Abstract
Evolution in the field of the total synthesis of natural products has led to exciting developments over the last decade. Numerous chemo-selective and enantioselective methodologies have emerged from total syntheses, resulting in efficient access to many important natural product targets. This Review highlights recent developments concerning dearomatization, a powerful strategy for the total synthesis of architecturally complex natural products wherein planar, aromatic scaffolds are converted to three-dimensional molecular architectures.
Keywords: arenes, biomimetic synthesis, natural products, dearomatization, total synthesis
1. Introduction
Since 1865 and the assignment of the benzene structure by the German chemist Friedrich A. Kekulé, suggesting that it is a six-membered ring of carbon atoms with alternating single and double bonds (1,3,5-cyclohexatriene), the physical properties and accurate structure of benzene have been studied in detail. The scientific community has been enthusiastic about the concept of aromaticity owing to a new understanding of benzene stabilization through resonance energy, and hence of all aromatic compounds, which proved to be extremely important for both fundamental and applied chemistry. A quantitative assessment of the degree of aromaticity may be approximated by the value of resonance energy[1] as well as structural and magnetic criteria.[2] Despite the high resonance energy of the benzene ring, a number of examples of dearomatization by microorganisms either through oxidation (oxygenases) or reduction (reductases) exist in nature.[3] Upon dearomatization of aromatic and heteroaromatic derivatives, highly reactive intermediates are generally produced leading to facile formation of carbon–carbon and carbon–heteroatom bonds, spontaneous cycloadditions, and cascade reactions.
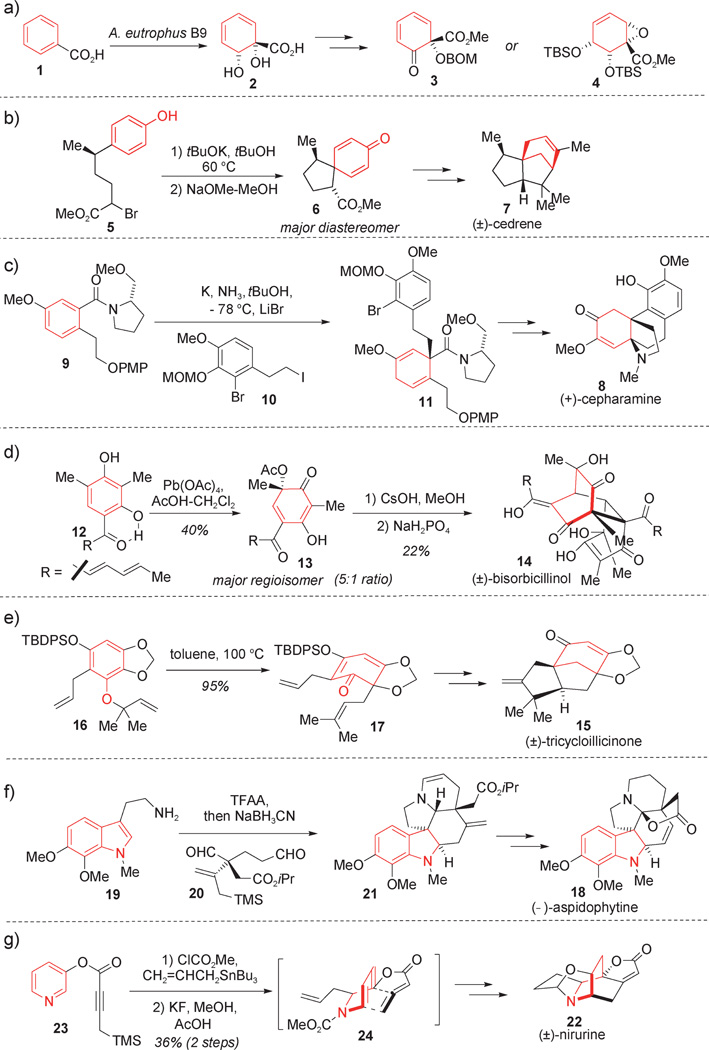
As shown in Figure 1, a number of strategies for dearomatization have been utilized by organic chemists during the course of complex total syntheses. Colored in red are aromatic nuclei that have been dearomatized during the course of these synthetic endeavors. In one classical example, Myers and co-workers applied a chemoenzymatic method for arene dearomatization by the dihydroxylation of benzoic acid (1) using the bacterium A. eutrophus affording 1,2-dihydroxycyclohexadiene 2 with high enantioselectivity (Figure 1 a).[4] The simple, dearomatized cyclohexadienone 2 was parlayed into a number of useful chiral building blocks (e.g. 3 and 4) for use in total synthesis and demonstrates the utility of enzymatic dearomatization. In the 1960s, Corey and coworkers employed the alkylative dearomatization of phenols[5] under basic conditions wherein the phenolate derived from 5 underwent intramolecular para-alkylation to access spirocycle 6 (Figure 1b). Installation of the quaternary center in 6 was crucial to prepare the natural product cedrene (7). Schultz and co-workers, pioneers of the diastereoselective Birch reduction/alkylative dearomatization process, reported the first enantioselective synthesis of the alkaloid (+)- cepharamine (8; Figure 1c).[6] Their route employed a diastereoselective Birch reduction of chiral benzamide 9 to access a chiral enolate intermediate which was alkylated in situ with iodide 10 to afford 1,4-cyclohexadiene 11 in 95% yield as a single diastereomer. In two separate studies, Corey and co-workers reported access to enantiopure (−)-trichodimerol,[7] and Nicolaou and co-workers described the biomimetic synthesis of numerous bisorbicillinoids employing in both cases dearomatization of sorbicillin (12; Figure 1d).[8] In the Nicolaou work, oxidative dearomatization with lead tetraacetate delivered the desired acetylated ortho-acetoxyquinol 13 along with its regioisomer (5:1). Mild and controlled deacetylation generated a highly reactive ortho-quinol which underwent different cascade events depending on the reaction conditions. Upon basic treatment, the reactive dearomatized ortho-quinol spontaneously dimerized in a Diels–Alder fashion to directly produce the natural product (±)-bisorbicillinol (14). In their synthesis of tricycloillicinone (15), Danishefsky and co-workers employed an underdeveloped dearomatization strategy involving an ortho-Claisen rearrangement (Figure 1e).[9] The rearrangement was conducted using the reverse O-prenylated derivative 16 to furnish the desired dearomatized cyclohexadienone 17, a precursor of the natural product. A classical example of indole dearomatization was achieved by Corey and co-workers during the synthesis of the indole alkaloid aspidophytine (18; Figure 1 f).[10] Condensation of tryptamine 19 with enantiopure dialdehyde 20 resulted in intramolecular indole alkylation at C3 generating a dearomatized indolinium that was trapped at C2 by a tethered allylsilane to furnish the aspidophytine core structure 21. This dearomatization cascade rapidly assembled the aspidophytine core bearing four stereocenters, two of which are quaternary. Finally, an impressive example of pyridinium dearomatization was reported in the early 1990s by Magnus and co-workers in their total synthesis of the pentacyclic alkaloid nirurine (22; Figure 1g).[11] Synthesis of the azabicyclo-[2.2.2]octane (isoquinuclidine) core of 22 commenced with the alkylative dearomatization at the C2 position of an acylpyridinium derived from pyridine 23 to introduce an allyl side chain.[12] Further desilylation generated allenoate intermediate 24 which participated in intramolecular [4+2] cycloaddition to furnish the nirurine core structure in two steps.
Figure 1.
Classical examples of dearomatization in complex synthesis: a) Myers et al.;[4] b) Corey et al.;[5] c) Schultz et al.;[6] d) Nicolaou et al.;[8] e) Danishefsky et al.;[9] f) Corey et al.;[10] g) Magnus et al.[11] Abbreviations of reagents and protecting groups are defined at the end of the Review.
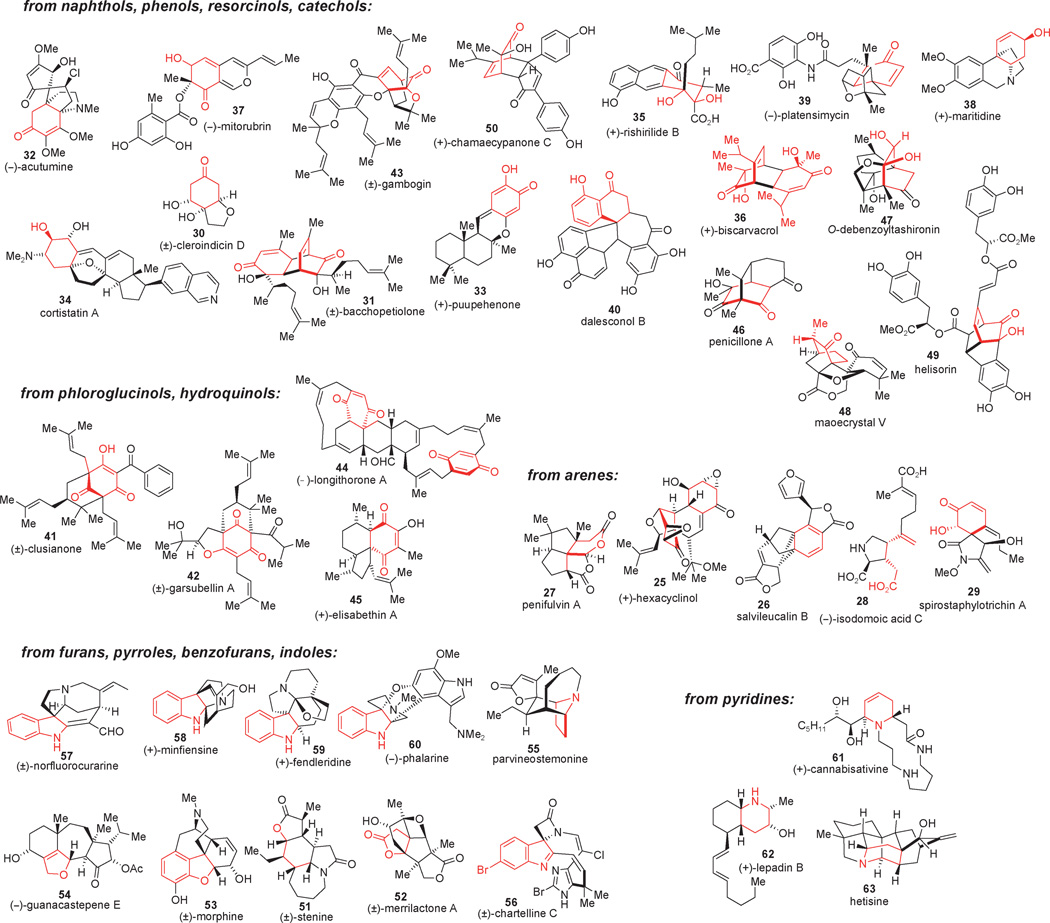
These classical and elaborated examples of dearomatization for the synthesis of complex targets have set the stage for new chemistry to be explored and for novel applications of dearomatization for the construction of complex scaffolds. In the early 1990s, Mander emphasized dearomatization strategies in a review concerning alkylative processes for the synthesis of polycyclic natural products.[13] Other important reviews have reported methodology developed for dearomatization using transition-metal complexation of simple arenes.[14] More recently, Pettus and co-workers have exhaustively reviewed the preparation and use of masked ortho- and para-benzoquinones (MOBs and MPBs) and their derived benzoquinol derivatives.[15] Quideau and co-workers have also comprehensively reviewed the oxidative dearomatization of phenols and anilines using both iodine(III) and iodine(V) reagents,[16] culminating in a recent review outlining applications of this chemistry in natural product synthesis.[17] In this review, we will focus on the dearomatization of arenes, phenols, and heteroarenes as part of total syntheses of complex natural products (cf. 25–63, Figure 2) from 2002 to the present. Natural product fragments derived from dearomatization are colored in red (Figure 2). In addition to outlining select dearomatization strategies in the context of complex natural product synthesis, we will also describe future perspectives in the field including prospects for the development of enantioselective dearomatization processes.
Figure 2.
Recent work highlighting syntheses of complex natural products using dearomatization strategies (2002–2010).
2. Dearomatization of Arenes
For decades, the dearomatization of arenes has been recognized as a chemical transformation of fundamental importance and provides a connection between a robust and abundant source of hydrocarbons and the alicyclic frameworks found in many biologicaly active products.[13, 14] Accordingly, benzene and its derivatives are attractive starting materials with great potential to deliver complex alicyclic building blocks containing unmasked functionality, new carbon–carbon bonds, and stereogenic centers.
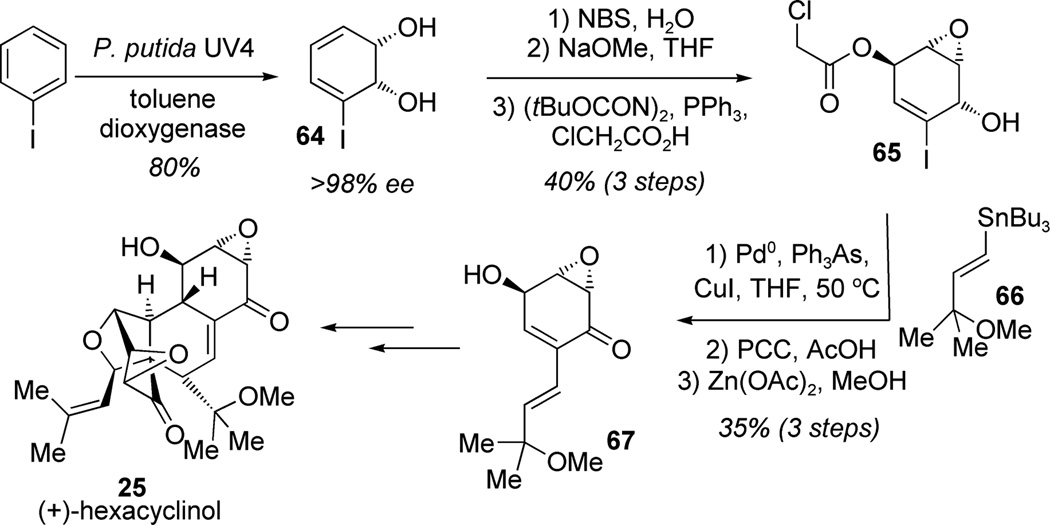
Although enzymatic and microbiological techniques are not often emphasized in organic chemistry laboratories, microorganisms are capable of useful transformations on a preparative scale for the production of enantiopure building blocks. For example, the enantiomerically pure cyclohexenediol 64 (>98% ee) was obtained from the enzymatic dihydroxylation of iodobenzene with the toluene dioxygenase P. putida UV4 (Scheme 1).[18] Banwell and co-workers employed the dearomatized synthon 64 in their synthesis of hexacyclinol (25; (Scheme 1).[19] Bromination of the dearomatized derivative 64 delivered the corresponding bromohydrin which was further converted in two steps to the chiral enantiopure building block 65 in 40% overall yield. The protected epoxydiol 65 was cross-coupled with vinyl stannane 66, oxidized, and deprotected to deliver the epoxyquinol monomer 67 which was dimerized and deprotected to yield the antiproliferative natural product (+)-hexacyclinol (25) in nine steps overall from iodobenzene.
Scheme 1.
Preparation of epoxyquinol synthons by enzymatic dihydroxylation of iodobenzene and elaboration to hexacyclinol (Banwell et al., 2009).[19]
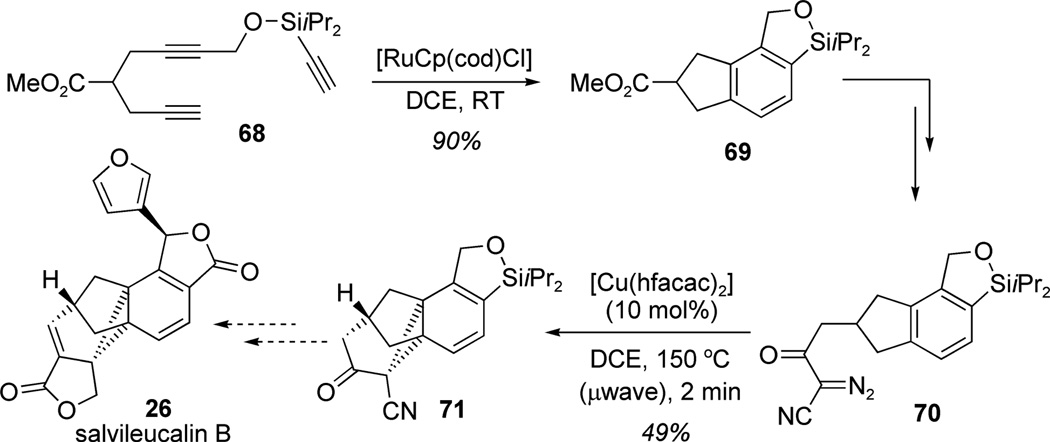
In the early 1990s, Mander and co-workers demonstrated the efficiency of carbene-based methodology for the dearomatization of arenes by means of cyclopropanation (Buchner reaction).[20] In a recent synthesis of the norcaradiene core of salvileucalin B (26), Reisman and co-workers also applied the Buchner reaction to assemble the natural product core (Scheme 2).[21] Exposure of triyne 68 to a catalytic amount of [RuCp(cod)Cl] enabled [2+2+2] cycloaddition leading to the production of indane derivative 69 in 90% yield. After a few manipulations, microwave thermolysis of α-diazo β-ketonitrile 70 in the presence of 10 mol%[Cu(hfacac)2] led to arene dearomatization to afford the fused cyclopropane 71 in 49% yield (Scheme 2). Late-stage intramolecular cyclopropanation of tricyclic arene 70 thereby provided rapid access to the norcaradiene core of the natural product and demonstrated the feasibility of the proposed route to salvileucalin B (26).
Scheme 2.
Approach to salvileucalin B: Preparation of the pentacyclic framework by Buchner dearomatization (Reisman et al., 2010).[21]
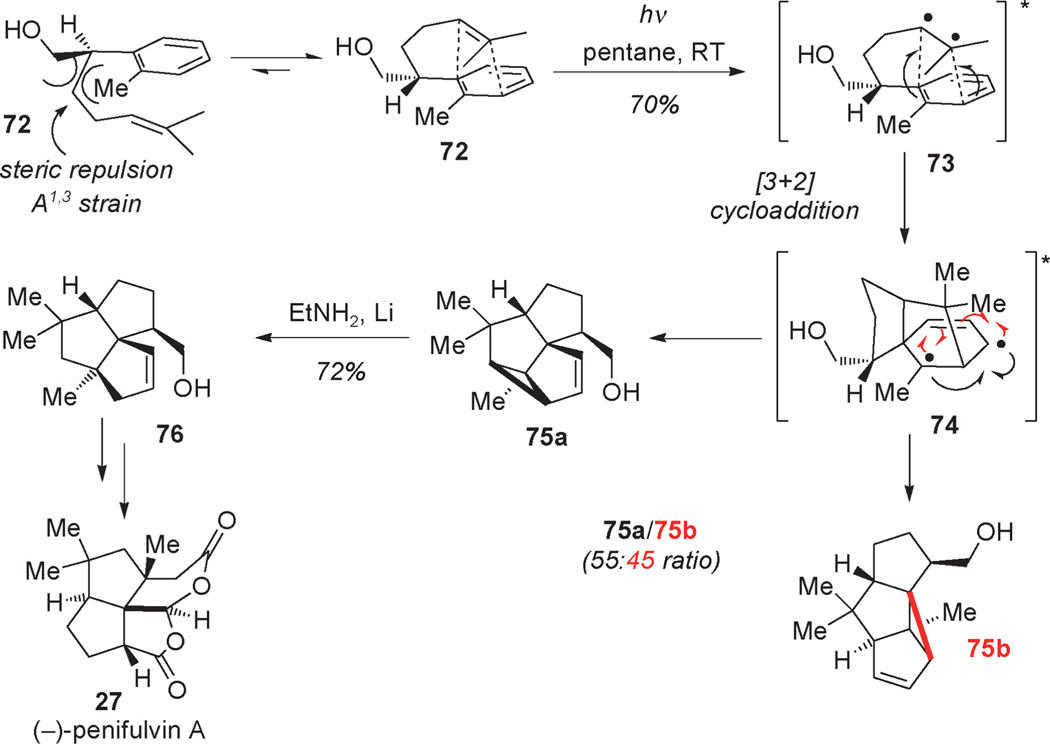
Wender’s remarkable synthesis of α-cedrene brought the attention of synthetic chemists to the alkene–arene meta-photocycloaddition, a formal [3+2] photocycloaddition.[22] Recently, Mulzer and co-workers highlighted the importance of this seminal work on the photoinduced intramolecular [3+2] cycloaddition of arenes and olefins in the enantioselective synthesis of the sesquiterpenoid (−)-penifulvin A (27; Scheme 3).[23] The asymmetric synthesis of (−)-27 was initiated by enantioselective alkylation using the Myers’ auxiliary which after several manipulations delivered enantiopure alcohol 72. Irradiation of arene substrate 72 to the singlet excited state generated excimer 73 which underwent meta-photocycloaddition to diradical 74 and produced a mixture of regioisomers 75a and 75b. In this example, π-facial stereocontrol is dictated by A1,3 strain involving steric interactions between the aryl methyl and the hydroxymethyl group as well as endo control in the photocycloaddition.[22] Final cyclization of diradical 74 delivered the tricyclic core bearing two contiguous quaternary centers as a mixture of constitutional isomers 75 a and 75b. Final Birch reduction of isomer 75 a afforded the desired triquinane framework 76 which was advanced to (−)-penifulvin A (27) using an efficient and innovative oxidative cascade sequence to form the two fused lactone rings in a single operation.
Scheme 3.
Total synthesis of penifulvin A using photoinduced [3+2] cycloaddition (Mulzer et al., 2009).[23]
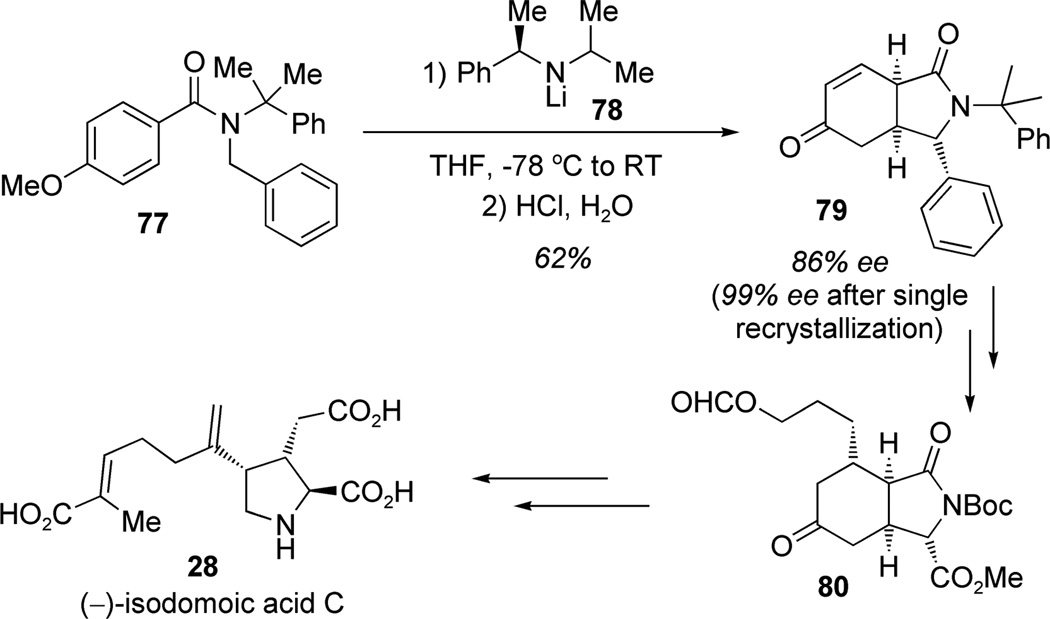
An important approach for arene dearomatization pioneered by Meyers and co-workers involves diastereoselective carbanion addition to aromatic rings.[24] Clayden and co-workers have further advanced this area and achieved the first enantioselectice total synthesis of (−)-isodomoic acid C (28) in 15 steps from benzamide 77 (Scheme 4).[25] Asymmetric deprotonation of N-benzyl benzamide 77 using the chiral lithium amide 78 was followed by dearomatizing anionic cyclization to afford bicyclic enone 79 (86%ee) after acidic hydrolysis. Further transformations led to ketone 80 which was ultimately advanced to (−)-isodomoic acid C (28).
Scheme 4.
Enantioselective synthesis of (−)-isodomoic acid C by de-aromatizing anionic cyclization (Clayden et al., 2005).[25]
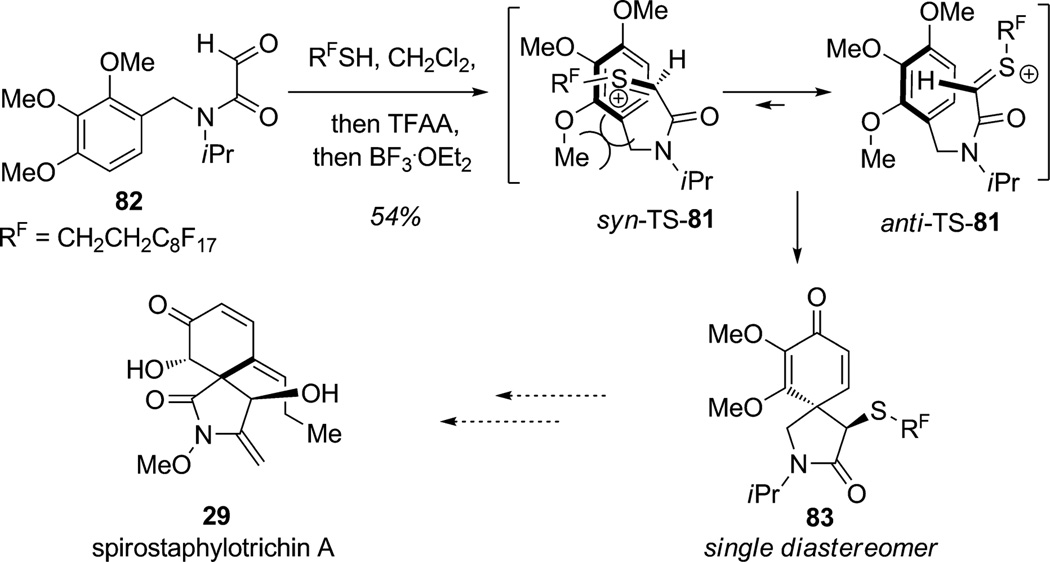
In 2008, Procter and co-workers reported thionium activation of aryl α-ketoamide substrates resulting in arene dearomatization (Scheme 5).[26] Specifically, thionium ion 81, generated by addition of thiols (RF=CH2CH2C8F17) to N-benzylglyoxamide derivative 82, underwent an unprecedented dearomatizing spirocyclization. The highly activated thionium species participated in diastereoselective spirocyclization; steric interactions between the ortho-methoxy group and the thionium moiety favored the anti transition state 81, leading to 2-azaspiro[4.5]decane 83 as a single diastereomer. This flexible methodology was applied to several substrates leading to simultaneous formation of carbon–carbon and carbon–sulfur bonds with two new stereocenters. This creative strategy may be applied in the future to the synthesis of spirocyclic alkaloids including spirostaphylotrichin A (29).
Scheme 5.
Alkylative dearomatization by thionium activation (Procter et al., 2008).[26]
3. Dearomatization of Phenols and Related Substrates
Phenols are by far the most frequently utilized substrates for dearomatization en route to complex natural products. The following section will be devoted to an overview of phenol dearomatization through the formation of C–X and C–C bonds. Catechol, resorcinol, and hydroquinone derivatives have been dearomatized to cyclohexadienones, whose reactivity varies depending on their intrinsic electronic stabilization. The resulting dearomatized cyclohexadienone intermediates generally react spontaneously or upon chemical activation to generate complex polycyclic frameworks. In addition, alkylative dearomatization of phenolate anions and sigmatropic, dearomatizing reactions will be presented along with oxidative dearomatization/Diels–Alder cascade strategies.
