Abstract
Widespread clinical use of acellular hemoglobin (Hb)-based O2 carriers (HBOCs) has been hampered by their ability to elicit both vasoconstriction and systemic hypertension. This is primarily due to the ability of acellular Hb to extravasate through the blood vessel wall and scavenge endothelial-derived nitric oxide (NO). Encapsulation of Hb inside the aqueous core of liposomes retards the rates of NO dioxygenation and O2 release, which should reduce or eliminate the vasoactivity of Hb. Our aim is to determine the extent of systemic and microvascular vasoactive responses (hypertension, vasoconstriction and hypoperfusion) after infusion of vesicle encapsulated Hbs, in which the encapsulated Hb is in either the deoxygenated or carbon monoxide (CO) state (HbV and COHbV, respectively). To investigate this hypothesis, we used the hamster window chamber model subjected to two successive hypervolemic infusions of HbV and COHbV solutions (each infusion represents 10% of the animal’s calculated blood volume) at Hb concentrations of either 7 or 10 g/dL. The hypervolemic infusion model used in this study has all the regulatory mechanisms responsible for predicting the vasoconstrictive responses of HBOCs. The results of this study demonstrate the absence of vasoconstrictive and hypertensive responses upon single and multiple infusions of HbV and COHbV solutions. The HbV and COHbV solutions increased the plasma O2 carrying capacity. However, COHbV delivered low therapeutic levels of CO without inducing any microcirculatory disturbances. Significance: Vesicles containing Hb can be used as a new therapeutic agent in transfusion medicine to treat anemia and revert hypoperfusion.
Keywords: Microcirculation, vesicle encapsulated hemoglobin, red blood cell substitute, hemoglobin-based oxygen carrier, functional capillary density, vasoconstriction, hypertension, blood pressure
INTRODUCTION
The initial treatment phase for severe blood loss and hemorrhagic shock begins with the infusion of plasma expanders in order to replace lost vascular volume and, upon continued blood loss, is followed by the restitution of oxygen (O2) carrying capacity with the transfusion of red blood cells (RBCs) [1]. However, there is no hemoglobin (Hb)-based O2 carrier (HBOC) that is approved for clinical use in the United States as a RBC substitute [2]. Worldwide, only two RBC substitutes are clinically used, namely: Hemopure® (OPK Biotech, Cambridge, MA), a glutaraldehyde polymerized bovine Hb, used in South Africa and Peftoran® (Peftoran Corp., Moscow), a perfluorocarbon-based emulsion, used in Russia [2, 3]. Therefore, the development of an effective RBC substitute that can simultaneously maintain blood volume and deliver O2 remains an area of global importance with obvious applications in routine surgery, emergency combat care and situations involving severe blood loss, associated with trauma.
Recently tested acellular HBOCs elicit vasoconstriction, an early event that occurs within minutes after infusion and which is sustained for hours upon infusion [4]. Vasoconstriction is evident as a rapid rise in peripheral vascular resistance and blood pressure, commonly associated with bradycardia, decreased cardiac output and subsequently affects blood perfusion to vital organs [5, 6]. Vasoconstriction is currently perceived to be the most critical barrier hampering HBOC commercial development [4, 7]. Vasoconstriction appears to be directly linked to nitric oxide (NO) scavenging, oversupply of O2 to the blood vessel wall, and HBOC extravasation through the blood vessel wall into the surrounding tissue space [8]. Regardless of the exact mechanism for the development of vasoconstriction, the presence of acellular Hb in the blood stream enables these hypothesized mechanisms of vasoconstriction [9].
These processes occur as NO and O2 traverse the RBC-free (or plasma) layer near the blood vascular-endothelium interface. NO and O2 diffusion across this interface occur due to an established concentration gradient. Therefore, the presence of acellular Hb in the RBC-free plasma layer scavenges NO, distorting the NO concentration field near the endothelial cell layer and diverts NO away from the smooth muscle layer. NO depletion deactivates smooth muscle guanylyl cyclase preventing vascular relaxation [10]. In the case of O2, the presence of acellular Hb in the RBC-free layer, affects O2 transport in two ways by augmenting the blood vessel wall partial pressure of O2 (pO2) gradient and oxygenated Hb (oxyHb) gradient, which therefore increases the net O2 flux through the blood/tissue interface and which causes O2-dependent vasoconstriction [11–13]. Therefore inspired by our results with large sized polymeric Hbs that can overcome these limitations associated with the use of small sized cell-free Hbs, we have developed liposome/vesicle encapsulated Hb as an alternate strategy to provide a safe, efficacious and cost-effective alternative to acellular HBOCs [14].
In this study, our aim is to determine the extent of systemic and microvascular vasoactive responses (hypertension, vasoconstriction and hypoperfusion) after infusion of vesicle encapsulated Hb (HbV), in which the encapsulated Hb is either deoxygenated (deoxyHb) or carbon monoxide saturated (COHb), in order to generate deoxygenated HbV (HbV) or CO saturated HbV (COHbV) solutions. We hypothesize that encapsulation of Hb within the vesicle prevents/limits the vasoactive responses of previously developed small sized acellular HBOCs. To investigate this hypothesis, we used the hamster window chamber model subjected to two successive hypervolemic infusions of HbV and COHbV solutions with Hb concentrations of either 7 or 10 g/dL. The volume infused was calculated as 10% of the animal’s calculated blood volume (BV). The 10% topload infusion strategy was used in this study, since it has been used as the standard experimental design to evaluate pharmacological and toxicological effects of HBOCs in Phase I clinical trials in the past. This model has been used to evaluate safety of HBOCs, as it exaggerates HBOCs’ vasoconstrictive and hypertensive effects [4].
MATERIALS AND METHODS
Materials
Hollow fiber (HF) filter modules were purchased from Spectrum Laboratories (Rancho Dominguez, CA). Cholesterol was purchased from Sigma-Aldrich (St. Louis, MO). Poly(ethyleneglycol)5000 distearoylphosphatidylethanolamine (PEG5000-DSPE) and distearoylphosphatidylcholine (DSPC) were obtained from Avanti Polar Lipids (Alabaster, AL). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and used as is.
Source of RBCs
Fresh bovine RBCs were purchased from QUAD 5 (Ryegate, MT).
Extraction and Purification of Bovine Hb from RBCs
Bovine Hb was purified from RBCs via the method of Palmer et al. using tangential flow filtration (TFF) utilizing HF cartridges [15]. Briefly, Hb was purified from fresh RBCs via a 3-stage hollow fiber filtration process. RBCs were initially washed 3 times with saline and subsequently lysed with phosphate buffer (3.75 mM, pH 7.2) on ice. The RBC lysate was then filtered through a glass column packed with glass wool to remove the majority of cell debris. The clarified RBC lysate was then passed through 50 nm and 500 kDa hollow fiber cartridges (Spectrum Labs, Rancho Dominguez, CA) in order to remove additional cell debris. The purified Hb was then collected and concentrated on a 100 kDa hollow fiber cartridge (Spectrum Labs). It is important to note that the purified Hb is in phosphate buffer (3.75 mM, pH 7.2).
Prior to using Hb in the preparation of vesicle dispersions described in this study, the Hb was converted into the HbCO form by thoroughly mixing/stirring Hb under a blanket of CO gas for 2 hours at 4°C. The conversion of Hb into the HbCO form was confirmed using UV-Vis spectroscopy and the known Soret band maxima for HbCO. After this, HbCO was buffer exchanged with phosphate-buffered saline (PBS) using 100 kDa hollow fiber cartridges (Spectrum Labs). To ensure complete buffer exchange, the process was repeated 3–4 times using a 1:10 v/v (HbCO:PBS) ratio.
