6 | 14.8 ± 0.3 | 14.8 ± 0.6 | 14.8 ± 0.7 | 14.4 ± 0.8 |
| Fct | % | - - | - - | - - | 1.7 ± 0.5 + | 1.6 ± 0.4 + |
| MetHb | % | - - | - - | - - | 2.6 ± 1.0 | - - |
|
| ||||||
| Blood Rheology and COP | ||||||
| Viscosity Blood | cP | 4.2 ± 0.2 | 4.2 ± 0.3 | 4.3 ± 0.3 | 4.4 ± 0.3 | |
| Plasma Viscsity | cP | 1.2 ± 0.1 | 1.2 ± 0.2 | 1.4 ± 0.3 | 1.4 ± 0.2 | |
| COP | mmHg | 17.8 ± 0.7 | 18.2 ± 0.5 | 17.6 ± 0.5 | 17.4 ±0.6 | |
Values are means ± SD. Baseline included three experimental groups. No significant differences were detected between the baseline values of each group. Hct, systemic hematocrit; Hb, hemoglobin content of blood; MAP, mean arterial blood pressure; PaO2, arterial partial O2 pressure; PaCO2, arterial partial pressure of CO2; Fct, fluorocrit; MetHb, methemoglobin.
P<0.05 compared to baseline;
P<0.05 compared to PFC.
Microhemodynamics
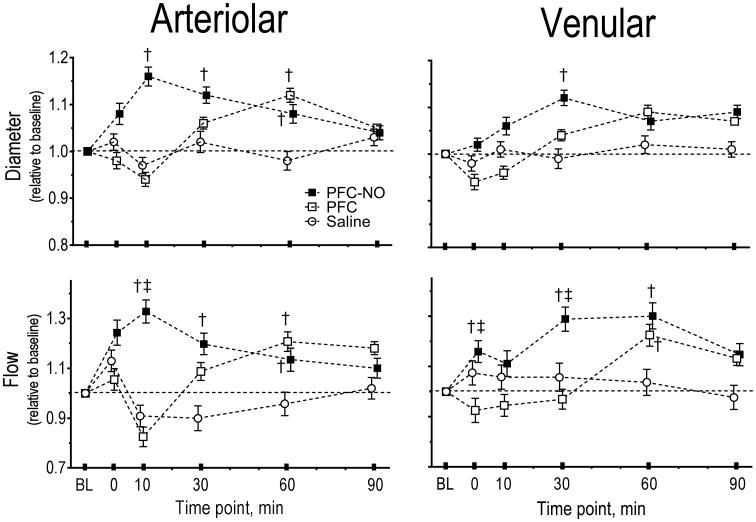
The changes in diameter and blood flow velocity for large feeding and small arterioles (range 45 - 75 μm) and small collecting venules (range 50 - 76 μm) were measured. The arteriolar and venular microhemodynamics parameters, including calculated volumetric blood flow rate are presented in Figure 2, for all the groups. The arteriolar diameter increased after infusion of PFC-NO, however, the vasodilation disappeared over time. Infusion of PFC, initially produced vasoconstriction and then produced vasodilation especially in arterioles. Saline infusion did not produce any significant changes in blood vessel diameter. Changes in the venular side were only significant 30 min after infusion for the PFC-NO group relative to baseline but not to the other groups. Arterioles have significant amounts of smooth muscle and rapidly responded to the changes in NO availability produced by the PFC, thus supporting the idea that the systemic and microvascular hemodynamics responses observed are due to release or uptake of NO by the PFC in circulation.
Figure 2. Microvascular vessel diameter and blood flow after infusion of PFC preloaded with NO.