3.1. Oxidative Dearomatization of Phenols by O-Alkylation
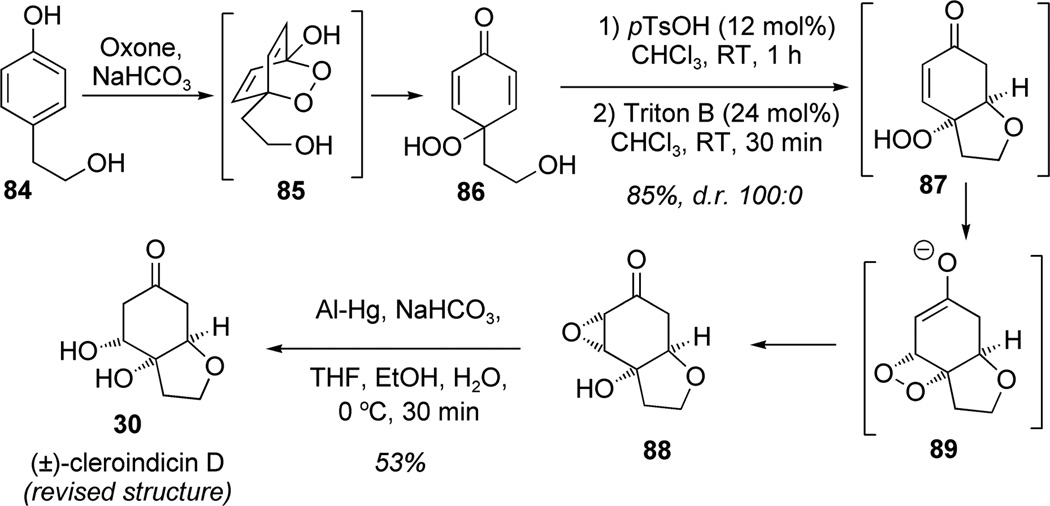
In general, dearomatization of phenols with oxygen-centered nucleophiles involves activation with hypervalent iodine or other oxidants resulting in either ortho-dearomatization (ortho-quinol or masked ortho-benzoquinone (MOBs)) or para-dearomatization (para-quinol or para-benzoquinone). Carreño and Urbano developed an efficient method for the selective oxidative dearomatization of para-alkyl phenols to access para-peroxyquinols and para-quinols using Oxone as a source of singlet oxygen.[27] This proved to be an attractive method for the synthesis of (±)-cleroindicin D (30; Scheme 6).[28] Dearomatization of para-(2-hydroxyethyl) phenol 84 with Oxone afforded the cyclic endoperoxide 85 which was readily ring-opened to the corresponding para-peroxyquinol 86. The resulting prochiral para-peroxyquinol 86 was treated sequentially with pTsOH and Triton-B to afford the cyclic peroxyenone 87 which rearranged diastereospecifically to the desired tricyclic epoxide 88 via proposed dioxetane intermediate 89. In this sequence, oxidative dearomatization and consecutive acidic/basic treatments efficiently created two new rings with four contiguous stereogenic centers in a diastereoselective manner. This methodology allowed for the synthesis and stereochemical assignment of (±)-cleroindicin D (30).
Scheme 6.
Total synthesis of cleroindicin using oxidative dearomatization with Oxone (Carreño/Urbano et al., 2007).[28]
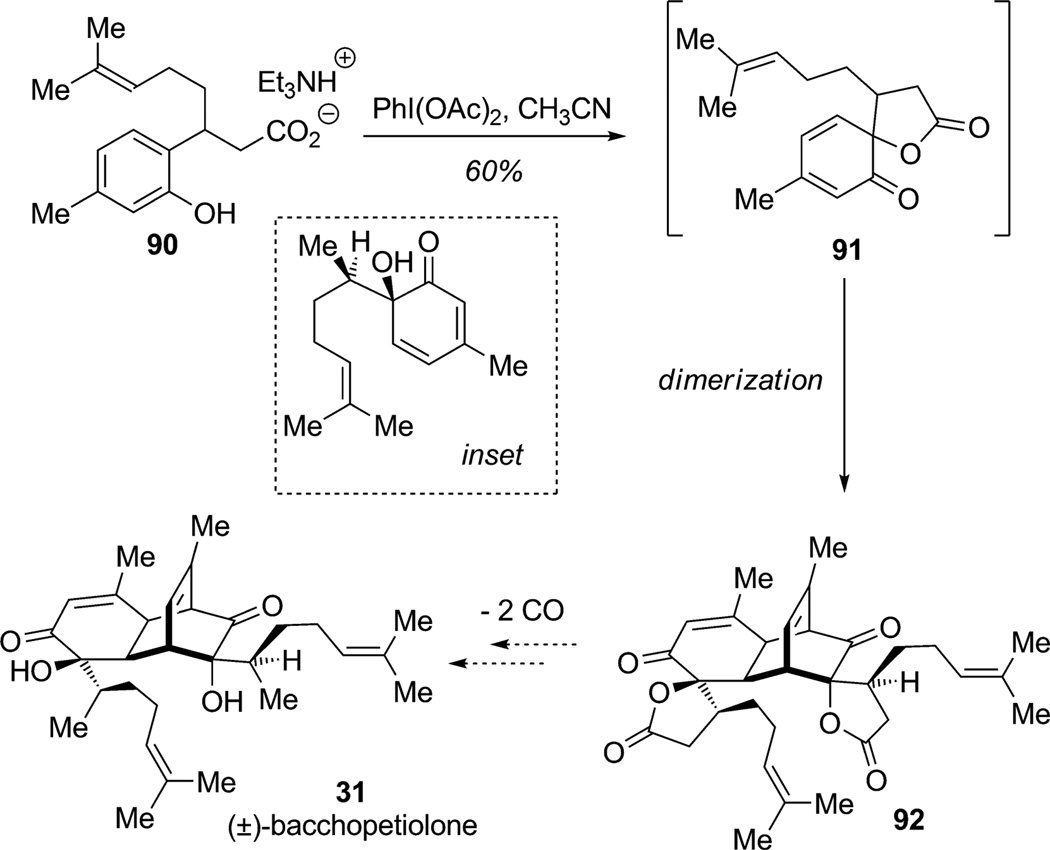
An underdeveloped oxidative dearomatization methodology involves oxidative spirolactonization with iodine(III) reagents.[29] This approach is exemplified by work conducted by Wood and co-workers en route to the natural product (±)-bacchopetiolone (31; Scheme 7).[29c] Indeed, bacchopetiolone may be derived from biosynthetic [4+2] dimerization of a bisabolene monomer (Scheme 7, see inset). The authors envisioned oxidative spirolactonization of the carboxylic salt 90 to access the monomeric subunit 91, a surrogate for the bisabolene monomer, which spontaneously dimerized to furnish the complex natural product framework 92 in a single step. Wood and co-workers also extensively examined lactone opening and Suárez fragmentation of the advanced intermediate 92.[29c] Further studies are underway targeting the removal of two carbon monoxide units to complete the synthesis of (±)-bacchopetiolone (31).
Scheme 7.
Approach to bacchopetiolone employing oxidative spirolactonization/dearomatization (Wood et al., 2006).[29c]
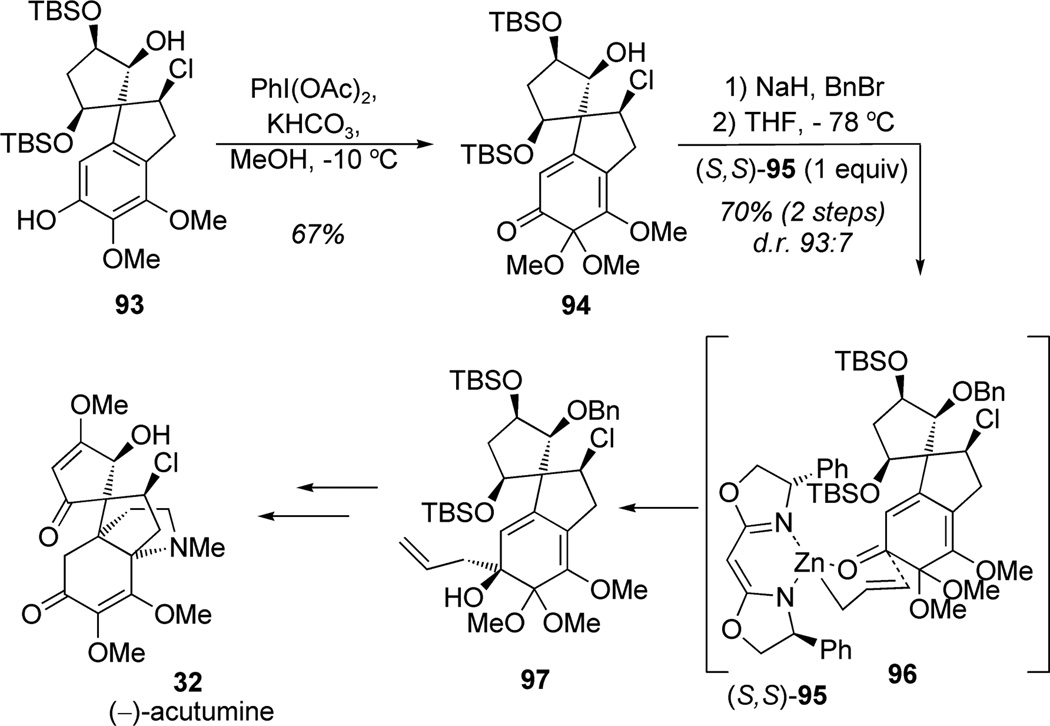
In the first total synthesis of (−)-acutumine (32), Castle and co-workers outlined the utility of the oxidative dearomatization of chiral chloroindane 93 to the corresponding MOB derivative 94 (Scheme 8).[30] Late-stage phenolic oxidation of phenol 93 was accomplished using bis(acetoxy)iodobenzene (PIDA) in 67% yield. This result highlights the utility of iodine(III) reagents for the oxidative dearomatization of highly complex substrates. Allylation of a protected form of ketone 94 was examined, first with allylmagnesium bromide, which showed modest diastereoselection (d.r. 7:3), and finally using Nakamura’s chiral allylzinc reagent (S,S)-95. Catalyst control in the latter instance led to allyl addition via the proposed transition state 96 to yield the desired allylic alcohol 97 (d.r. 93:7). Further manipulations, including an anionic oxy-Cope rearrangement, led to an elegant asymmetric synthesis of (−)-acutumine (32).
Scheme 8.
Total synthesis of acutumine including late-stage dearomatization (Castle et al., 2009).[30]
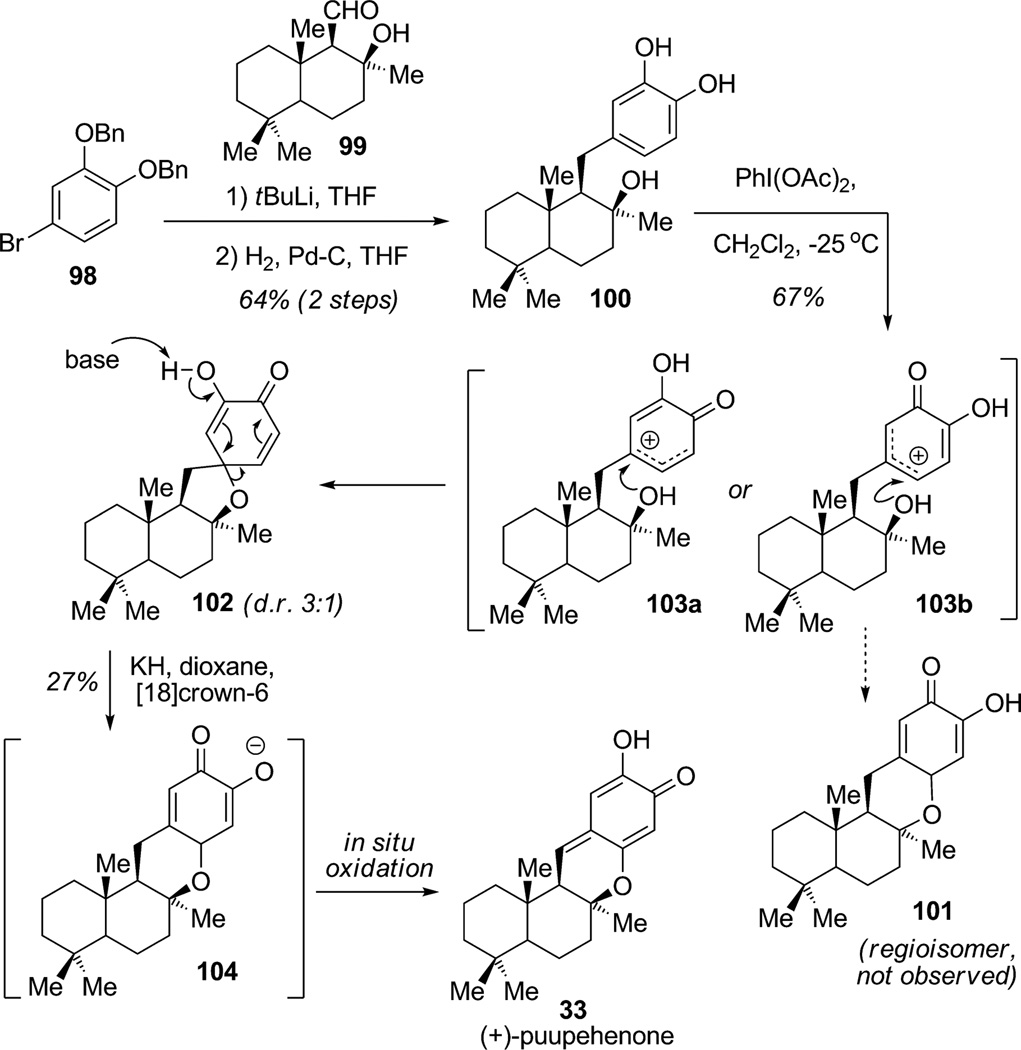
Quideau and co-workers reported the biomimetic synthesis of (+)-puupehenone (33), a drimane sesquiterpene appended with a shikimate-derived hydroxyquinone fragment (Scheme 9).[31] In this interesting study, the authors examined the regioselectivity of the oxidative dearomatization of an unsymmetrical catechol. Addition of the anion of protected bromocatechol 98 to enantiopure aldehyde 99 produced catechol 100 in 64% overall yield after hydrogenolysis. The key regioselective oxidative activation of catechol 100 using hypervalent iodine (PIDA) did not afford the desired tetracyclic fused-ring system 101 but led instead to spirocycle 102 (d.r. 3:1). Accordingly, the authors proposed that the drimane framework exhibits an electron-releasing effect stabilizing the more substituted carbocation intermediate 103 a versus 103b thereby triggering exclusive 5-exo-trig spiroannulation. Further rearrangement under basic conditions permitted a 1,2-oxyalkyl shift to the fused heterocyclic intermediate 104, which spontaneously oxidized in situ to furnish synthetic (+)-puupehenone (33) in 27% yield.
Scheme 9.
Total synthesis of (+)-puupehenone involving the regio-selective oxidative dearomatization of a catechol (Quideau et al., 2002).[31]
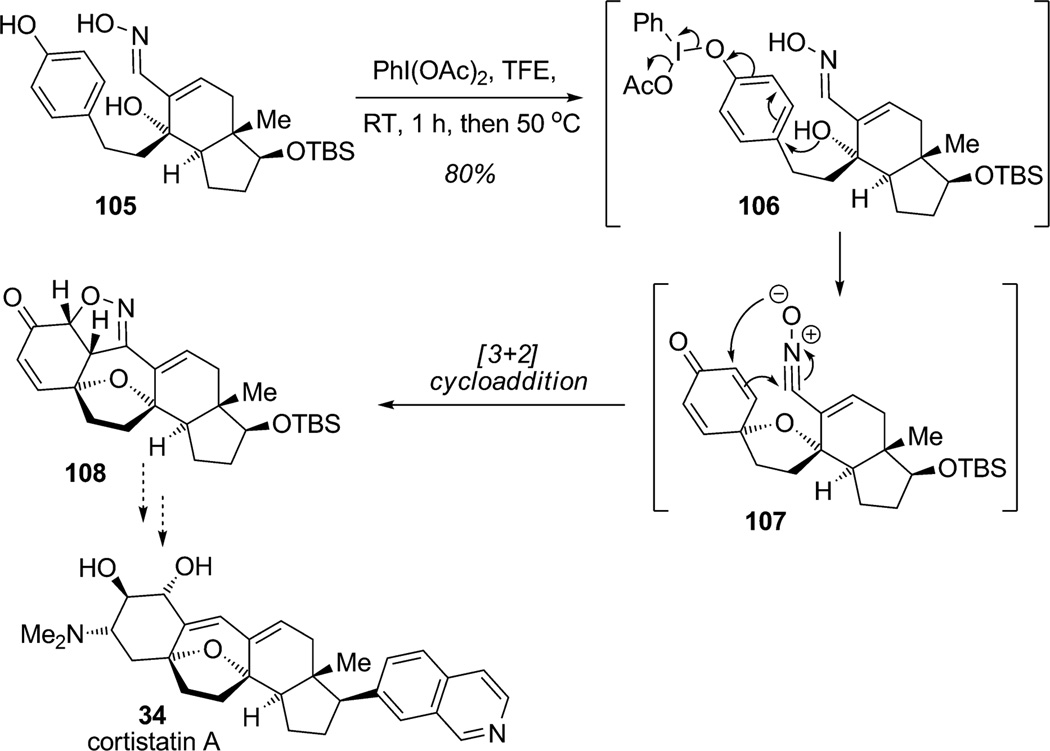
Recently, a cascade process triggered by intramolecular oxidative dearomatization was reported by Sorensen and coworkers for construction of the pentacyclic core of cortistatin A (34; Scheme 10).[32] This elegant sequence illustrates the power of oxidative dearomatization in complex settings employing tandem intramolecular oxidative para-dearomatization/intramolecular dipolar cycloaddition. Exposure of the advanced phenol 105 to PIDA led to phenol activation followed by nucleophilic attack of the proximate tertiary alcohol via intermediate 106. Further oxidation of the oxime to a nitrile oxide generated both reaction partners for intramolecular [3+2] dipolar cycloaddition of substrate 107.[33] These two successive transformations carried out in the same reaction vessel produced the hexacyclic product 108 as a single diastereomer, a compound presenting the pentacyclic core architecture of cortistatin A (34).
Scheme 10.
Approach to the cortisatin A framework through tandem oxidative dearomatization/[3+2] cycloaddition (Sorensen et al., 2010).[32]
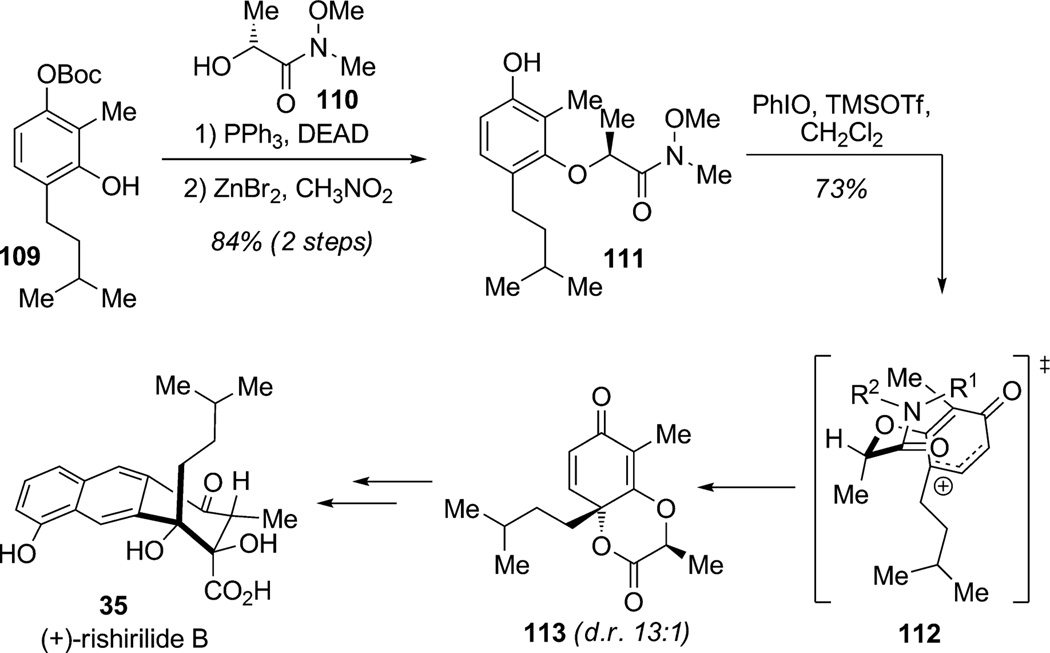
In their studies, the Quideau and Pettus research groups have developed significant oxidative methods involving ortho-dearomatization[34] of catechols and para-dearomatization[35] of resorcinols (Scheme 11 and Scheme 12). A concise asymmetric synthesis of (+)-rishirilide B (35) reported by Pettus and co-workers highlights two highly efficient methods: 1) coupling of ortho-quinone methides and 2) diastereoselective dearomatization of resorcinols providing access to densely functionalized bicyclic enones (Scheme 11).[36] Resorcinol 109 was coupled through a Mitsunobu reaction to lactate derivative 110 to afford substrate 111 after deprotection. Diasteroselective oxidative dearomatization of 111 using in situ generated PhI[OTMS]OTf[35] presumably proceeded via the chairlike transition state 112 and led to chirality transfer from the chiral auxillary to afford 1,4-dioxan-2-one 113 in high diastereoselectivity. Further transformations of 113 completed an efficient asymmetric total synthesis of (+)-rishirilide B (35) in 15 steps and 12.5% overall yield.
Scheme 11.
Total synthesis of (+)-rishirilide B by means of the diastereoselective para-oxidative dearomatization of a resorcinol substrate (Pettus et al., 2006).[36]
Scheme 12.
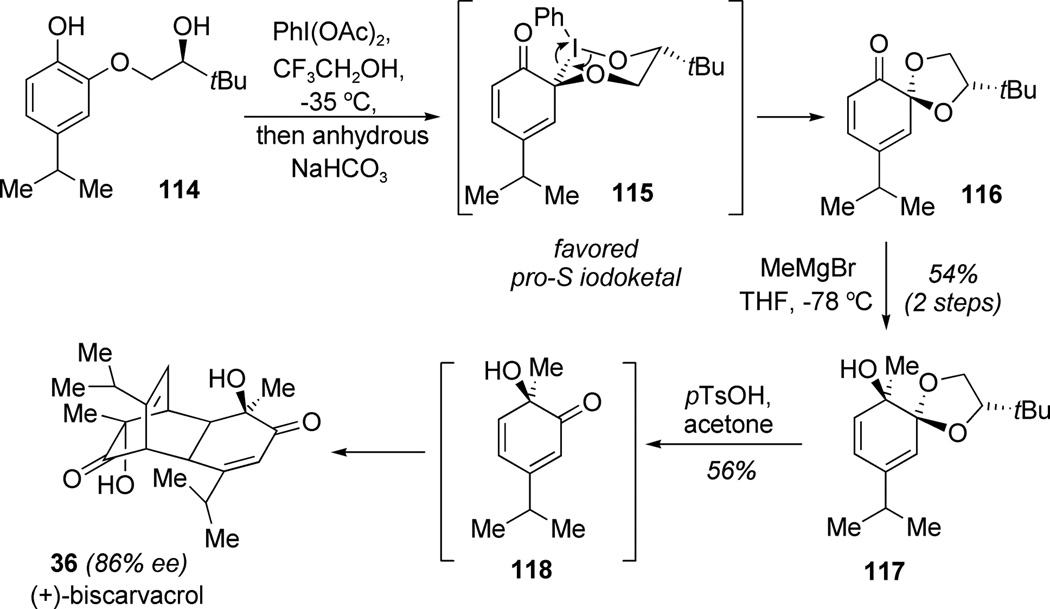
Total synthesis of (+)-biscarvacrol using a tethered chiral auxiliary for ortho-oxidative dearomatization (Quideau et al., 2008).[37]
Quideau and co-workers developed an efficient route to masked ortho-benzoquinones (MOBs) in chiral, nonracemic form through the λ3-iodane (PIDA)-mediated ortho-oxidative dearomatization of catechols. The methodology was successfully applied to the asymmetric total synthesis of the bis(monoterpene) (+)-biscarvacrol (36; Scheme 12).[37] During this synthesis, the authors demonstrated the utility of a chiral tether for the diastereoselective spiroketalization of resorcinol 114. A chairlike iodine(III) intermediate 115, which may be stabilized by orbital interactions between the iodine and spiro-carbon atom, was proposed to explain the stereochemical outcome of the reaction and production of (S,S)-MOB diastereomer 116. The addition of methyl Grignard to ketone 116 from the less hindered face was found to be highly diastereoselective (d.r. >95:5) and afforded alcohol 117 in 54% yield over two steps. The ketal auxiliary was finally removed under acidic conditions which initiated spontaneous and regioselective cyclodimerization of ortho-quinol intermediate 118 to furnish (+)-biscarvacrol (36) as the major stereoisomer (86% ee).
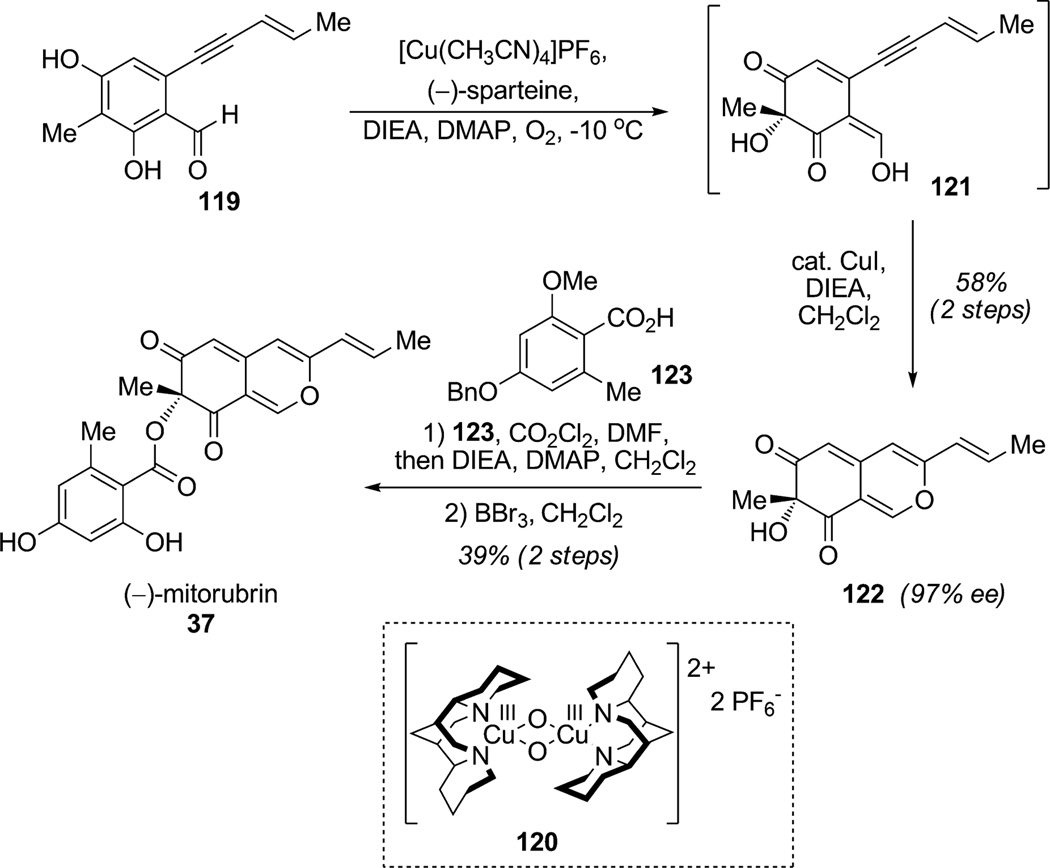
Porco and co-workers described the synthesis of (−)-mitorubrin (37) and related azaphilone natural products employing enantioselective oxidative dearomatization of resorcinols (Scheme 13).[38] Dearomatization of the resorcinol aldehyde 119 using the [{(−)-sparteine}2Cu2O2] complex 120 was achieved in a regioselective manner with high enantioslectivity to afford vinylogous acid 121. Enyne 121 was subjected to CuI-catalyzed cycloisomerization to afford the mitorubrin core structure 122 (58% for two steps, 97%ee). Further esterification with acid 123 and final deprotection afforded the desired azaphilone (−)-mitorubrin (37). This convergent synthesis featured the highly enantioselective oxidative dearomatization of resorcinol aldehyde 119 using a readily accessible chiral bis-μ-oxo copper complex.
Scheme 13.