HbV and COHbV Preparation
COHbV dispersions were prepared as previously reported in the literature [16]. Briefly, DSPC and cholesterol (1:1 molar ratio) were completely dissolved in 30–40 mL of chloroform. The organic solution was then removed to form a dry lipid film using a rotary evaporator. The lipid film was further dried under vacuum for 48 hrs and then hydrated with 100 mL of carbonyl Hb (HbCO) ([Hb] > 300 mg/mL) that was buffer exchanged in PBS (0.1 M, pH 7.4) all inside a round bottom flask. The contents of the flask were thoroughly mixed at room temperature for 12–14 hours under a blanket of CO gas to form a homogeneous suspension. The lipid/HbCO solution was then passed through an EmulsiFlex-C5 homogenizer (Avestin Inc, Ontario, Canada) 6–7 times in batch mode. The homogenizing pressure was adjusted in the range of 10,000–15,000 psi. After completion of each homogenization cycle, the vesicle dispersion size distribution was measured using dynamic light scattering (DLS). The homogenization process was terminated once the vesicles reached a size < 200 nm. For PEGylation, PEG5000-DSPC (1% w/v) dispersed in 0.1 M PBS, pH 7.4 was added to the homogenized COHbV solution (1 mL to every 2 mL of Hb vesicle/solution, respectively) to maintain the PEG-lipid below its critical micelle concentration. The solution was then gently stirred at 55–58°C for a period of one hour under a CO saturated atmosphere, in order to facilitate the incorporation of PEG-lipid into the outer leaflet of the COHbVs.
Unencapsulated Hb was removed from COHbV particles at room temperature via diafiltration using a 500 kDa HF cartridge (Spectrum Laboratories, Rancho Dominguez, CA), using PBS as the diafiltration buffer. To ensure complete removal of free Hb from the COHbV solution, the process was repeated 7–8 times using a 1:10 v/v (COHbV:PBS) ratio until no free Hb was observed in the filtrate. The filtrate was assayed for the presence of Hb using UV-Vis spectroscopy and the Winterbourn equation [17]. Finally, the COHbV solution was concentrated using a 500 kDa HF cartridge to a Hb concentration > 10 g/dL. Strict aseptic conditions were maintained throughout the preparation of COHbVs. Prior to all experiments, tubing, filters and glassware used for the preparation of COHbVs were immersed in 1 M NaOH solution overnight and rinsed with deionized water. The diafiltration and concentration of COHbVs was performed inside a laminar flow hood.
In the case of deoxygenated HbVs, CO bound to Hb was photodissociated via irradiation of the COHbV solution with visible light under an O2 saturated atmosphere at 4°C. This process converts HbCO to HbO2 in the vesicle dispersion. The conversion was confirmed by measuring the absorption spectra of lysed Hb obtained from the HbV dispersion using UV-Vis spectroscopy. Consequently, the oxygenated HbV dispersion was placed inside a sealed serum bottle. A long needle was then used to degas the solution for 15–20 minutes. Afterwards, a blanket of argon gas was introduced into the bottle using the same long needle for 30 mins at 4°C. The cycle was repeated once again to make sure that all of the HbO2 in the vesicle dispersion was converted into the deoxygenated form. Finally, the pO2 of the deoxygenated Hb vesicle dispersion was measured using a RapidLab 248® Blood Gas Analyzer (Siemens Healthcare Diagnostics Inc., Tarrytown, NY).
Hb and Methemoglobin Concentration in HbV and COHbV Dispersions
The concentration of Hb and methemoglobin (metHb) in HbV dispersions was assayed after their lysis via the method of Rameez and Palmer [16]. Briefly, the HbV solution was diluted with PBS in a 1:9 v/v ratio. The solution was then heated to 5–10°C above the phase transition temperature of DSPC (55° C), for 15–20 min. Then a 10% v/v solution of Triton X-100 solution was added to the heated HbV solution and thoroughly mixed for 1 minute. The solution was then centrifuged at 14,000 rpm for 2–3 minutes. Finally, the supernatant was collected and the concentration of total released Hb and metHb were assayed via UV-Vis spectroscopy using the Winterbourn equation [17].
O2-HbV Equilibrium
CO bound to Hb was photodissociated via irradiation of the COHbV solution with visible light via the method of Rameez and Palmer [16]. O2-HbV equilibrium curves were then measured using a Hemox™ Analyzer (TCS Scientific Corp., New Hope, PA, USA) at 37°C as described in the literature [15].
HbV and COHbV Size Distribution
The size distribution of HbVs and COHbVs was measured using a Zetasizer Nano DLS spectrometer (Malvern Instruments Ltd, Worcestershire, United Kingdom) at 37°C.
Viscosity and Colloid Osmotic Pressure of HbV and COHbV Solutions
The viscosity of HbV and COHbV solutions was measured using a DV-II plus rheometer (Brookfield Engineering Laboratories, Middleboro, MA) at a shear rate of 150/sec, while the colloid osmotic pressure (COP) was measured using a 4420 Colloid Osmometer (Wescor, Logan, UT).
Experimental Groups
The experimental groups were labeled as HbV7, HbV10, COHbV7 and COHbV10. Therefore, HbV7 and HbV10 represent HbVs composed of deoxygenated Hb at Hb concentrations of 7 and 10 g/dL, respectively, while COHbV7 and COHbV10 represent HbVs composed of HbCO at Hb concentrations of 7 and 10 g/dL, respectively. A total of sixteen animals were entered into the study. All animals tolerated the entire protocol without visible signs of discomfort. Four animals were assigned to each experimental group.
Animal Preparation
Experiments were performed in 55 – 65 g male Golden Syrian Hamsters (Charles River Laboratories, Boston, MA) fitted with a dorsal skinfold window chamber. The hamster window chamber model is widely used for microvascular studies in the unanesthetized state. The complete surgical technique is described in detail elsewhere [18]. Arterial and venous catheters filled with a heparinized saline solution (30 IU/mL) were implanted into the carotid and jugular vessels. Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and securely attached to the window frame. Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee.
Inclusion Criteria
The microvasculature was examined 3 to 4 days after window implantation surgery, and only animals passing an established systemic and microcirculatory inclusion criteria were used in the study. Animals were considered suitable for experiments if: 1) systemic parameters were within the normal range, namely, heart rate (HR) > 340 beats/min, mean arterial blood pressure (MAP) > 80 mm Hg, systemic Hct > 45%, and arterial O2 partial pressure (pAO2) > 50 mm Hg; and 2) microscopic examination of the tissue in the chamber observed under 650× magnification did not reveal signs of low perfusion, inflammation, edema or bleeding.
Experimental Setup
The unanesthetized animal was placed in a restraining tube with a longitudinal slit from which the window chamber protruded, then fixed to the microscopic stage for transillumination with the intravital microscope (BX51WI, Olympus, New Hyde Park, NY). Animals were given 20 min to adjust to the tube environment before any measurements were made. The tissue image was projected onto a charge-coupled device camera (4815, COHU, San Diego, CA) connected to a videocassette recorder and viewed on a monitor. Measurements were carried out using a 40× (LUMPFL-WIR, numerical aperture 0.8, Olympus) water immersion objective.
Test Solutions
Hb concentrations in the test solutions were adjusted to 7 and 10 gHb/dL. The biophysical properties of all HbV and COHbV solutions are presented in Table 1 and 2.
Table 1.
Biophysical characteristics of the Hb and HbVs used in this study.
| Sample | Size Diameter |
P50 mm Hg |
n | % metHb level |
CO mM |
|---|---|---|---|---|---|
|
Hb | |||||
| Hb (TFF) | 64 kDa; 5nm | 25.47 | 2.66 | <0.5 | 0 |
|
Hb Vesicles | |||||
| COHbV7 | 167.4 nm | 20.58 | 1.43 | <1.0 | 4.3 |
| COHbV10 | 194.4 nm | 20.01 | 1.41 | <1.0 | 6.2 |
| HbV7 and HbV10 | 175.2 nm | 22.55 | 1.55 | <1.0 | 0 |
Table 2.
Rheological characteristics of the HbV solutions.
| Hb Vesicles | ||
|---|---|---|
| Sample | Viscosity cP* |
COP mm Hg |
| COHbV7 | 7.2 | 3.1 |
| COHbV10 | 9.8 | 4.4 |
| HbV7 | 6.8 | 2.4 |
| HbV10 | 9.3 | 4.7 |
measured at 160 s−1 and 37 °C.
