Abstract
Background
A reduction of dopamine release or D2 receptor blockade in the terminal fields of the mesolimbic system clearly reduces conditioned fear. Injections of haloperidol, a preferential D2 receptor antagonist, into the inferior colliculus (IC) enhance the processing of unconditioned aversive information. However, a clear characterization of the interplay of D2 receptors in the mediation of unconditioned and conditioned fear is still lacking.
Methods
The present study investigated the effects of intra-IC injections of the D2 receptor-selective antagonist sulpiride on behavior in the elevated plus maze (EPM), auditory-evoked potentials (AEPs) to loud sounds recorded from the IC, fear-potentiated startle (FPS), and conditioned freezing.
Results
Intra-IC injections of sulpiride caused clear proaversive effects in the EPM and enhanced AEPs induced by loud auditory stimuli. Intra-IC sulpiride administration did not affect FPS or conditioned freezing.
Conclusions
Dopamine D2-like receptors of the inferior colliculus play a role in the modulation of unconditioned aversive information but not in the fear-potentiated startle response.
Introduction
Several studies have suggested modulatory roles for γ-aminobutyric acid (GABA), serotonin, opioid peptides, excitatory amino acids, and neuropeptides in the so-called encephalic aversion system (EAS), which includes the dorsal periaqueductal gray (dPAG), deep layers of the superior colliculus, amygdala, medial hypothalamus, and inferior colliculus (IC) [1]–[4]. However, little is known about the role of dopamine (DA) in the mediation of aversive states in the EAS.
The IC is a primary acoustic structure of the brainstem and the most caudal structure of the EAS that integrates sensory information of an aversive nature. Chemical and electrical stimulation of the IC causes unconditioned defensive behavior [2], [3], [5], [6]. We showed that fear-evoking stimuli increase the magnitude of auditory-evoked potentials (AEPs) that were directly recorded from the ventral aspects of the IC in response to an aversive auditory stimulus (AAS) [7], [8]. Local application of the excitatory amino acids glutamate and N-methyl-d-aspartate in the central and external nuclei of the IC also increased the acoustically evoked and spontaneous firing of most neurons in this region [9], [10].
Studies of DA-mediated mechanisms of fear/anxiety have considerable relevance with regard to the organism's reactivity following exposure to external acute stressors [11]–[14]. However, investigations of DA's mediation of the defense reaction in the midbrain tectum have been scarce. Aversive stimulation of the midbrain tectum at the escape threshold enhances DA release in the prefrontal cortex [15], and intracollicular injections of haloperidol, a nonselective DA receptor antagonist, enhance AEPs recorded directly from the IC [16]. These results support a previous study that showed that systemic injections of the DA D2 receptor antagonist sulpiride enhanced escape responses in rats subjected to a switch-off procedure [17]. In contrast, the association between changes in DA transmission and conditioned fear has been previously demonstrated in numerous studies. Thus, recent studies demonstrated that systemic and intra-amygdala injections of sulpiride attenuated the expression of conditioned fear [18]–[20].
As indicated above, numerous studies have reported that the activation of DA pathways that arise from the ventral tegmental area (VTA) and project to structures of the mesolimbic system increases learned anxiety, but the role played by DA mechanisms in innate fear is not entirely understood. Based on previous evidence from this laboratory, DA that acts through D2 receptors may play a distinct role in mediating the processing of aversive information of an unconditioned nature. To test this hypothesis, we microinjected sulpiride into the IC and evaluated exploratory behavior in rats in the elevated plus maze (EPM), which assesses the fear of animals to height and open spaces. We also recorded AEPs in response to aversive stimulation (loud sounds) recorded directly from electrodes implanted in the ventral aspects of the IC, an area of the IC that contains the neural substrates of fear [21] and as such would be associated with the processing of aversive information [8]. Furthermore, we evaluated conditioned fear by assessing fear-potentiated startle (FPS) and freezing responses to a light (conditioned stimulus) that was previously paired with footshock during training sessions. When the rats were returned to the test chamber and presented with startle-eliciting stimuli in either the presence or absence of the light, startle amplitude in light-startle trials (fear-potentiated startle) was significantly greater than startle amplitude in startle-alone trials (baseline).
Methods and Materials
Animals
A total of 206 male Wistar rats, weighing 250–300 g, from the animal facility of the University of São Paulo at Ribeirão Preto were used. The animals were housed in groups of four in plastic boxes (40×33×26 cm) and maintained under a 12 h/12 h light/dark cycle (lights on at 7:00 AM) at 23±1°C. The rats were allowed free access to food and water throughout the experiment. The experiments were performed during the light phase of the cycle. All of the experiments received formal approval (Process n°. 10.1.595.53.7 and 12.1.909.53.3) from the Committee on Animal Research and Ethics of the University of São Paulo.
Surgery
The animals were anesthetized with a mixture of ketamine/xylazine (100/7.5 mg/kg, intraperitoneal) and fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). The upper incisor bar was set 3.0 mm below the interaural line, such that the skull was horizontal between bregma and lambda. Guide cannulae (0.6 mm outer diameter) for drug injections and/or a brain electrode (160 µm diameter) were implanted into the midbrain, aimed at the IC. The guide cannulae and electrodes were introduced vertically using the following coordinates, with lambda as the reference: anterior/posterior, −1.0 mm; medial/lateral, ±1.5 mm; dorsal/ventral, −4.0 mm [22]. The cannulae and electrodes were fixed to the skull with acrylic resin and two stainless-steel screws. Each guide cannula was sealed with a stainless steel wire to protect it from blockage. Afterward, the rats were allowed 5 days to recover from the surgical procedure.
Elevated plus maze
An EPM, described in detail elsewhere [23], was used, consisting of two open arms (50×10 cm) crossed at right angles with two closed arms of the same size. The two closed arms were enclosed by 40 cm high walls, with the exception of the central part of the maze (10×10 cm) where the open and closed arms crossed. The entire apparatus was elevated 50 cm above the floor. The experimental room was illuminated (30 lux). The experimental sessions were recorded by a video camera interfaced with a monitor and a DVD recorder in an adjacent room. Five days after surgery, the rats received intra-IC bilateral administration of dopaminergic drugs or vehicle. After 15 min, they were individually placed in the central area of the EPM with their nose facing one of the closed arms. The rats were then allowed to freely explore the maze for 5 min. Conventional EPM measures were recorded, including the number of entries into the closed and open arms and time spent in the open arms [24]. Additional behavioral categories were evaluated as previously described, including grooming, scanning, head dipping, rearing, end-arm exploration, peeping out, flat-back approach, and stretched-attend posture [24], [25].