Total synthesis of (−)-mitorubin employing enantioselective ortho-oxidative dearomatization (Porco, Jr. et al., 2006).[38]
3.2. Oxidative and Alkylative Dearomatization of Phenols with Concomittant C–C Bond Formation
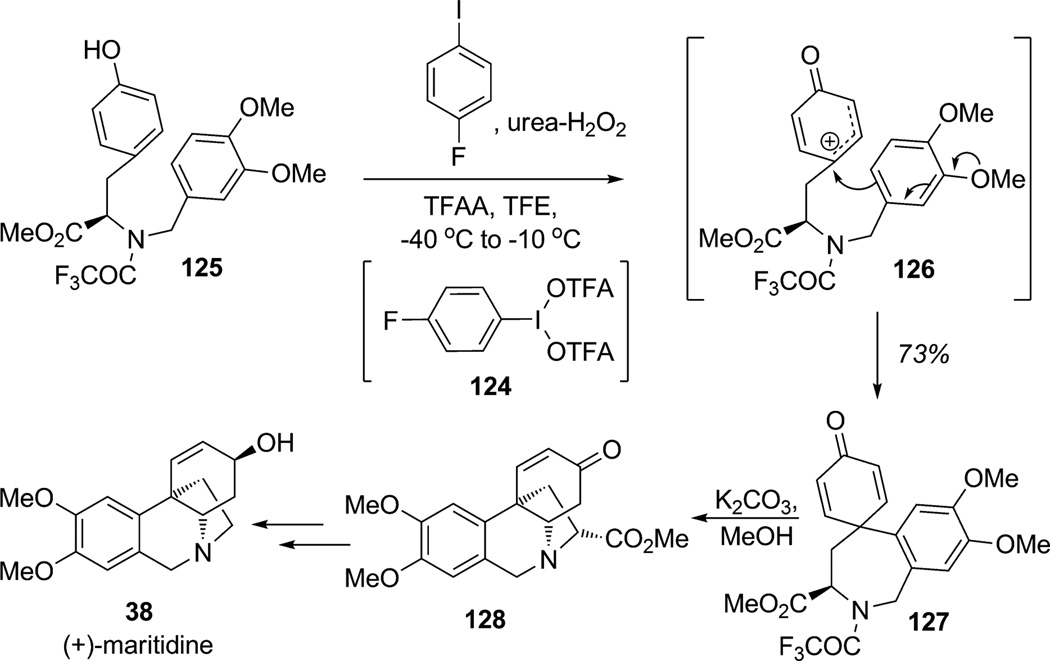
As highlighted in the previous section, many oxidative dearomatizations involve the introduction of soft heteroatomic nucleophiles.[39] Despite a few sparse examples, C–C bond formation during oxidative dearomatization is of significant interest in complex natural product synthesis.[40] Recently, Kita and co-workers have developed catalytic processes for iodoarene-catalyzed C–C bond formations of phenols.[41] Use of the novel and recyclable iodine(III) reagent (bisacetoxy)iodozo(4-fluoro)benzene 124 (prepared in situ from 1-fluoro-4-iodobenzene and urea–H2O2 (UHP) with trifluoroacetic anhydride (TFAA)) should allow new possibilities for oxidative dearomatization (Scheme 14).[41] In fact, the highly reactive iodine(III) reagent 124 can be produced catalytically and was shown to react with phenolic substrate 125 to produce the discrete carbocation intermediate 126 which was selectively trapped by the pendant aromatic ring to afford the desired spirocyclic amino ester 127. After trifluoroacetamide removal, further cyclizaton to 128 was accomplished completing a formal synthesis of (+)-maritidine (38).
Scheme 14.
Formal synthesis of (+)-maritidine employing oxidative dearomatization/C-arylation (Kita et al., 2008).[41]
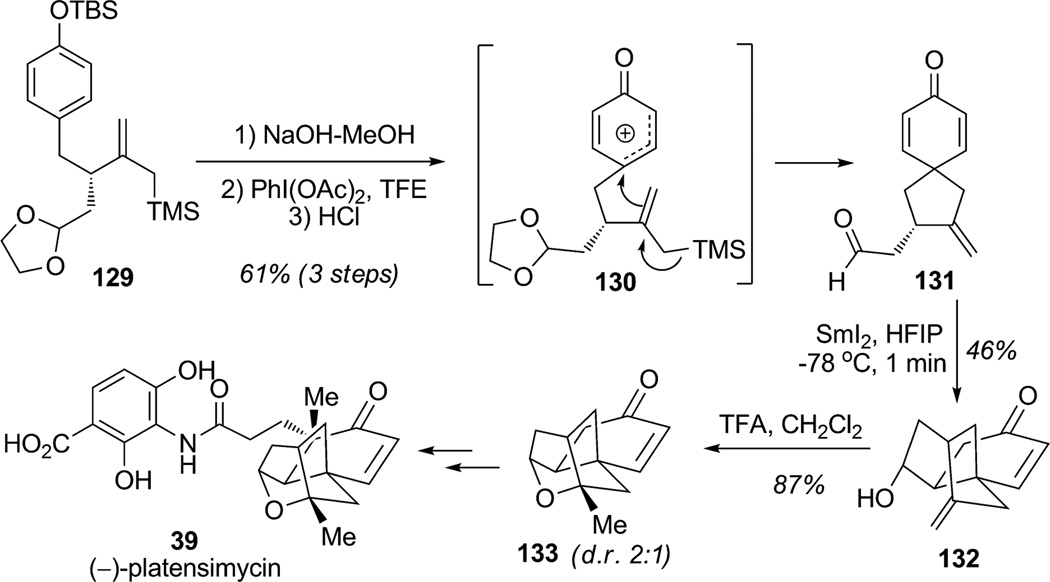
An important example of the oxidative dearomatization of a phenolic substrate with concomittant C–C bond formation in the context of complex total synthesis was reported by Nicolaou and co-workers in their enantioselective synthesis of (−)-platensimycin (39; Scheme 15).[42] The authors employed oxidative dearomatization with intramolecular para-spiroannulation of a pendant allylsilane[43] using hypervalent iodine activation to assemble the first two rings of the natural product. Allylsilane 129 was prepared enantioselectively using the Myers’ auxiliary for asymmetric alkylation. After silyl deprotection under basic conditions, the key spirocyclization/dearomatization was then investigated using iodine- (III) reagents. Only few examples of nonaromatic carboncentered nucleophiles in iodine(III)-mediated oxidative dearomatizations have been reported. Activation of the free phenol moiety by PIDA in a polar solvent (TFE) afforded the activated intermediate 130 bearing a delocalized carbocation which reacted internally with the allylsilane to furnish the desired spirocyclic dienone. Subsequent removal of the ethylene acetal group led to the free aldehyde substrate 131 in 61% overall yield. Further radical-mediated cyclization using samarium diodide furnished tricyclic product 132 which underwent acid-mediated etherification to efficiently produce the tetracyclic core of platensimycin 133.
Scheme 15.
Total synthesis of (−)-platensimycin involving an oxidative para-spiroannulation strategy (Nicolaou et al., 2007).[42]
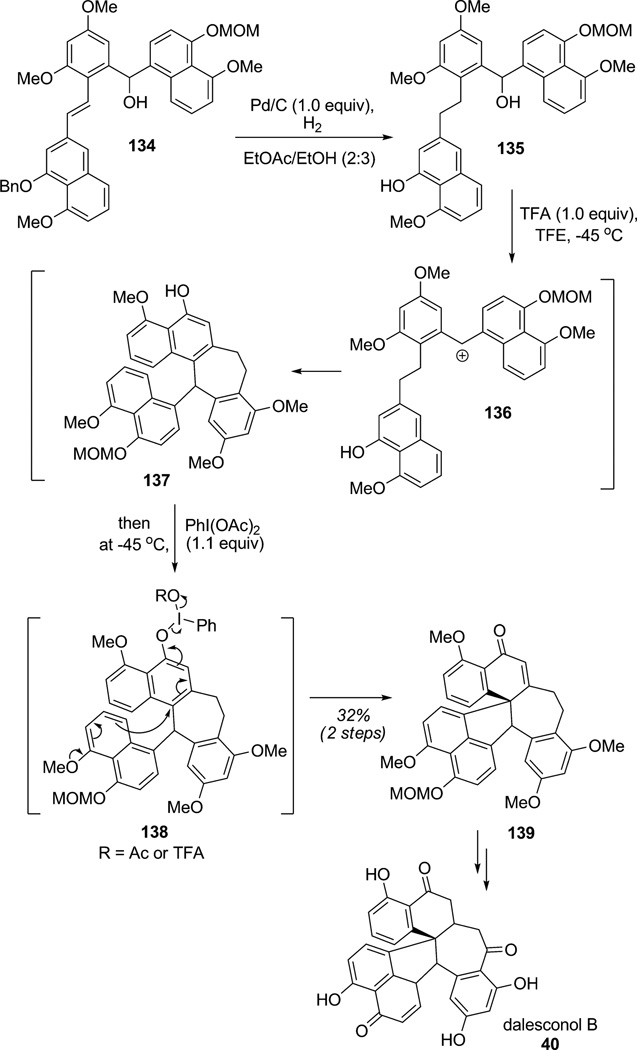
In a recent synthesis of the immunosuppressive polyketide dalesconol B (40), Snyder and co-workers employed oxidative dearomatization as part of a clever tandem process (Scheme 16).[44] In this sequence, finetuning of the two phenol protecting groups and the acid sources were required for the sequence of Friedel–Crafts cyclizations. Indeed, in such complex syntheses of polyphenolic structures, stabilized carbocations from retro-Friedel–Crafts or skeletal rearrangements may occur and generate multiple polycyclic structures. Hydrogenation of substrate 134 delivered the isolable free phenol 135. The latter compound was first treated with TFA producing carbocation 136 thereby triggering intramolecular Friedel–Crafts alkylation to 137 in a controlled manner. Subsequent oxidation of naphthol 137 in the same reaction vessel using PIDA afforded 138 which initiated oxidative dearomatization and assembly of the polycyclic dalesconol precursor 139 in 32%overall yield. This remarkable access to the complex heptacyclic core structure through tandem Friedel–Crafts/oxidative dearomatization paved the way for the total synthesis of dalesconol B after a few additional steps.
Scheme 16.
Total synthesis of dalesconol B employing a Friedel–Crafts/oxidative para-cyclization tandem sequence (Snyder et al., 2010).[44]
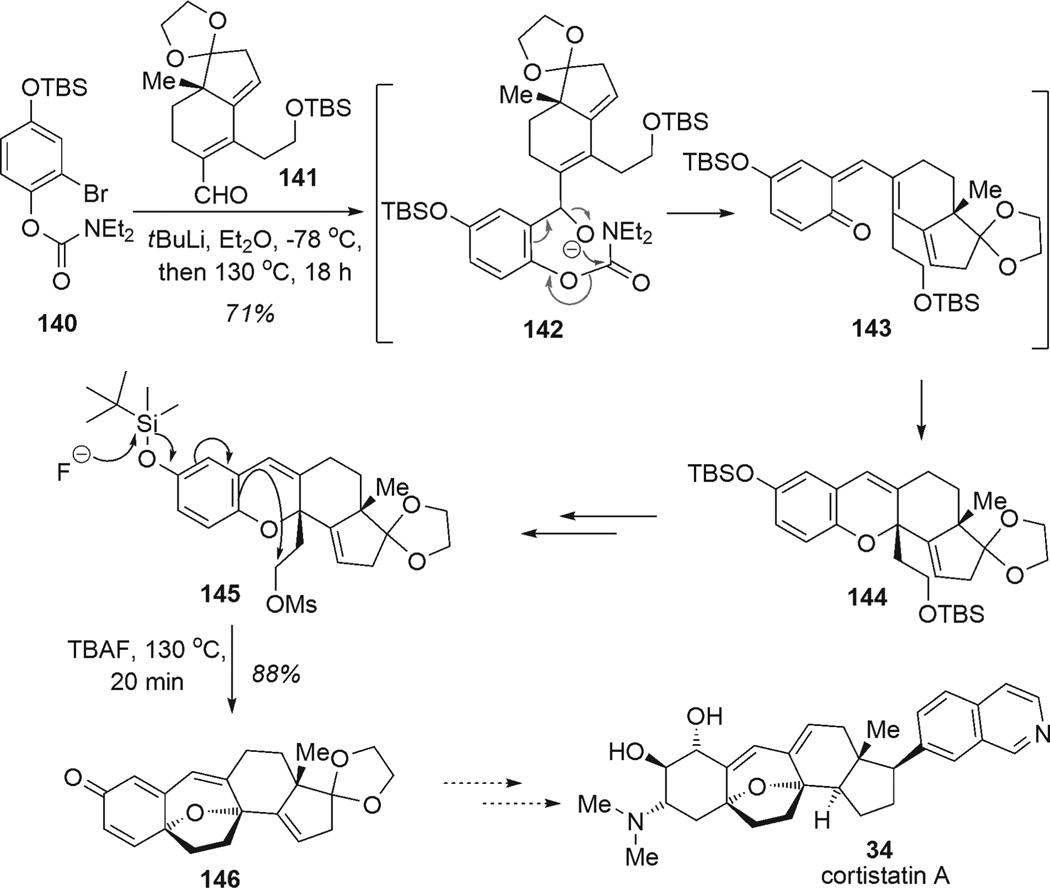
Other useful methodologies have emerged for the construction of polycyclic frameworks involving para-alkylation of phenols under basic conditions.[45] Danishefsky and coworkers recently reported their efforts toward cortistatin A (34) using a cascade sequence involving electrocyclization followed by alkylative para-dearomatization of a phenol (Scheme 17).[46] Addition of the aryllithium derived from aryl bromide 140 to aldehyde 141 initiated a cascade process involving acyl transfer, elimination to ortho-quinone methide 143, and 6π-electrocyclization leading to isolation of dihydropyran 144 in 71% yield. After protecting group manipulations, alkylative dearomatization of substrate 145 was accomplished using a fluoride source at elevated temperature to promote regioselective spirocyclization and form the last ether ring of the cortistatin core 146 (88% yield).
Scheme 17.
Approach to cortistatin A by means of para-alkylative phenol dearomatization (Danishefsky et al., 2008).[46]
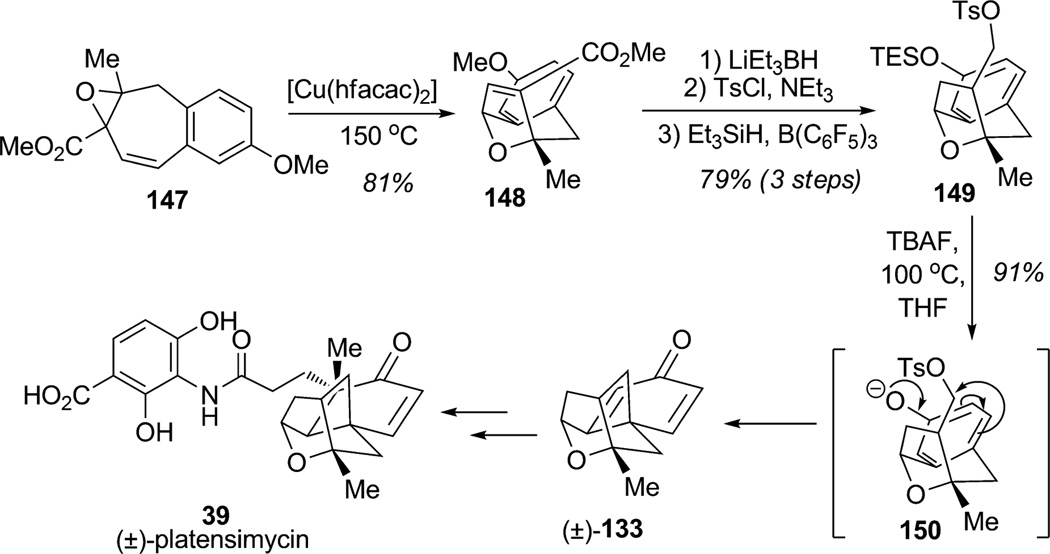
Utilizing a similar para-alkylation of a phenolate, Njardarson and co-workers developed a short route to dienone (±)-133, a key intermediate in the synthesis of platensimycin (39; Scheme 18).[47] For this purpose, the vinyl oxirane substrate 147 was subjected to ring-expansion catalyzed by a copper(II) catalyst to effect rearrangement to the desired bicyclo[2.3.1] ether 148 in 81% yield. Further reduction of 148, followed by tosylation of the primary alcohol and silylation, afforded silyl ether 149. Treatment of the protected phenol 149 with a fluoride source under thermal conditions promoted smooth spirocyclization via 150 which generated the tetracyclic framework and the crucial quaternary stereocenter of the platensimycin core 133 (91% yield).
Scheme 18.
Approach to (±)-platensimycin using intramolecular para-alkylative phenol dearomatization (Njardarson et al., 2009).[47]
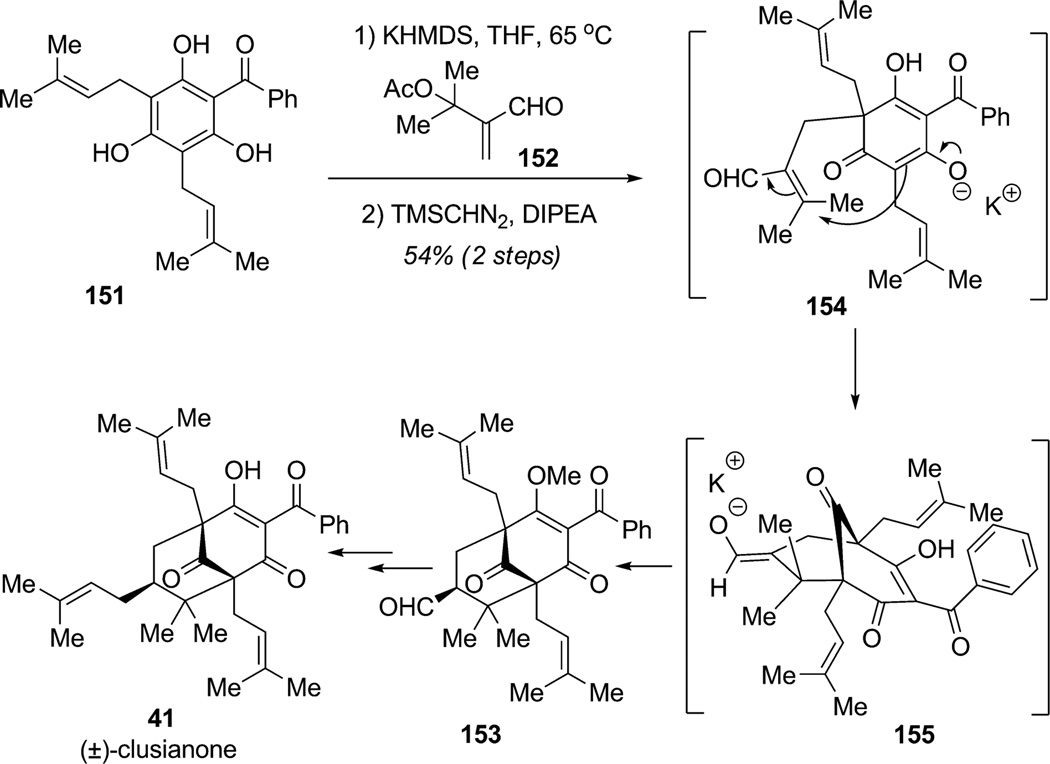
Porco and co-workers reported efforts towards the synthesis of the bicyclo[3.3.1] framework of the polycyclic polyprenylated acylphloroglucinol (PPAP) (±)-clusianone (41; Scheme 19).[48] The convergent stategy involved a three-step cascade sequence involving Michael addition/elimination/Michael addition (MEM) of the readily accessible and naturally occurring acylphloroglucinol clusiaphenone B (151). This proposed biomimetic approach delivered the bicyclic framework of the natural product in a single step. In the dearomatization event, treatment of 151 with KHMDS followed by addition of α-acetoxymethyl acrylate 152 led to an efficient and highly diastereoselective MEM cascade sequence to produce the desired product 153 after methyl enol formation (54% overall yield). This MEM cascade entails three steps: 1) Michael addition of the phenolate derived from phloroglucinol 151 to α,β-unsaturated aldehyde 152, 2) elimination of the tertiary acetate to afford intermediate 154, and 3) a second Michael addition and annulation to core structure 155 with the formation of two contiguous quaternary centers. Final diastereselective reprotonation of enolate 155 was found to be under thermodynamic control and yielded exclusively aldehyde 153. Extension of the aldehyde to a prenyl group and final deprotection afforded (±)-clusianone (41) in seven steps overall. The authors separately reported an efficient method for the dearomatization of phloroglucinol derivatives through alkylation of allylic bromides and primary alkyl triflates.[49]
Scheme 19.
Total synthesis of (±)-clusianone using a MEM sequence (Porco, Jr. et al., 2007).[48]
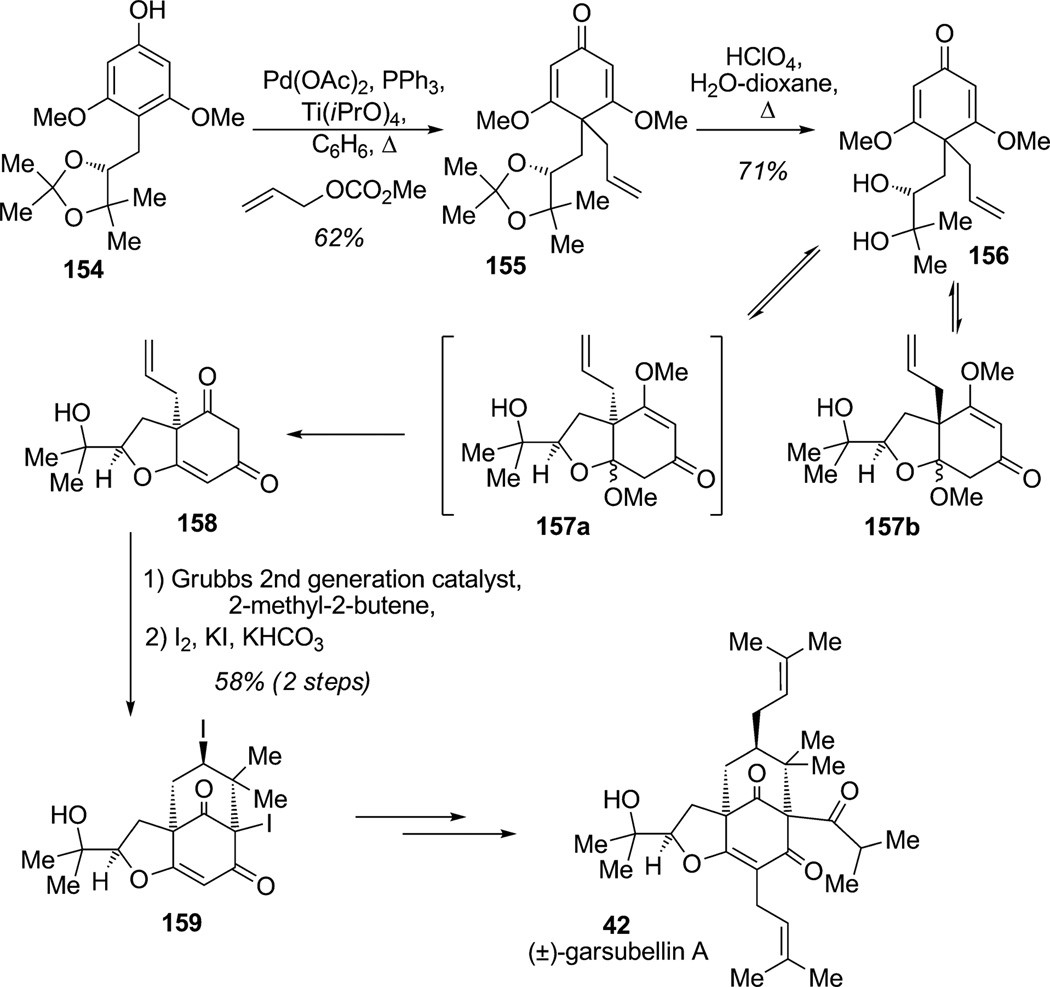
Another strategy for alkylative dearomatization with concomittant C–C bond formation is based on [3,3]-sigma-tropic rearrangement. In 2006, Danishefsky and co-workers reported the construction of the triterpene (±)-garsubellin A (42; Scheme 20).[50] The bicyclo[3.3.1]nonane framework is present in numerous natural products exhibiting important biological properties including neurotrophic activity. One of the challenges to access the core structure of (±)-garsubellin A (42) is the generation of a para-dearomatized product from phloroglucinol derivative 154. To address this issue, Danishefsky and co-workers explored an unusual dearomatizing strategy through allylation of phenol 154 at the para position, which may may proceed either by direct para C-allylation by via O-allylation followed by tandem Claisen–Cope rearrangements under Lewis acidic conditions.[51] This sequence delivered the desired dienenone 155 in 62% yield. Further treatment of acetonide 155 under acidic conditions led to diol 156 which spontaneously cyclized to give a mixture of Michael-type products 157 a and 157b. Longer reaction times promoted an additional step of the cascade with the transformation of intermediate 157 a to the corresponding ketone 158 (71% overall yield). After installation of the isoprene side chain via cross metathesis, Danishefsky and co-workers achieved carbocyclization using an iodonium intermediate under basic conditions to produce the desired bicyclo-[3.3.1]nonane framework 159. Further synthetic manipulations completed an impressive synthesis of (±)-garsubellin A (42).
Scheme 20.
Total synthesis of (±)-garsubellin A by means of para-alkylative dearomatization of a phloroglucinol (Danishefsky et al., 2006).[50]
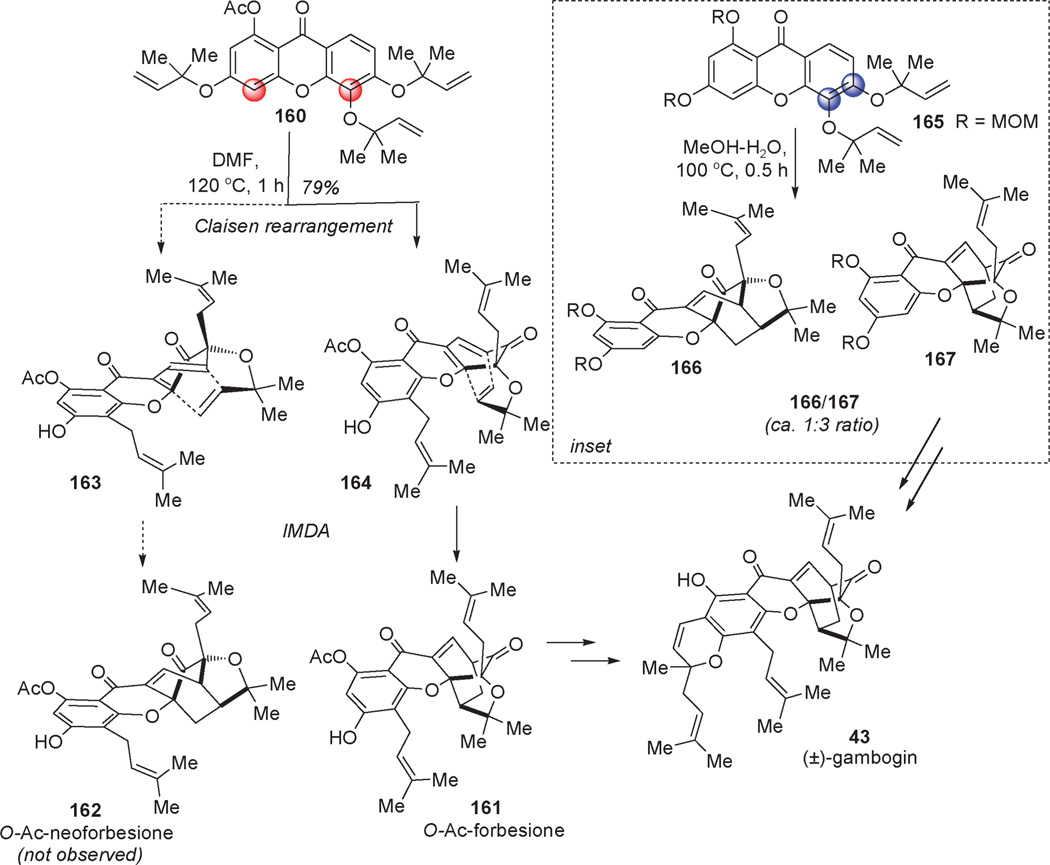
Dearomatization by means of a Claisen rearrangement has been sparsely used in total synthesis.[52] The most impressive examples are presented in Scheme 21 wherein Theodorakis et al.[53] and Nicolaou et al.[54] independently demonstrated the validity of a biomimetic process[55] through Claisen rearrangement/dearomatization to access the natural product (±)-gambogin (43). In the Theodorakis synthesis, exposure of acetylated xanthone 160 bearing reverse O-prenylated side chains to thermal conditions in polar protic solvents led to a remarkable Claisen dearomatization/Diels– Alder (CDDA) cascade. In this study, the authors evaluated the impact of different xanthone protecting groups and determined that electronic factors govern the regioselectivity of the Claisen rearrangement. Using an acetate protecting group, the authors were able to exclusively produce the desired CDDA isomer O-Ac-forbesione 161 in 79% yield in which case the constitutional isomer O-Ac-neoforbesione 162 was not observed. Regioselective dearomatization of xanthone 160 by means of two Claisen rearrangements presumably delivered intermediate 164 which underwent intramolecular Diels–Alder (IMDA) cycloaddition. Final deprotection and condensation with citral concluded an efficient biomimetic synthesis of (±)-gambogin (43).