Hypervolemic Infusion (Top-Load) Protocol
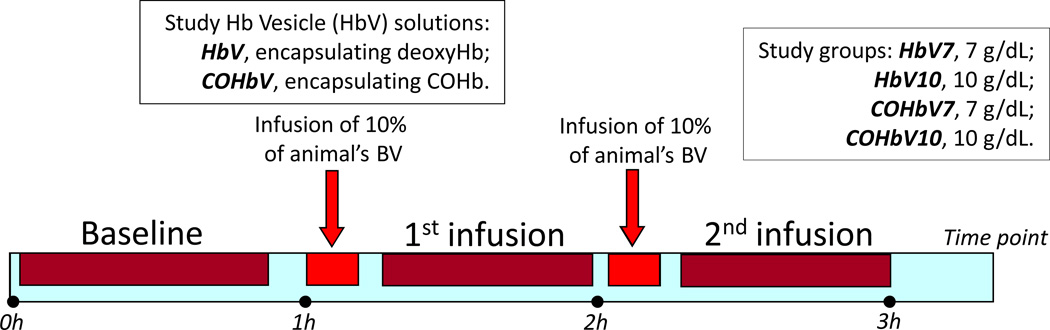
HbVs or COHbVs were infused as 10% of the animal’s calculated blood volume (BV, estimated as 7% of the body weight). After each infusion, animals were allowed 20–30 min to stabilize prior to systemic and microvascular characterization. All infusions were completed i.v. at a flow rate of 100 µL/min (Figure 1).
Figure 1. Multiple hypervolemic infusion protocol.
Animals were instrumented with the dorsal window chamber and catheters. Two days after recuperation they were divided into four experimental groups, and administered either HbV or COHbV solutions at a Hb concentration of either 7 or 10 g/dL. Solutions were labeled: HbV7, HbV10, COHbV7 and COHbV10. Each infusion was calculated as 10% of the animal’s blood volume (BV).
Systemic Parameters
MAP and HR were recorded continuously (MP 150, Biopac System; Santa Barbara, CA). Hct was measured from centrifuged arterial blood samples taken in heparinized capillary tubes. Hb content was determined spectrophotometrically (B-Hemoglobin, Hemocue, Stockholm, Sweden).
Blood Chemistry and Biophysical Properties
Arterial blood was collected in heparinized glass capillaries (50 µL) and immediately analyzed for pO2, partial pressure of CO2 (pCO2), base excess (BE) and pH (Rapidlab 248, Bayer, Norwood, MA).
Microhemodynamics
Arteriolar and venular blood flow velocities were measured on-line using the photodiode cross-correlation method (Photo Diode/Velocity Tracker Model 102B, Vista Electronics, San Diego, CA). The measured centerline velocity (V) was corrected according to blood vessel size to obtain the mean RBC velocity (V/Rv), where Rv represents the ratio between the blood vessel centerline velocity and average blood velocity based on data obtained in glass tubes [19]. According to Lipowsky and Zweifach, Rv = 1.6 for blood vessels between 15 and 90 µm diameter, but not for larger blood vessels [19]. A video image-shearing method was used to measure blood vessel diameter (D) [20]. Blood flow (Q) was calculated from measured values as Q = π × (V/Rv) (D/2)2.
Functional Capillary Density (FCD)
Functional capillaries, defined as those capillary segments that have RBC transit of at least a single RBC in a 45 s period in 10 successive microscopic fields were assessed, totaling a region of 0.46 mm2. The relative change in FCD from baseline levels after each intervention is indicative of the extent of capillary perfusion [21].
Data Analysis
Tabular results are presented as the mean ± standard deviation. Data within each group were analyzed using analysis of variance for repeated measurements (ANOVA, Kruskal-Wallis test). When appropriate, post hoc analyses were performed with the Dunns multiple comparison test. Comparison between HbVs in different liganded states with different Hb concentrations was analyzed using two-way ANOVA; post hoc analyses were performed with the Bonferroni post tests. Microhemodynamic data are presented as absolute values and ratios relative to baseline values. A ratio of 1.0 signifies no change from baseline, while lower and higher ratios are indicative of changes proportionally lower and higher than baseline (i.e., 1.5 would mean a 50% increase from the baseline level). The same blood vessels and capillary fields were followed so that direct comparisons to their baseline levels could be performed, allowing for more robust statistics on small sample populations. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, Inc., San Diego, CA). Changes were considered statistically significant if P<0.05.
RESULTS
Biophysical Properties of HbVs and COHbVs
The biophysical properties of the HbVs and COHbVs, such as, Hb solution concentration, metHb level, molecular diameter, regressed O2 affinity (P50) and cooperativity coefficient (n), as well as CO concentration (i.e. [CO]) are shown in Table 1. The initial Hb solution concentration (before dilution in preparation for the hypervolemic studies) was always above 10 g/dL for HbVs and COHbVs. The objective was to prepare HbVs and COHbVs with Hb concentrations similar to that of whole blood (15 g/dL), in order for these solutions to be used as efficacious HBOCs. The metHb level of HbV and COHbV dispersions prepared in this study was less than 1% as compared to ~ 0.5% for purified Hb. This shows that the metHb level of encapsulated Hb did not increase significantly during the preparation of HbV or COHbV dispersions. In addition, the size distribution of HbVs ranged from 167–195 nm. Therefore, the vesicle diameters are in the ideal range (160–220 nm) required to achieve long circulatory lifetimes [22].
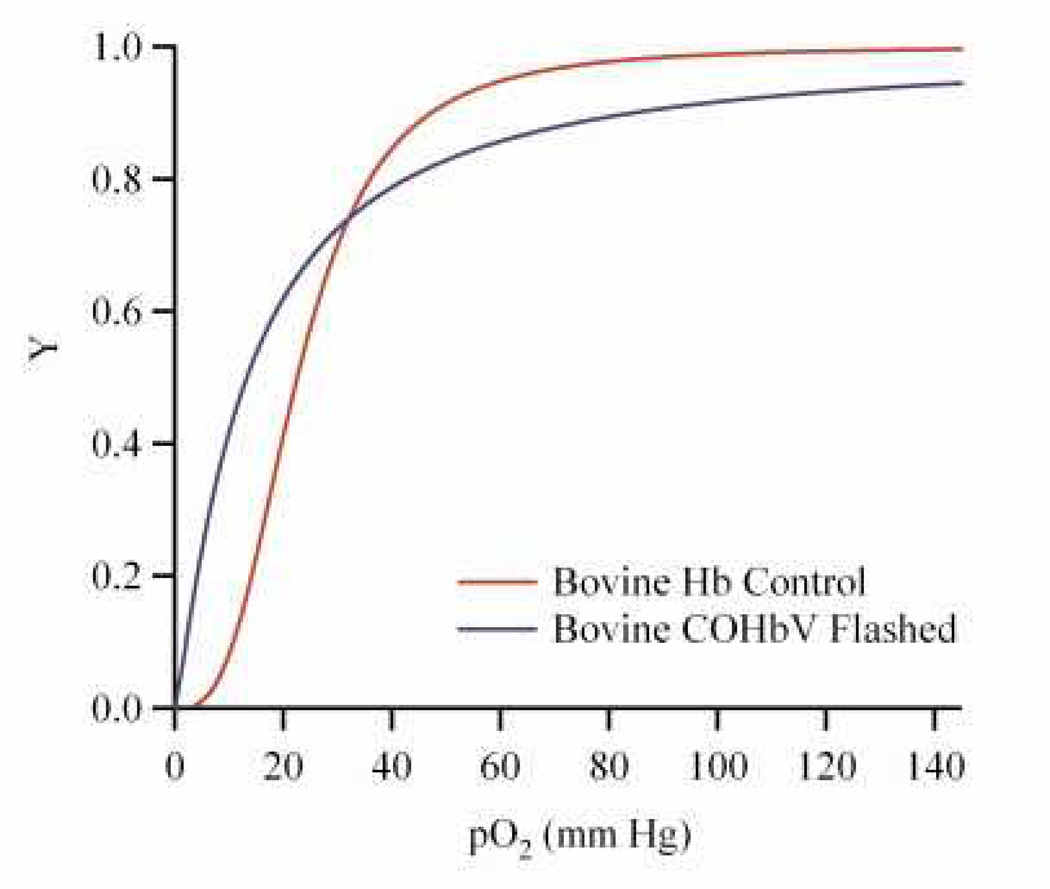
Figure 2 shows equilibrium O2 binding curves of a representative COHbV dispersion where the CO bound to Hb was photodissociated via irradiation with visible light. The P50 for HbVs was in the range of 20–22.55 mm Hg, lower than that of bovine Hb (P50 ≈ 26.03 mm Hg) [15]. Similarly, the cooperativity coefficient for HbVs was in the range of 1.43–1.55 much lower than that of bovine Hb (n ≈ 2.7). The changes in the P50 and n values of HbVs as compared to unencapsulated Hbs has been attributed to encapsulation of Hb inside the aqueous core of liposomes and subsequent protein crowding which reduces the cooperativity of the encapsulated Hb [16]. The viscosity and COP of HbV and COHbV solutions is presented in Table 2. HbVs and COHbVs exhibited increasing solution viscosity and COP, as a function of increasing Hb concentration.