Auditory-evoked potentials
An experimental wire mesh cage (19×13.5×9 cm), located inside an insulated Faraday system and surrounded by a ventilated sound-attenuating plywood chamber (64×60×40 cm), was used. A loudspeaker located 10 cm behind the cage delivered continuous background noise (55 dB). Acoustic stimuli (100 clicks, 50 ms duration; 3000 Hz square-wave pulses) presented at a rate of 0.33 Hz (one every 3 s) were delivered via two piezoelectric speakers (12 Ω, 200 W; LeSon, São Paulo, SP, Brazil) mounted on the lateral walls of the sound-insulating box, 15 cm from the wire mesh cage. The acoustic stimulus was a pure tone (92.5 dB sound pressure level). Software and an appropriate interface (Lynx Electronics, São Paulo, SP, Brazil) controlled the presentation and sequence of the acoustic stimuli. Sound pressure levels were measured at the level of the ears of the animals using a 0.125 inch microphone and type 2636 DK-2580 measuring amplifier (Bruel and Kjaer Sound & Vibration Measurement A/S, Naerum, Denmark).
Brainstem AEPs are very small electrical voltage potentials that are recorded from electrodes in response to a repetitive stimulus along a specific brainstem auditory pathway. These potentials reflect neuronal activity in the auditory complex, mainly in the cochlear nucleus, superior olive, and IC [26]. Previous studies have shown that AEPs generated in the IC are sensitive to aversive manipulations [7], [27], [28]. The average value was obtained at the end of the sessions. Auditory-evoked potentials were recorded as the voltage difference between the tips of an insulated wire (150 µm) inserted through the cannula and tip of the guide cannula implanted in the IC. This voltage difference was fed into a TX001 amplifier (20–200 Hz bandwidth; Lynx Electronics, São Paulo, SP, Brazil) through two noiseless shielded cables that were passed through a hole in the roof of the Faraday cage. A previous study from our laboratory indicated no hemispheric differences in AEPs recorded under the present experimental conditions [27]. The output of the amplifier was connected to one of four channels on an analog/digital converter (CAD 12/36). The filtering, amplification, and digitalization of the signals were performed using the Sysdin system (Lynx Electronics, São Paulo, SP, Brazil). The potential signals were filtered (high-pass filter, 20 Hz; low-pass filter, 200 Hz) and sampled at a rate of 0.33 kHz. Sysdin software was programmed to sum individual AEP amplitudes. The data acquisition sweep began 10 ms before the onset of the sound stimulus (i.e., the latency to switch on the sound plus sound propagation) and continued for 200 ms after termination of the sound stimulus. During recording, the animals were monitored via a camera system placed in the experimental room. N1 was visually identified as the first negative wave, and P1 was identified at the first positive wave approximately 15 ms after the sound presentation. The positive peak P1 is considered an early component of the collicular response. Its amplitude was measured peak to peak, with peak latency between 5 and 8 ms [29]. The AEPs elicited from the IC were recorded from the ventrocaudal portions of the nucleus. This method of analysis is similar to previous studies from our and other laboratories [16], [29]–[31]. Peak amplitudes were defined as the maximum amplitude measured between N1 and the end of P1, similar to previous studies from our laboratory [7], [27], [31]. The computer output was graphically displayed on an XY plotter (Hewlett-Packard, Palo Alto, CA, USA).
Fear-potentiated startle
Matching
The test cages were two wire-grid cages (16.5×7.5×7.5 cm) fixed to response platforms by four thumb screws each. The cages and response platforms were located inside ventilated, sound-attenuating plywood chambers (64×60×40 cm). A loudspeaker located 10 cm behind the test cages delivered both the startle stimulus (100 dB, 50 ms burst of white noise) and continuous background noise (55 dB). The startle reaction of the rats generated pressure on the platform, and the analog signals were amplified, digitized, and analyzed by Startle Reflex software (Med Associates, St. Albans, VT, USA). The startle reaction was recorded within a time window of 100 ms after the onset of the startle stimulus. For the first 2 days, the rats were placed in the test cage for a 5 min habituation period and afterward received a total of 30 startle stimuli with an interstimulus interval of 30 s. Each matching session was 20 min in duration. The rats were assigned to the different control and drug groups, such that each group had similar average startle amplitudes based on the last matching day.
Training
After recovery from surgery, the rats were conditioned to a light conditioned stimulus (CS) in cages (20×20×25 cm) with stainless-steel sides and back walls, transparent Plexiglas ceilings and front doors, and grid floors that consisted of stainless-steel rods. These cages were located within ventilated and sound-attenuated chambers (45×45×45 cm) that were different from the test cages to avoid conditioning to the context. The rats were placed in the training cage and received 10 CS-unconditioned stimulus (US) pairings after an habituation phase of 5 min using a 4 s, 6 W light CS that co-terminated with a 1 s, 0.6 mA footshock US. The intertrial interval varied randomly between 60 and 180 s. The duration of each training session was approximately 25 min.
Testing
The test session was conducted without footshock presentation in the same cages that were used for matching. Twenty-four hours after training, the rats received an intra-IC injection of sulpiride or vehicle and were placed in the startle test cage 5 min later. After 5 min of habituation, the rats received 60 startle stimuli (noise bursts) with a 30 s interstimulus interval. Half of the startle stimuli were presented in the absence of the CS (i.e., noise-alone trials) to provide a baseline, and the other half of the startle stimuli were presented in the presence of the CS (i.e., light-noise trials). In the light-noise trials, the startle stimulus was presented during the last second of a 4 s light presentation. The noise-alone and light-noise trials were interspersed randomly. The duration of the test session was 37 min.
Drugs
The drugs, doses, and injection times were based on previous studies from our laboratory [19], [32]. The drugs used were (−)-quinpirole hydrochloride (D2 agonist), (S)-(−)-sulpiride (D2 antagonist), (±)-SKF-38393 hydrochloride (D1 agonist), and R-(+)-SCH-23390 hydrochloride (D1 antagonist). All of the drugs were purchased from Sigma (St. Louis, MO, USA) and dissolved in physiological saline (0.9%) shortly before use, with the exception of sulpiride that was first mixed with 2% Tween 80 (Sigma, St. Louis, MO, USA). Physiological saline or saline plus 2% Tween were used as vehicle control. Doses of 0.5, 1.0, 2.0, 3.0, and/or 4.0 µg were microinjected in a constant volume of 0.2 µl/site into the IC.
Microinjection procedure
The injection needles were thin dental needles (0.3 mm outer diameter) connected to 5 µl Hamilton syringes with polyethylene tubes (Becton-Dickinson, Franklin Lakes, NJ, USA). Each rat remained free in a polypropylene box (28×17×13 cm), and the needles were introduced into the guide cannula until its lower end reached 1 mm below it. The solutions were injected into the IC in a volume of 0.2 µl using an infusion pump (Harvard Apparatus, Holliston, MA, USA). As noted previously, the radius of diffusion of a 0.5 µl volume is approximately 1.0 mm [33]. In the present study, we used a 0.2 µl drug volume, so the drug diffusion is presumed to be restricted to the target structure. Previous studies from our laboratory also showed that a volume of 0.2 µl did not spread in a tissue diameter greater than 0.4 mm [21], [34], so the drug injections in the present study unlikely spread beyond the target region. The microinjection procedure used here has been described in previous papers from this laboratory [18], [19], [20], [32].