PFC-NO, PFC preloaded with NO; (solid square); PFC, PFC without NO (hollow square); and Saline, volume control without NO (hollow circle). †, P<0.05 compared to saline. ‡, P<0.05 compared to PFC. Diameters (μm, mean ± SD) at baseline (BL) in each animal group were as follows: PFC (arterioles (A): 59.2 ± 6.3, n = 34; venules (V): 62.5 ± 7.3, n = 28). PFC-NO (A: 57.6 ± 5.8, n = 32; V: 62.6 ± 6.5, n = 38). Saline (A: 56.4 ± 7.1, n = 33, V: 65.0 ± 7.3, n = 32). n = number of vessels studied. RBC velocities (mm/s, mean ± SD) at baseline in each animal group were as follows: PFC (A: 4.4 ± 1.2; V: 2.3 ± 0.8); PFC-NO (A: 4.2 ± 0.9; V: 2.4 ± 0.9); Saline (A: 4.5 ± 0.7; V: 2.1 ± 0.8). Flow (nl/s, mean ± SD) at baseline in each animal group were as follows: PFC (A: 12.2 ± 2.8; V: 6.7 ± 2.4); PFC-NO (A: 11.7 ± 3.0, V: 7.3 ± 2.6); Saline (A: 11.4 ± 3.2; V: 7.1 ± 2.8).
Capillary Perfusion
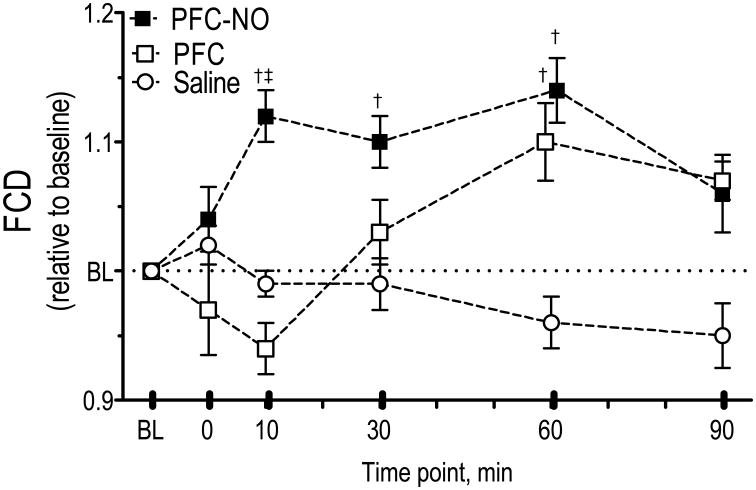
FCD for all groups is presented in Figure 3. FCD increased from baseline for the PFC-NO group at 10, 30 and 60 min after infusion. The PFC group showed and initial decrease in capillary perfusion and then it presented an increase compared to baseline at 60 min after infusion. Saline did not significantly change FCD over the entire observation time.
Figure 3. Functional capillary density (FCD) after infusion of PFC preloaded with NO.
PFC-NO, PFC preloaded with NO; (solid square); PFC, PFC without NO (hollow square); and Saline, volume control without NO (hollow circle). †, P<0.05 compared to saline. ‡, P<0.05 compared to PFC. FCD (cm-1) at baseline for each group were as follows: PFC (107 ± 11); PFC-NO (112 ± 10); and Saline (102 ± 7).
Plasma NO forms
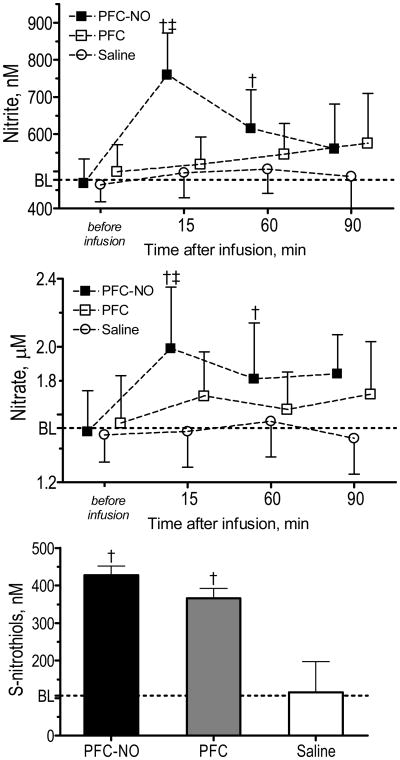
Plasma bioactive (nitrite and S-nitrosothiols) and bioinactive (nitrate) NO forms concentration are presented in Figure 4. Untreated hamster plasma nitrite and nitrate concentrations were 477 ± 63 nM and 1.52 ± 0.24 μM, respectively. Plasma nitrite and nitrate significantly increased 15 min after infusion of PFC-NO compared to PFC and Saline, and then they decreased over time. Plasma nitrite and nitrate slowly increased in the PFC group, to be no different compared to Saline or baseline. Plasma S-nitrosothiols concentration measured at the end of the experiment were higher for PFC-NO and PFC compared to Saline. Untreated hamster plasma S-nitrosothiols was determined to be 107 ± 25 nM.