Scheme 21.
Total syntheses of gambogin involving Claisen rearrangement/dearomatization (Theodorakis et al., 2004; Nicolaou et al., 2005).[53, 54]
In their study, Nicolaou and co-workers observed that a related cascade sequence could be conducted in polar protic solvents (methanol–water) leading to a remarkable rate acceleration (see inset in Scheme 21). Reaction of xanthone 165 afforded in this case the pentacyclic structural isomers 166 and 167 in a 1:3 ratio and 100% yield. Unfortunately, use of the MOM-protected xanthone 165 revealed poor electronic differentiation of the two prenyl groups and afforded a mixture of both isomers 166 and 167. Further DFT studies from the authors in collaboration with Houk and co-workers revealed that in the CDDA cascade, the Claisen rearrangement is reversible and relatively unselective, while the Diels– Alder cycloaddition dictats regioselectivity favoring formation of the five-membered-ring ether.[56] Further deprotections, prenyl installation, and cyclization led to the second total synthesis of (±)-gambogin (43).
3.3. Oxidative Dearomatization of Phenol Triggering Diels–Alder Cycloaddition
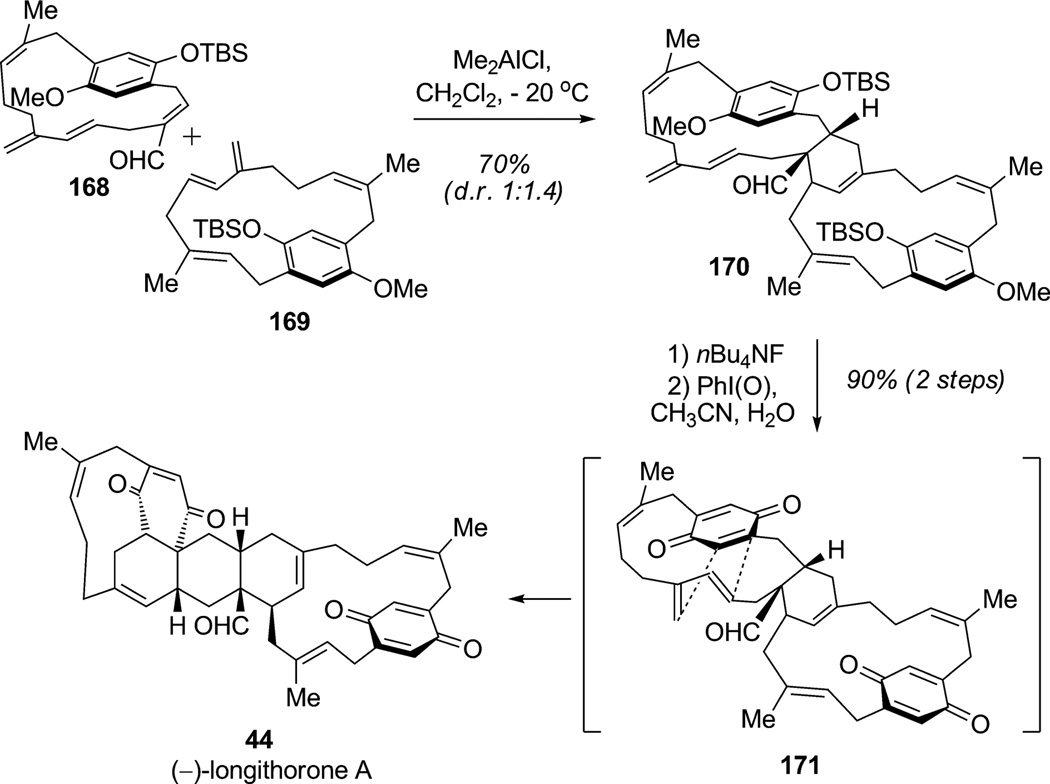
Studies regarding Diels–Alder cycloadditions in nature have been of great interest to the organic chemistry community. Few examples of spontaneous Diels–Alder reactions in biosyntheses and some examples of enzymatically promoted Diels–Alder reactions have been reported.[57] According to this concept, Schmitz and co-workers proposed a biosynthesis of (−)-longithorone A (44) involving consecutive inter- and intramolecular Diels–Alder cycloadditions.[58] A few years later, Shair and co-workers disclosed an elegant biomimetic synthesis of (−)-longithorone A (44) involving an intermolecular Diels–Alder cycloaddition between [12]paracyclophanes 168 and 169 promoted by a Lewis acid to generate cyclohexene derivative 170 in 70% yield (d.r. 1:1.4; Scheme 22).[59] Further oxidation of both hydroquinone moieties led to the corresponding dearomatized bis(para-benzoquinone) intermediate 171 which spontaneously underwent transannular Diels–Alder cycloaddition to simultaneously forge the last three rings of the natural product (90% yield). This synthesis was highlighted by two consecutive key transformations first constructing the cyclohexene ring by [4+2] cycloaddition and then assembling the last three rings in a single oxidative dearomatization/Diels–Alder cycloaddition sequence.
Scheme 22.
Total synthesis of (−)-longithorone A through oxidation with a hypervalent iodine reagent and Diels–Alder cycloaddition (Shair et al., 2002).[59]
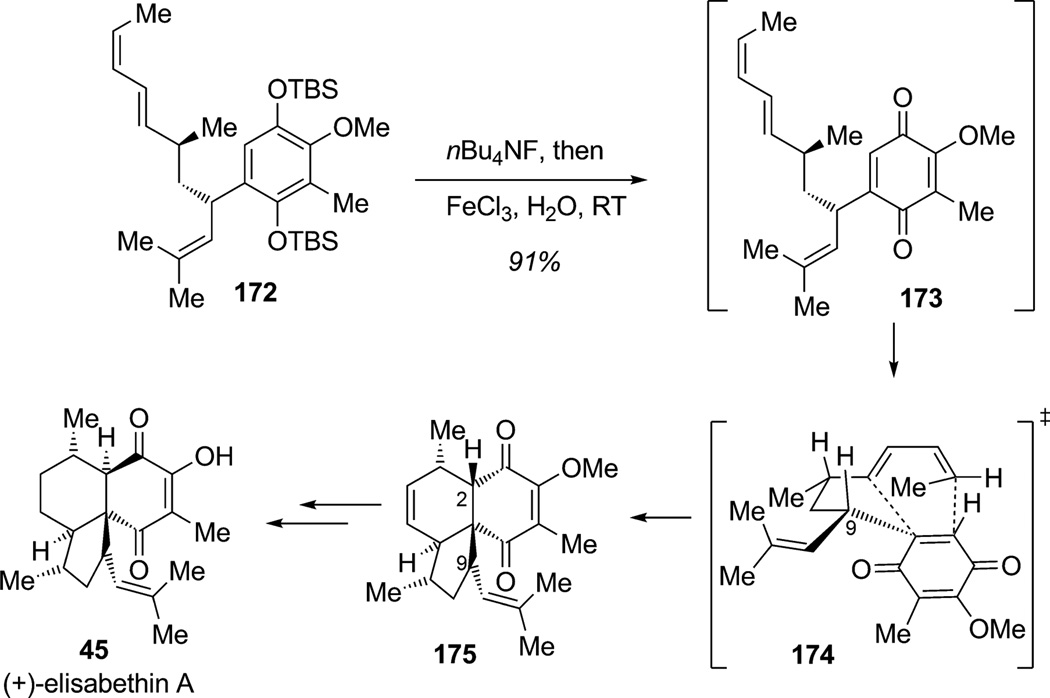
In 2001, Nicolaou and co-workers reported a concise construction of colombiasin A using the intrinsic reactivity of a dearomatized quinone to participate in an efficient IMDA cycloaddition to produce the natural product core.[60] Other elegant examples of this approach were reported separately in the total syntheses of (+)-elisabethin A (45) by the Mulzer group[61a] and elisapterosin B by the Rawal group,[61b] both employing oxidative dearomatization/IMDA cycloaddition cascade sequences (Scheme 23). In the first synthesis by Mulzer and co-workers, bis(silyl) hydroquinone 172 was cleanly deprotected by tetrabutylamonium fluoride to give the corresponding hydroquinone which was subject to the dearomatization-IMDA cascade under the action of iron trichloride. This “one-pot” procedure generated in situ the desired trisubstituted para-benzoquinone 173 which spontaneously cyclized via exo-transition state 174 to produce the desired tricyclic product 175 in 91% yield. This late-stage oxidative dearomatization/IMDA cascade relied on the Z configuration of the terminal olefin to induce the desired stereochemistry. The facial selectivity of the diene–quinone cycloaddition is presumably dictated by the minimization of allylic strain between the substituents at C9 and the quinone carbonyl moiety in endo transition state 174 such that cycloadduct 175 is produced as a single diastereoisomer. Required chemoselective removal of the endocyclic alkene, epimerization at C2, and deprotection afforded elisabethin A in an elegant manner.
Scheme 23.
Total synthesis of (+)-elisabethin A through oxidative dearomatization/intramolecular Diels–Alder cycloaddition (Mulzer et al., 2003).[61a]
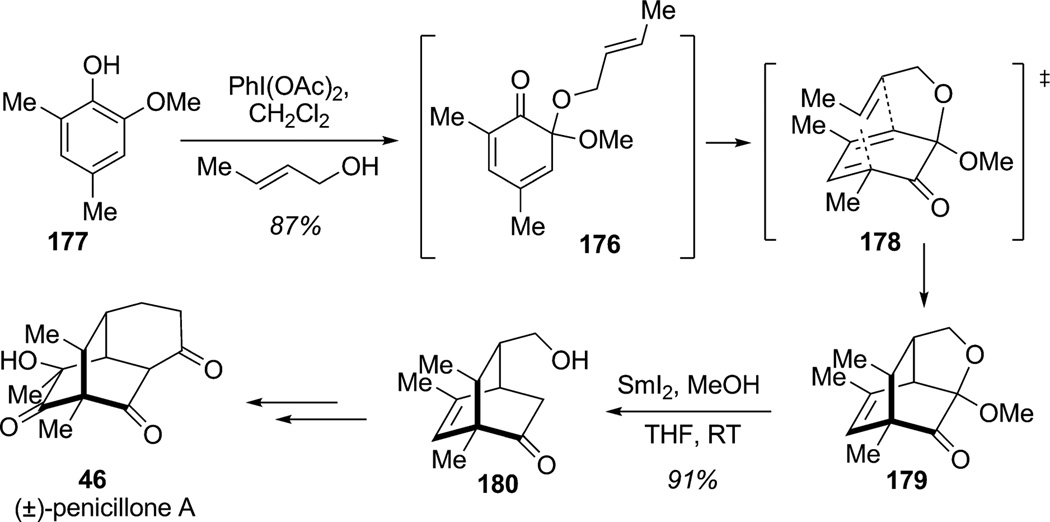
The ortho-oxidative dearomatization of phenols has also provided opportunities for the construction of complex scaffolds using Diels–Alder cycloaddition. Liao and co-workers have conducted extensive studies regarding sequential oxidative dearomatization/Diels–Alder cycloaddition.[62] A recent example from this group is shown in Scheme 24 and illustrates the formation of a highly functionalized bicyclo-[2.2.2]octane ring system in a single operation en route to (±)-penicillone A (46; Scheme 24).[63] Masked ortho-benzoquinone (MOB) derivative 176 was prepared by regioselective ortho-oxidative dearomatization of phenol 177 with PIDA and trans-crotyl alcohol. The highly reactive, dearomatized intermediate 176 readily reacted in an IMDA cycloaddition at room temperature via the presumed endo transition state 178. The reactivity of MOB 176 as a diene allowed the formation of bicyclo[2.2.2]octane 179 in a single step in 87% yield. Further reduction of the cyclic ketal with samarium iodide provided alcohol 180 which was elaborated in six steps to the natural product (±)-penicillone A (46).
Scheme 24.
Total synthesis of (±)-penicillone A using an ortho-oxidative dearomatization/IMDA cascade (Liao et al., 2007).[63]
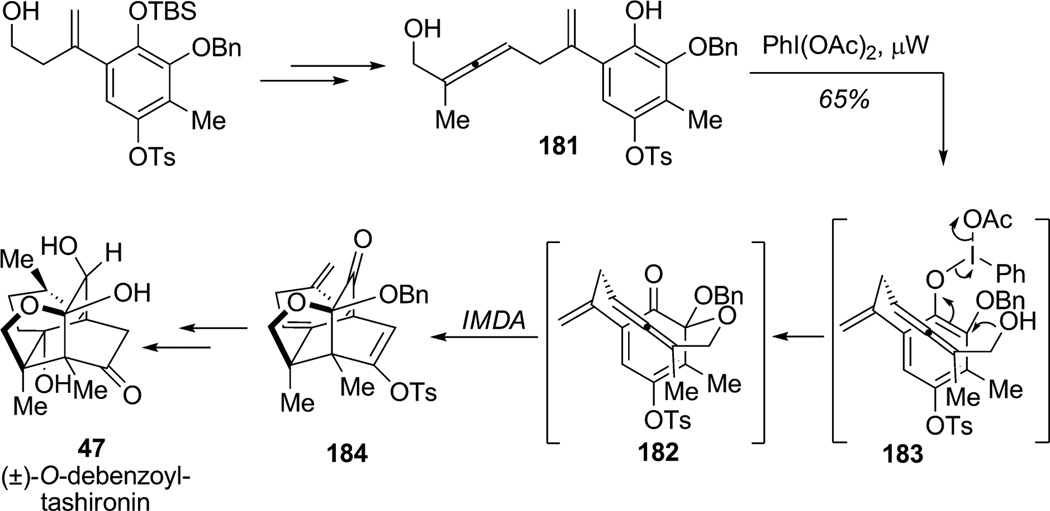
(±)-11-O-Debenzoyltashironin (47) is a tetracyclic caged structure possessing three quaternary centers, two of which are positioned at junctions of the [2.2.2]bicyclic framework (Scheme 25). The strategy proposed by Danishefsky and coworkers to access 11-O-debenzoyltashironin (47) featured an original ortho-oxidative dearomatization/IMDA cycloaddition sequence (Scheme 25).[64] Allenic substrate 181 was prepared from the corresponding homoallylic alcohol in 60% yield over five steps. Regioselective ortho-oxidative dearomatization of phenol 181 delivered a highly reactive MOB 182 via activated intermediate 183 leading to transannular Diels–Alder cycloaddition upon heating to produce the desired tetracyclic adduct 184 in 65% yield. This remarkable transformation employed an allene fragment as a dienophile for a transannular cycloaddition cascade which produced all four rings of this highly congested and challenging molecule.
Scheme 25.
Total synthesis of (±)-O-debenzoylttashironin through oxidative dearomatization/Diels–Alder cycloaddition (Danishefsky et al., 2006).[64]
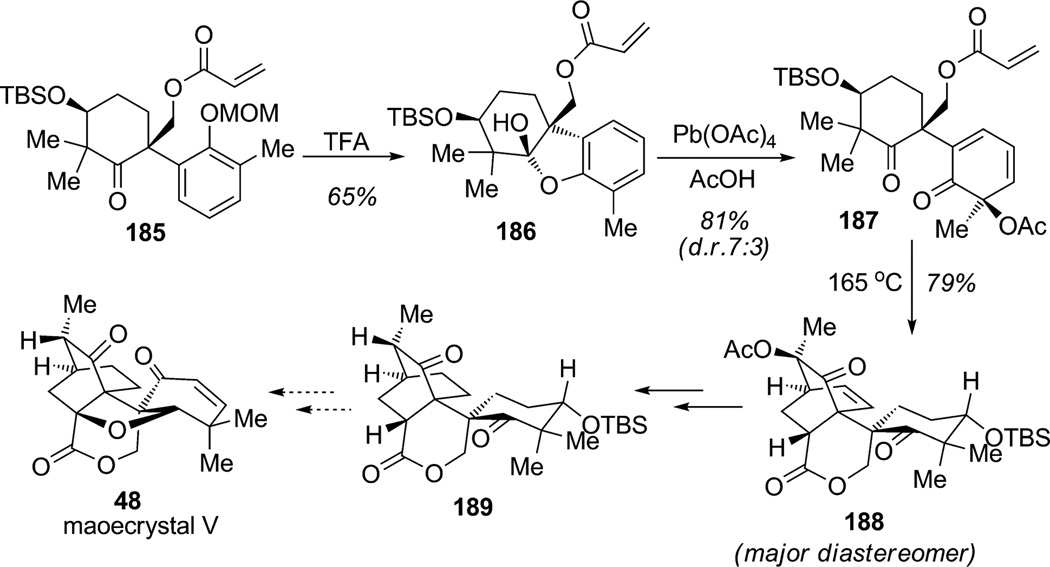
In an early approach to maoecrystal V (48), Baran and coworkers also executed an ortho-oxidative dearomatization/IMDA sequence to create the core of the natural product (Scheme 26).[65] Treatment of the advanced aryl ketone 185 with TFA simultaneously removed the ether protecting group and induced cyclization to afford hemiketal 186 in 65%yield. Wessely oxidation[66] of 186 employing Pb(OAc)4 led to ortho-oxidative dearomatization with modest diastereoselectivity (d.r. 7:3), thereby generating the dearomatized ortho-quinol derivative 187 in 81% yield. Diels–Alder cycloaddition was successfully conducted at high temperature to deliver both endo cycloadducts (major diastereomer 188 shown). Further hydrogenation and cleavage of the α-acetoxyketone and diastereoselective reprotonation (d.r. 17:3) yielded the tricyclic product 189, a compound bearing the carbon skeleton of maoecrystal V (48).
Scheme 26.
Approach to maeocrystal V using oxidative ortho-dearomatization/IMDA cycloaddition (Baran et al., 2009).[65]
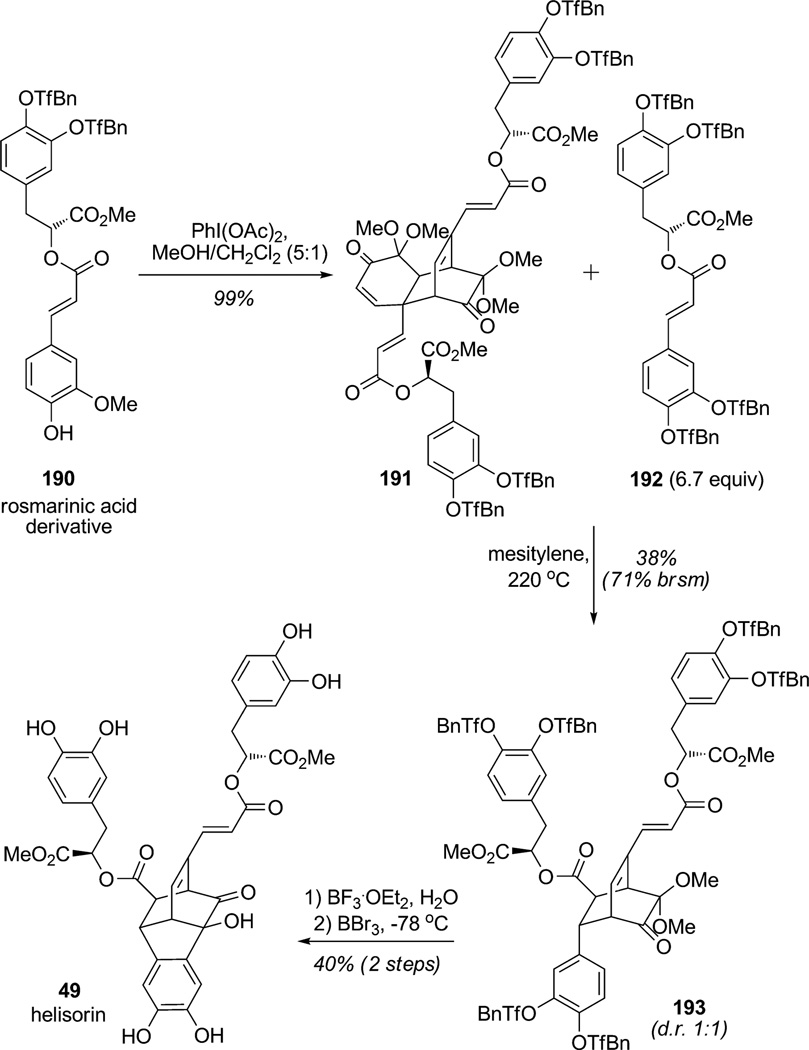
Flavonoids, polyphenols, and heteroarenes present in nature are sources of a significant number of complex dearomatized metabolites. Indeed, the propensity for dearomatization of polyphenols through single electron transfer (SET) and oxidative dearomatization has led to the generation of a number of highly complex structures. In this regard, Snyder and co-workers examined biomimetic access to helisorin and related neolignan natural products from rosmarinic acid (Scheme 27).[67] Oxidative homodimerization of the rosmarinic methyl ester derivative 190 using PIDA by Diels–Alder cycloaddition yielded the tricyclic product 191. In this type of ortho-oxidative dearomatization with substrates lacking stabilizing substituents at the para position, the resulting MOB intermediates are well known to spontaneously dimerize.[68] Dimer 191 was engaged in a retro-Diels– Alder/Diels–Alder sequence at elevated temperature with a large excess of the dienophile partner 192 which afforded the desired Diels–Alder heteroadduct 193 in 38%yield (d.r. 1:1). This example illustrates that the remote stereocenter of rosmarinic derivative 192 did not induce high stereoselectivity during the Diels–Alder cycloaddition. Nevertheless, chromatographic separation of the cycloadduct diastereomers afforded pure bicyclo[2.2.2]octenone 193 which was next treated with BF3·OEt2 to achieve intramolecular Friedel–Crafts reaction and deliver the tetracyclic core of helisorin. Controlled exposure to BBr3 at low temperature resulted in the full deprotection of the para-(CF3)-benzyl ethers providing helisorin (49) in 40% yield (two steps). This elegant synthesis of helisorin (49) not only emphasizes a common biomimetic pathway towards several neolignan natural products, but also the careful handling required to access the highly oxygenated and sensitive intermediates.
Scheme 27.
Total synthesis of helisorin with an ortho-oxidative dearomatization and a retro-Diels–Alder/Diels–Alder sequence (Snyder et al., 2009).[67]
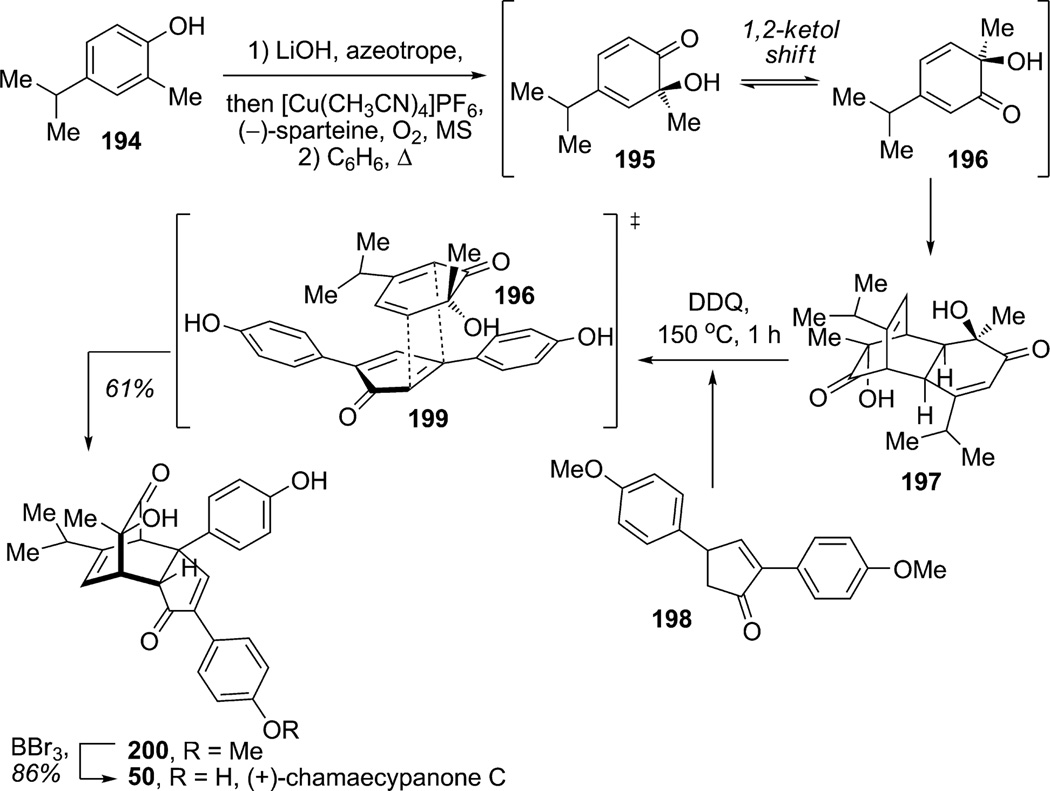
Porco and co-workers recently demonstrated the utility of related ortho-quinol derivatives in an enantioselective synthesis of (+)-chamaecypanone C (50; Scheme 28).[69] In this study, the authors outlined use of chiral, nonracemic ortho-quinols obtained by enantioselective ortho-oxidative dearomatization of lithium phenolates with a copper bis(oxo) complex derived from (−)-sparteine. This chiral copper complex mediated the chemoselective ortho-dearomatization of 2,4-disubstituted phenol 194 to afford ortho-quinol 195 which equilibrated by means of a [1,2]-ketol shift to isomer 196. ortho-Quinol 196 spontaneously underwent Diels–Alder dimerization to generate bicyclo[2.2.2]octenone 197 in 80% overall yield (>99%ee). The dimeric chiral ortho-quinol 197 underwent retro-Diels–Alder cycloaddition upon exposure to elevated temperature and was further reacted through Diels–Alder cycloaddition with the in situ generated diarylcyclopentadienone 198. This three-step cascade thus involved 1) thermolytic generation of chiral ortho-quinol 196, 2) oxidation of cyclopentenone 198 to a highly reactive 2,4-diarylcyclopentadienone 199 and 3) their union through an endo- and face-selective Diels–Alder cycloaddition to afford the desired enantiopure cycloadduct 200 in 61% yield. Final, demethylation of 200 using BBr3 afforded (+)-chamaecypanone C (48) in 86% yield.
Scheme 28.
Total synthesis of (+)-chamaecypanone C by means of enantioselective copper-mediated oxidative dearomatization/[4+2] cycloaddition (Porco, Jr. et al., 2009).[69]
4. Dearomatization of Electron-Rich Heteroarenes: Furans, Pyrroles, Benzofurans, and Indoles
Dearomatization of electron-rich arenes has often been employed as a key part of complex total synthesis endeavors. For instance, furans have been extensively used in intramolecular Diels–Alder (IMDA)[70] cycloadditions and the hetero-Diels–Alder (HDA)[71] counterpart to generate complex ring structures. Other applications, including vinylogous aldol reactions, are prominent for 2-silyloxyfurans to forge C–C bonds and introduce butenolide fragments in a single step.[72] In this section, we outline a number of representative examples in total synthesis involving the dearomatization of furans, pyrroles, indoles, and related heterocycles. In addition, examples of modern diastereo- and enantioselectivedearomatization of electron-rich heteroarenes to build complex chiral frameworks will be presented.