Histology
Upon completion of the experiments, the rats were anesthetized with a lethal dose of urethane (3 g/kg) and transcardially perfused with 0.9% saline followed by 4% formaldehyde. The brains were removed from the skulls and maintained in formaldehyde solution for 2 h, after which the brains were cryoprotected in 30% sucrose for 48 h. Serial 60 µm coronal brain sections were cut using a cryostat, mounted on gelatin-coated slides, and stained with Cresyl violet (5%; Sigma-Aldrich, St. Louis, MO, USA) to localize the positions of the microinjection sites according to the atlas of Paxinos and Watson [22].
Statistical analysis
The data are expressed as mean + SEM. The conditioned freezing response was analyzed using Student's t-test. The EPM and AEP data were subjected to one-way analysis of variance (ANOVA). Two-way repeated-measures ANOVA was conducted on the FPS data. Post hoc differences were tested using the Newman-Keuls test. Values of p<0.05 were considered significant.
Results
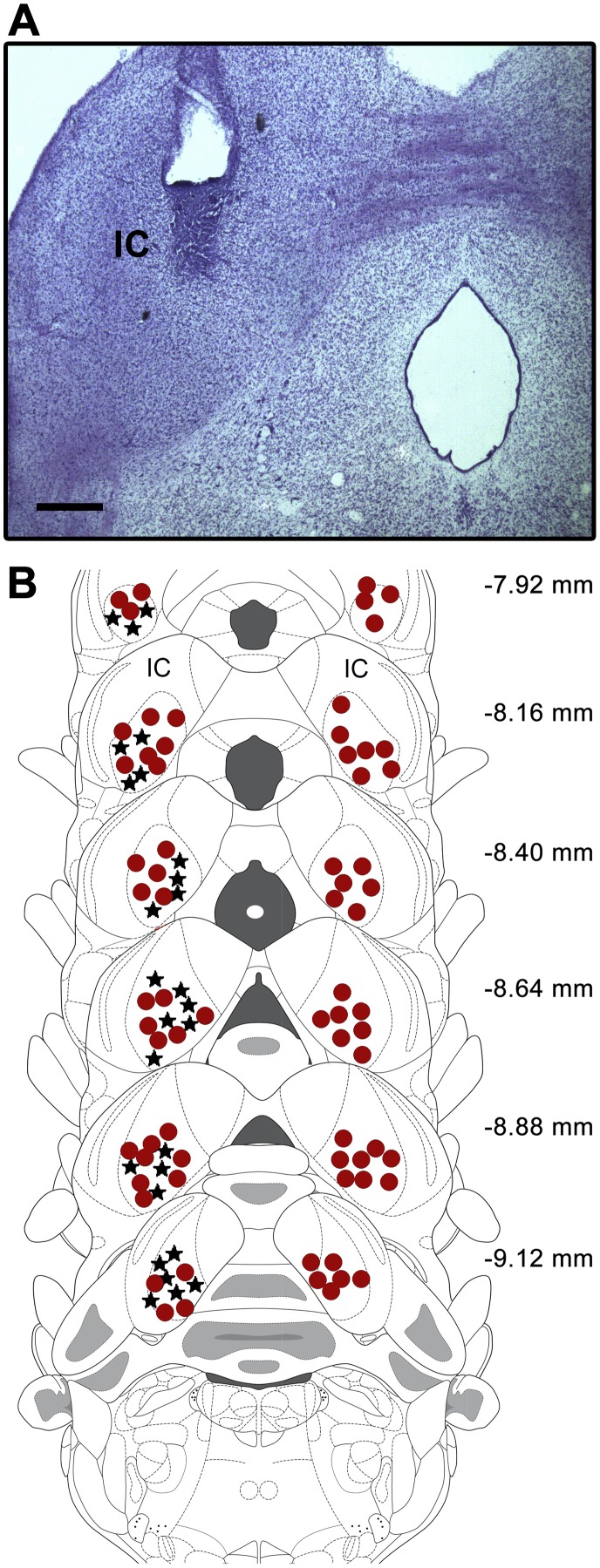
All of the microinjections and recording sites in the present study were situated in the ventral aspects of the IC. Fig. 1 depicts the histological localization of the microinjection/recording sites in the IC, based on the diagrams in the atlas of Paxinos and Watson [22].
Figure 1. Target microinjection/recording sites in the inferior colliculus.
(A) Photomicrograph showing a representative microinjection site in the IC. (B) Outlines of the microinjection and recording sites on coronal brain sections from the Paxinos and Watson atlas (2007) are shown. Symbols of each group were represented in the same side for the sake of clarity. • – injection sites, ★ – recording electrode implants. The number of points in the figure is less than the total number of rats used because several points overlap. Scale bar = 0.5 mm. IC = inferior colliculus.
Elevated plus maze
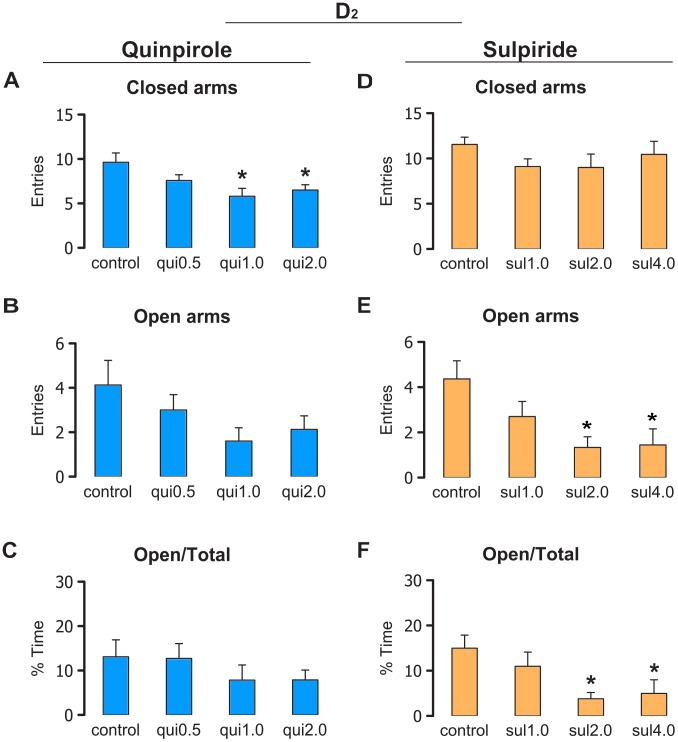
Fig. 2A–C show the mean behavioral responses for rats that received intra-IC injections of vehicle (Control; n = 8), 0.5 µg/0.2 µl quinpirole (n = 7), 1.0 µg/0.2 µl quinpirole (n = 10), and 2.0 µg/0.2 µl quinpirole (n = 8) before the testing session in the EPM. The ANOVA did not indicate a statistically significant effect of treatment on the frequency of open arm entries (F 3,29 = 2.14, p>0.05) or percentage of time spent in the open arms (F 3,29 = 0.78, p>0.05). The ANOVA indicated statistically significant effects of treatment on the frequency of closed arm entries (F 3,29 = 4.03, p<0.05). The Newman-Keuls post hoc test revealed a reduction of closed arm entries in the groups treated with 1.0 and 2.0 µg/0.2 µl quinpirole compared with the control group (p<0.05). The analysis of the complementary ethological categories also indicated a motor effect of quinpirole in the IC (Figure S1 in File S1).