Figure 4. Plasma levels of nitrite, nitrate and S-nitrosothiols.
Baseline (BL) levels values were obtained from a different group of animals (n = 6). PFC-NO, PFC preloaded with NO; (solid square); PFC, PFC without NO (hollow square); and Saline, volume control without NO (hollow circle). †, P<0.05 compared to saline. ‡, P<0.05 compared to PFC. S-nitrosothiols were only measured at the end of the experiments. †, P<0.05 compared to saline. ‡, P<0.05 compared to PFC.
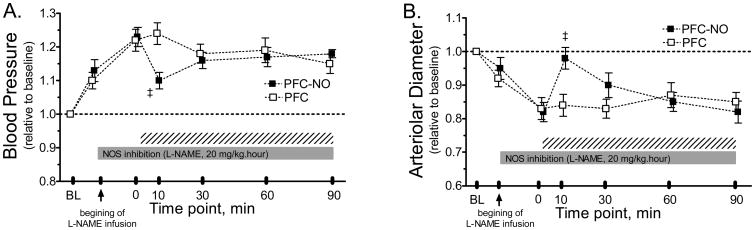
PFC effects during NO synthase inhibition
Six animals were randomly assigned to the experimental groups as follows: PFC (n = 3; 62 ± 4 g); PFC-NO (n = 3; 65 ± 7 g). MAP was statistically increased from baseline by NO synthase inhibition, infusion of PFC-NO statistically decreased MAP compared to the group treated with PFC (Figure 5A). Changes in arteriolar diameter during NO synthase inhibition and the effects of PFC and PFC-NO infusion are presented in Figure 5B. NO synthase inhibition induced vasoconstriction. Infusion of PFC-NO produced significant vasodilation compared to infusion of PFC, although the effects of PFC-NO only lasted for less than 30 min.
Figure 5. Changes in mean arterial blood pressure microvascular vessel diameter during NO synthase inhibition after infusion of PFC preloaded with NO.
PFC-NO, PFC preloaded with NO; (solid square) and PFC, PFC without NO (hollow square). The solid horizontal bar indicated the perdios of L-Name infusion and the hashed bar indicates the time after the hypervolemic infusion of the test solutions. ‡, P<0.05 compared to PFC. L-NAME was infused at 20 mg/kg per hour over the entire observation period. PFC with or without NO were infused when L-NAME induced an steady state (∼20 min). Mean arterial pressure (MAP, mmHg, mean ± SD) at baseline (BL) in each animal group were as follows: PFC (109 ± 7, n = 3) and PFC-NO (A: 107 ± 8, n = 3). Arterioles diameters (μm, mean ± SD) at baseline (BL) in each animal group were as follows: PFC ((56.8 ± 7.1, n = 14) and PFC-NO (59.1 ± 6.2, n = 12).
Discussion
The principal finding of the study is that the infusion of a PFC emulsion (3 mL/kg, 1 g/kg) equilibrated with NO at atmospheric pressure causes vascular relaxation, increased blood flow and decreased vascular hindrance for 30 min after administration. Whereas infusion of a PFC emulsion alone (without NO) produced acute vasoconstriction and decreased blood flow in the short term. However, these changes were transient and both groups infused with PFC (with or without NO) presented similar hemodynamics 90 min after infusion. PFC-induced vasoconstriction was not due to osmotic changes or volume effects (hypervolemia) since PFC had physiological osmolarity and the rate of infusion was slow. PFC infusion during NO synthase inhibition did not affected blood pressure or arteriolar diameter, confirming that initial vasoactive effects of PFC are related to NO. In addition, PFC with NO infusion during NO synthase inhibition validates that PFC can be a tool to deliver NO to the vasculature. Bioactive forms of NO (nitrite and S-nitrosothiols) are part of long term PFC induced vascular changes. Plasma nitrite increased after infusion of PFC-NO and S-nitrosothiols increased in all animals treated with PFC. In summary, the initial vasoactivity (relaxation) of PFC-NO is due to NO release or an increase in plasma nitrite concentrations, while the PFC vasoactivity (constriction) is due to capturing of NO by PFC micelles. Then, the initial NO release and capture processes, are followed by hemodynamic responses due to NO bioactive forms (nitrite and S-nitrosothiols) redistributing the effects of NO throughout different regions of the cardiovascular system.