Figure 2. Representative O2 equilibrium curves of cell-free bovine Hb and HbV.
Here, Y is the fraction of Hb saturated with O2 and pO2 is the partial pressure of O2. “Flashed,” implies that the CO bound to the Hb was photodissociated via irradiation with visible light.
Systemic Parameters
All groups were statistically similar (P>0.40) in terms of systemic and microcirculatory parameters at baseline. Absolute values for MAP, HR, Hct, Hb and body weight at baseline are presented in Table 3. For each HbV/COHbV solution, the total Hb concentration, volume of test solution infused, Hct, COHb and metHb levels are also presented in Table 3. The Hct decreased as a function of the infused test solution volume, although the total Hb concentration increased with the volume of infused HbV/COHbV solutions. COHb was only detected in the COHbV groups and its concentration increased with increasing infusion volume of test solution. MetHb was only detected after the second infusion of HbV10.
Table 3.
Blood pressure, heart rate and blood chemistry.
| Hb vesicles | ||||
|---|---|---|---|---|
| Baseline | HbV7 | HbV10 | COHbV7 | COHbV10 |
| N | 4 | 4 | 4 | 4 |
| MAP, mmHg | 112 ± 7 | 114 ± 8 | 110 ± 8 | 109 ± 6 |
| HR, bpm | 432 ± 24 | 428 ± 25 | 430 ± 20 | 426 ± 21 |
| Hct, % | 49 ± 1 | 48 ± 2 | 48 ± 2 | 49 ± 1 |
| Hb, g/dl | 14.9 ± 0.7 | 14.7 ± 0.6 | 14.8 ± 1.0 | 14.8 ± 0.6 |
| Body weight, g | 64.3 ± 4.2 | 65.2 ± 4.1 | 65.6 ± 6.0 | 64.7 ± 5.8 |
| HbV and COHbV infusion | ||||
| 1st infusion of 10% of the animal's BV | ||||
| Hct, % | 46 ± 1 | 46 ± 2 | 45 ± 2 | 47 ± 1 |
| Total Hb, g/dl | 15.6 ± 0.8 | 15.9 ± 0.8 | 15.6 ± 0.6 | 15.8 ± 0.7 |
| COHb,% of total Hb | - - | - - | 2.0 ± 1.2† | 2.6 ± 1.6† |
| MetHb, % of total Hb | - - | - - | - - | - - |
| 2nd infusion of 10% of the animal's BV | ||||
| Hct, % | 45 ± 2 | 44 ± 2 | 44 ± 2 | 44 ± 1 |
| Hb, g/dl | 16.3 ± 0.9 | 16.9 ± 0.8 | 16.0 ± 1.2 | 16.5 ± 0.6 |
| COHb,% of total Hb | - - | - - | 3.4 ± 0.9† | 4.6 ± 1.2† |
| MetHb, % of total Hb | - - | 3.6 ± 0.5† | - - | - - |
| Blood Viscosity, cP | 4.7 ± 0.4† | 4.9 ± 0.5† | 4.8 ± 0.4† | 5.1 ± 0.4† |
| Plasma Viscosity, cP | 1.7 ± 0.2† | 1.9 ± 0.2† | 1.8 ± 0.1† | 2.0 ± 0.3† |
Absolute values for mean arterial pressure (MAP), heart rate (HR), hematocrit (Hct), Hb and body weight at baseline. The total Hb concentration, Hct, COHb and metHb levels after the 1st infusion and total Hb concentration, Hct, COHb and metHb levels and blood/plasma viscosities after the 2nd infusion are also presented.
measured at 160 s−1 and 37°C.
P<0.05 compared to baseline.
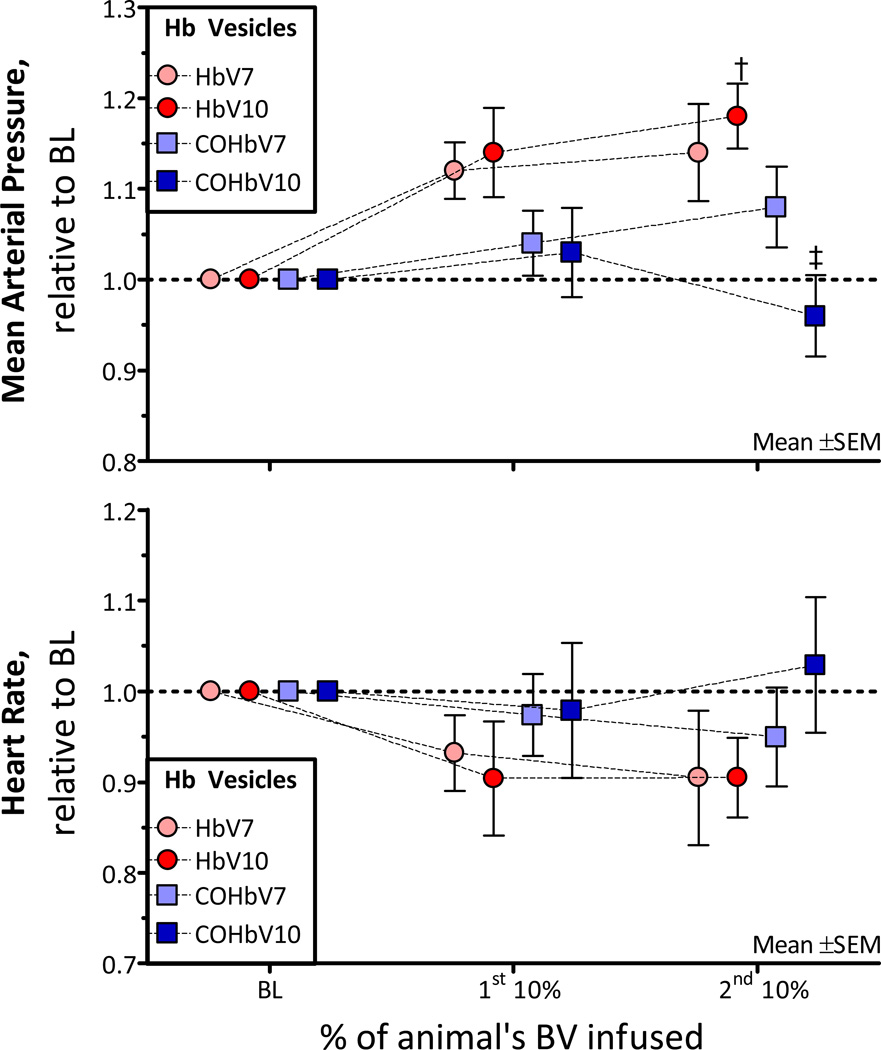
Changes in MAP
MAP and HR are presented in Figure 3. The MAP after the 1st infusion was not different from baseline. However after the 2nd infusion, the MAP was increased from baseline for the HbV solutions. The MAP after infusion of COHbV10 was significantly lower than that of HbV10. The HR was not different compared to baseline after the infusion of HbV solutions. However, the general trend of the data shows minor hemodynamic changes in animals infused with HbV compared to the COHbV, suggesting that the CO present in the COHbV produces pharmacological effects in the microcirculation. In addition, the HbV and COHbV solutions were considered biocompatible; since few leukocytes and macrophages were observed in the microcirculation and the hemodynamics were stable after each infusion.
Figure 3. Relative changes from baseline in mean arterial pressure (MAP) and heart rate (HR) after infusion of HbV or COHbV solutions.