4.1. Dearomatization of Furans, Pyrroles, and Benzofurans in Total Synthesis
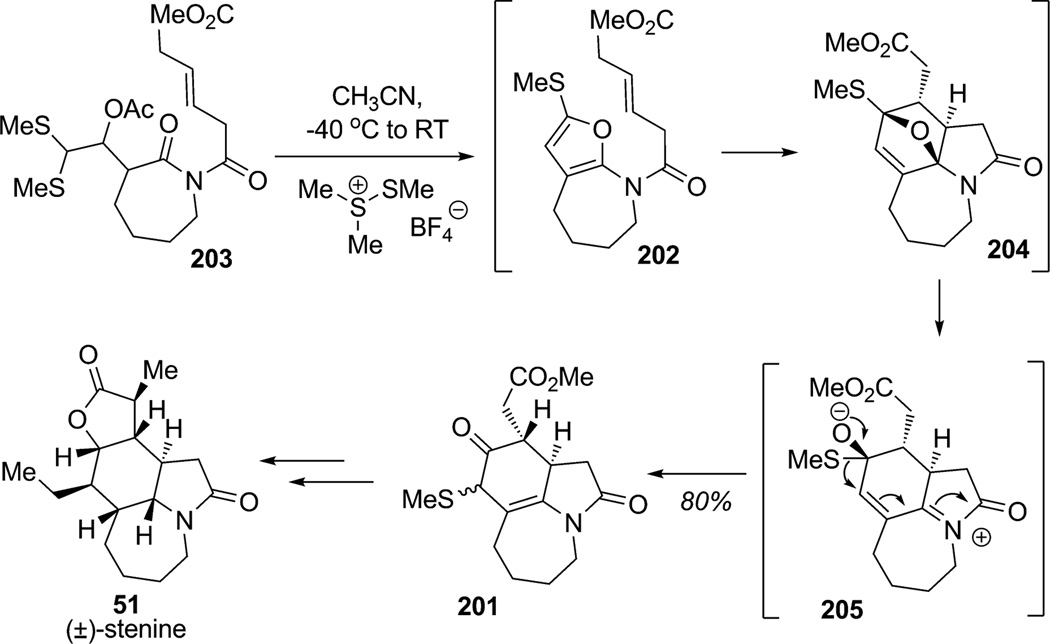
The hydrooxindole core skeleton of stenine (51) contains seven contiguous stereocenters and is a structural motif shared by several members of the Stemona alkaloids (Scheme 29). During their studies, Padwa and co-workers demonstrated the feasibility of a domino amido-Pummerer/Diels–Alder sequence which provided access to complex hetreocycles in a single step with concomittant formation of three new rings.[73] Extensive studies showed that 2,5-disubstituted furans bearing heteroatoms have a great propensity for dearomatization, especially through cascade reactions initiated by IMDA cycloadditions.[74] In their recent campaign toward the total synthesis of (±)-stenine (51), Padwa and Ginn found the IMDA/N-acyliminium strategy to be of great utility for construction of the highly functionalized hydrooxindole core 201 (Scheme 29).[75] The requisite 2-methylthio-5-amidofuran 202 necessary for the intramolecular [4+2] cycloaddition was prepared by cyclization of imidodithioacetal 203 using dimethyl(methylthio)sulfonium tetrafluoroborate (DMTSF). The 2-methylthio-5-amidofuran intermediate 202 could not be isolated[75] and participated directly in intramolecular [4+2] cycloaddition to afford the dearomatized, cyclic hemiaminal 204. Ring opening of hemiaminal 204 led to the production of the N-acyliminium intermediate 205 which further rearranged by a 1,2-shift to afford azepinoindole 201 in 80% overall yield (d.r. 1:1). The total synthesis of (±)-stenine (51) was subsequently accomplished in sixteen steps and 2.1% overall yield from ε-caprolactam.
Scheme 29.
Total synthesis of (±)-stenine through dearomatization of a 2-thiomethylfuran (Padwa et al., 2002).[75]
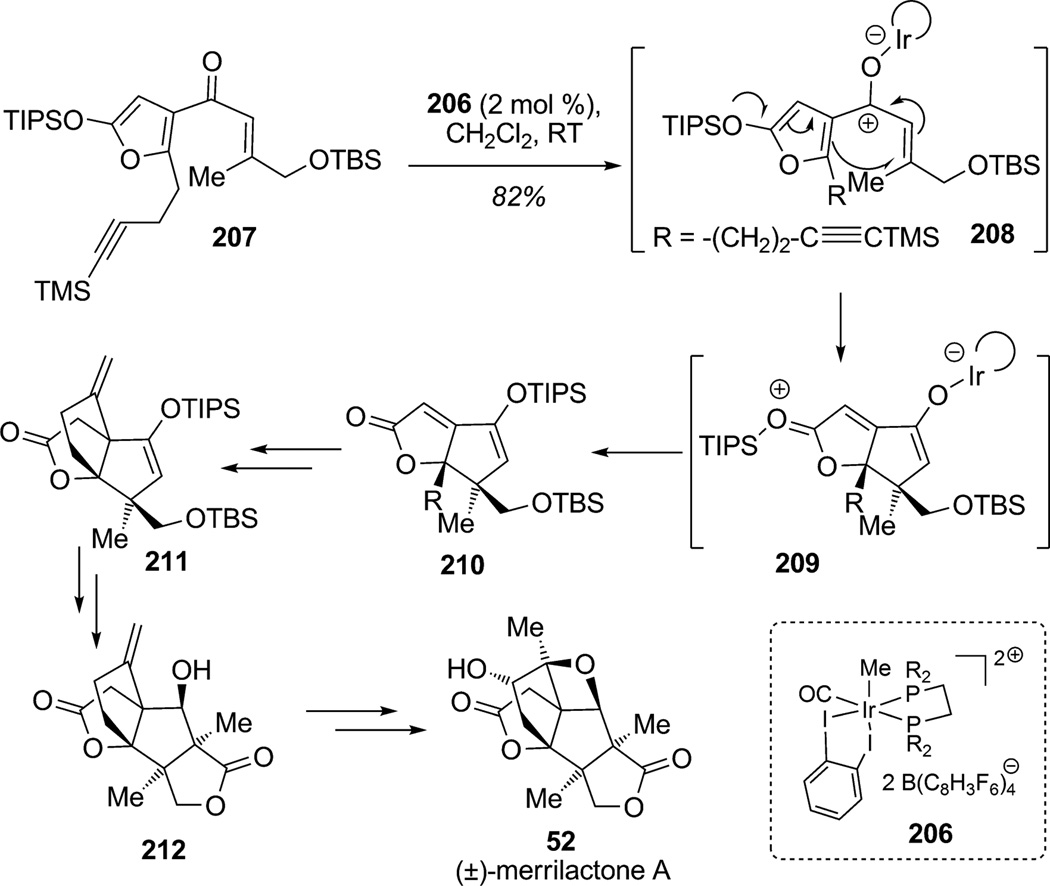
Frontier and co-workers reported the stereoselective total synthesis of the pentacyclic sesquiterpene dilactone (±)-merrilactone A (52) featuring the catalytic Nazarov cyclization of 2-silyloxyfurans (Scheme 30).[76] Recently, catalytic Nazarov cyclizations have been reported employing a wide range of transition-metal complexes and have demonstrated that dienones with high electron density at one terminus of the pentadienyl cation intermediate exhibit high cyclization reactivity. In this context, Frontier and co-workers employed iridium complex 206 to catalyze the Nazarov cyclization of trialkyl-2-silyloxyfuranyl enone 207 (Scheme 30). Indeed, the authors suggested that iridium(III) complex 206 or a Lewis acidic silicon species could be involved in the dearomatization of silyloxyfuran via 208 and activation of the enone moiety leading to the bicyclic framework 209 which may be followed by silyl transfer to produce bicyclic enone product 210 in 82% yield. This approach for catalytic enone activation triggered the dearomatization of the 2-silyoxyfuran and afforded the desired trisubstituted Nazarov product 210 bearing two adjacent, highly crowded stereocenters. The third ring was installed by radical cyclization to access scaffold 211 in a few steps. Finally, generation of an ether from the exocyclic alkene in 212 to install the angular oxetane concluded an elegant synthesis of (±)-merrilactone A (52).
Scheme 30.
Total synthesis of (±)-merrilactone A: catalytic Nazarov cyclization using the dearomatization of a 2-silyloxyfuran (Frontier et al., 2008).[76]
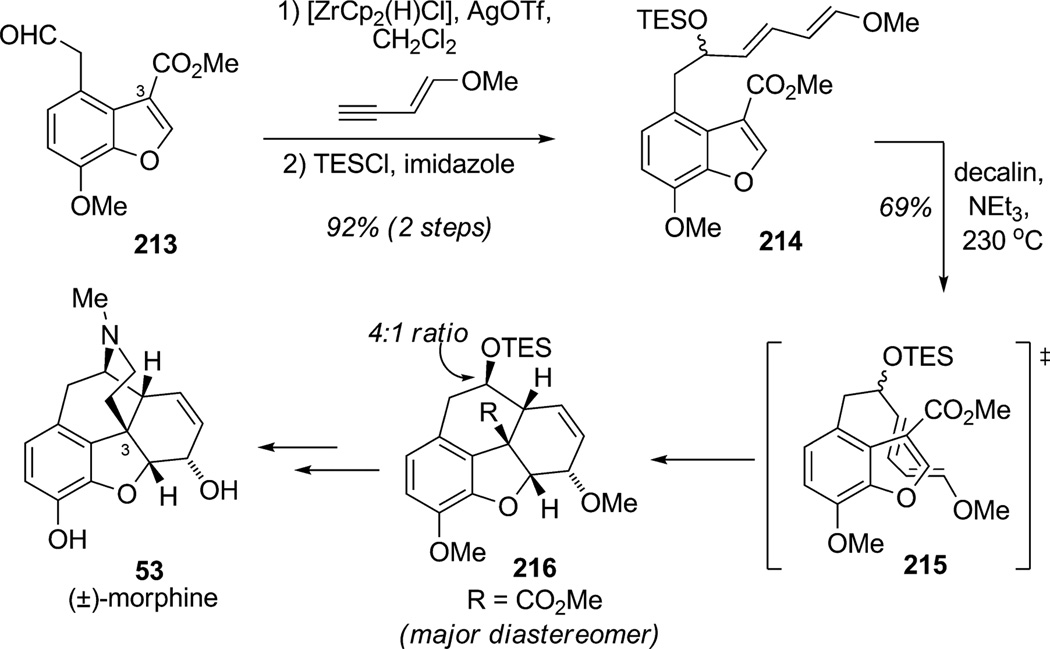
In the case of the morphine alkaloids, a key structural challenge involves the construction of four rings sharing the same carbon atom C3 having a high propensity for skeletal rearrangement. A number of research groups have devoted significant efforts to studying the Diels–Alder reactions of indoles and benzofurans,[77] which proved to be fruitful for the formal synthesis of morphine by Stork and co-workers (Scheme 31).[78] A Suzuki reaction sequence involving hydrozirconation and in situ addition to benzofuranacetaldehyde 213 produced silyl ether 214 in 95% yield. IMDA cycloaddition of 214 occurred via endo transition state 215 affording control of five contiguous asymmetric stereocenters of tetracyclic product 216. Specifically, cycloaddition in decalin at elevated temperature afforded a 69% yield of cycloadduct 216 (d.r. 4:1) along with recovered starting material. This endo intramolecular Diels–Alder cycloaddition installed four of the five required stereocenters of morphine (53) and greatly facilitated final manipulations including construction of the tertiary amine bridge to afford after a few additional steps the natural product (±)-codeine, a precursor to the alkaloid (±)-morphine (53).
Scheme 31.
Formal synthesis of (±)-morphine highlighted by an IMDA dearomatization of a benzofuran (Stork et al., 2009).[78]
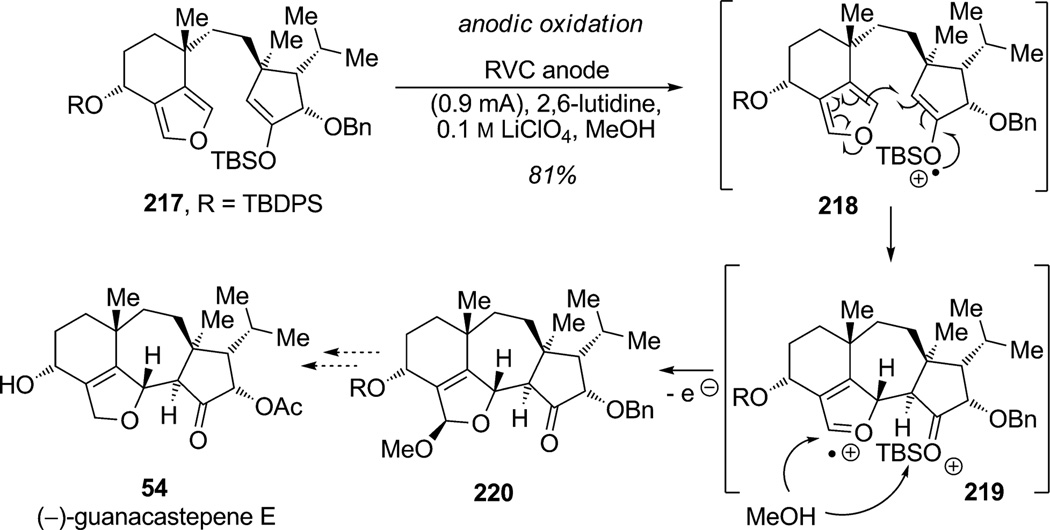
Several research groups have studied and demonstrated the utility of electrochemical, oxidative furan dearomatization with enol ether partners.[79] Indeed, electrochemical oxidation of the silyl enol ether should occur preferentially as determined by cyclic voltammetry studies (TMS enol ether Eox=0.87 V (vs. Ag/Ag+) when monosubstituted furan Eox= 1.31 V (vs. Ag/Ag+)). In their approach toward the guanacastepene family of natural products ((−)-guanacastepene E (54)), Trauner and co-workers established that an oxidative radical cation cascade could be accomplished in a complex setting (Scheme 32).[80] Late-stage anodic oxidation of polycyclic substrate 217 presumably generated the radical cation 218 which was then trapped by the pendant bicyclic furan to forge a new C–C bond and generate intermediate 219. Quenching of the oxonium ion 219 with methanol, followed by a second oxidation and desilylation, generated acetal 220 in 81%overall yield. This radical cation cascade triggered the dearomatization of furan through “umpolung-like” reactivity allowing two nucleophiles to enter successively at the C2 and C4 positions of the furan moiety and elegantly afforded the highly functionalized tetracyclic guanacastepene core 220.
Scheme 32.
Synthetic approach toward (−)-guanacastepene E: oxidative radical annulation by means of furan dearomatization (Trauner et al., 2005).[80]
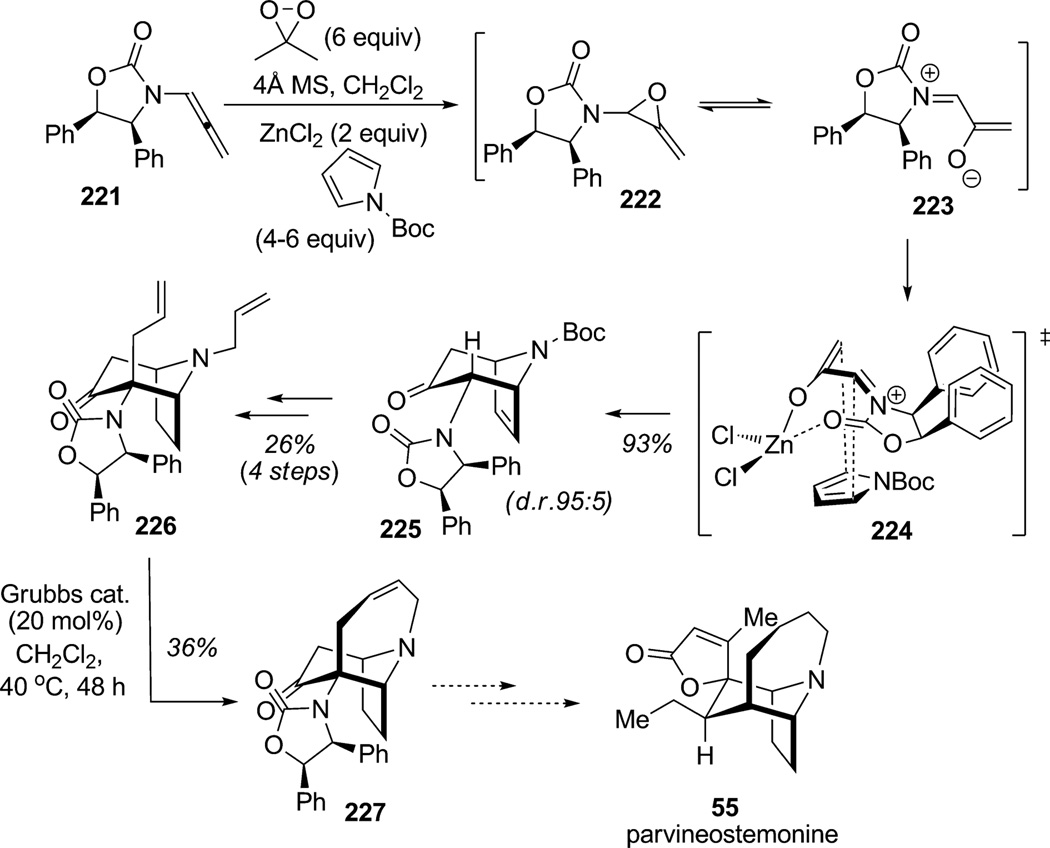
[4+3] Cycloaddition of furans and pyrroles is a powerful dearomatizing reaction for the construction of bridged bicyclic frameworks.[81] Highly stereoselective [4+3] cycloadditions of N-substituted pyrroles with allenamide-derived, nitrogen-stabilized chiral oxyallyl cations were reported by Hsung and co-workers (Scheme 33).[82] This diastereoselective methodology provides an efficient means for construction of the chiral tropinone alkaloid parvineostemonine (55). Reaction of allenamide 221 with dimethyldioxirane in the presence of N-Boc-pyrrole afforded the allene oxide intermediate 222 which subsequently tautomerized to the highly reactive oxyallyl cation species 223. First, the Seebach chiral auxiliary (not shown)[83] was found to perform well and induce high diasterereoselection (81% yield, d.r. 95:5). The stereochemical outcome obtained upon use of allenamide 221 derived from the (1R,2S)-diphenyloxazolidinone auxiliary can be rationalized from proposed transition state 224, wherein the bottom face of the zinc-chelated oxyallyl cation is open for [4+3] cycloaddition to afford bicyclic derivative 225 (93% yield, d.r. 95:5). After hydrogenation, protecting group removal, and allyl group installation, the bicyclic piperidine derivative 226 was obtained in 26% yield over four steps. Transannular ring-closing metathesis of substrate 226 delivered the tricyclic core 227 of the natural product parvineostemonine in 36% yield.
Scheme 33.
Approach to parvineostemonine employing asymmetric oxyallyl cation [4+3] cycloaddition for pyrrole dearomatization (Hsung et al., 2007).[82]
4.2. Indole Dearomatization in Total Synthesis
As outlined in the introduction of this Review, many indolic alkaloid frameworks can be accessed from either Diels–Alder cycloaddition or stepwise nucleophilic dearomatization at C3 followed by successive trapping at C2. In this regard, impressive examples of indole dearomatization have emerged and will be summarized in the following section.
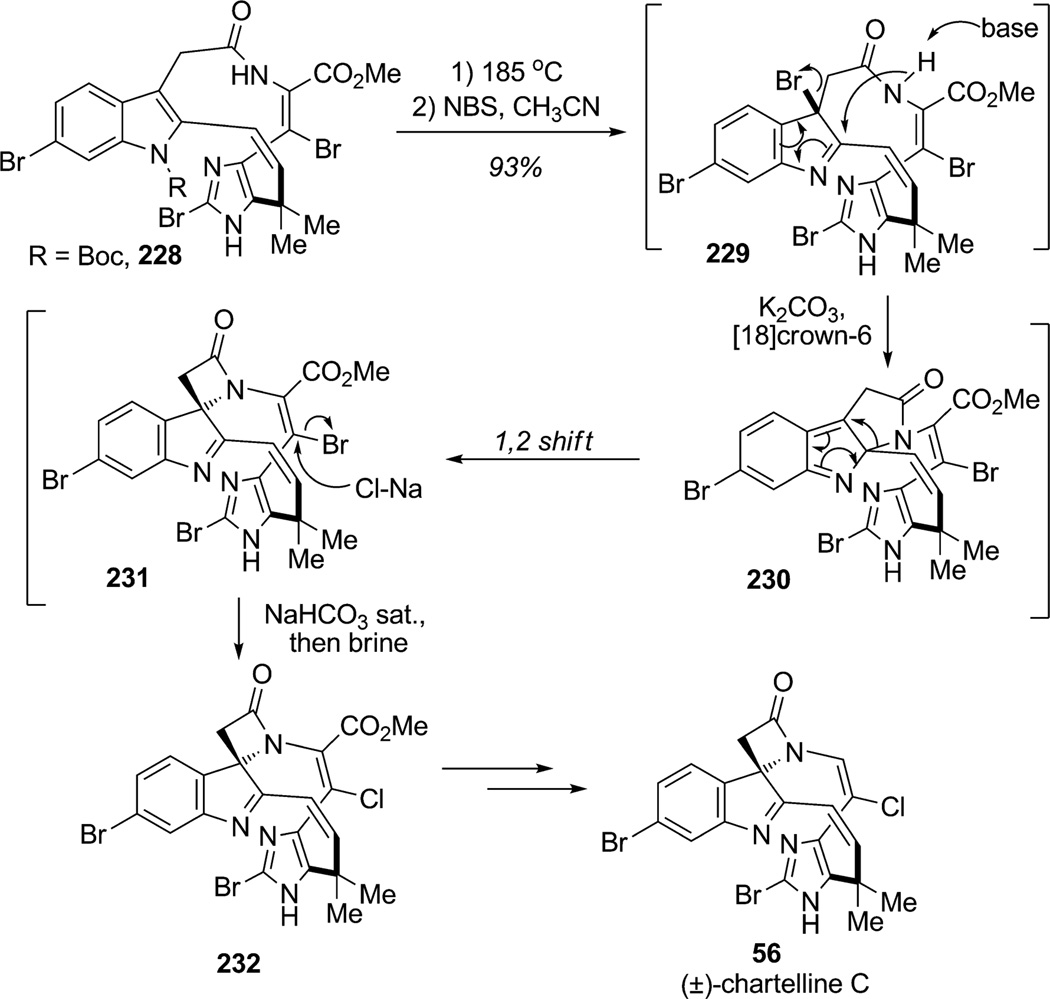
Recently, Baran and co-workers reported several total syntheses in which indolic cores were efficiently dearomatized.[84] In the synthesis of (±)-chartelline C (56), Baran and co-workers developed a biomimetic strategy involving brominative dearomatization followed by amide trapping (Scheme 34).[85] Initial carbamate deprotection under thermal conditions was followed by a cascade process promoted by N-bromosuccinimide (NBS). Bromoindolenine 229 was presumably formed upon exposure of macrocycle 228 to NBS with base and trapped at the C2 position by the proximate amide nitrogen to afford pyrroloindoline 230 which underwent ring contraction to produce the β-lactam 231. Aqueous workup resulted in bromine displacement to afford the chloroenamide 232 in 93% overall yield. Further ester hydrolysis and thermolytic decarboxylation concluded an elegant synthesis of (±)-chartelline C (56).
Scheme 34.
Total synthesis of chartelline C by brominative indole dearomatization/amide trapping (Baran et al., 2006).[85]
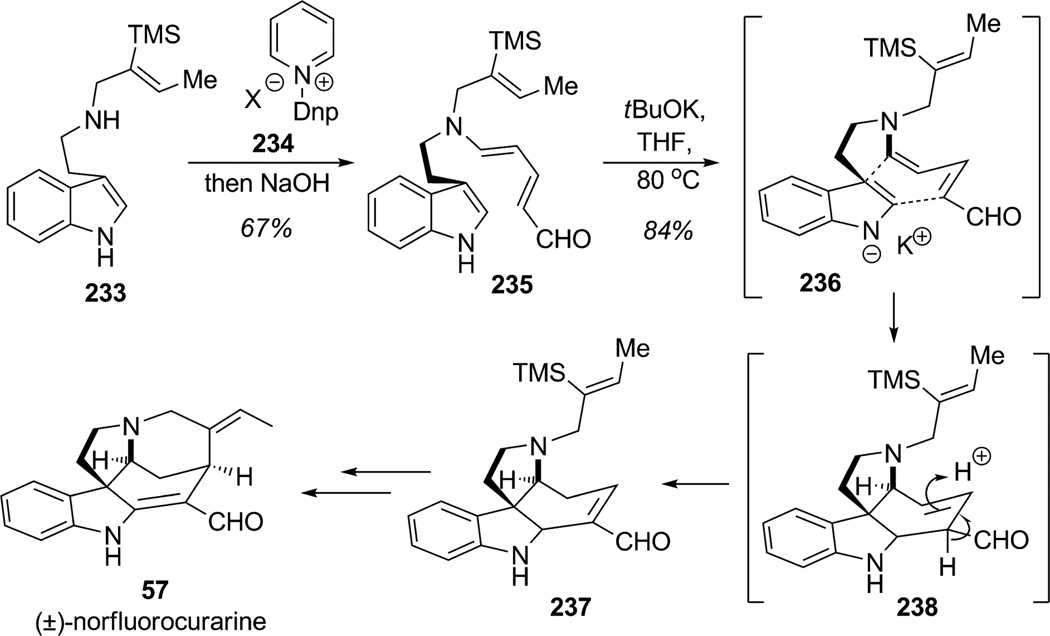
In a recent synthesis of the Strychnos alkaloid (±)-norfluorocurarine (57), Vanderwal and co-workers manipulated the indole nucleus without employing protecting groups to implement IMDA cycloaddition leading to rapid construction of the functionalized tetracyclic ring system (Scheme 35).[86] The secondary amine substrate 233 (readily obtained from tryptamine) was condensed with the dinitrophenylpyridinium salt 234 using the Zincke protocol to produce the corresponding dienamine aldehyde 235 after ring-opening of the pyridinium salt intermediate.[87] As reported earlier by Marko and co-workers,[88] bis-cyclization of indole anion 236 was achieved by treatment of indole derivative 235 with tBuOK in THF at 80°C in a sealed tube to afford the tetracyclic alkaloid structure 237 as a single diastereomer in 84% yield through formal Diels–Alder cycloaddition and olefin isomerization of aldehyde 238. Even though a stepwise or concerted mechanism for this dearomatization cascade has not yet been elucidated, this spectacular dearomatization through the anionic bicyclization of 235 established, in a single dearomatization step, the tetracyclic core of the Strychnos-type alkaloids. Installation of the iodine onto the vinylsilane and final Heck cyclization completed the total synthesis of (±)-norfluorocurarine (57) in 16% overall yield (nine steps in the longest linear sequence).
Scheme 35.
Total synthesis of (±)-norfluorocurarine using IMDA indolic dearomatization (Vanderwal et al., 2009).[86]
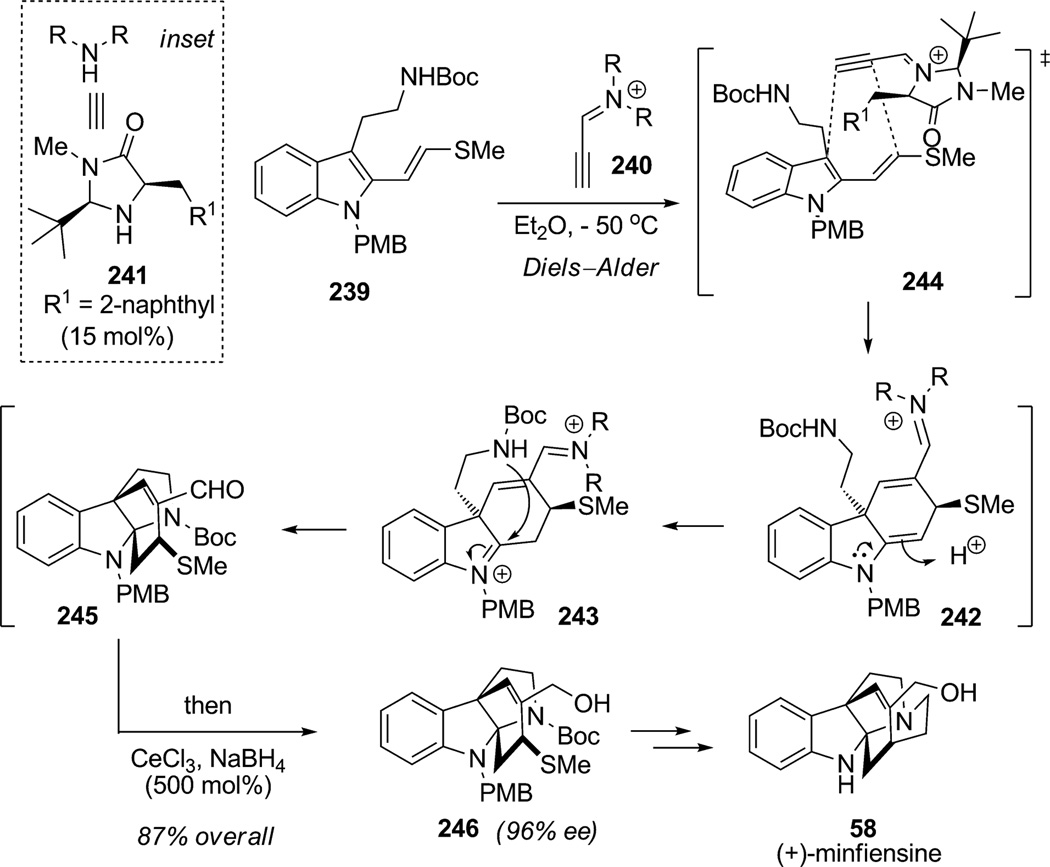
The total synthesis of (+)-minfiensine (58) has been reported several times prior to 2009. However, the organocatalytic enantioselective cascade underlined by indole dearomatization by means of Diels–Alder cycloaddition is a major achievement in modern alkaloid synthesis (Scheme 36).[89] MacMillan and co-workers reported a remarkable synthesis with the following key features: 1) an organocatalytic [4+2] cycloaddition coupled with hemiaminal isomerization/cyclization to allow rapid access to the desired tetracyclic framework and 2) a second radical cyclization to install the last ring of the alkaloid. In the first event, protected tryptamine 239 participated in a dearomatization cascade by means of a catalytic Diels–Alder reaction with propyne 4-imidazolidonium 240 (from imidazolidinone catalyst 241) to generate the enamine intermediate 242 which underwent isomerization to indolinium 243. Further cyclization of the pendant protected amine in a 5-exo-trig manner furnished pyrroloindoline 245. The authors proposed a specific arrangement for the Diels–Alder cycloaddition wherein the acetylenic group of the iminium intermediate 244 may be positioned away from the tert-butyl substituent of catalyst 241, thereby facilitating endo selectivity during the cycloaddition (cf. 244) and establishing the C3 stereocenter of indoline intermediate 242. Reductive workup in the same pot delivered product 246 in 87% yield and 96%ee. This organocatalytic cascade and tandem reduction sequence allowed MacMillan and co-workers to produce the tetracyclic core of minfiensine 246 in a single step with high enantioselectivity and diastereocontrol. Further transformations (with a 6-exo-dig radical cyclization) completed an efficient nine-step total synthesis of (+)-minfiensine (58).