Figure 2. Effects of D2 drugs in the elevated plus maze.
The figure shows the effects of intra-IC injections of the vehicle for quinpirole (Control; n = 8), 0.5 µg/0.2 µl quinpirole (n = 7), 1.0 µg/0.2 µl quinpirole (n = 10), 2.0 µg/0.2 µl quinpirole (n = 8), the vehicle for sulpiride (Control; n = 11), 1.0 µg/0.2 µl sulpiride (n = 10), 2.0 µg/0.2 µl sulpiride (n = 9), and 4.0 µg/0.2 µl sulpiride (n = 9) on exploratory behavior in rats subjected to the elevated plus maze. (A, D) Number of entries into the closed arms of the elevated plus maze. (B, E) Number of entries into the open arms of the elevated plus maze. (C, F) Percentage of time spent in the open arms relative to total time. The data are expressed as mean + SEM. *p<0.05, compared with control group (Newman-Keuls test).
Fig. 2D–F show the mean behavioral responses for rats that received intra-IC injections of vehicle (Control; n = 11), 1.0 µg/0.2 µl sulpiride (n = 10), 2.0 µg/0.2 µl sulpiride (n = 9), and 4.0 µg/0.2 µl sulpiride (n = 9) before being tested in the EPM. The ANOVA did not indicate statistically significant effects of treatment on the frequency of closed arm entries (F 3,35 = 1.18, p>0.05) but revealed statistically significant effects of treatment on the frequency of open arm entries (F 3,35 = 4.35, p<0.05) and percentage of time spent in the open arms (F 3,35 = 3.69, p<0.05). The Newman-Keuls post hoc test revealed a reduction of open arm exploration in the groups treated with 2.0 and 4.0 µg/0.2 µl sulpiride compared with controls (p<0.05). The analysis of the complementary ethological categories in the EPM also indicated an anxiogenic-like effect of sulpiride in the IC (Figure S1 in File S1).
The mean behavioral responses for rats that received intra-IC injections of the vehicle for SKF-38393 (Control; n = 10), 1.0 µg/0.2 µl SKF-38393 (n = 9), 2.0 µg/0.2 µl SKF-38393 (n = 8), the vehicle for SCH-23390 (Control; n = 17), 1.0 µg/0.2 µl SCH-23390 (n = 10), 2.0 µg/0.2 µl SCH-23390 (n = 12), and 4.0 µg/0.2 µl SCH-23390 (n = 10) before being tested in the EPM did not show significant differences (Figures S2 and S3 in File S1).
Auditory-evoked potentials
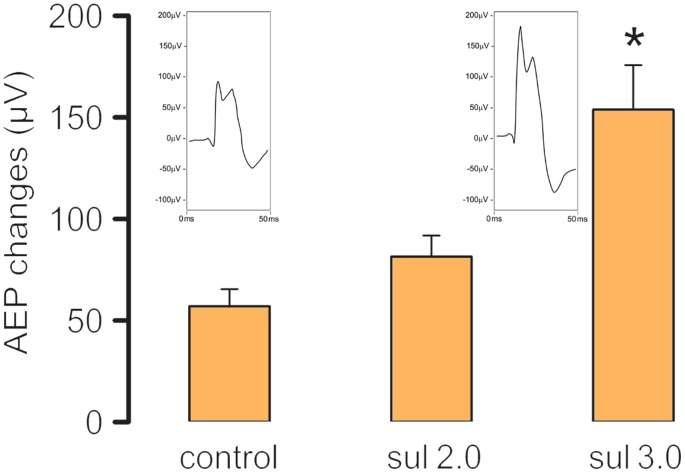
Fig. 3 shows the mean AEP changes recorded for rats that received an intra-IC injection of vehicle (Control; n = 6), 2.0 µg/0.2 µl sulpiride (n = 7), and 3.0 µg/0.2 µl sulpiride (n = 8) before AAS presentation. The one-way ANOVA revealed a significant effect of treatment (F 2, 18 = 5.91, p<0.05). The Newman-Keuls post hoc test revealed that 3.0 µg/0.2 µl sulpiride significantly increased AEPs compared with the control group (p<0.05). In the present study, we also found that loud tones (92.5 dB, AAS), in contrast to tones presented at low intensity, increased Fos distribution in structures that are responsible for the integration of defense reactions to unconditioned aversive stimulation, such as the periaqueductal gray (Figures S4, S5, S6 in File S1).
Figure 3. Effect of sulpiride on the auditory-evoked potentials.
The figure shows the effects of intra-IC injections of vehicle (Control; n = 6), 2.0 µg/0.2 µl sulpiride (n = 7), and 3.0 µg/0.2 µl sulpiride (n = 8) on the amplitude of AEPs recorded in the IC. The data are expressed as mean + SEM. *p<0.05, compared with control group (Newman-Keuls test). Representative collicular AEPs recorded from the central and external nuclei of the inferior colliculus following local injections of saline (control) or 3.0 µg/0.2 µl sulpiride are also illustrated.
Fear-potentiated startle
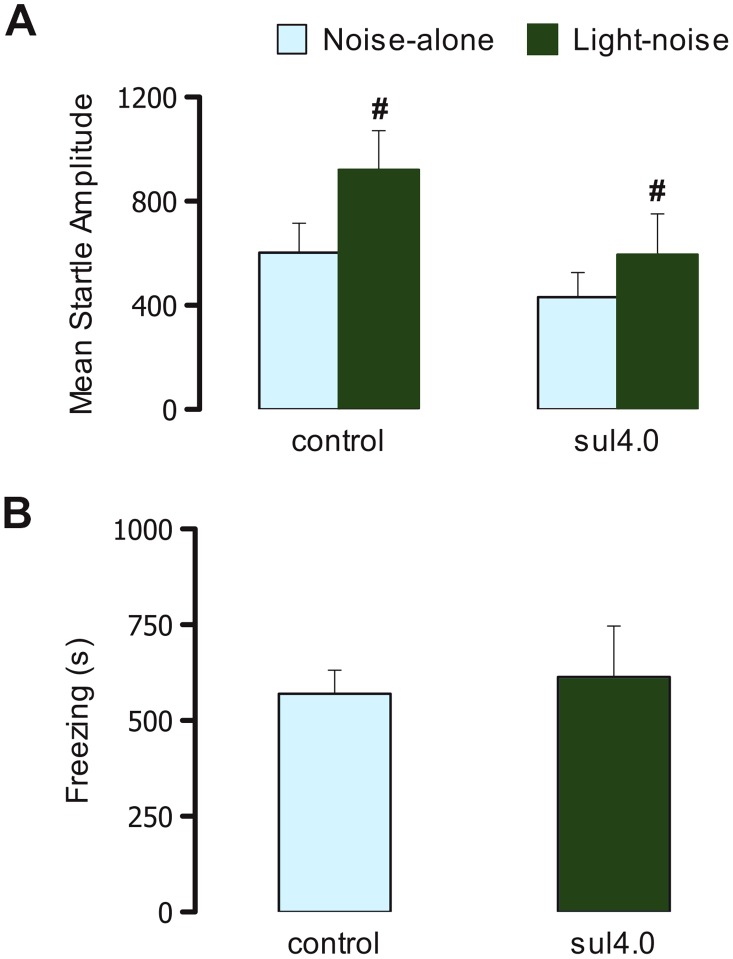
Fig. 4A shows the startle amplitude in rats that received intra-IC injections of vehicle (Control; n = 12) and 4.0 µg/0.2 µl sulpiride (n = 11). The two-way repeated-measures ANOVA revealed a significant effect of trial type (F 1,45 = 14.27, p<0.05) but no significant effect of treatment (F 1,21 = 2.02, p>0.05) and no treatment×trial type interaction (F 1,45 = 1.51, p>0.05). The Newman-Keuls post hoc test revealed an overall enhancement of the startle response in light-noise trials compared with noise-alone trials (p<0.05). Fig. 4B shows the conditioned freezing response during the test session in the same rats. Student's t-test revealed no significant effects of intra-IC injections of sulpiride on the conditioned freezing response (t = 0.31, p>0.05).