The broken line represents the baseline level. †, P < 0.05 relative to baseline; ‡, P<0.05 compared to HbV at an equal Hb concentration. A. Changes in MAP. The baseline MAP (mm Hg, mean ± SD) for each group was as follows: HbV7: 112 ± 7; HbV10: 114 ± 8; COHbV7: 110± 8; COHbV10: 109 ± 6. B. Changes in HR. The baseline HR (bpm, mean ± SD) for each group was as follows: HbV7: 432 ± 24; HbV10: 428 ± 25; COHbV7: 430± 20; COHbV10: 426 ± 21.
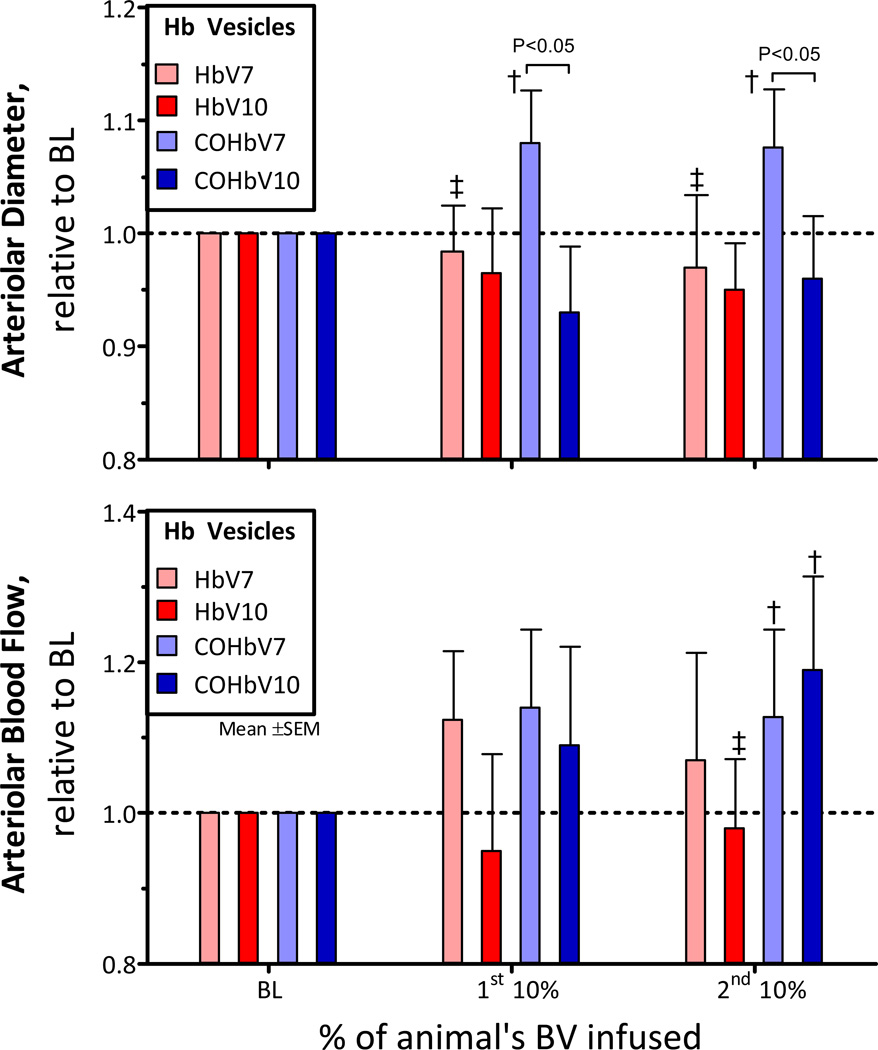
Changes in Arteriolar Diameter and Blood Flow
The arteriole diameters after infusion of HbV and COHbV solutions are presented in Figure 4A, and changes in arteriole blood flows are shown in Figure 4B. The first and 2nd infusion of HbV and COHbV solutions did not affect arteriole diameter. However, the COHbV7 solution showed consistent vasodilation compared to baseline (P<0.10). Arteriole blood flows were not different from baseline after the 1st infusion of HbV and COHbV solutions. However, the 2nd infusion of COHbV solutions (COHb7 and COHb10) increased arteriole blood flow compared to baseline.
Figure 4. Relative changes from baseline in arteriolar diameter and blood flow after infusion of HbV or COHbV solutions.
The broken line represents the baseline level. †, P < 0.05 relative to baseline; ‡, P<0.05 compared to HbV at an equal Hb concentration. A. Changes in arteriolar diameter. The baseline diameter (µm, mean ± SD) for each group was as follows: HbV7: 62 ± 16; HbV10: 63 ± 15; COHbV7: 67 ± 12; COHbV10: 65 ± 14. B. Changes in volumetric flow rate. The baseline blood flow (nL/s, mean ± SD) for each group was as follows: HbV7: 12 ± 4; HbV10: 11 ± 5; COHbV7: 12 ± 5; COHbV10: 11 ± 5.
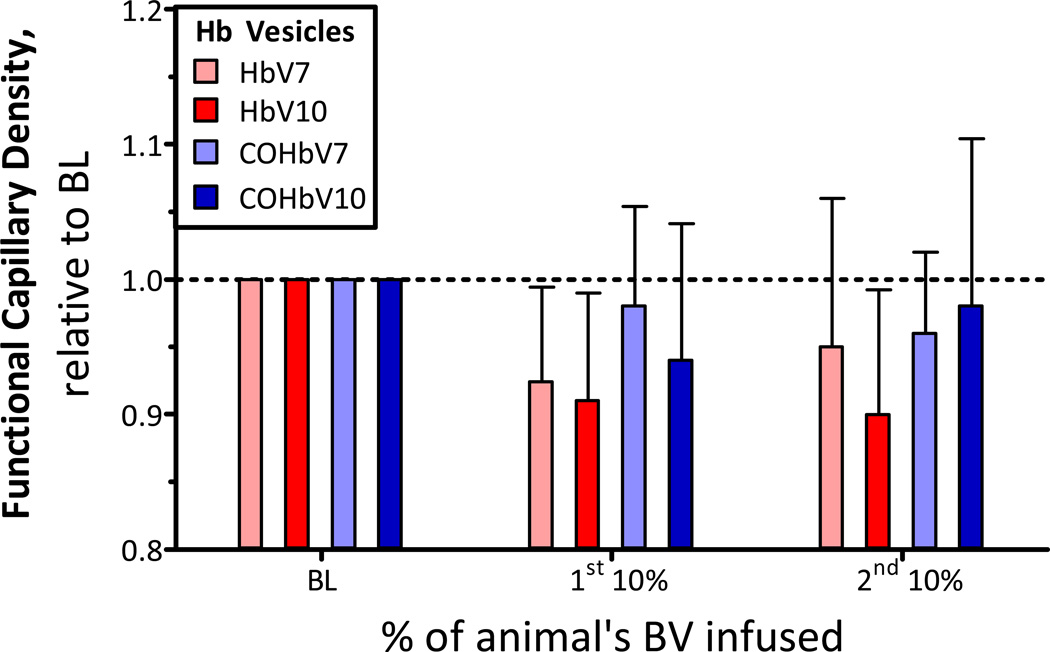
Changes in Functional Capillary Density (FCD)
The FCD after infusion of HbV and COHbV solutions is presented in Figure 5. At all concentrations evaluated for HbV and COHbV solutions, the FCD decreased from baseline, but the change was not statistically significant.
Figure 5. Relative changes from baseline in functional capillary density (FCD) after infusion of HbV or COHbV solutions.
The broken line represents the baseline level. The baseline FCD (cm−1, mean ± SD) for each group was as follows: HbV7: 110 ± 14; HbV10: 104 ± 11; COHbV7: 107 ± 13; COHbV10: 110 ± 8.