Scheme 36.
Total synthesis of (+)-minfiensine: enantioselective iminium-catalyzed Diels–Alder dearomatization of indoles (MacMillan et al., 2009).[89]
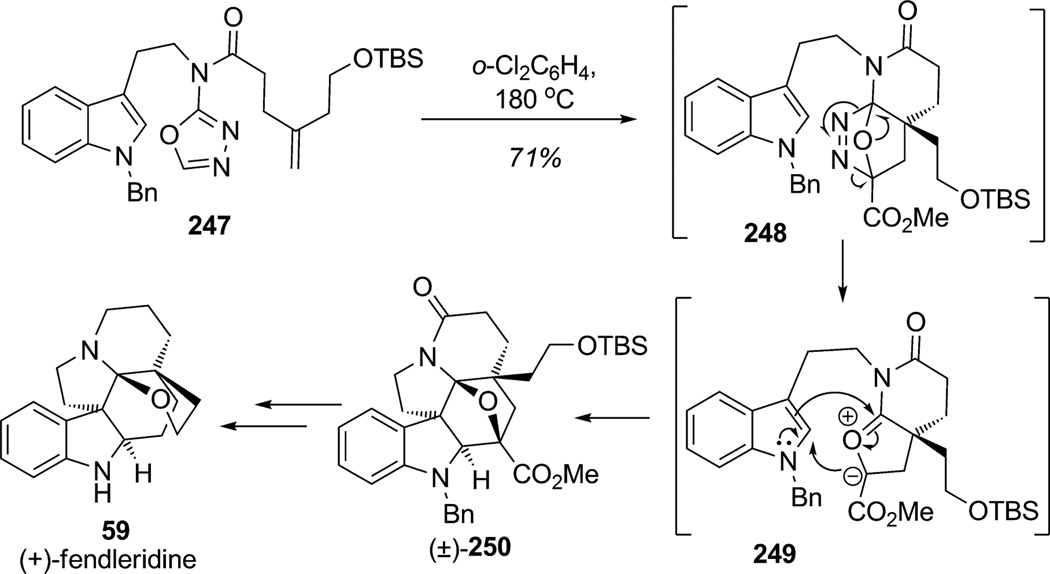
As described in the introduction, approaches to the tricyclic ring system containing the angular tertiary amine of the Aspidosperma alkaloids have been a central objective for the synthesis of various indole alkaloids. Boger and coworkers have developed an impressive methodology to create such a ring system in a single step involving [4+2]/[3+2] cycloaddition of an 1,3,4-oxadiazole substrate.[90] In their recent synthesis of (+)-fendleridine (59; Scheme 37),[91] the Boger group reported an elegant reaction cascade from tryptamine derivative 247 (prepared in four steps from N-benzyltryptamine) by means of the intramolecular and regioselective [4+2] cycloaddition of the 1,3,4-oxadiazole moiety with the tethered 1,1-disubstituted alkene. Diels– Alder cycloadduct 248 spontaneously extruded nitrogen to provide 1,3-dipole intermediate 249 which is stabilized by substitution at the dipole termini. 1,3-Dipole intermediate 249 subsequently triggered indole dearomatization through regio- and diasteroselective [3+2] cycloaddition to produce the hexacyclic alkaloid structure 250 as a racemate in 71% yield. The intrinsic regioselectivity of the ensuing 1,3-dipolar cycloaddition is reinforced by the linking tether and the relative stereochemistry dictated by endo cycloaddition wherein the dipolarophile is sterically directed to the opposite face of the newly formed fused lactam. In total, four C–C bonds, three rings, five relative stereogenic centers including the C19 N,O-ketal and the complete natural product skeleton are assembled in a single step affording product 250. It is noteworthy that the use of a 1,1-disubstituted alkene as the dienophile in the inverse-demand [4+2] cycloaddition allowed the formation of the crucial quaternary stereocenter. Subsequent modifications of the N,O-ketal moiety, redox adjustments, and separation of the enantiomers by HPLC finalized the synthesis of (+)-fendleridine (59).
Scheme 37.
Total synthesis of (+)-fendleridine using tandem [4+2]/[3+2] cycloadditions of 1,3,4-oxadiazoles with indole dearomatization (Boger et al., 2010).[91]
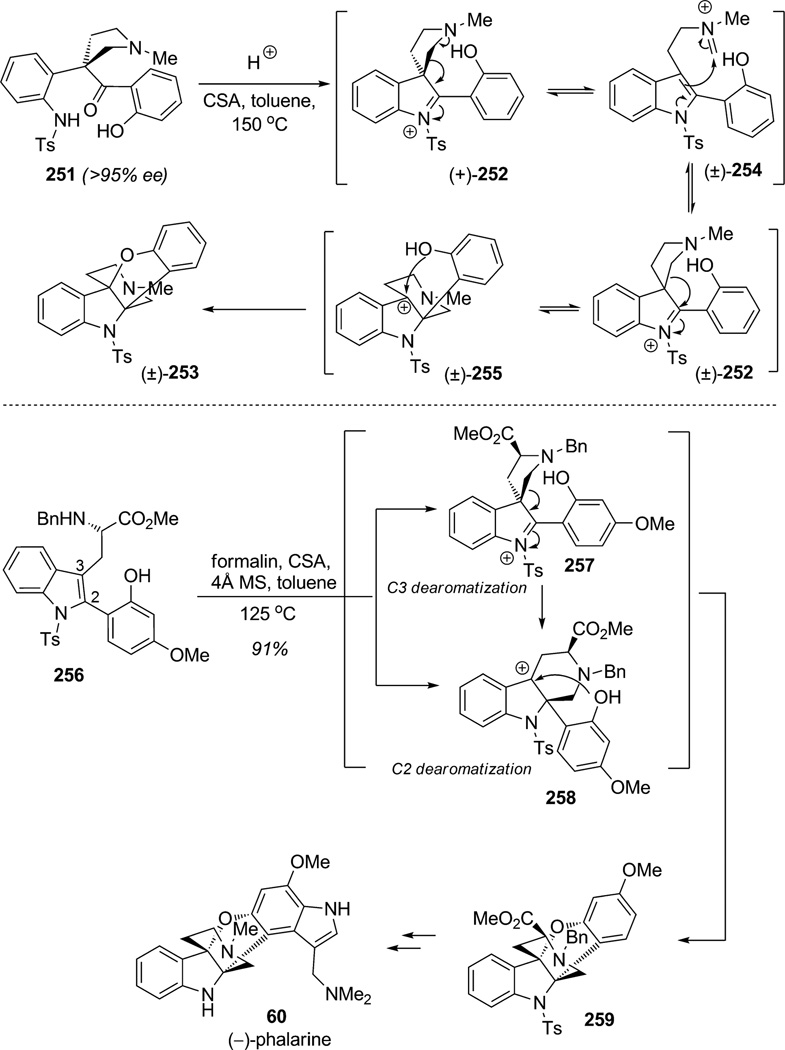
In their quest to synthesize the alkaloid (−)-phalarine (60), Danishefsky and co-workers developed a new pathway involving the interception of a Pictet–Spengler intermediate with a tethered nucleophile (Scheme 38).[92] In their approach, the enantiopure aniline derivative 251 (>95%ee) was subjected to acid to initiate cyclization to the chiral, nonracemic spirocyclic indolinium (+)-252. As proposed by the authors, a possible pathway for the production of the pentacyclic core of phalarine 253 may involve a Wagner–Meerwein-type 1,2-shift. This structural rearrangement is expected to proceed in a suprafacial, diastereoselective fashion and deliver product (+)-253 with preservation of enantiomeric integrity. Unfortunately, Danishefsky and coworkers obtained the desired core of phalarine (±)-253 in racemic form which suggested that racemization occurred, most likely by means of a retro-Mannich reaction leading to the achiral aromatized tryptamine iminium 254. This aromatic intermediate was set for dearomatization (Pictet–Spengler reaction) and regeneration of spirocyclic indolinium intermediate (±)-252 as racemate. A subsequent 1,2-shift created benzylic carbocation (±)-255 which was trapped by the proximate phenol to give the racemic pentacyclic structure (±)-253 in 52%yield. To solve this problem, Danishefsky and co-workers conducted a diastereoselective Pictet–Spengler reaction using the enantiopure tryptophan derivative 256.[92c] Accordingly, dearomatization was conducted using iminium chemistry either at C2 or C3 (via intermediates 257 or 258, respectively) to afford pentacycle 259 as a single diastereomer in 91% yield. Both pathways via iminium 257 or stabilized carbocation 258 are plausible and provide a good handle for chirality transfer at both the C2 and C3 positions during the dearomatization. The total synthesis of (−)-phalarine (60) was further completed with reductive decarboxylation and installation of the tryptamine portion by means of the Gassman oxindole synthesis. In these studies, the authors demonstrated the versatility of dearomatization using the Pictet–Spengler reaction coupled with nucleophilic trapping to generate the desired core structure of the natural product in a single step.
Scheme 38.
Total synthesis of (−)-phalarine through indole dearomatization: Pictet–Spengler cyclization and intramolecular carbocation trapping (Danishefsky et al., 2010).[92c]
5. Dearomatization of Electron-Poor Heteroarenes (Pyridinium and Related Compounds)
Pyridines and their activated pyridinium counterparts provide a class of interesting substrates for dearomatization chemistry and syntheses of complex piperidine, quinoline, and alkaloidal structures. Pyridinium compounds present high electronic deficiency at both the C2 and C4 positions leading to two possible regioselective dearomatization pathways.[93] It is noteworthy that only few methodologies for the alkylative dearomatization of pyridinium/isoquinolinium compounds have been reported in a catalytic, enantioselective context.[94]
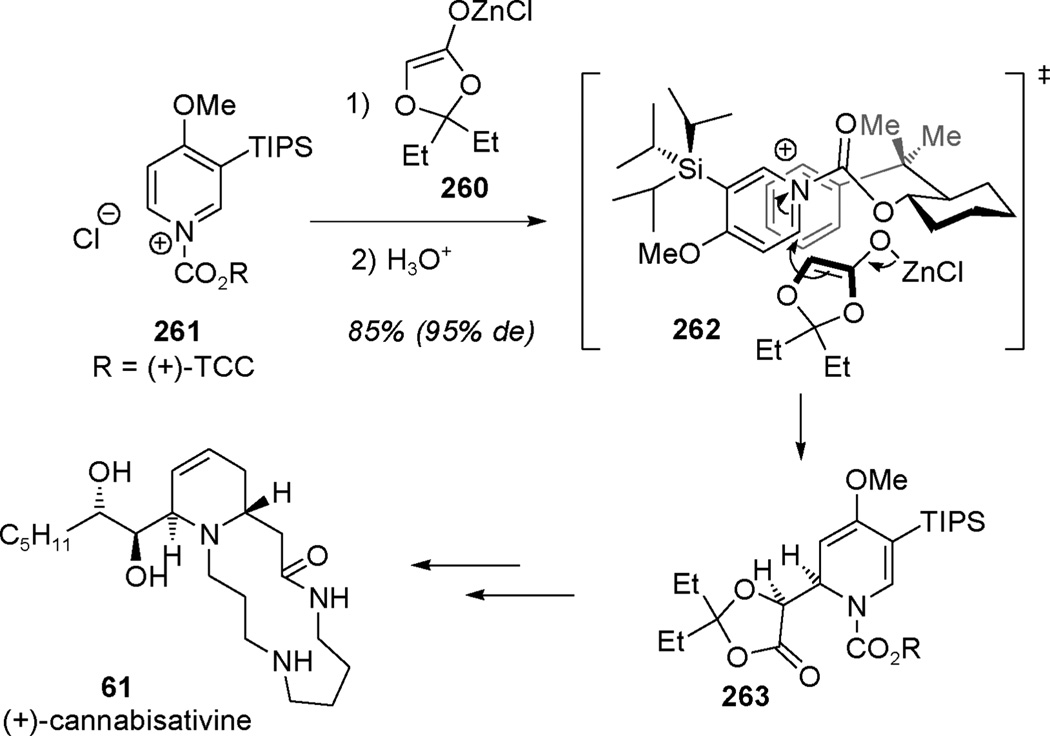
For chirality control in the dearomatization of pyridinium compounds, most methodologies reported up to date are diastereoselective. In the early stage of the stereoselective synthesis of (+)-cannabisativine (61), Comins and co-workers demonstrated the high selectivity of their alkylative methodology with the addition of the prochiral zinc enolate 260 to pyridinium electrophile 261 (Scheme 39).[95] The preformed E zinc enolate 260 added stereoselectively to pyridinium 261, thereby differentiating the diastereotopic faces created by the trans-2-(R)-cumylcyclohexyl chiral auxiliary ((+)-TCC) through proposed transition state 262[96] to afford dihydropyridone 263 as the major diastereomer (95%de) in 85% yield. In this case, the authors presumed involvement of acyclic transition state 262 with a synclinal orientation wherein the TCC auxiliary blocks the back face of pyridinium 261. Further manipulations were used to generate the dihydroxylated side chain of the natural product followed by macrocyclization to achieve an elegant synthesis of (+)-cannabisativine (61).
Scheme 39.
Total synthesis of (+)-cannabisativine using the diastereoselective C2-alkylation of pyridiniums (Comins et al., 2004).[95]
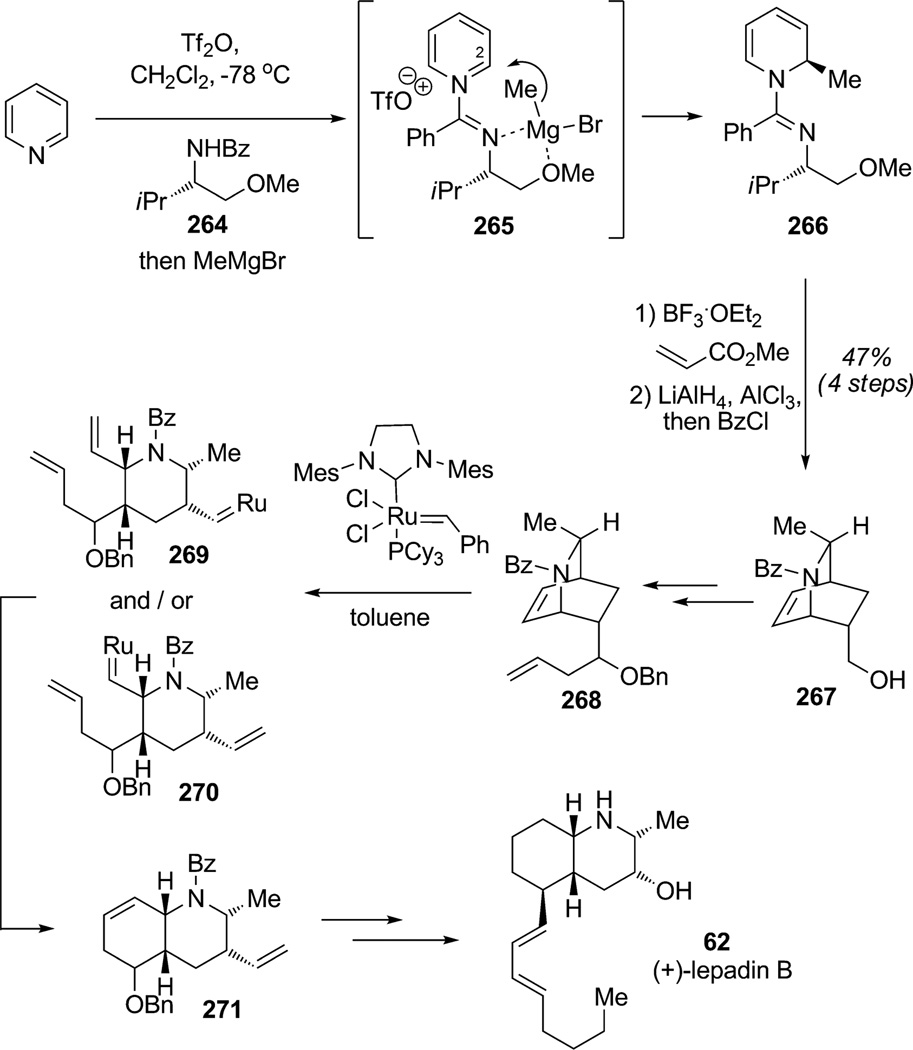
Recently, Charette and co-workers relied on a similar diastereoselective dearomatization strategy based on the alkylation of a pyridinium species for the synthesis of the alkaloid (+)-lepadin B (62; Scheme 40).[97] Reaction of pyridine with the triflic amidate of l-(OMe)-valinol 264 generated amidinium 265 which underwent regio- and diastereoselective alkylation at C2.[98] In fact, the bulky phenyl group discouraged nucleophilic attack at C4 by chelation of the valinol auxiliary with organomagnesium or zincate reagents leading to dearomatization (shown for methyl Grignard) to afford the amidine product 266. endo-Selective Diels–Alder cycloaddition and further reduction/protection afforded the isoquinoline skeleton 267 in 47% overall yield (three steps) and 84%ee. After few manipulations, the derived isoquinoline substrate 268 was successfully subjected to tandem metathesis (ROM–RCM) which proceeded via a presumed mixture of intermediates 269/270 to furnish the desired cis-fused decahydroquinoline 271 having the framework of the natural product. This stereoselective total synthesis of (+)-lepadin B (62) represents a key strategy for the synthesis of fused piperidines by combining diastereoselective alkylative dearomatization/Diels–Alder cycloaddition and tandem ROM–RCM.
Scheme 40.
Total synthesis of (+)-lepadin B using the diastereoselective alkylation of a pyridinium (Charette et al., 2008).[97]
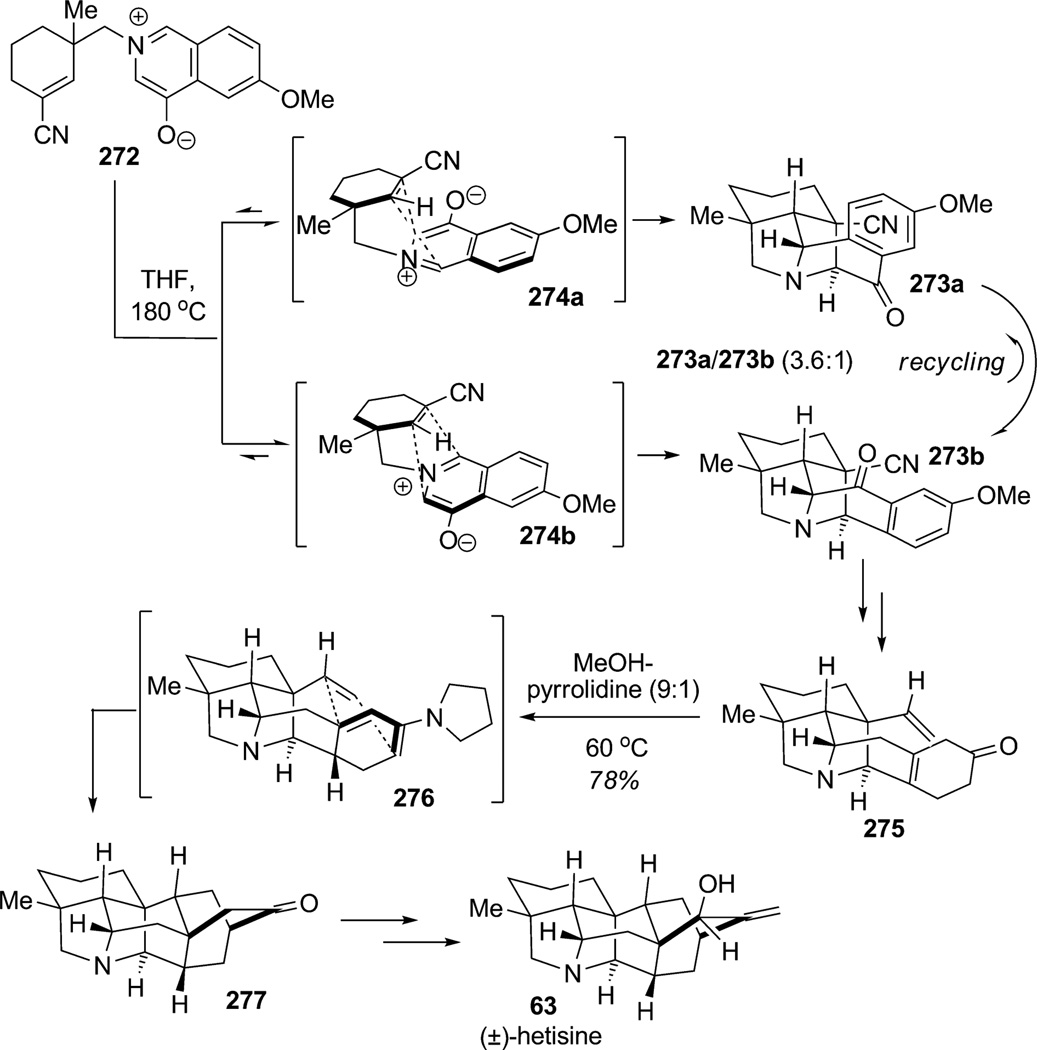
1,3-Dipolar cycloadditions involving oxidopyridinium betaines have previously been used in alkaloid synthesis.[99] Gin and co-workers fully illustrated the value of this dearomatization approach in a complex setting during their elegant synthesis of (±)-hetisine (63; Scheme 41).[100] In this work, the authors reported dearomatization of oxidoisoquinolinium betaine 272 at 180°C through intramolecular 1,3-dipolar cycloaddition to provide pyrrolidines 273 a and 273b (constitutional isomers, 3.6:1 ratio). The two constitutional isomers arose from the different facial approaches of the dipolarophile partner in arrangements 274 a and 274b. The authors confirmed that the 1,3-dipolar cycloaddition was reversible and under thermodynamic control which enabled the recycling of the undesired isomer 273 a to increase the production of the desired pentacyclic core 273b. After a few steps, the β,γ -unsaturated cyclohexenone 275 was obtained and transformed upon exposure to pyrrolidine into dienamine 276 which readily underwent intramolecular Diels–Alder cylcoaddition to afford the desired heptacyclic core structure 277 in 78% yield. (±)-Hetisine (63) was ultimately prepared in 15 steps from commercially available starting materials, underscoring the utility of the dearomatization of oxidoisoquinolinium betaine through 1,3-dipolar cycloaddition for the construction of complex alkaloids.
Scheme 41.
Total synthesis of (±)-hetisine employing the intramolecular 1,3-dipolar cycloaddition of oxidoisoquinolium betaines (Gin et al., 2006).[100]
6. Future Opportunities and Perspectives for Asymmetric Dearomatization
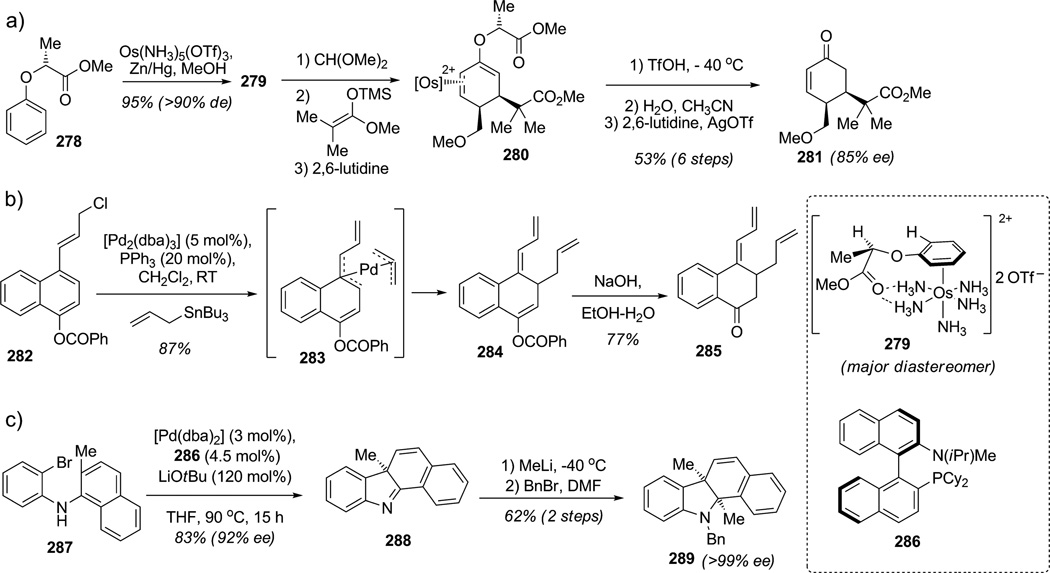
As described in this Review (Section 2), the dearomatization of arenes is a highly important chemical transformation for the preparation of polycyclic frameworks of many biologically active natural products. Transition-metal-catalyzed dearomatization has been developed in the past few years employing arene coordination (Scheme 42).[101] For instance, Harman and co-workers have developed a useful approach to arene dearomatization. Simple coordination of arenes to a transition metal, in particular, π-basic transitionmetal complexes, has been found to disrupt aromatic stabilization through η2 coordination (Scheme 42 a).[102] Such coordination between the chiral enantiopure phenol ether 278 and osmium could be made in a well-defined complex such as 279 (>90%de) which promotes diastereoselective addition toward electrophiles (e.g. formate) followed by addition of a silyl ketene acetal to afford the highly substituted cyclohexadiene 280. Further acidic treatment promoting auxiliary removal and decomplexation of the transition metal furnished the useful cyclohexenone building block 281 in 53% overall yield and 85%ee. In another study concerning π-allyl reactivity, Yamamoto and co-workers discovered the unexpected reactivity of benzyl chlorides and naphthalene allyl chlorides such as 282 (Scheme 42b).[103] Indeed, both systems when treated with catalytic palladium(0) reacted via π-allyl species to deliver different dearomatized products. In the case of the naphthalene allyl chloride derivative 282, the authors believe that the original bis(η3-allyl)palladium intermediate may isomerize to the more stable η3-benzylpalladium complex 283, which after reductive elimination regenerates the original palladium(0) catalyst and delivers the dearomatized compound 284 possessing both butadiene and allyl moieties. Final deprotection of the carbonate moiety delivers the cyclohexanone fragment 285. This chemistry highlights the possibility of synthesizing fused six-membered-ring systems from simple naphthalenes or phenanthrenes by means of extremely mild palladiumcatalyzed allylative dearomatization reactions with allyltributylstannane. Recently, Rawal and co-workers also reported a palladium-mediated alkylative dearomatization of numerous indolic substrates at C3 postion using allyl methylcarbonate.[104]
Scheme 42.
New strategies for arene dearomatization using transition-metal catalysis (Harman,[102] Y. Yamamoto,[103] Buchwald[105] et al., 2008–2010).