Figure 4. Effects of D2 drugs on fear-potentiated startle and conditioned freezing.
The figure shows the effects of intra-IC injections of vehicle (Control; n = 12) and 4.0 µg/0.2 µl sulpiride (n = 11) on the mean startle amplitude (A) and conditioned freezing (B) in rats subjected to the conditioned fear test. The data are expressed as mean + SEM. # p<0.05, compared with noise-alone (Newman-Keuls test).
Discussion
GABA and excitatory amino acids have been shown to have opposing actions in the mediation of defensive behavior generated and organized in the IC [35]–[39]. Serotonin and opioid peptides play inhibitory roles in the neural substrates of aversion in this structure [3], [4], [36], [38]. Little is known about the role of DA in mediating unconditioned and conditioned fear in the IC. In the present study, local injections of the D2 receptor antagonist sulpiride into the IC in rats caused proaversive effects in the EPM and enhanced AEPs in response to loud sounds. We also found that local infusion of sulpiride into the IC did not affect the startle response (whether unconditioned or conditioned).
Although the precise neural circuitry of DA transmission involved in aversive states remains unclear, pharmacological and neurochemical studies appear to implicate prefrontal cortex [40], [41] and nucleus accumbens DA terminals in the response to acute stressors [42]–[49]. With regard to DA's mediation of conditioned fear, an increase in DA metabolism in the mesolimbic system is correlated with conditioned fear, and a decrease in DA activity in the basolateral amygdala causes a reduction of the expression of conditioned fear responses [19], [20], [34], [50], [51]. In fact, intraperitoneal injections of low doses of the D2 receptor agonist quinpirole act at autoreceptors on VTA neurons, slowing DA release at their terminals and also causing a reduction of conditioned fear responses [19], [34], [51]. Therefore, the fear response to a light-CS appears to depend on the activation of mesolimbic dopaminergic connections and can be specifically modulated by manipulating these projection neurons. In the present study, intra-IC sulpiride injections did not affect FPS or conditioned freezing in response to a light-CS.
With regard to filtering processes associated with aversive information, accumulating evidence suggests the involvement of DA mechanisms in structures of the EAS. Although DA-mediated mechanisms that are involved in the generation of aversion at the level of the IC have not been extensively studied, this region has appreciable dopaminergic activity [52], [53]. In the present study, intra-IC injections of sulpiride exerted clear anxiogenic-like effects in rats subjected to the EPM and enhanced the amplitude of AEPs in response to loud auditory stimuli recorded from the ventral aspects of the IC. The lack of effects of intra-IC injections of the D1 receptor agonist SKF-38393 and antagonist SCH-23390 in rats in the EPM is consistent with the much higher density of D2 receptors than D1 receptors in the IC [53].
A question that arises is the way in which the IC is involved in DA-mediated fear. The IC is distinguished from other auditory centers in the brainstem by its connections with motor systems [54]. Support for a functional link between the activation of these neurons and behavioral responses induced by aversive stimulation of the IC has also been reported [15]. According to several studies, the IC is positioned to send auditory information to motor centers that participate in prey capture and predator avoidance behaviors, such as the intermediate and deep layers of the superior colliculus, which controls head, eye, and pinnae movements for the orientation of objects in space [54]–[58]. Evidence also indicates that motor systems project to the IC, in which projections from the substantia nigra pars reticulata [59], [60] and globus pallidus [61] to the IC have been reported in rats. Additionally, lesions of the substantia nigra pars reticulata increase defensive responses (e.g., escape thresholds) induced by electrical or chemical stimulation of the IC [62]–[64]. In the present study, we compared outcomes when the stimuli (loud sounds) were immediately available to the IC for sensory processing (AEPs) and action outcomes when cortical or limbic processing of the signal was also required (EPM). We previously showed that conditioned fear, which recruits the mesocorticolimbic system, was significantly impaired by the DA receptor antagonist sulpiride [19], [34], [51]. In contrast, when the stimulus was eligible for IC processing as in the present study, the role of DA neurons associated with aversive situations that activated afferent sensory pathways via the IC appeared to be primary and possibly instrumental, and sulpiride increased the aversiveness of these stimuli. Thus, unconditioned and conditioned responses were differentially affected by the pharmacological manipulations. Indirect support for these results was previously provided by studies that demonstrated that systemic injections of the DA receptor antagonist sulpiride increased the switch-off response to light presented as an aversive US and enhanced fear-like behavior in the open arms of the EPM [17], [65]. Moreover, our recent study found that systemic administration of haloperidol strongly reduced ultrasonic vocalizations emitted during the testing session of a contextual fear conditioning paradigm [66], but increased the magnitude of AEPs in response to the unconditioned auditory aversive stimulation [16].
The startle reflex test measures a motor response whereas the AEPs recordings from electrodes directly implanted into the IC reflect the processing of sensory information. Depending on the aversive condition the startle reflex may be even reduced when the acoustic evoked potentials are enhanced [27]. Sensorimotor filtering is commonly activated in the midbrain tectum when acoustic or visual stimuli are presented to animals. As an explanation for the present findings, ascending projections from the IC would be first activated by aversive stimuli, and descending pathways would be sequentially recruited. This alternate circuit is associated with the processing of auditory information of an aversive nature, which triggers fear-like behavior [64], [67]. In fact, the IC is a key pathway for auditory information, and disturbances at this level may alter transmission to cortical centers. Abnormal cortical areas are known to exist in schizophrenia patients and may account for the abnormal processing of auditory information that results in auditory hallucinations and decreased responsiveness to sounds [68]. Interestingly, the lack of effect of intra-IC sulpiride administration on the startle reflex in response to noise alone is also consistent with the notion that the IC is not part of direct startle reflex circuitry [69], [70].