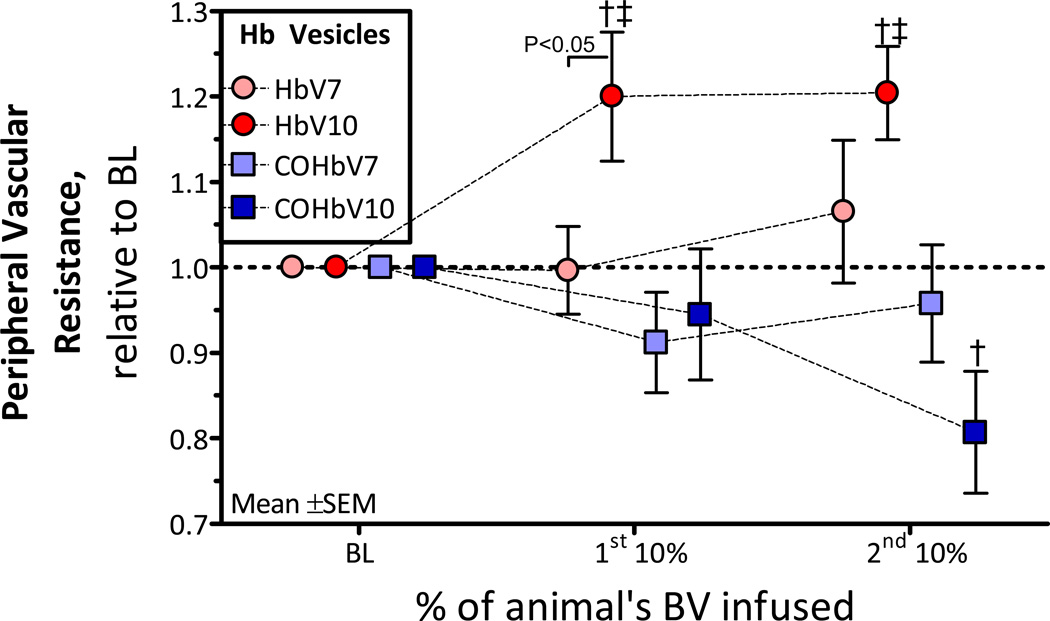
Changes in Peripheral Vascular Resistance (PVR)
The PVR was calculated as the ratio between the MAP and arteriolar blood flow. Figure 6 presents changes in PVR after infusion of HbV and COHbV solutions. At all concentrations evaluated, all HbV and COHbV solutions did not affect the PVR compared to baseline (P<0.05). Although, HbV and COHbV solutions exhibited completely different trends. For instance, HbV solutions tended to increase the PVR, while COHbV solutions tended to decrease the PVR.
Figure 6. Relative changes from baseline in calculated peripheral vascular resistance (PVR) after infusion of HbV or COHbV solutions.
†, P < 0.05 relative to baseline; ‡, P<0.05 compared to HbV at an equal Hb concentration.
DISCUSSION
Biophysical Properties of HbV Solutions
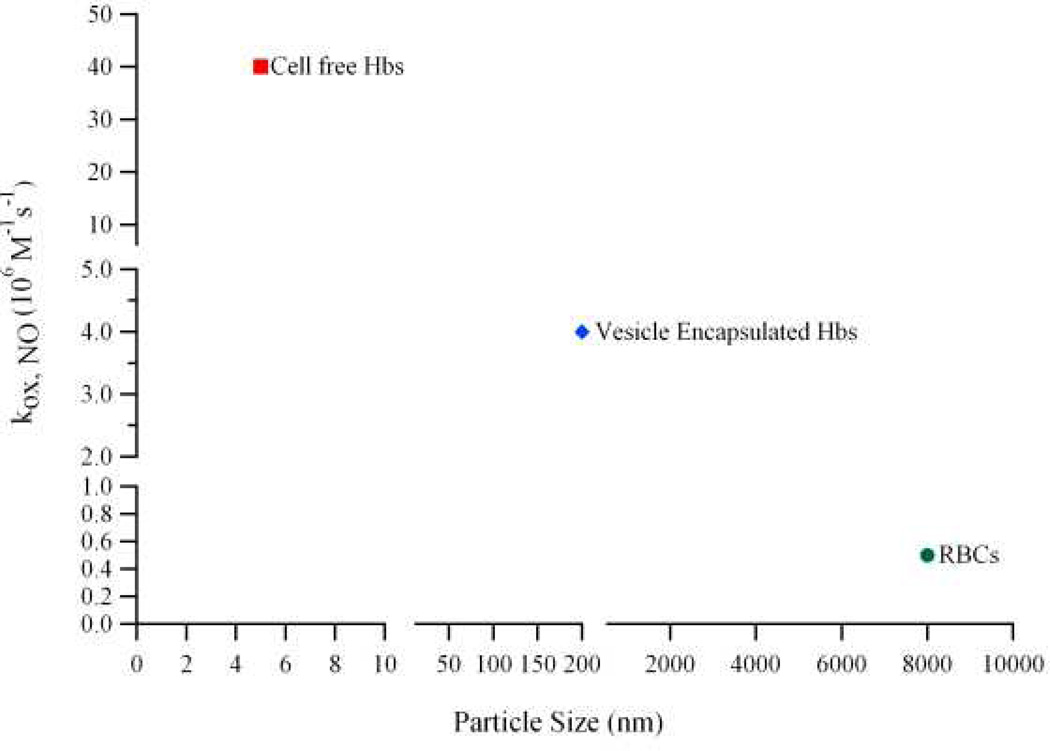
The goal of this study is to examine the role of encapsulated Hb (in different liganded states), in regulating Hb-induced vasoconstriction in vivo. A recent study by Rameez and Palmer (2011), demonstrated that reduction in the magnitude of the NO dioxygenation rate constant (kox,NO) is due to the encapsulation of concentrated Hb inside the aqueous core of vesicles, which provides a large intracellular barrier against NO diffusion. Moreover, this effect became more pronounced as the diameter of the HBOC increased in size. Figure 7 shows the dependence of kox,NO on HBOC size. The values for kox,NO shown in the Figure 7 were previously reported by Rameez and Palmer [16]. Thus HbV has unique physicochemical properties, including normal O2 affinity and cooperativity, reduced rates of O2 dissociation and increased molecular dimensions compared to molecular Hb (Table 1).
Figure 7. Dependence of the NO dioxygenation rate constant on HBOC size.
NO dioxygenation rate constants were taken from the study of Rameez and Palmer [16].
We hypothesize that by encapsulating different liganded states of Hb inside vesicles, we have another strategy for controlling vasoconstriction. In order to test our hypothesis, COHbV and HbV solutions were prepared at two different Hb concentrations for in vivo evaluation of systemic and microcirculatory parameters.
In vivo Response of Hb Vesicle Solutions
The principal finding of this study is that infusion of single and multiple infusions of HbV and COHbV solutions produced limited hypertension and vasoconstriction. Only after the second infusion of the higher concentration HbV, blood pressure increased compared to baseline without inducing vasoconstriction, which can be explained by the increases in blood viscosity. Additionally, infusion of COHbV solutions increased blood flow. Therefore, COHb infusion decreased PVR. The concentration of Hb in the HbV solution did not have significant effects after infusion, in terms of vasoconstriction and hypertension. However, the higher concentration HbV solution increased PVR. The observed increase in PVR after infusion of 10 gHb/dL of HbV decreased the transmission of perfusion pressure to the microcirculation [23]. The principal microvascular complication associated with the infusion of HbV and COHbV solutions included a minor decrease in FCD (i.e. capillaries perfused with RBCs), a critical parameter that ensures tissue homeostasis. An important difference between HbV and COHbV solutions are their effects on PVR, which suggest a significant pharmacological effect due to the Hb liganded state and the release of CO.