Asymmetric dearomatization methods are underdeveloped and significant potential for chemical synthesis. Therefore, the development of new catalytic and enantioselective dearomatization methods such as that recently reported by Buchwald and co-workers will set the bar for further applications in the total synthesis of complex molecules (Scheme 42c).[105] The authors found that a palladium(0) complex bearing the chiral P,N ligand 286 catalyzed asymmetric, intramolecular dearomatization of naphthalene derivatives such as 287 to produce the fused tetracyclic indolenine 288 (83% yield, 92%ee) which contains two contigous nonaromatic rings proximal to a quaternary stereocenter. Indolenine 288 was further functionalized at C2 by highly diastereoselective addition of methyllithium (d.r. 9:1) and directly protected on the indoline nitrogen to deliver tetracyclic product 289 in 62% overall yield and 99%ee. This elegant work should inspire future studies in the area of transition-metal-catalyzed, enantioselective dearomatization.
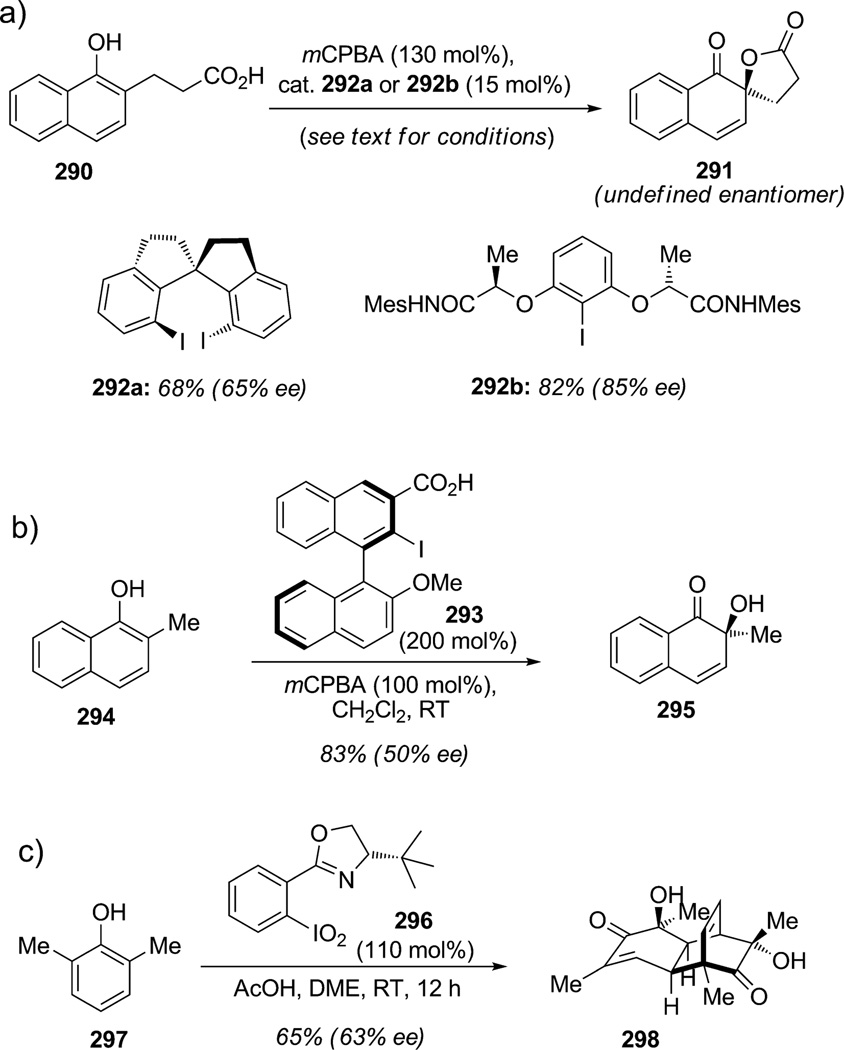
As described in Section 3 of this Review, three main challenges remain in the development of oxidative dearomatization processes of phenols induced by hypervalent iodine reagents: 1) addition of carbon-centered nucleophiles, 2) development of catalytic processes for dearomatization, and finally 3) improvement of catalytic, enantioselective dearomatization methods (Scheme 43). A number of research groups are currently working in the area of enantioselective oxidative dearomatization employing hypervalent iodine reagents. A recent example by Kita and co-workers described the first catalytic enantioselective oxidative dearomatization of naphthol 290 to produce chiral ortho-spirolactone 291 (Scheme 43a).[106] In this study, the authors evaluated the activity of a new chiral spirocyclic organoiodine(III) reagent formed in situ from 292 a and the effect of polar solvents on the yield and enantioselectivity of the dearomatization. Indeed, particular conditions were needed to avoid formation of a discrete carbocation that may result in diminished enantioselectivity and the spirolactone 291 was obtained in 68%yield and 65% ee. Dichloromethane or chloroform were found to be suitable solvents for this reaction in the presence of stoichiometric amounts of acetic and meta-chloroperbenzoic acid to regenerate the iodine(III) catalyst from reagent 292 a. At the beginning of 2010, Ishihara and co-workers examined the same reaction using a new catalytic C2- symmetric iodine source 292b and were able to isolate the desired spirolactone 291 in 82% yield and increased 85% ee.[107] Reactions were optimized using dichloromethane/nitromethane as the solvent mixture without any acid additive leading to high levels of enantioselectivity in the intramolecular oxidative dearomatization of naphthols. Previously, Quideau and co-workers developed the iodine reagent 293 based on a binaphthyl framework (Scheme 43 b).[108] To date, this approach has not been successfully applied to the enantioselective dearomatization of naphthol 294 in a catalytic fashion, but showed promising results for enantioselective, intermolecular dearomatization to access αhydroxy ketone 295 in 83%yield and 50% ee. New iodine(V) reagents have also been developed notably by Zhdankin and co-workers[109] and IBX derivatives have been successfully used for the dearomatization of phenols. Additionally, Birman and co-workers have explored the potential of new chiral organoiodine(V) reagents (Scheme 43c).[110] Indeed, the ortho-substituted iodoxybenzene derivatives 296 possessing chiral oxazoline groups were utilized for the enantioselective oxidative dearomatization of ortho-alkyl phenols. The authors were able to dearomatize phenolic substrates such as 2,6-disubstituted phenol 297 with a stoichiometric amount of a chiral hypervalent iodine(V) reagent and acetic acid to deliver an ortho-quinol that spontaneously dimerized to produce enantioenriched tricyclic products such as 298 in 29–68% yield (62–77%ee).
Scheme 43.
Enantioselective and catalytic oxidative dearomatization methodologies (Kita,[106] Ishihara,[107] Quideau,[108] Birman[110] et al., 2008–2010).
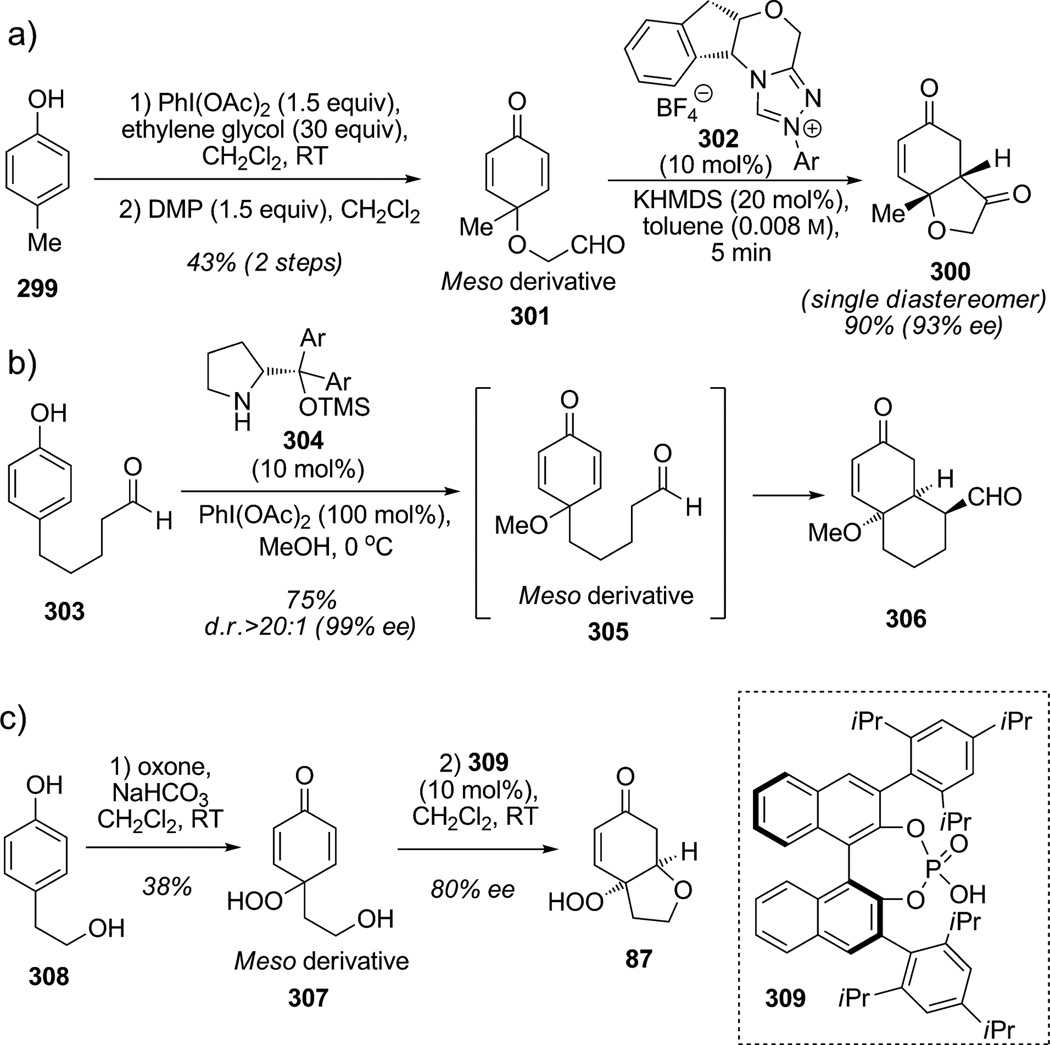
Another promising area for oxidative dearomatization relies on the desymmetrization of derived meso dienones to afford chiral enantiopure polycyclic building blocks (Scheme 44).[111] Rovis and co-workers reported a three-step sequence involving dearomatization, oxidation, and Stetter condensation starting from para-alkylphenol 299 to deliver in high diastereo- and enantioselectivity various complex lactones 300 (Scheme 44a).[112] This strategy involved para-oxidative dearomatization of phenols 299 with ethylene glycol to generate after oxidation with Dess–Martin periodinane (DMP) the meso-cyclohexadienone aldehydes 301 (20–50% yield), thereby establishing a powerful maneuver for further enantioselective desymmetrization using the Stetter reaction. Next, the key asymmetric intramolecular Stetter reaction using a chiral enantiopure N-heterocyclic carbene (NHC) catalyst (triazolium 302) afforded hydrobenzofuranone 300 in good yield as a single diastereomer with excellent enantioselectivity (93%ee). Gaunt and co-workers also reported their efforts for the direct conversion of para-substituted phenols 303 to highly functionalized chiral building blocks by means of para-oxidative dearomatization with methanol and other protic/soft nucleophiles followed by proline-catalyzed enantioselective desymmetrization (Scheme 44 b).[113] This creative transformation demonstrated the possibility of cascade sequences using hypervalent iodine with an organocatalyst. In the presence of proline catalyst 304, meso-cyclohexadieneone 305 cyclized via enamine chemistry to yield bi- or tricyclic frameworks such as 306 in 75% yield with high diastereo- and enantioselectivity. Finally, a recent example from You and co-workers, based on the previous work of Urbano and Carreño,[27] demonstrated the possibilty for desymmetrization of meso-peroxyquinol 307 throug an enantioselective oxa-Michael addition (Scheme 44 c).[114] Dearomatization of para-alkylphenol 308 with Oxone delivered meso para-peroxyquinol 307 which was subsequently desymmetrized in a presumed thermodynamic oxa-Michael process catalyzed by the chiral Brønsted acid 309 to deliver the enantioenriched bicyclic ether 310 in 80%ee.
Scheme 44.
Organocatalytic desymmetrization of meso dienones obtained through oxidative dearomatization (Rovis,[112] Gaunt,[113] You[114] et al., 2006–2010).
7. Summary
This Review has illustrated a number of examples and possibilities for the dearomatization of arenes, phenols, and heteroaromatic compounds which in the appropriate setting may be used to generate complex natural product frameworks in a stereocontrolled fashion. Further understanding of aromaticity and thus dearomatization will continue to evolve and provide new opportunities for methodology development and applications in total synthesis. As outlined in this Review, oxidative, but also reductive and alkylative dearomatization methodologies may be further developed as powerful tools for chemical synthesis. In addition, examples of reagent-controlled and catalytic enantioselective dearomatization of arenes, indoles, and pyridines are somewhat limited to date. Future research and emphasis in this area should lead to additional strategies and methods for the construction of polycyclic scaffolds which should be of great value to the field of natural products total synthesis.
Acknowledgments
We thank the National Institutes of Health (GM-073855) and the National Science Foundation (0848082) for financial support. We also would like to thank present and former members of the Porco research group for their many contributions to the field of dearomatization used in total synthesis, in particular Dr. Ji Qi, Dr. Jianglong Zhu, and Dr. Suwei Dong. We gratefully acknowledge Dr. Brad Balthaser, Dr. Matthew Medeiros, Dr. Benedikt Crone, and Dr. Andrew Kleinke for helpful discussions and careful proofreading of this review.
Abbreviations
- Ac
acyl
- acac
acetylacetonate
- Bn
benzyl
- Boc
tert-butoxycarbonyl
- brsm
based on recovered starting material
- CDDA
Claisen dearomatization/Diels–Alder
- cod
cyclooctadiene
- Cp
cyclopentadienyl
- Cy
cyclohexyl
- CSA
camphorsulfonic acid
- dba
trans,trans-dibenzylideneacetone
- DCE
dichloroethane
- DDQ
2,3-dichloro-5,6-dicyanobenzoquinone
- DIEA
diisopropylethylamine
- DMAP
4-dimethylaminopyridine
- DMP
Dess–Martin periodinane
- Dnp
3,5-dinitrophenyl
- hfacac
hexafluoroacetylacetonate
- HFIP
hexafluoroisopropanol
- IBX
2-iodoxybenzoic acid
- IMDA
intramolecular Diels–Alder
- KHMDS
potassium hexamethyldisilazide
- mCPBA
meta-chloroperbenzoic acid
- MES
mesitylene
- MOB
masked ortho-benzoquinone
- MOM
methoxymethyl ether
- MS
molecular sieves
- NBS
N-bromosuccinimide
- PCC
pyridinium chlorochromate
- PIDA
bis(acetoxy)iodobenzene
- Piv
pivaloyl
- PMB
para-methoxybenzyl
- PMP
para-methoxyphenyl
- TBAF
tetrabutylammonium fluoride
- TBDPS
tert-butyldiphenylsilyl
- TBS
tert-butyldimethylsilyl
- TES
triethylsilyl
- Tf
trifluoromethansulfonyl, SO2CF3
- TFA
trifluoroacetic acid
- TFAA
trifluoroacetic anhydride
- TFE
2,2,2-trifluoroethanol
- THF
tetrahydrofuran
- TIPS
triisopropylsilyl
- Ts
tosyl, toluenesulfonyl
- TS
transition state
Biographies

Stéphane P. Roche was born in Thiers (France) in 1979. He received his PhD degree (2006) in chemistry from the Blaise Pascal University under the supervision of Professor D. J. Aitken. He then joined the Institute of Chemical and Engineering Sciences (ICES, @Star) in Singapore, as a research fellow with Professor K. C. Nicolaou (2006–2008). At the end of 2008, he joined the Porco research group at Boston University. His research interests include the development of new “bio-inspired” methodologies and dearomatization strategies and their application to the concise, total syntheses of biologically active natural products.

John A. Porco, Jr. was born in Danbury, Connecticut (USA) in 1963. He received his PhD in 1992 from Harvard University under the direction of Professor Stuart Schreiber. After a period in industry he joined the Department of Chemistry at Boston University in 1999 as Assistant Professor and was promoted to Professor of Chemistry in September 2004. His research is focused in two major areas: the development of new synthetic methodologies for efficient chemical synthesis of complex molecules and the synthesis of complex chemical libraries.
References
- 1.Franklin JL. J. Am. Chem. Soc. 1950;72:4278–4280. [Google Scholar]
- 2.a) Krygowski TM, Cyraňski MK. Chem. Rev. 2001;101:1385–1419. doi: 10.1021/cr990326u. [DOI] [PubMed] [Google Scholar]; b) Balaban AT, Oniciu DC, Katritzky AR. Chem. Rev. 2004;104:2777–2812. doi: 10.1021/cr0306790. [DOI] [PubMed] [Google Scholar]; c) Chen Z, Wannere CS, Corminboeuf C, Puchta R, von P, Schleyer R. Chem. Rev. 2005;105:3842–3888. doi: 10.1021/cr030088+. [DOI] [PubMed] [Google Scholar]
- 3.a) Boll M. J. Mol. Microbiol. Biotechnol. 2005;10:132–142. doi: 10.1159/000091560. [DOI] [PubMed] [Google Scholar]; b) Thiele B, Rieder O, Golding BT, Müller M, Boll M. J. Am. Chem. Soc. 2008;130:14050–14051. doi: 10.1021/ja805091w. [DOI] [PubMed] [Google Scholar]; c) Kung JW, Baumann S, Von Bergen M, Müller M, Hagedoorn P-L, Hagen WR, Boll M. J. Am. Chem. Soc. 2010;132:9850–9856. doi: 10.1021/ja103448u. [DOI] [PubMed] [Google Scholar]
- 4. Myers AG, Siegel DR, Buzard DJ, Charest MG. Org. Lett. 2001;3:2923–2926. doi: 10.1021/ol010151m. For a recent example, see: Mihovilovic MD, Leisch HG, Mereiter K. Tetrahedron Lett. 2004;45:7087–7090.
- 5.Corey EJ, Girotra NN, Mathew CT. J. Am. Chem. Soc. 1969;91:1557–1559. [Google Scholar]
- 6. Schultz AG, Wang A. J. Am. Chem. Soc. 1998;120:8259–8260. For review on asymmetric Birch reduction, see Schultz AG. Chem. Commun. 1999:1263–1271.
- 7.Barnes-Seeman D, Corey EJ. Org. Lett. 1999;1:1503–1504. doi: 10.1021/ol991070h. [DOI] [PubMed] [Google Scholar]
- 8.a) Nicolaou KC, Simonsen KB, Vassilikogiannakis G, Baran PS, Vidali VP, Pitsinos EN, Couladouros EA. Angew. Chem. 1999;111:3762–3766. doi: 10.1002/(sici)1521-3773(19991203)38:23<3555::aid-anie3555>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 1999;38:3555–3559. doi: 10.1002/(sici)1521-3773(19991203)38:23<3555::aid-anie3555>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Vassilikogiannakis G, Simonsen KB, Baran PS, Zhong Y-L, Vidali VP, Pitsinos EN, Couladouros EA. J. Am. Chem. Soc. 2000;122:3071–3079. doi: 10.1002/(sici)1521-3773(19991203)38:23<3555::aid-anie3555>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9. Pettus TRR, Chen X-T, Danishefsky SJ. J. Am. Chem. Soc. 1998;120:12684–12685. Pettus TRR, Inoue M, Chen X-T, Danishefsky SJ. J. Am. Chem. Soc. 2000;122:6160–6168. for new developments of prenylic dearomatization, see: Lei X, Dai M, Hua Z, Danishefsky SJ. Tetrahedron Lett. 2008;49:6383–6385. doi: 10.1016/j.tetlet.2008.07.184.
- 10.He F, Bo Y, Altom JD, Corey EJ. J. Am. Chem. Soc. 1999;121:6771–6772. [Google Scholar]
- 11.a) Magnus P, Rodriguez-López J, Mulholland K, Matthewst I. J. Am. Chem. Soc. 1992;114:382–383. [Google Scholar]; b) Magnus P, Rodriguez-López J, Mulholland K, Matthews I. Tetrahedron. 1993;49:8059–8072. [Google Scholar]
- 12.Yamaguchi R, Moriyasu M, Yoshioka M, Kawanisi M. J. Org. Chem. 1988;53:3507–3512. [Google Scholar]
- 13.Mander LN. Synlett. 1991:134–144. [Google Scholar]
- 14.a) Pape AR, Kaliappan KP, Kündig EP. Chem. Rev. 2000;100:2917–2940. doi: 10.1021/cr9902852. [DOI] [PubMed] [Google Scholar]; b) Smith PL, Chordia MD, Harman WD. Tetrahedron. 2001;57:8203–8225. [Google Scholar]; c) Kündig EP, Pape A. Top. Organomet. Chem. 2004;7:71–94. [Google Scholar]; d) Keane JM, Harman WD. Organometallics. 2005;24:1786–1798. [Google Scholar]; e) López Ortiz F, Iglesias MJ, Fernández I, Andújar Sánchez CM, Gómez GR. Chem. Rev. 2007;107:1580–1691. doi: 10.1021/cr030207l. [DOI] [PubMed] [Google Scholar]
- 15.Magdziak D, Meek SJ, Pettus TRR. Chem. Rev. 2004;104:1383–1429. doi: 10.1021/cr0306900. [DOI] [PubMed] [Google Scholar]
- 16.a) Quideau S, Pouységu L. Org. Prep. Proced. Int. 1999;31:617–680. [Google Scholar]; b) Quideau S, Pouységu L, Deffieux D. Synlett. 2008:467–495. [Google Scholar]
- 17.Pouységu L, Deffieux D, Quideau S. Tetrahedron. 2010;66:2235–2261. [Google Scholar]
- 18. Boyd DR, Sharma ND, Byrne B, Hand MV, Malone JF, Sheldrake GN, Blacker J, Dalton H. J. Chem. Soc. Perkin Trans. 1. 1998:1935–1943. For reviews on biocatalysis for oxidative enantioselective dearomatization, see: Hudlicky T, Gonzalez D, Gibson DT. Aldrichimica Acta. 1999;32:35–62. Hudlicky T, Reed JW. Synlett. 2009:685–702.
- 19.Pinkerton DM, Banwell MG, Willis AC. Org. Lett. 2009;11:4290–4293. doi: 10.1021/ol9016657. [DOI] [PubMed] [Google Scholar]
- 20.a) Mander LN, Pyne SG. J. Am. Chem. Soc. 1979;101:3373–3375. [Google Scholar]; b) Mander LN. Acc. Chem. Res. 1983;16:48–54. [Google Scholar]; c) King GR, Mander LN, Monck NJT, Morris JC, Zhang H. J. Am. Chem. Soc. 1997;119:3828–3829. [Google Scholar]; d) Frey B, Wells AP, Rogers DH, Mander LN. J. Am. Chem. Soc. 1998;120:1914–1915. [Google Scholar]
- 21.Levin S, Nani RR, Reisman SE. Org. Lett. 2010;12:780–783. doi: 10.1021/ol902848k. [DOI] [PubMed] [Google Scholar]
- 22. Wender PA, Howbert JJ. J. Am. Chem. Soc. 1981;103:688–690. For reviews about the dearomatizing cycloaddition of arenes, see: Cornelisse J. Chem. Rev. 1993;93:615–669. Hoffmann N. Synthesis. 2004:481–495. Chappell D, Russell AT. Org. Biomol. Chem. 2006;4:4409–4430. doi: 10.1039/b614011b. Mattay J. Angew. Chem. 2007;119:674–677. Angew. Chem. Int. Ed. 2007;46:663–665. doi: 10.1002/anie.200603337.
- 23.a) Gaich T, Mulzer J. J. Am. Chem. Soc. 2009;131:452–453. doi: 10.1021/ja8083048. [DOI] [PubMed] [Google Scholar]; b) Gaich T, Mulzer J. Org. Lett. 2010;12:272–275. doi: 10.1021/ol902594b. [DOI] [PubMed] [Google Scholar]
- 24.a) Beak P, Meyers AI. Acc. Chem. Res. 1986;19:356–363. [Google Scholar]; b) Meyers AI, Higashiyama K. J. Org. Chem. 1987;52:4592–4597. [Google Scholar]; c) Andrews RC, Teague SJ, Meyers AI. J. Am. Chem. Soc. 1988;110:7854–7858. [Google Scholar]; d) Robichaud AJ, Meyers AI. J. Org. Chem. 1991;56:2607–2609. [Google Scholar]
- 25. Clayden J, Knowles FE, Baldwin IR. J. Am. Chem. Soc. 2005;127:2412–2413. doi: 10.1021/ja042415g. For a review on intramolecular dearomatization through alkylation with nucleophilic anions, see: Clayden J, Kenworthy MN. Synthesis. 2004:1721–1736.
- 26.Ovens C, Martin NG, Procter DJ. Org. Lett. 2008;10:1441–1444. doi: 10.1021/ol8002095. [DOI] [PubMed] [Google Scholar]
- 27.Carreño MC, González-Lápez M, Urbano A. Angew. Chem. 2006;118:2803–2807. doi: 10.1002/anie.200504605. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:2737–2741. doi: 10.1002/anie.200504605. [DOI] [PubMed] [Google Scholar]
- 28.Barradas S, Carreño MC, González-López M, Latorre A, Urbano A. Org. Lett. 2007;9:5019–5022. doi: 10.1021/ol702236e. [DOI] [PubMed] [Google Scholar]
- 29.a) Cox C, Danishefsky SJ. Org. Lett. 2000;2:3493–3496. doi: 10.1021/ol006531+. [DOI] [PubMed] [Google Scholar]; b) Wood JL, Graeber JK, Njardarson JT. Tetrahedron. 2003;59:8855–8858. [Google Scholar]; c) Bérubé A, Drutu I, Wood JL. Org. Lett. 2006;8:5421–5424. doi: 10.1021/ol061737h. [DOI] [PubMed] [Google Scholar]; d) Morton JGM, Kwon LD, Freeman JD, Njardarson JT. Synlett. 2009:23–27. [Google Scholar]
- 30.Li F, Tartakoff SS, Castle SL. J. Am. Chem. Soc. 2009;131:6674–6675. doi: 10.1021/ja9024403. [DOI] [PubMed] [Google Scholar]
- 31.Quideau S, Lebon M, Lamidey A-M. Org. Lett. 2002;4:3975–3978. doi: 10.1021/ol026855t. [DOI] [PubMed] [Google Scholar]
- 32.Frie JL, Jeffrey CS, Sorensen EJ. Org. Lett. 2010;12:5394–5397. doi: 10.1021/ol902168g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.a) Mendelsohn BA, Lee S, Kim S, Teyssier F, Aulakh VS, Ciufolini MA. Org. Lett. 2009;11:1539–1542. doi: 10.1021/ol900194v. [DOI] [PubMed] [Google Scholar]; b) Mendelsohn BA, Ciufolini MA. Org. Lett. 2009;11:4736–4739. doi: 10.1021/ol901914u. [DOI] [PubMed] [Google Scholar]
- 34.Quideau S, Fabre I, Deffieux D. Org. Lett. 2004;6:4571–4573. doi: 10.1021/ol048030k. [DOI] [PubMed] [Google Scholar]
- 35.a) Van DeWater RW, Hoarau C, Pettus TRR. Tetrahedron Lett. 2003;44:5109–5113. [Google Scholar]; b) Mejorado LH, Hoarau C, Pettus TRR. Org. Lett. 2004;6:1535–1538. doi: 10.1021/ol0498592. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hoarau C, Pettus TRR. Org. Lett. 2006;8:2843–2846. doi: 10.1021/ol061000s. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wenderski TA, Huang S, Pettus TRR. J. Org. Chem. 2009;74:4104–4109. doi: 10.1021/jo900401k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mejorado LH, Pettus TRR. J. Am. Chem. Soc. 2006;128:15625–15631. doi: 10.1021/ja062987w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pouységu L, Chassaing S, Dejugnac D, Lamidey A-M, Miqueu K, Sotiropoulos J-M, Quideau S. Angew. Chem. 2008;120:3608–3611. doi: 10.1002/anie.200705816. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:3552–3555. doi: 10.1002/anie.200705816. [DOI] [PubMed] [Google Scholar]
- 38.a) Zhu J, Grigoriadis NP, Lee JP, Porco JA., Jr J. Am. Chem. Soc. 2005;127:9342–9343. doi: 10.1021/ja052049g. [DOI] [PubMed] [Google Scholar]; b) Zhu J, Porco JA., Jr Org. Lett. 2006;8:5169–5171. doi: 10.1021/ol062233m. [DOI] [PubMed] [Google Scholar]
- 39.For select examples of soft nucleophiles used in oxidative dearomatization, see: Ousmer M, Braun NA, Ciufolini MA. Org. Lett. 2001;3:765–767. doi: 10.1021/ol015526i. b) See also Ref. [33b] Liang H, Ciufolini MA. Org. Lett. 2010;12:1760–1763. doi: 10.1021/ol1003669. For a review of nitrogen nucleophiles in oxidative dearomatization, see: Ciufolini MA, Braun NA, Canesi S, Ousmer M, Chang J, Chai D. Synthesis. 2007:3759–3772.