In summary, the present results show that D2-like receptors of the IC plays a role in the defensive behavior displayed by rats subjected to the elevated-plus maze, but not to the fear-potentiated startle test. Also, the reactivity of the neural substrates of fear in the inferior colliculus measured by AEPs to loud sounds is enhanced by the D2 receptor antagonist sulpiride locally injected into this structure. Dopamine appears to regulate unconditioned fear at the IC level, likely by reducing the sensorimotor gating of aversive events. Taking also into account that much evidence has shown that dopamine plays a role in the mediation of conditioned fear in the amygdala and nucleus accumbens the theory proposed by McNaughton and Corr [71] needs to be considered in future studies in this line of research. They consider that there is a rostrocaudal gradient in the brain, with unconditioned fear mapping at more caudal structures, such as the IC and dPAG, whereas conditioned fear is mapped at more rostral levels, such as the amygdala and prefrontal cortex. Thus, because the density of D2 receptors appears to largely predominate over other DA receptors in the IC [53], the potential use of the IC as a target to investigate unconditioned fear processes associated with DA mechanisms that are mediated by these receptors is unique. The present findings may stimulate further studies from this and other laboratories to provide further data on the differential role of DA in conditioned fear and the processing of unconditioned aversive information.
Supporting Information
Supporting figures. Figure S1, Table summary of the effects of D2 drugs on the complementary categories of the elevated plus-maze. Effects of intra-IC injections of vehicle, quinpirole 1.0 µg/0.2 µL or 2.0 µg/0.2 µL and vehicle and 1.0, 2.0 or 4.0 µg/0.2 µL sulpiride on the complementary ethological categories of rats submitted to the elevated plus-maze. Figure S2, Table summary of the effects of D1 drugs on the complementary categories of the elevated plus-maze. Effects of intra-IC injections of vehicle, 1.0 or 2.0 µg/0.2 µL SKF-38393, and vehicle or 1.0, 2.0 or 4.0 µg/0.2 µL SCH-23390 on the complementary ethological categories of rats submitted to the elevated plus-maze. Figure S3, D1 drugs in the elevated plus-maze (EPM). Effects of intra-IC injections of vehicle, 1.0 and 2.0 µg/0.2 µL SKF-38393 or vehicle, 1.0, 2.0 or 4.0 µg/0.2 µL SCH-23390 on exploratory behavior of rats submitted to the elevated plus-maze. (A and D) Number of entries in the closed arms of the maze. (B and E) Number of entries in the open arms of the maze. (C and F). % of time spent into the open arms in relation to total. Figure S4, Fos-positive immunohistochemistry in response to aversive acoustic stimuli (AAS). Number of Fos-positive cells in midbrain (A) and telencephalic (B) structures in rats exposed to testing sessions with or without (Control) presentation of AASs. Figure S5, Fos-positive immunohistochemistry in midbrain structures in response to aversive acoustic stimuli (AAS). Photomicrographs of Fos-positive cells (dark dots) in the dmPAG, dlPAG, vlPAG, and IC in rats exposed to testing sessions with and without (Control) AAS presentation. Figure S6, Fos positive immunohistochemistry in telencephalic structures in response to aversive acoustic stimuli (AAS). Photomicrographs of Fos-positive cells (dark dots) in the Cg1, CPu, NAcC and NAcSh of rats exposed to testing sessions with (AAS) or without (Control) AAS presentation.
(PDF)
Acknowledgments
A.R. de Oliveira holds a postdoctoral fellowship from FAPESP (Proc. no. 2010/50669-6). S. Muthuraju is a recipient of a postdoctoral scholarship from FAPESP (Proc. no. 2011/14686-6). R.C. Almada holds a postdoctoral fellowship from FAPESP (Proc. no. 2012/22681-7). A.C. Colombo holds a Master scholarship from FAPESP (Proc. no. 2012/06546-2).
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by FAPESP (Proc. no. 11/00041-3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brandão ML, Aguiar JC, Graeff FG (1982) GABA mediation of the anti-aversive action of minor tranquilizers. Pharmacol Biochem Behav 16: 397–402. [DOI] [PubMed] [Google Scholar]
- 2. Brandão ML, Cardoso SH, Melo LL, Motta V, Coimbra NC (1994) The neural substrate of defensive behavior in the midbrain tectum. Neurosci Biobehav Rev 18: 339–346. [DOI] [PubMed] [Google Scholar]
- 3. Brandão ML, Anseloni VZ, Pandossio JE, De Araujo JE, Castilho VM (1999) Neurochemical mechanisms of the defensive behavior in the dorsal midbrain. Neurosci Biobehav Rev 23: 863–875. [DOI] [PubMed] [Google Scholar]
- 4. Brandão ML, Borelli KG, Nobre MJ, Santos JM, Albrechet-Souza L, et al. (2005) Gabaergic regulation of the neural organization of fear in the midbrain tectum. Neurosci Biobehav Rev 29: 1299–1311. [DOI] [PubMed] [Google Scholar]
- 5. Schmitt P, Carrive P, Discala G, Jenck F, Brandao M, et al. (1986) A neuropharmacological study of the periventricular neural substrate involved in flight. Behav Brain Res 22: 181–190. [DOI] [PubMed] [Google Scholar]
- 6. Brandão ML, Melo LL, Cardoso SH (1993) Mechanisms of defense in the inferior colliculus. Behav Brain Res 58: 49–55. [DOI] [PubMed] [Google Scholar]
- 7. Brandão ML, Coimbra NC, Osaki MY (2001) Changes in the auditory-evoked potentials induced by fear-evoking stimulations. Physiol Behav 72: 365–372. [DOI] [PubMed] [Google Scholar]
- 8. Sandner G, Canal NM, Brandao ML (2002) Effects of ketamine and apomorphine on inferior colliculus and caudal pontine reticular nucleus evoked potentials during prepulse inhibition of the startle reflex in rats. Behav Brain Res 128: 161–168. [DOI] [PubMed] [Google Scholar]
- 9. Faingold CL, Hoffmann WE, Caspary DM (1989) Effects of excitant amino acids on acoustic responses of inferior colliculus neurons. Hear Res 40: 127–136. [DOI] [PubMed] [Google Scholar]
- 10. Li Y, Evans MS, Faingold CL (1998) In vitro electrophysiology of neurons in subnuclei of rat inferior colliculus. Hearing Research 121: 1–10. [DOI] [PubMed] [Google Scholar]
- 11. Fadda F, Argiolas A, Melis MR, Tissari AH, Onali PL, et al. (1978) Stress-induced increase in 3,4-dihydroxyphenylacetic acid (DOPAC) levels in the cerebral cortex and in n. accumbens: reversal by diazepam. Life Sci 23: 2219–2224. [DOI] [PubMed] [Google Scholar]