PFCs use as intravascular oxygen carriers, has suggested several advantageous properties in the microcirculation.18-20 However, these studies dealt with conditions of extreme hemodilution, hemorrhagic shock, and 100% oxygen inhalation. Therefore, the subtle effects due to changes in NO levels along the circulation might have been masked. PFCs are well tolerated in animals and humans, but their pulmonary and systemic hemodynamic effects have limited their clinical use.21 At high doses, PFC use has been associated with hypertension and decreased heart rate 22; in addition to beneficial anti-inflammatory and antithrombotic effects 19, 20, which were solely associated to the PFC ability to transport oxygen. This study shows that PFC are vasoactive in a bimodal way (Figure 6), an initial vasoconstriction attributed to NO sequestering by the PFC followed by vasodilation caused by NO redistribution, and that PFC can transport NO from zones with high NO to zones low NO, as well as to promote the generation of nitrite and S-nitrosothiols.
Figure 6. Mechanism of NO regulation by PFC emulsion.
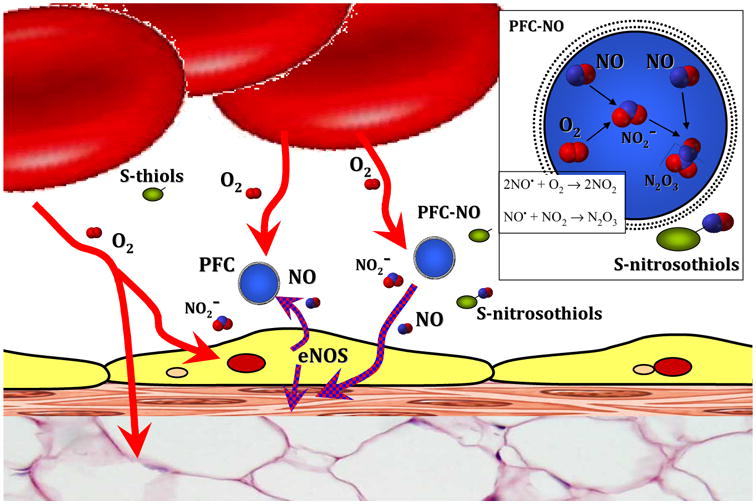
PFC-NO transported NO in a bimodal way, 1) PFC-NO releases NO (relaxing smooth muscle and produces vasodilation) and 2) PFC traps the NO produced by endothelial cells throw endothelial NO synthase (eNOS) (preventing relaxation of smooth muscle and produces vasoconstriction). Sequestration of NO by PFC in the presence of oxygen, facilitates the reactions of NO with oxygen, which results in the formation of dinitrogen trioxide (N2O3), a strong thiol nitrosating agent that increases S-nitrosothiols.
Sequestration of NO by PFC differs from hemoglobin scavenging of NO, because PFC preserves NO vasoactivity in other forms, such as nitrite and S-nitrosothiols, while hemoglobin scavenging of NO produces nitrate and methemoglobin. Increased levels of nitrite and S-nitrosothiols induced systemic hemodynamic effects in conduit and resistance vessels and the reduction of circulating nitrite to NO alone is an important physiological reaction aimed to increase vasodilation during hypoxia.23, 24 Increased NO reaction products in plasma, nitrite and nitrate, can be recycled in blood and tissues to form again NO and other bioactive nitrogen oxides with effects in vascular homestasis.25 The increase in S-nitrosothiols by PFC and PFC-NO infusion can be due to the production of nitrated lipid derivatives, which later react with thiols.26 In addition, NO reacts readily with oxygen forming dinitrogen trioxide (N2O3), in a process called as NO autoxidation.27 N2O3 is decomposed rapidly to nitrosonium ion (NO+) and nitrite, the nitrosonium ion is responsible for the nitrosylation of thiols.27 Inside the perfluorocarbon micelle a high concentration of NO and oxygen leads to NO autoxidation and formation of N2O3 (Figure 6).11 The rate of autoxidation is mainly dependent on the concentrations of NO and oxygen, and it is dramatically accelerated within lipid membranes.27 Therefore, oxygen and NO levels are interestingly link to the mechanisms associated with PFC vasoactivity, in terms of generation of bioactive NO and their vasoactivity. The rate of formation of N2O3 after infusion of PFC without NO is high at the site of NO synthesis, as physiological concentrations of oxygen are higher than NO in the circulation while, the infusion of NO preloaded PFC favors the formation of N2O3 in areas different than those of high enough NO synthesis. In support of this concept, physiological levels of plasma S-nitrosothiols were increased by the presence of PFC in circulation independent of the NO preload.