- 40.For recent examples of oxidative dearomatization with C–C bond formation, see: Swenton JS, Callinan A, Chen Y, Rohde JJ, Kearns ML, Morrow GW. J. Org. Chem. 1996;61:1267–1274. Tohma H, Harayama Y, Hashizume M, Iwata M, Kiyono Y, Egi M, Kita Y. J. Am. Chem. Soc. 2003;125:11235–11240. doi: 10.1021/ja0365330. Ozanne-Beaudenon A, Quideau S. Angew. Chem. 2005;117:7227–7231. doi: 10.1002/anie.200501638. Angew. Chem. Int. Ed. 2005;44:7065–7069. doi: 10.1002/anie.200501638. Honda T, Shigehisa H. Org. Lett. 2006;8:657–659. doi: 10.1021/ol052841m. Shigehisa H, Takayama J, Honda T. Tetrahedron Lett. 2006;47:7301–7306. Nicolaou KC, Majumder U, Roche SP, Chen DY-K. Angew. Chem. 2007;119:4799–4802. doi: 10.1002/anie.200701947. Angew. Chem. Int. Ed. 2007;46:4715–4718. doi: 10.1002/anie.200701947. Guérard KC, Sabot C, Racicot L, Canesi SJ. J. Org. Chem. 2009;74:2039–2045. doi: 10.1021/jo802728u. Eastman K, Baran PS. Tetrahedron. 2009;65:3149–3154.
- 41.Dohi T, Minamitsuji Y, Maruyama A, Hirose S, Kita Y. Org. Lett. 2008;10:3559–3562. doi: 10.1021/ol801321f. [DOI] [PubMed] [Google Scholar]
- 42.Nicolaou KC, Edmonds DJ, Li A, Tria GS. Angew. Chem. 2007;119:4016–4019. doi: 10.1002/anie.200700586. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:3942–3945. doi: 10.1002/anie.200700586. [DOI] [PubMed] [Google Scholar]
- 43.a) Quideau S, Looney MA, Pouységu L. Org. Lett. 1999;1:1651–1654. [Google Scholar]; b) Quideau S, Pouységu L, Oxoby M, Looney MA. Tetrahedron. 2001;57:319–329. [Google Scholar]; c) Sabot C, Commare B, Duceppe M-A, Nahi S, Guérard KC, Canesi S. Synlett. 2008:3226–3230. [Google Scholar]; d) Sabot C, Guérard KC, Canesi S. Chem. Commun. 2009:2941–2943. doi: 10.1039/b903530c. [DOI] [PubMed] [Google Scholar]
- 44.Snyder SA, Sherwood TC, Ross AG. Angew. Chem. 2010;122:5272–5276. doi: 10.1002/anie.201002264. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2010;49:5146–5150. doi: 10.1002/anie.201002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.a) Masamune S. J. Am. Chem. Soc. 1961;83:1009–1010. [Google Scholar]; b) Boger DL, Machiya K. J. Am. Chem. Soc. 1992;114:10056–10058. [Google Scholar]; c) Lalic G, Corey EJ. Org. Lett. 2007;9:4921–4923. doi: 10.1021/ol702323s. [DOI] [PubMed] [Google Scholar]
- 46.Dai M, Danishefsky SJ. Tetrahedron Lett. 2008;49:6610–6612. doi: 10.1016/j.tetlet.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGrath NA, Bartlett ES, Sittihan S, Njardarson JT. Angew. Chem. 2009;121:8695–8698. doi: 10.1002/anie.200903347. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2009;48:8543–8546. doi: 10.1002/anie.200903347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi J, Porco JA., Jr J. Am. Chem. Soc. 2007;129:12682–12683. doi: 10.1021/ja0762339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitasev B, Porco JA., Jr Org. Lett. 2009;11:2285–2288. doi: 10.1021/ol900590t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siegel DR, Danishefsky SJ. J. Am. Chem. Soc. 2006;128:1048–1049. doi: 10.1021/ja057418n. [DOI] [PubMed] [Google Scholar]
- 51.a) Borgulya J, Hansen HJ, Barner R, Schmid H. Helv. Chim. Acta. 1963;46:2444–2445. [Google Scholar]; b) Satoh T, Ikeda M, Miura M, Nomura M. J. Org. Chem. 1997;62:4877–4879. doi: 10.1021/jo962387n. [DOI] [PubMed] [Google Scholar]
- 52. Tisdale EJ, Chowdhury C, Vong BG, Li H, Theodorakis EA. Org. Lett. 2002;4:909–912. doi: 10.1021/ol017278w. Tisdale EJ, Vong BG, Li H, Kim SH, Chowdhury C, Theodorakis EA. Tetrahedron. 2003;59:6873–6887. Tisdale EJ, Slobodov I, Theodorakis EA. Org. Biomol. Chem. 2003;1:4418–4422. doi: 10.1039/b311833a. For review on caged Garcinia xanthones, see: Chantarasriwong O, Batova A, Chavasiri W, Theodorakis EA. Chem. Eur. J. 2010;16:9944–9962. doi: 10.1002/chem.201000741.
- 53.Tisdale EJ, Slobodov I, Theodorakis EA. Proc. Natl. Acad. Sci. USA. 2004;101:12030–12035. doi: 10.1073/pnas.0401932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicolaou KC, Hao X, Wartmann M. Angew. Chem. 2005;117:766–771. doi: 10.1002/anie.200462211. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:756–761. doi: 10.1002/anie.200462211. [DOI] [PubMed] [Google Scholar]
- 55.a) Hilvert D, Nared KD. J. Am. Chem. Soc. 1988;110:5593–5594. [Google Scholar]; b) Gouverneur VE, Houk KN, de Pascual-Teresa B, Beno B, Janda KD, Lerner RA. Science. 1993;262:204–208. doi: 10.1126/science.8211138. [DOI] [PubMed] [Google Scholar]; c) Chook YM, Gray JV, Ke H, Lipscomb WN. J. Mol. Biol. 1994;240:476–500. doi: 10.1006/jmbi.1994.1462. [DOI] [PubMed] [Google Scholar]; d) Lee AY, Karplus PA, Ganem B, Clardy J. J. Am. Chem. Soc. 1995;117:3627–3628. [Google Scholar]
- 56.Hayden AE, Hao X, Nicolaou KC, Houk KN. Org. Lett. 2006;8:2989–2992. doi: 10.1021/ol060917o. [DOI] [PubMed] [Google Scholar]
- 57. Stocking EM, Williams RM. Angew. Chem. 2003;115:3186–3223. doi: 10.1002/anie.200200534. Angew. Chem. Int. Ed. 2003;42:3078–3115. doi: 10.1002/anie.200200534. Ose T, Watanabe K, Mie T, Honma M, Watanabe H, Yao M, Oikawa H, Tanaka I. Nature. 2003;422:185–189. doi: 10.1038/nature01454. Auclair K, Sutherland A, Kennedy J, Witter DJ, Van den Heever JP, Hutchinson CR, Vederas JC. J. Am. Chem. Soc. 2000;122:11519–11520. Oikawa H, Kobayashi T, Katayama K, Suzuki Y, Ichihara A. J. Org. Chem. 1998;63:8748–8756. For a review on biomietic Diels–Alder reaction, see: Pindur U, Schneider GH. Chem. Soc. Rev. 1994;23:409–415.
- 58.a) Fu X, Hossain MB, Schmitz FJ, van der Helm D. J. Org. Chem. 1997;62:3810–3819. [Google Scholar]; b) Fu X, Hossain MB, Van der Helm D, Schmitz FJ. J. Am. Chem. Soc. 1994;116:12125–12126. [Google Scholar]
- 59.a) Layton ME, Morales CA, Shair MD. J. Am. Chem. Soc. 2002;124:773–775. doi: 10.1021/ja016585u. [DOI] [PubMed] [Google Scholar]; b) Morales CA, Layton ME, Shair MD. Proc. Natl. Acad. Sci. USA. 2004;101:12036–12041. doi: 10.1073/pnas.0401952101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicolaou KC, Vassilikogiannakis G, Mägerlein W, Kranich R. Angew. Chem. 2001;113:2543–2547. doi: 10.1002/1521-3773(20010702)40:13<2482::AID-ANIE2482>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2001;40:2482–2486. [Google Scholar]
- 61. Heckrodt TJ, Mulzer J. J. Am. Chem. Soc. 2003;125:4680–4681. doi: 10.1021/ja034397t. For similar strategy using a different oxidative dearomatization, see Waizumi N, Stankovic AR, Rawal VH. J. Am. Chem. Soc. 2003;125:13022–13023. doi: 10.1021/ja035898h.
- 62.For a review concerning oxidative dearomatization/IMDA cascade sequences, see Liao C-C, Peddiniti RK. Acc. Chem. Res. 2002;35:856–866. doi: 10.1021/ar000194n. Liao C-C. Pure Appl. Chem. 2005;77:1221–1234.
- 63.Hsu D-S, Liao C-C. Org. Lett. 2007;9:4563–4565. doi: 10.1021/ol702062p. [DOI] [PubMed] [Google Scholar]
- 64.Cook SP, Polara A, Danishefsky SJ. J. Am. Chem. Soc. 2006;128:16440–16441. doi: 10.1021/ja0670254. [DOI] [PubMed] [Google Scholar]
- 65.Krawczuk PJ, Schöne N, Baran PS. Org. Lett. 2009;11:4774–4776. doi: 10.1021/ol901963v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.For recent examples of the Wessely oxidation, see Mehta G, Maity P. Tetrahedron Lett. 2007;48:8865–8868. also see references [7] and [28a]
- 67.Snyder SA, Kontes F. J. Am. Chem. Soc. 2009;131:1745–1752. doi: 10.1021/ja806865u. [DOI] [PubMed] [Google Scholar]
- 68.Gagnepain J, Méreau R, Dejugnac D, Léger J-M, Castet F, Deffieux D, Pouységu L, Quideau S. Tetrahedron. 2007;63:6493–6505. [Google Scholar]
- 69.Dong S, Hamel E, Bai R, Covell DG, Beutler JA, Porco JA., Jr Angew. Chem. 2009;121:1522–1525. doi: 10.1002/anie.200805486. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2009;48:1494–1497. doi: 10.1002/anie.200805486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.For select recent examples of furan dearomatization through Diels–Alder cycloaddition, see: Matsumoto T, Sohma T, Yamaguchi H, Kurata S, Suzuki K. Synlett. 1995:263–265. Kaelin DE, Jr, Lopez OD, Martin SF. J. Am. Chem. Soc. 2001;123:6937–6938. doi: 10.1021/ja0108640. Toró A, Deslongchamps P. J. Org. Chem. 2003;68:6847–6852. doi: 10.1021/jo034123o. Dadwal M, Kesharwani MK, Danayak V, Ganguly B, Mobin SM, Muruganantham R, Namboothiri INN. Eur. J. Org. Chem. 2008:6106–6118. Zubkov FI, Ershova JD, Orlova AA, Zaytsev VP, Nikitina EV, Peregudov AS, Gurbanov AV, Borisov RS, Khrustalev VN, Maharramov AM, Varlamov AV. Tetrahedron. 2009;65:3789–3803. Magnus P, Littich R. Org. Lett. 2009;11:3938–3941. doi: 10.1021/ol901537n.
- 71.For recent examples of the photoxidative dearomatization of furan in total synthesis, see: Vassilikogiannakis G, Stratakis M. Angew. Chem. 2003;115:5623–5626. doi: 10.1002/anie.200352180. Angew. Chem. Int. Ed. 2003;42:5465–5468. doi: 10.1002/anie.200352180. Vassilikogiannakis G, Margaros I, Montagnon T. Org. Lett. 2004;6:2039–2042. doi: 10.1021/ol0493610. Miyashita M, Sasaki M, Hattori I, Sakai M, Tanino K. Science. 2004;305:495–499. doi: 10.1126/science.1098851. Yoshimura F, Sasaki M, Hattori I, Komatsu K, Sakai M, Tanino K, Miyashita M. Chem. Eur. J. 2009;15:6626–6644. doi: 10.1002/chem.200900310.
- 72.For recent examples of the dearomatization of 2-silyloxyfuran to generate butenolides, see: Burghart J, Brückner R. Angew. Chem. 2008;120:7777–7782. doi: 10.1002/anie.200801638. Angew. Chem. Int. Ed. 2008;47:7664–7668. doi: 10.1002/anie.200801638. Simmons B, Walji AM, MacMillan DWC. Angew. Chem. 2009;121:4413–4417. Angew. Chem. Int. Ed. 2009;48:4349–4353. doi: 10.1002/anie.200900220.
- 73.For a review on Pummerer/Diels–Alder cascade reactions highlighting furan dearomatization, see: Padwa A. Chem. Commun. 1998:1417–1424. Bur SK, Padwa A. Chem. Rev. 2004;104:2401–2432. doi: 10.1021/cr020090l.
- 74.a) Ginn JD, Lynch SM, Padwa A. Tetrahedron Lett. 2000;41:9387–9391. [Google Scholar]; b) Bur SK, Lynch SM, Padwa A. Org. Lett. 2002;4:473–476. doi: 10.1021/ol016804g. [DOI] [PubMed] [Google Scholar]; c) Zhang H, Boonsombat J, Padwa A. Org. Lett. 2007;9:279–282. doi: 10.1021/ol062728b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ginn JD, Padwa A. Org. Lett. 2002;4:1515–1517. doi: 10.1021/ol025746b. [DOI] [PubMed] [Google Scholar]
- 76.He W, Huang J, Sun X, Frontier AJ. J. Am. Chem. Soc. 2008;130:300–308. doi: 10.1021/ja0761986. [DOI] [PubMed] [Google Scholar]
- 77.For recent examples of benzofuran dearomatization using Diels–Alder cycloaddition, see: Chen C-H, Rao PD, Liao C-C. J. Am. Chem. Soc. 1998;120:13254–13255. Marrocchi A, Minuti L, Taticchi A, Scheeren HW. Tetrahedron. 2001;57:4959–4965. France S, Boonsombat J, Leverett CA, Padwa A. J. Org. Chem. 2008;73:8120–8123. doi: 10.1021/jo8016956. For recent examples of indole dearomatization using Diels–Alder cycloaddition, see: Kobayashi S, Ueda T, Fukuyama T. Synlett. 2000:883–886. Crawley SL, Funk RL. Org. Lett. 2003;5:3169–3171. doi: 10.1021/ol034407v. For the catalytic enantioselective dearomatization of indole through the Diels–Alder cycloaddition, see: Gioia C, Bernardi L, Ricci A. Synthesis. 2010:161–170.
- 78.Stork G, Yamashita A, Adams J, Schulte GR, Chesworth R, Miyazaki Y, Farme JJ. J. Am. Chem. Soc. 2009;131:11402–11406. doi: 10.1021/ja9038505. [DOI] [PubMed] [Google Scholar]
- 79.For review on the electrochemistry of furans, see: Sperry JB, Wright DL. Chem. Soc. Rev. 2006;35:605–621. doi: 10.1039/b512308a. Moeller KD. Synlett. 2009:1208–1218.
- 80.Hughes CC, Miller AK, Trauner D. Org. Lett. 2005;7:3425–3428. doi: 10.1021/ol047387l. [DOI] [PubMed] [Google Scholar]
- 81.For use of nitrogen-stabilized oxyallyl cations in [4+3] cycloadditions, see: Walters MA, Arcand HR. J. Org. Chem. 1996;61:1478–1486. MaGee DI, Godineau E, Thornton PD, Walters MA, Sponholtz DJ. Eur. J. Org. Chem. 2006:3667–3680. For the use of oxyallyl cations in stereoselective [4+3] cycloadditions, see: Harmata M, Ghosh SK, Hong X, Wacharasindu S, Kirchhoefer P. J. Am. Chem. Soc. 2003;125:2058–2059. doi: 10.1021/ja029058z. Huang J, Hsung RP. J. Am. Chem. Soc. 2005;127:50–51. doi: 10.1021/ja044760b.
- 82.Antoline JE, Hsung RP, Huang J, Song Z, Li G. Org. Lett. 2007;9:1275–1278. doi: 10.1021/ol070103n. [DOI] [PubMed] [Google Scholar]
- 83.a) Hintermann T, Seebach D. Helv. Chim. Acta. 1998;81:2093–2121. [Google Scholar]; b) Gaul C, Seebach D. Org. Lett. 2000;2:1501–1504. doi: 10.1021/ol0000410. [DOI] [PubMed] [Google Scholar]
- 84.a) Baran PS, Richter JM. J. Am. Chem. Soc. 2004;126:7450–7451. doi: 10.1021/ja047874w. [DOI] [PubMed] [Google Scholar]; b) Baran PS, Guerrero CA, Hafensteiner BD, Ambhaikar NB. Angew. Chem. 2005;117:3960–3963. doi: 10.1002/anie.200461864. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:3892–3895. doi: 10.1002/anie.200500655. [DOI] [PubMed] [Google Scholar]; c) Baran PS, Maimone TJ, Richter JM. Nature. 2007;446:404–408. doi: 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]
- 85.a) Baran PS, Shenvi RA, Mitsos CA. Angew. Chem. 2005;117:3780–3783. doi: 10.1002/anie.200500522. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:3714–3717. doi: 10.1002/anie.200500522. [DOI] [PubMed] [Google Scholar]; b) Baran PS, Shenvi RA. J. Am. Chem. Soc. 2006;128:14028–14029. doi: 10.1021/ja0659673. [DOI] [PubMed] [Google Scholar]
- 86.Martin DBC, Vanderwal CD. J. Am. Chem. Soc. 2009;131:3472–3473. doi: 10.1021/ja900640v. [DOI] [PubMed] [Google Scholar]
- 87.For a recent example of the ring-opening of a pyridinium with a secondary amine, see: Steinhardt SE, Silverston JS, Vanderwal CD. J. Am. Chem. Soc. 2008;130:7560–7561. doi: 10.1021/ja8028125.
- 88.a) Turet L, Markó IE, Tinant B, Declercq J-P, Touillaux R. Tetrahedron Lett. 2002;43:6591–6595. [Google Scholar]; b) Heureux N, Wouters J, Markó IE. Org. Lett. 2005;7:5245–5248. doi: 10.1021/ol0521127. [DOI] [PubMed] [Google Scholar]
- 89.Jones SB, Simmons B, MacMillan DWC. J. Am. Chem. Soc. 2009;131:13606–13607. doi: 10.1021/ja906472m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wilkie GD, Elliott GI, Blagg BSJ, Wolkenberg SE, Soenen DR, Miller MM, Pollack S, Boger DL. J. Am. Chem. Soc. 2002;124:11292–11294. doi: 10.1021/ja027533n. Elliott GI, Fuchs JR, Blagg BSJ, Ishikawa H, Tao H, Yuan Z-Q, Boger DL. J. Am. Chem. Soc. 2006;128:10589–10595. doi: 10.1021/ja0612549. Kato D, Sasaki Y, Boger DL. J. Am. Chem. Soc. 2010;132:3685–3687. doi: 10.1021/ja910695e. For a review on the HDA reaction showing the utility of nitrogen extrusion, see: Boger DL. Chem. Rev. 1986;86:781–793. For seminal work on HDA reactions with indole triggering nitrogen extrusion, see: Benson SC, Lee L, Yang L, Snyder JK. Tetrahedron. 2000;56:1165–1180.
- 91.Campbell EL, Zuhl AM, Liu CM, Boger DL. J. Am. Chem. Soc. 2010;132:3009–3012. doi: 10.1021/ja908819q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.a) Li C, Chan C, Heimann AC, Danishefsky SJ. Angew. Chem. 2007;119:1466–1469. [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:1444–1447. doi: 10.1002/anie.200604071. [DOI] [PubMed] [Google Scholar]; b) Li C, Chan C, Heimann AC, Danishefsky SJ. Angew. Chem. 2007;119:1470–1472. [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:1448–1450. doi: 10.1002/anie.200604072. [DOI] [PubMed] [Google Scholar]; c) Trzupek JD, Lee D, Crowley BM, Marathias VM, Danishefsky SJ. J. Am. Chem. Soc. 2010;132:8506–8512. doi: 10.1021/ja1030968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.For a recent example of pyridinium dearomatization, see: Legault C, Charette AB. J. Am. Chem. Soc. 2003;125:6360–6361. doi: 10.1021/ja0348647. Arnott G, Brice H, Clayden J, Blaney E. Org. Lett. 2008;10:3089–3092. doi: 10.1021/ol801092s. Barbe G, Pelletier G, Charrette AB. Org. Lett. 2009;11:3398–3401. doi: 10.1021/ol901264f. Mousseau JJ, Fortier A, Charrette AB. Org. Lett. 2010;12:516–519. doi: 10.1021/ol902710f. For reviews about pyridinium dearomatization, see: Lavilla R. J. Chem. Soc. Perkin Trans. 1. 2002:1141–1156. Comins DL. J. Heterocycl. Chem. 1999;36:1491–1500.
- 94.For example of enantioselective pyridinium/isoquinolinium dearomatization, see: Frisch K, Landa A, Saaby S, Jørgensen KA. Angew. Chem. 2005;117:6212–6217. Angew. Chem. Int. Ed. 2005;44:6058–6063. doi: 10.1002/anie.200501900. Taylor MS, Tokunaga N, Jacobsen EN. Angew. Chem. 2005;117:6858–6862. doi: 10.1002/anie.200502277. Angew. Chem. Int. Ed. 2005;44:6700–6704. doi: 10.1002/anie.200502277. Yamaoka Y, Miyabe H, Takemoto Y. J. Am. Chem. Soc. 2007;129:6686–6687. doi: 10.1021/ja071470x.
- 95.Kuethe JT, Comins DL. J. Org. Chem. 2004;69:5219–5231. doi: 10.1021/jo049724+. [DOI] [PubMed] [Google Scholar]
- 96.a) Comins DL, Hong H. J. Am. Chem. Soc. 1993;115:8851–8852. [Google Scholar]; b) Comins DL, Hong H. J. Org. Chem. 1993;58:5035–5036. [Google Scholar]; c) Comins DL, Kuethe JT, Hong H, Lakner FJ. J. Am. Chem. Soc. 1999;121:2651–2652. [Google Scholar]; d) Comins DL, Sahn JJ. Org. Lett. 2005;7:5227–5228. doi: 10.1021/ol052068v. [DOI] [PubMed] [Google Scholar]
- 97.Barbe G, Charette AB. J. Am. Chem. Soc. 2008;130:13873–13875. doi: 10.1021/ja8068215. [DOI] [PubMed] [Google Scholar]
- 98.a) Charette AB, Grenon M, Lemire A, Pourashraf M, Martel J. J. Am. Chem. Soc. 2001;123:11829–11830. doi: 10.1021/ja017136x. [DOI] [PubMed] [Google Scholar]; b) Lemire A, Grenon M, Pourashraf M, Charrette AB. Org. Lett. 2004;6:3517–3520. doi: 10.1021/ol048624n. [DOI] [PubMed] [Google Scholar]; c) Focken T, Charrette AB. Org. Lett. 2006;8:2985–2988. doi: 10.1021/ol0609006. [DOI] [PubMed] [Google Scholar]
- 99. Kozikowski AP, Araldi GL, Ball RG. J. Org. Chem. 1997;62:503–509. doi: 10.1021/jo961957g. Sung MJ, Lee HI, Chong Y, Cha JK. Org. Lett. 1999;1:2017–2019. doi: 10.1021/ol9911932. For a review on oxidopyridinium dearomatization, see: Radhakrishnan KV. Top. Heterocycl. Chem. 2008;13:71–98.
- 100.a) Peese KM, Gin DY. Org. Lett. 2005;7:3323–3325. doi: 10.1021/ol051184v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Peese KM, Gin DY. J. Am. Chem. Soc. 2006;128:8734–8735. doi: 10.1021/ja0625430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.For recent and innovative examples of transition-metal-catalyzed dearomatization, see: Pigge FC, Coniglio JJ, Dalvi R. J. Am. Chem. Soc. 2006;128:3498–3499. doi: 10.1021/ja058342y. Lavy S, Pérez-Luna A, Kündig EP. Synlett. 2008:2621–2624. Lian Y, Davies HML. J. Am. Chem. Soc. 2010;132:440–441. doi: 10.1021/ja9078094. Fructos MR, Álvarez E, Díaz-Requejo MM, Pérez PJ. J. Am. Chem. Soc. 2010;132:4600–4607. doi: 10.1021/ja1006614.
- 102.For selected examples of π-basic dearomatizing agents, see: Chordia MD, Harman WD. J. Am. Chem. Soc. 2000;122:2725–2736. Stokes SM, Jr, Ding F, Smith PL, Keane JM, Kopach ME, Jervis R, Sabat M, Harman WD. Organometallics. 2003;22:4170–4171. Lis EC, Jr, Salomon RJ, Sabat M, Myers WH, Harman WD. J. Am. Chem. Soc. 2008;130:12472–12476. doi: 10.1021/ja803605m. For a recent review on this topic, see: d) For a recent review on this topic, see Ref. [14d]
- 103. Bao M, Nakamura H, Yamamoto Y. J. Am. Chem. Soc. 2001;123:759–760. doi: 10.1021/ja003718n. Lu S, Xu Z, Bao M, Yamamoto Y. Angew. Chem. 2008;120:4438–4441. doi: 10.1002/anie.200800529. Angew. Chem. Int. Ed. 2008;47:4366–4369. doi: 10.1002/anie.200800529. For DFT calculations on palladium-catalyzed dearomatization, see: Ariafard A, Lin Z. J. Am. Chem. Soc. 2006;128:13010–13016. doi: 10.1021/ja063944i.
- 104.Kagawa N, Malerich JP, Rawal VH. Org. Lett. 2008;10:2381–2384. doi: 10.1021/ol8006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.García-Fortanet J, Kessler F, Buchwald SL. J. Am. Chem. Soc. 2009;131:6676–6677. doi: 10.1021/ja9025193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dohi T, Maruyama A, Takenaga N, Senami K, Minamitsuji Y, Fujioka H, Caemmerer SB, Kita Y. Angew. Chem. 2008;120:3847–3850. doi: 10.1002/anie.200800464. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:3787–3790. doi: 10.1002/anie.200800464. [DOI] [PubMed] [Google Scholar]
- 107.Uyanik M, Yasui T, Ishihara K. Angew. Chem. 2010;122:2221–2223. doi: 10.1002/anie.200907352. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2010;49:2175–2177. doi: 10.1002/anie.200907352. [DOI] [PubMed] [Google Scholar]
- 108.Quideau S, Lyvinec G, Marguerit M, Bathany K, Ozanne-Beaudenon A, Buffeteau T, Cavagnat D, Chénedé A. Angew. Chem. 2009;121:4675–4679. doi: 10.1002/anie.200901039. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2009;48:4605–4609. doi: 10.1002/anie.200901039. [DOI] [PubMed] [Google Scholar]
- 109.a) Ladziata U, Koposov AY, Lo KY, Willging J, Nemykin VN, Zhdankin VV. Angew. Chem. 2005;117:7289–7293. doi: 10.1002/anie.200502707. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:7127–7131. doi: 10.1002/anie.200502707. [DOI] [PubMed] [Google Scholar]; b) Ladziata U, Willging J, Zhdankin VV. Org. Lett. 2006;8:167–170. doi: 10.1021/ol052684r. [DOI] [PubMed] [Google Scholar]
- 110.Boppisetti JK, Birman VB. Org. Lett. 2009;11:1221–1223. doi: 10.1021/ol8029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.a) Breuning M, Corey EJ. Org. Lett. 2001;3:1559–1562. [Google Scholar]; b) Imbos R, Minnaard AJ, Feringa BL. J. Am. Chem. Soc. 2002;124:184–185. doi: 10.1021/ja017200a. [DOI] [PubMed] [Google Scholar]
- 112.a) Liu Q, Rovis T. J. Am. Chem. Soc. 2006;128:2552–2553. doi: 10.1021/ja058337u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu Q, Rovis T. Org. Process Res. Dev. 2007;11:598–604. doi: 10.1021/op600278f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vo NT, Pace RDM, O’Hara F, Gaunt MJ. J. Am. Chem. Soc. 2008;130:404–405. doi: 10.1021/ja077457u. [DOI] [PubMed] [Google Scholar]
- 114.Gu Q, Rong Z-Q, Zheng C, You S-L. J. Am. Chem. Soc. 2010;132:4056–4057. doi: 10.1021/ja100207s. [DOI] [PubMed] [Google Scholar]