NO Scavenging and Blood Vessel Wall Hyper Oxygenation
The presence of CO bound to Hb inside Hb vesicles determined in vivo responses towards infusion of HbV and COHbV solutions, with minor to no effect on the biophysical properties of the Hb vesicles, e.g. viscosity, COP and equilibrium O2 transport properties (P50 and cooperativity coefficient). Hb encapsulation prevents the vasoactive and hypertensive effects of acellular Hb by reducing the Hb diffusion coefficient (via the relative size of the Hb vesicle [~200 nm] compared to cell-free Hb [~5.5 nm]) and by compartmentalizing the Hb inside the vesicle while it is circulating in the intravascular space. Additionally, encapsulating Hb inside the vesicle aqueous core prevents NO scavenging and blood vessel hypeRoxygenation. As these solutions are expected to be used after trauma and hemorrhage, the maintenance of blood perfusion and the increase in O2 transport by Hb vesicles can prevent the development of end-organ damage. Hb encapsulation inside the aqueous core of the vesicle has a direct impact on Hb’s proximity to the vascular endothelium and Hb’s molecular diffusivity. In the absence of acellular Hb, the rate of O2 delivery in blood is limited by the diffusion of dissolved O2, and the low solubility of O2 in plasma. However, in the presence of acellular HBOCs, these molecules enhance premature O2 delivery to the resistance arterioles [9, 24]. In the case of HbV and COHbV solutions, Hb encapsulation reduces the vesicle’s molecular diffusivity compared to tetrameric Hb or small sized chemically modified Hb formulations. Therefore, Hb encapsulation inside the vesicle increases the HBOC molecular size and reduces the O2 flux. In this context, the size of HbV and COHbV vesicles are anticipated to attenuate the rate of O2 transport from the central blood vessel RBC column to the vascular wall across the plasma layer.
Exclusion of HbV from the Endothelium Glycocalyx
In the absence of acellular Hb in the circulation, the plasma layer adjacent to the vascular endothelial wall regulates NO scavenging by the Hb encapsulated inside the erythrocytes [8]. When acellular HBOCs formulations are introduced in the circulation, they penetrate within the plasma layer and act as a sink for the NO generated by the endothelium. However, HbV’s and COHbV’s molecular size and corpuscular nature partially prevents the penetration of HbV within the plasma layer, weakening the Hb scavenging effects of the Hb encapsulated inside the HbV, similarly as in the case of erythrocytes. The paradox surrounding how NO can escape HbV and COHbV scavenging can partially be explained by the presence of the endothelial glycocalyx. This unstirred layer surrounding the abluminal side of the vascular endothelium potentially decreases overall NO uptake by HbV and COHbV particles by limiting their proximity to the blood vessel wall [8]. The endothelial glycocalyx ranges from 0.4 to 0.5 µm thick, and its permeability depends on the size and charge of the molecule in proximity with it [25, 26]. In our previous study, we showed that the endothelial glycocalyx of the blood vessels in the hamster window model is impermeable to macromolecules larger that 70 kDa, and that it becomes permeable when its integrity is compromised [27]. HbV and COHbV solutions prevent the extravasation of Hb tetramers and αβ dimers and their subsequent intercalation between the endothelium and the smooth muscle layers, which would lead to inflammatory responses due to oxidative tissue injury [28]. While the vasoactivity of small acellular HBOCs could be satisfactorily explained by NO scavenging, hyper-oxygenation of the arterioles and extravasation, the absence of vasoconstriction with HbV and COHbV solutions re-enforces the importance of Hb encapsulation as a simple strategy for increasing HBOC molecular size and preventing the mechanisms that can induce an increase in vascular resistance.
Hyperviscous Oxygen Carriers
HbV and COHbV solutions possess high solution viscosities and increased plasma viscosities, leading to their characterization as hyperviscous O2 carriers. In this context, blood vessel wall shear stress and endothelial cell mechanotransduction are important factors that regulate tissue perfusion. The migration of RBCs to the axial core in blood vessels (Fåhraeus effect) leads to the formation of a high-viscosity, RBC-rich core and a low-viscosity, mostly RBC-free plasma layer, resulting in a nonlinear blood flow velocity profile [29]. The vasoinactivity of HbV and COHbV solutions could also be explained in part by their increase in microcirculatory shear stress, leading to the increased production of endothelium derived autocoids, principally NO [30]. Changes in plasma viscosity by high viscosity plasma expanders increase blood vessel wall shear stress and perivascular levels of NO [30]. Current mathematical models describing the NO balance in the presence of acellular Hbs that include shear stress dependent endothelial cell NO production, predict that significant changes in shear stress will affect the smooth muscle NO concentration profile, and produce major changes in the perivascular NO concentration [31]. The increase in plasma viscosity after infusion of HbV and COHbV solutions redistributes viscosity in the circulation, causing it to increase to a greater extent in the central circulation, explaining in part, the increase in blood pressure, measured without vasoconstriction. Higher concentrations of HbV have more elevated viscosity than the formulation evaluated here, and further increase plasma and blood viscosity, thus increasing vascular resistance when infused without hemodilution.
Pharmacological Activity of CO
Carbon monoxide (CO), an infamous toxic air pollutant, is normally produced in biological systems from the degradation of heme catalyzed by the enzyme heme oxygenase [32]. Heme oxygenase invokes cellular protection in response to cell injury, inflammation, oxidative stress, etc. The protective response of heme oxygenase is ascribed to its end products namely, biliverdin and CO. CO exerts various cellular effects by modulating intracellular signaling pathways, from cytoprotective, immunomodulatory, and anti-inflammatory responses by modulating cytokines and activating mitogen-activated protein kinases [33]. CO uptake by inhalation has shown beneficial actions, however it is not controllable. COHbV solutions represent CO-releasing formulations with the unique ability to release CO in a controlled manner to produce vasorelaxant pharmacological effects, as indicated by the changes in PVR. Therefore, the therapeutic potential of COHbV is three-fold. It could help in increasing blood perfusion by delivering small amounts of vasodilatory CO to the surrounding vasculature, and eventually as the CO is released, the COHbV will convert into HbV in the circulation and will then be able to transport oxygen to surrounding tissues. Additionally, the bond between the heme iron and CO has increased stability with respect to that with O2. This implies that the CO saturated Hb complex is more stable than deoxy-Hb or oxy-Hb by inhibiting autoxidation, thus COHb has increased stability during storage and should limit methemoglobin (met-Hb) formation. Our findings lead to the new concept that transfusing COHbV might add a new function to HBOCs, as CO-delivery agents. On the other hand, CO poisoning is the leading cause of poisoning worldwide. The pathophysiologic mechanisms of CO toxicity are related to hypoxia. The affinity of CO for heme protein is approximately 200 times that of O2, thus CO binds rapidly to Hb forming COHb and reducing O2 carrying capacity leading to tissue hypoxia. The threshold for CO poisoning in human has been established to be when HbCO level reaches 40% [34]. In our experiment, COHb levels were well below this limit, in addition blood gas and hemodynamic parameters were stable. Moreover, our results confirmed that CO did not accumulated, and the HbCO levels in the COHbV decreased rapidly and became less than 5% within less than 1 h after two infusions of 10g/dL of COHbV. There seems to be a rapid ligand exchange reaction from COHbV to oxyHbV. In vivo a rapid CO exchange reaction between HbV and RBC is expected due to HbV CO binding kinetics [16].
In summary, our study shows a lack of vasoconstrictive and hypertensive responses upon multiple infusions of HbV and COHbV solutions into the circulation. The COHbV solutions can increase plasma O2 carrying capacity and deliver therapeutic low levels of CO without inducing microcirculatory disturbances. It is important to note that the hypervolemic infusion model used to evaluate vasoconstrictive responses induced by HbV and COHbV solutions in this study, has all the regulatory mechanisms responsible for vasoconstrictive responses fully presented. Therefore, it anticipates the physiological response to HbV and COHbV solutions more so than clinically relevant conditions, like exchange transfusion, where HbV and COHbV solutions would be utilized. Under anemic conditions, O2 delivered to the tissues becomes insufficient to meet their O2 demand, and allogeneic blood transfusion cannot be avoided. At this point, an attractive alternative to allogeneic blood transfusion can include infusion of HbV and COHbV solutions at 10 g/dL, which roughly has 70% of the O2 carrying capacity of whole blood (non-diluted blood). The smaller size of HbV and COHbV particles compared to erythrocytes can also increase O2 release to tissues via the process of facilitated O2 diffusion. This accomplishment provides for a new therapeutic approach in transfusion medicine, the treatment of ischemia, and clinical conditions where tissue oxygenation is affected. Future research on HbV and COHbV solutions will be directed towards understanding their volume expansion and O2 transport characteristics. Finally, the net results of this work and further microvascular evaluation should result in the development of a universal transfusion fluid that lowers the transfusion trigger, and is more efficacious and economic than blood, in order to save and reduce our reliance on blood transfusions.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01HL078840 and R01DK070862 to AFP. Additional support for this work was provided by bioengineering research partnership grant R24-HL64395, program project P01 HL071064 and grants R01-HL52684, R01-HL078840, R01-HL62354, R01-HL62318 and R01-HL76182. We acknowledge Dr. Robert Lee, Department of Pharmacy, The Ohio State University, Columbus OH, for his willingness to let us use his homogenizer. The authors also thank Froilan P. Barra and Cynthia Walser for surgical preparation of the animals.