The vasoactivity of PFC generated NO bioactive forms dependes on oxygen levels, as nitrite reductase activity depends on erythrocytic oxyhemoglobin saturation.28 The rate of nitrite reduction to NO by hemoglobin is determined by a balance between hemoglobin hemes in the oxygenated and dexoxygenated conformation. Thus resulting in a maximal nitrite reduction rate when hemoglobin saturation is near 50% (near the Hb P50), an ideal set point for hypoxia-responsive NO generation.28 S-nitrosothiols release of NO is variable and dependent on temperature, pH, the presence of transition metals, and the presence of oxygen and reactive oxygen species.29 This study was performed in hamsters which are fossorials animals with low systemic arterial oxygen tension compared to other rodents. Their low arterial oxygen concentration and low oxygen saturation may favour central vasodilatory responses and the infusion of PFC may exacerbate this condition. Further experimentation is required to test this hypothesis, as well as experimentation with non-fossorial species to evaluate if systemic changes with NO preloaded PFC depend on arterial oxygen content. However, the responses observed are expected to be preserved in the microcirculation across species, since the oxygen tensions in the hamster window chamber model are similar to the oxygen tensions in the mice window chamber model.30
PFC captures NO and oxygen from the aqueous phase and accelerates NO oxidation to nitrite, nitrate and S-nitrosothiols (Figure 4). According to the theory of micellar catalysis, the rate of NO oxidation and S-nitrosothiols formation depends on the volume of the hydrophobic phase. Larger amounts of PFC could be progressively less effective producing S-nitrosothiols 11. Notably, the administration of a large dose of PFC caused extensive vasoconstriction, reduction of erythrocyte velocity in postcapillary venules and increased venular leukocyte sticking, which is probably due to rapid sequestration of NO.31 Conversely, a small dose of PFCs, as used in the present study, induced long-lasting vascular relaxation.
Clinically, NO gas is used as an inhalable drug. However, inhaled NO increases MetHb levels.32 MetHb does not transport oxygen and is potentially cytotoxic due to heme and heme-derived iron oxidative reaction.32 Inhaled NO therapy works for limited periods and in controls scenarios, but its cost and potential toxicity have generated interest in alternatives. Synthetic agents that release NO spontaneously or after enzymatic cleavage have been proposed.33 Few NO releasing molecules (NO donors) are clinically used, with the exception of nitroglycerine. PFC can be loaded with a finite amount of NO, and it can transport and release NO exerting vasoactive effects, preventing toxicity due to over exposure by inhalation or NO donors. The current limitations of NO delivery systems have stimulated an extraordinary interest in the development of compounds that delivery NO in a controlled and sustained manner. We have developed and evaluated NO releasing nanoparticles based on hydrogel matrix that when exposed to moisture slowly releases therapeutic levels of NO, previously trapped within the dry particles.34 Based on the observed physiological effects of PFC in circulation, it will be interesting to explore the therapeutic potential of NO releasing nanoparticles and PFC together, since the nanoparticles can increase circulating NO levels and the PFC can promote the formation of bioactive NO forms, via N2O3, nitrite and S-nitrosothiols. Future studies with NO preloaded PFC should explore other signalling pathways were NO is involved including coagulation and vascular inflammation.