- 12. Biggio G, Concas A, Corda MG, Giorgi O, Sanna E, et al. (1990) GABAergic and dopaminergic transmission in the rat cerebral cortex: effect of stress, anxiolytic and anxiogenic drugs. Pharmacol Ther 48: 121–142. [DOI] [PubMed] [Google Scholar]
- 13.Anisman H, Zalcman S, Shanks N, Zucharko R (1991) Multisystem regulation of performance deficits induced by stressors: an animal model of depression. In: Boulton AA, Baker GB, Martin-Iverson MT, editors. Animal Models in Psychiatry, vol. II. Clifton: Humana Press, pp. 1–59. [Google Scholar]
- 14. Feenstra MGP, Botterblom MHA, Van Uum JFM (1995) Novelty-induced increase in dopamine release in the rat prefrontal cortex in vivo: inhibition by diazepam. Neurosci Lett 189: 81–84. [DOI] [PubMed] [Google Scholar]
- 15. Cuadra G, Zurita A, Macedo CE, Molina VA, Brandao ML (2000) Electrical stimulation of the midbrain tectum enhances dopamine release in the frontal cortex. Brain Res Bull 52: 413–418. [DOI] [PubMed] [Google Scholar]
- 16. Muthuraju S, Nobre MJ, Saito VMN, Brandão ML (2014) Distinct effects of haloperidol in the mediation of conditioned fear in the mesolimbic system and processing of unconditioned aversive information in the inferior colliculus. Neuroscience 261: 195–206. [DOI] [PubMed] [Google Scholar]
- 17. Reis FL, Masson S, de Oliveira AR, Brandao ML (2004) Dopaminergic mechanisms in the conditioned and unconditioned fear as assessed by the two-way avoidance and light switch-off tests. Pharmacol Biochem Behav 79: 359–365. [DOI] [PubMed] [Google Scholar]
- 18. de Souza Caetano KA, de Oliveira AR, Brandao ML (2013) Dopamine D2 receptors modulate the expression of contextual conditioned fear: role of the ventral tegmental area and the basolateral amygdala. Behav Pharmacol 24: 264–274. [DOI] [PubMed] [Google Scholar]
- 19. de Oliveira AR, Reimer AE, de Macedo CE, de Carvalho MC, Silva MA, et al. (2011) Conditioned fear is modulated by D2 receptor pathway connecting the ventral tegmental area and basolateral amygdala. Neurobiol Learn Mem 95: 37–45. [DOI] [PubMed] [Google Scholar]
- 20. de Oliveira AR, Reimer AE, Reis FM, Brandão ML (2013) Conditioned fear response is modulated by a combined action of the hypothalamic-pituitary-adrenal axis and dopamine activity in the basolateral amygdala. Eur Neuropsychopharmacol 23: 379–389. [DOI] [PubMed] [Google Scholar]
- 21. Ferreira-Netto C, Borelli KG, Brandao ML (2007) Distinct Fos expression in the brain following freezing behavior elicited by stimulation with NMDA of the ventral or dorsal inferior colliculus. Exp Neurol 204: 693–704. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates, 6th ed. San Diego: Academic Press. [Google Scholar]
- 23. Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167. [DOI] [PubMed] [Google Scholar]
- 24. Anseloni VZ, Brandao ML (1997) Ethopharmacological analysis of behaviour of rats using variations of the elevated plus-maze. Behav Pharmacol 8: 533–540. [DOI] [PubMed] [Google Scholar]
- 25. Rodgers RJ, Nikulina EM, Cole JC (1994) Dopamine D1 and D2 receptor ligands modulate the behaviour of mice in the elevated plus-maze. Pharmacol Biochem Behav 49: 985–995. [DOI] [PubMed] [Google Scholar]
- 26. Long KJ, Allen N (1984) Abnormal brain-stem auditory evoked potentials following Ondine's curse. Arch Neurol 41: 1109–1110. [DOI] [PubMed] [Google Scholar]
- 27. Nobre MJ, Sandner G, Brandao ML (2003) Enhancement of acoustic evoked potentials and impairment of startle reflex induced by reduction of GABAergic control of the neural substrates of aversion in the inferior colliculus. Hear Res 184: 82–90. [DOI] [PubMed] [Google Scholar]
- 28. Baas JM, Milstein J, Donlevy M, Grillon C (2006) Brainstem correlates of defensive states in humans. Biol Psychiatry 59: 588–593. [DOI] [PubMed] [Google Scholar]
- 29. Hall RD, Mark RG (1967) Fear and the modification of acoustically evoked potentials during conditioning. J Neurophysiol 30: 893–910. [DOI] [PubMed] [Google Scholar]
- 30. Szczepaniak WS, Moller AR (1995) Evidence of decreased GABAergic influence on temporal integration in the inferior colliculus following acute noise exposure: a study of evoked potentials in the rat. Neurosci Lett 196: 77–80. [DOI] [PubMed] [Google Scholar]
- 31. Cabral A, De Ross J, Castilho VM, Brandao ML, Nobre MJ (2009) Glutamate receptor antagonism in inferior colliculus attenuates elevated startle response of high anxiety diazepam-withdrawn rats. Neuroscience 161: 707–717. [DOI] [PubMed] [Google Scholar]
- 32. de Oliveira AR, Reimer AE, Brandao ML (2009) Role of dopamine receptors in the ventral tegmental area in conditioned fear. Behav Brain Res 199: 271–277. [DOI] [PubMed] [Google Scholar]
- 33. Myers RD (1966) Injection of solutions into cerebral tissue: Relation between volume and diffusion. Physiol Behav 1: 171–174. [Google Scholar]
- 34. Borelli KG, Brandao ML (2008) Effects of ovine CRF injections into the dorsomedial, dorsolateral and lateral columns of the periaqueductal gray: a functional role for the dorsomedial column. Horm Behav 53: 40–50. [DOI] [PubMed] [Google Scholar]
- 35. Cardoso SH, Coimbra NC, Brandao ML (1994) Defensive reactions evoked by activation of NMDA receptors in distinct sites of the inferior colliculus. Behav Brain Res 63: 17–24. [DOI] [PubMed] [Google Scholar]
- 36. Cardoso SH, Melo L, Coimbra NC, Brandao ML (1992) Opposite effects of low and high doses of morphine on neural substrates of aversion in the inferior colliculus. Behav Pharmacol 3: 489–495. [PubMed] [Google Scholar]
- 37. Pandossio JE, Brandao ML (1999) Defensive reactions are counteracted by midazolam and muscimol and elicited by activation of glutamate receptors in the inferior colliculus of rats. Psychopharmacology 142: 360–368. [DOI] [PubMed] [Google Scholar]
- 38. Castilho VM, Brandao ML (2001) Conditioned antinociception and freezing using electrical stimulation of the dorsal periaqueductal gray or inferior colliculus as unconditioned stimulus are differentially regulated by 5-HT2A receptors in rats. Psychopharmacology 155: 154–162. [DOI] [PubMed] [Google Scholar]
- 39. Brandao ML, Tomaz C, Borges PC, Coimbra NC, Bagri A (1988) Defense reaction induced by microinjections of bicuculline into the inferior colliculus. Physiol Behav 44: 361–365. [DOI] [PubMed] [Google Scholar]