Contributor Information
Pedro Cabrales, Email: pcabrales@ucsd.edu.
Shahid Rameez, Email: rameez.1@osu.edu.
Andre F. Palmer, Email: palmer.351@osu.edu.
REFERENCES
- 1.Persson J, Grande PO. Volume expansion of albumin, gelatin, hydroxyethyl starch, saline and erythrocytes after haemorrhage in the rat. Intensive Care Med. 2005;31(2):296–301. doi: 10.1007/s00134-004-2510-3. [DOI] [PubMed] [Google Scholar]
- 2.Winslow RM. New transfusion strategies: Red cell substitutes. Ann Rev Med. 1999;50:337–353. doi: 10.1146/annurev.med.50.1.337. [DOI] [PubMed] [Google Scholar]
- 3.Moore EE, Cheng AM, Moore HB, et al. Hemoglobin-based oxygen carriers in trauma care: scientific rationale for the US multicenter prehosptial trial. World J Surg. 2006;30(7):1247–1257. doi: 10.1007/s00268-005-0499-6. [DOI] [PubMed] [Google Scholar]
- 4.Alayash AI. Oxygen therapeutics: Can we tame haemoglobin? Nature Reviews Drug Discovery. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 5.Matheson B, Kwansa HE, Bucci E, et al. Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol. 2002;93(4):1479–1486. doi: 10.1152/japplphysiol.00191.2002. [DOI] [PubMed] [Google Scholar]
- 6.Gaucher-Di Stasio C, Paternotte E, Prin-Mathieu C, et al. The importance of the effect of shear stress on endothelial cells in determining the performance of hemoglobin based oxygen carriers. Biomaterials. 2009;30(4):445–451. doi: 10.1016/j.biomaterials.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 7.Winslow RM. aa-crosslinked hemoglobin: Was failure predicted by preclinical testing? Vox Sanguinis. 2000;79:1–20. doi: 10.1159/000031200. [DOI] [PubMed] [Google Scholar]
- 8.Cabrales P, Sun G, Zhou Y, et al. Effects of the molecular mass of tense-state polymerized bovine hemoglobin on blood pressure and vasoconstriction. J Appl Physiol. 2009;107(5):1548–1558. doi: 10.1152/japplphysiol.00622.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy MR, Vandegriff KD, Winslow RM. The role of facilitated diffusion in oxygen transport by cell-free hemoglobins: implications for the design of hemoglobin-based oxygen carriers. Biophys Chem. 2001;92:103–117. doi: 10.1016/s0301-4622(01)00194-6. [DOI] [PubMed] [Google Scholar]
- 10.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med. 2004;36(6):707–717. doi: 10.1016/j.freeradbiomed.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Johnson PC. Flow measurment techniques in the microcirculation. In: Baker CH, Nastuk WL, editors. Microcirc Tech. London: Academic; 1986. pp. 149–159. [Google Scholar]
- 12.Nishide H, Chen XS, Tsuchida E. Facilitated oxygen transport with modified and encapsulated hemoglobins across non-flowing solution membrane. Artif Cells Bloos Substit Immobil Biotechnol. 1997;25:335–346. doi: 10.3109/10731199709118924. [DOI] [PubMed] [Google Scholar]
- 13.Bouwer ST, Hoofd L, Kreuzer F. Diffusion coefficients of oxygen and hemoglobin measured by facilitated oxygen diffusion through hemoglobin solutions. Biochim Biophys Acta. 1997;1338:127–136. doi: 10.1016/s0167-4838(96)00197-5. [DOI] [PubMed] [Google Scholar]
- 14.Svedberg T, Eriksson IB. Molecular weights of the blood pigments of Arenicola and of Lumbricus. Nature. 1932;130:434–435. [Google Scholar]
- 15.Palmer AF, Sun G, Harris DR. Tangential flow filtration of hemoglobin. Biotechnology Progress. 2009;25(1):189–199. doi: 10.1002/btpr.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rameez S, Palmer AF. Simple Method for Preparing Poly(ethylene glycol)-Surface-Conjugated Liposome-Encapsulated Hemoglobins: Physicochemical Properties, Long-Term Storage Stability, and Their Reactions with O2, CO, and NO. Langmuir. 2011 doi: 10.1021/la201246m. null-null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CC W. CRC Handbook of Methods for Oxygen Radical Research. 1985. [Google Scholar]
- 18.Endrich B, Asaishi K, Götz A, et al. Technical report: A new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med. 1980;177:125–134. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- 19.Lipowsky HH, Zweifach BW. Application of the "two-slit" photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 20.Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res. 1973;5(3):309–312. doi: 10.1016/0026-2862(73)90042-3. [DOI] [PubMed] [Google Scholar]
- 21.Cabrales P, Tsai AG, Frangos JA, et al. Oxygen delivery and consumption in the microcirculation after extreme hemodilution with perfluorocarbons. Am J Physiol. 2004;287(1):H320–H330. doi: 10.1152/ajpheart.01166.2003. [DOI] [PubMed] [Google Scholar]
- 22.Awasthi VD, Garcia D, Goins BA, et al. Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int J Pharm. 2003;253(1–2):121–132. doi: 10.1016/s0378-5173(02)00703-2. [DOI] [PubMed] [Google Scholar]
- 23.Cabrales P, Tsai AG, Winslow RM, et al. Effects of extreme hemodilution with hemoglobin-based O2 carriers on microvascular pressure. Am J Physiol Heart Circ Physiol. 2005;288(5):H2146–H2153. doi: 10.1152/ajpheart.00749.2004. [DOI] [PubMed] [Google Scholar]
- 24.Winslow RM, Vandegriff KD. In: Hemoglobin oxygen affinity and the design of red cell substitutes. Winslow RM, Vandegriff KD, Intaglietta M, editors. Boston: Birkhäuser; 1997. [Google Scholar]
- 25.Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000;278(1):H285–H289. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- 26.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79(3):581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- 27.Frietsch T, Gassmann M, Groth G, et al. Excessive erythrocytosis does not elevate capillary oxygen delivery in subcutaneous mouse tissue. Microcirculation. 2007;14(2):111–123. doi: 10.1080/10739680601131200. [DOI] [PubMed] [Google Scholar]
- 28.Dull RO, DeWitt BJ, Dinavahi R, et al. Quantitative assessment of hemoglobin-induced endothelial barrier dysfunction. J Appl Physiol. 2004;97(5):1930–1937. doi: 10.1152/japplphysiol.00102.2004. [DOI] [PubMed] [Google Scholar]
- 29.Reinke W, Gaehtgens P, Johnson PC. Blood viscosity in small tubes: effect of shear rate, aggregation, and sedimentation. Am J Physiol. 1987;253(3 Pt 2):H540–H547. doi: 10.1152/ajpheart.1987.253.3.H540. [DOI] [PubMed] [Google Scholar]
- 30.Tsai AG, Acero C, Nance PR, et al. Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol Heart Circ Physiol. 2005;288(4):H1730–H1739. doi: 10.1152/ajpheart.00998.2004. [DOI] [PubMed] [Google Scholar]
- 31.Sriram K, Vazquez BY, Yalcin O, et al. The effect of small changes in hematocrit on nitric oxide transport in arterioles. Antioxid Redox Signal. 2011;14(2):175–185. doi: 10.1089/ars.2010.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodgers PA, Vreman HJ, Dennery PA, et al. Sources of carbon monoxide (CO) in biological systems and applications of CO detection technologies. Semin Perinatol. 1994;18(1):2–10. [PubMed] [Google Scholar]
- 33.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 34.Gorman D, Drewry A, Huang YL, et al. The clinical toxicology of carbon monoxide. Toxicology. 2003;187(1):25–38. doi: 10.1016/s0300-483x(03)00005-2. [DOI] [PubMed] [Google Scholar]