The use of NO loaded PFCs could have an important therapeutic use to treat temporal alterations in NO signalling to restore microvascular function, prevent organ damage and resolve acute disruption vascular homeostasis.2 For example, PFC micelles are approximately 30 times smaller than erythrocytes, NO preloaded micelles will reach critically ischemic tissue flowing through stenotic vessels where only plasma flow is maintained and promoting local vasodilation and increased blood flow. In summary, the present study shows that PFC emulsions act as NO carriers and releasers with acute direct beneficial effects on microvascular function. Our study confirms the possible use of PFC as plasma bioactive NO forms regulator and presents some of the systemic and microvascular hemodynamic response of PFC infusion. NO preloaded PFC and PFC (without NO) can provide a pharmacological approach to regulate NO bioactivity in the circulation.
Acknowledgments
This investigation was supported by the following grants: NIH R01 HL52684, R01 HL064395 and R01 HL062318; ARMY: W81XWH-11-2-0012 and COLCIENCIAS: 237-210. The author thanks Froilan P. Barra and Cynthia Walser for the surgical preparation of the animals.
Footnotes
Disclaimer: Author declares no competing financial interests by the results presented in this manuscript.
References
- 1.Clark LC, Jr, Becattini F, Kaplan S, Obrock V, Cohen D, Becker C. Perfluorocarbons having a short dwell time in the liver. Science. 1973;181(4100):680–2. doi: 10.1126/science.181.4100.680. [DOI] [PubMed] [Google Scholar]
- 2.Spiess BD. Perfluorocarbon emulsions as a promising technology: a review of tissue and vascular gas dynamics. J Appl Physiol. 2009;106(4):1444–52. doi: 10.1152/japplphysiol.90995.2008. [DOI] [PubMed] [Google Scholar]
- 3.Clark MC, Weiman DS, Pate JW, Gir S. Perfluorocarbons: future clinical possibilities. J Invest Surg. 1997;10(6):357–65. doi: 10.3109/08941939709099599. [DOI] [PubMed] [Google Scholar]
- 4.Lowe KC. Perfluorocarbons as oxygen-transport fluids. Comp Biochem Physiol A Comp Physiol. 1987;87(4):825–38. doi: 10.1016/0300-9629(87)90001-6. [DOI] [PubMed] [Google Scholar]
- 5.Kvietys PR, Granger DN. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med. 2012;52(3):556–92. doi: 10.1016/j.freeradbiomed.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 7.Ignarro LJ. Nitric oxide-mediated vasorelaxation. Thromb Haemost. 1993;70(1):148–51. [PubMed] [Google Scholar]
- 8.Wink DA, Darbyshire JF, Nims RW, Saavedra JE, Ford PC. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1993;6(1):23–7. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- 9.Lancaster JR., Jr Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A. 1994;91(17):8137–41. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR., Jr Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci U S A. 1998;95(5):2175–9. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafikova O, Sokolova E, Rafikov R, Nudler E. Control of plasma nitric oxide bioactivity by perfluorocarbons: physiological mechanisms and clinical implications. Circulation. 2004;110(23):3573–80. doi: 10.1161/01.CIR.0000148782.37563.F8. [DOI] [PubMed] [Google Scholar]
- 12.Endrich B, Asaishi K, Götz A, Messmer K. Technical report: A new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med. 1980;177:125–134. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- 13.Cabrales P, Briceño JC. Delaying Blood Transfusion in Experimental Acute Anemia with a Perfluorocarbon Emulsion. Anesthesiology. 2011;114(4):901. doi: 10.1097/ALN.0b013e31820efb36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakata M, Yoshida A, Haga M. Methemoglobin in blood as determined by double-wavelength spectrophotometry. Clin Chem. 1982;28(3):508–11. [PubMed] [Google Scholar]
- 15.Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 16.MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851(1-2):93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Cabrales P, Tsai AG. Plasma viscosity regulates systemic and microvascular perfusion during acute extreme anemic conditions. American Journal of Physiology-Heart and Circulatory Physiology. 2006;291(5):H2445–H2452. doi: 10.1152/ajpheart.00394.2006. [DOI] [PubMed] [Google Scholar]
- 18.Cabrales P, Tsai AG, Frangos JA, Briceno JC, Intaglietta M. Oxygen delivery and consumption in the microcirculation after extreme hemodilution with perfluorocarbons. Am J Physiol. 2004;287(1):H320–30. doi: 10.1152/ajpheart.01166.2003. [DOI] [PubMed] [Google Scholar]
- 19.Paxian M, Keller SA, Huynh TT, Clemens MG. Perflubron emulsion improves hepatic microvascular integrity and mitochondrial redox state after hemorrhagic shock. Shock. 2003;20(5):449–57. doi: 10.1097/01.shk.0000090601.26659.87. [DOI] [PubMed] [Google Scholar]
- 20.Heard SO, Puyana JC. The anti-inflammatory effects of perfluorocarbons: let's get physical. Crit Care Med. 2000;28(4):1241–2. doi: 10.1097/00003246-200004000-00067. [DOI] [PubMed] [Google Scholar]
- 21.Faithfull NS, King CE, Cain SM. Peripheral vascular responses to fluorocarbon administration. Microvasc Res. 1987;33(2):183–93. doi: 10.1016/0026-2862(87)90016-1. [DOI] [PubMed] [Google Scholar]
- 22.Johnson EC, Erickson BK, Podolsky A, Birks EK, Keipert PE, Faithfull NS, Wagner PD. Effects of a perfluorocarbon emulsion for enhanced O2 solubility on hemodynamics and O2 transport in dogs. J Appl Physiol. 1995;79(5):1777–86. doi: 10.1152/jappl.1995.79.5.1777. [DOI] [PubMed] [Google Scholar]
- 23.Jensen FB. The role of nitrite in nitric oxide homeostasis: a comparative perspective. Biochim Biophys Acta. 2009;1787(7):841–8. doi: 10.1016/j.bbabio.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Rassaf T, Kleinbongard P, Preik M, Dejam A, Gharini P, Lauer T, Erckenbrecht J, Duschin A, Schulz R, Heusch G, Feelisch M, Kelm M. Plasma nitrosothiols contribute to the systemic vasodilator effects of intravenously applied NO: experimental and clinical Study on the fate of NO in human blood. Circ Res. 2002;91(6):470–7. doi: 10.1161/01.res.0000035038.41739.cb. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 26.Lima ES, Bonini MG, Augusto O, Barbeiro HV, Souza HP, Abdalla DS. Nitrated lipids decompose to nitric oxide and lipid radicals and cause vasorelaxation. Free Radic Biol Med. 2005;39(4):532–9. doi: 10.1016/j.freeradbiomed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Nedospasov AA. Is N2O3 the main nitrosating intermediate in aerated nitric oxide (NO) solutions in vivo? If so, where, when, and which one? J Biochem Mol Toxicol. 2002;16(3):109–20. doi: 10.1002/jbt.10029. [DOI] [PubMed] [Google Scholar]
- 28.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115(8):2099–107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gow AJ, Buerk DG, Ischiropoulos H. A novel reaction mechanism for the formation of S-nitrosothiol in vivo. J Biol Chem. 1997;272(5):2841–5. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]
- 30.Cabrales P, Tsai AG, Frangos JA, Intaglietta M. Role of endothelial nitric oxide in microvascular oxygen delivery and consumption. Free Radic Biol Med. 2005;39(9):1229–37. doi: 10.1016/j.freeradbiomed.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Nolte D, Pickelmann S, Lang M, Keipert P, Messmer K. Compatibility of different colloid plasma expanders with perflubron emulsion: an intravital microscopic study in the hamster. Anesthesiology. 2000;93(5):1261–70. doi: 10.1097/00000542-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Lowson SM. Inhaled alternatives to nitric oxide. Crit Care Med. 2005;33(3 Suppl):S188–95. doi: 10.1097/01.ccm.0000156792.40298.5a. [DOI] [PubMed] [Google Scholar]
- 33.Morley D, Keefer LK. Nitric oxide/nucleophile complexes: a unique class of nitric oxide-based vasodilators. J Cardiovasc Pharmacol. 1993;22(Suppl 7):S3–9. [PubMed] [Google Scholar]
- 34.Cabrales P, Han G, Roche C, Nacharaju P, Friedman AJ, Friedman JM. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med. 2010;49(4):530–8. doi: 10.1016/j.freeradbiomed.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]