- 40. Espejo EF, Minano FJ (1999) Prefrontocortical dopamine depletion induces antidepressant-like effects in rats and alters the profile of desipramine during Porsolt's test. Neuroscience 88: 609–615. [DOI] [PubMed] [Google Scholar]
- 41. Morrow BA, Elsworth JD, Rasmusson AM, Roth RH (1999) The role of mesofrontal dopamine neurons in the acquisition and expression of conditioned fear in the rat. Neuroscience 92: 553–564. [DOI] [PubMed] [Google Scholar]
- 42. Salamone JD (1994) The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res 61: 117–133. [DOI] [PubMed] [Google Scholar]
- 43. Salamone JD (1996) The behavioral neurochemistry of motivation: methodological and conceptual issues in studies of the dynamic activity of nucleus accumbens dopamine. J Neurosci Methods 64: 137–149. [DOI] [PubMed] [Google Scholar]
- 44. McCullough LD, Salamone JD (1992) Anxiogenic drugs beta-CCE and FG 7142 increase extracellular dopamine levels in nucleus accumbens. Psychopharmacology 109: 379–382. [DOI] [PubMed] [Google Scholar]
- 45. Tidey JW, Miczek KA (1996) Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res 721: 140–149. [DOI] [PubMed] [Google Scholar]
- 46. Datla KP, Ahier RG, Young AM, Gray JA, Joseph MH (2002) Conditioned appetitive stimulus increases extracellular dopamine in the nucleus accumbens of the rat. Eur J Neurosci 16: 1987–1993. [DOI] [PubMed] [Google Scholar]
- 47. Young AM (2004) Increased extracellular dopamine in nucleus accumbens in response to unconditioned and conditioned aversive stimuli: studies using 1 min microdialysis in rats. J Neurosci Methods 138: 57–63. [DOI] [PubMed] [Google Scholar]
- 48. Marinelli S, Pascucci T, Bernardi G, Puglisi-Allegra S, Mercuri NB (2005) Activation of TRPV1 in the VTA excites dopaminergic neurons and increases chemical- and noxious-induced dopamine release in the nucleus accumbens. Neuropsychopharmacology 30: 864–870. [DOI] [PubMed] [Google Scholar]
- 49. Anstrom KK, Miczek KA, Budygin EA (2009) Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience 161: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martinez RC, Oliveira AR, Macedo CE, Molina VA, Brandao ML (2008) Involvement of dopaminergic mechanisms in the nucleus accumbens core and shell subregions in the expression of fear conditioning. Neurosci Lett 446: 112–116. [DOI] [PubMed] [Google Scholar]
- 51. de Oliveira AR, Reimer AE, Brandao ML (2006) Dopamine D2 receptor mechanisms in the expression of conditioned fear. Pharmacol Biochem Behav 84: 102–111. [DOI] [PubMed] [Google Scholar]
- 52. Melo LL, Santos P, Medeiros P, Mello RO, Ferrari EA, et al. (2010) Glutamatergic neurotransmission mediated by NMDA receptors in the inferior colliculus can modulate haloperidol-induced catalepsy. Brain Res 1349: 41–47. [DOI] [PubMed] [Google Scholar]
- 53. Hurd YL, Suzuki M, Sedvall GC (2001) D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J Chem Neuroanat 22: 127–137. [DOI] [PubMed] [Google Scholar]
- 54. Casseday JH, Covey E (1996) A neuroethological theory of the operation of the inferior colliculus. Brain Behav Evol 47: 311–336. [DOI] [PubMed] [Google Scholar]
- 55. Covey E, Hall WC, Kobler JB (1997) Subcortical connections of the superior colliculus in the mustache bat, Pteronotus parnellii . J Comp Neurol 263: 179–197. [DOI] [PubMed] [Google Scholar]
- 56. Masino T, Knudsen EI (1993) Orienting head movements resulting from electrical microstimulation of the brainstem tegmentum in the barn owl. J Neurosci 13: 351–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. García Del Caño G, Gerrikagoitia I, Alonso-Cabria A, Martínez-Millán L (2006) Organization and origin of the connection from the inferior to the superior colliculi in the rat. J Comp Neurol 499: 716–731. [DOI] [PubMed] [Google Scholar]
- 58. Aparicio MA, Viñuela A, Saldaña E (2010) Projections from the inferior colliculus to the tectal longitudinal column in the rat. Neuroscience 166: 653–664. [DOI] [PubMed] [Google Scholar]
- 59. Olazábal UE, Moore JK (1989) Nigrotectal projection to the inferior colliculus: horseradish peroxidase transport and tyrosine hydroxylase immunohisto-chemical studies in rats, cats, and bats. J Comp Neurol 282: 98–118. [DOI] [PubMed] [Google Scholar]
- 60. Castellan-Baldan L, da Costa Kawasaki M, Ribeiro SJ, Calvo F, Correa VM, et al. (2006) Topographic and functional neuroanatomical study of GABAergic disinhibitory striatum-nigral inputs and inhibitory nigrocollicular pathways: neural hodology recruiting the substantia nigra, pars reticulata, for the modulation of the neural activity in the inferior colliculus involved with panic-like emotions. J Chem Neuroanat 32: 1–27. [DOI] [PubMed] [Google Scholar]
- 61. Moriizumi T, Hattori T (1991) Pallidotectal projection to the inferior colliculus of the rat. Exp Brain Res 87: 223–226. [DOI] [PubMed] [Google Scholar]
- 62. Coimbra NC, Brandão ML (1993) GABAergic nigro-collicular pathways modulate the defensive behaviour elicited by midbrain tectum stimulation. Behav Brain Res 59: 131–139. [DOI] [PubMed] [Google Scholar]
- 63. Nobre MJ, Lopes MG, Brandão ML (2004) Defense reaction mediated by NMDA mechanisms in the inferior colliculus is modulated by GABAergic nigro-collicular pathways. Brain Res 999: 124–131. [DOI] [PubMed] [Google Scholar]
- 64. Maisonnette SS, Kawasaki MC, Coimbra NC, Brandao ML (1996) Effects of lesions of amygdaloid nuclei and substantia nigra on aversive responses induced by electrical stimulation of the inferior colliculus. Brain Res Bull 40: 93–98. [DOI] [PubMed] [Google Scholar]
- 65. Garcia AM, Martinez R, Brandao ML, Morato S (2005) Effects of apomorphine on rat behavior in the elevated plus-maze. Physiol Behav 85: 440–447. [DOI] [PubMed] [Google Scholar]
- 66. Colombo AC, de Oliveira AR, Reimer AE, Brandão ML (2013) Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res 257: 201–207. [DOI] [PubMed] [Google Scholar]
- 67. LeDoux JE, Sakagushi A, Reis DJ (1986) Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci 4: 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. David AS, Woodruff PWR, Howard R, Mellers JDC, Brammer M, et al. (1996) Auditory hallucinations inhibit exogenous activation of auditory association cortex. Neuroreport 7: 932–936. [DOI] [PubMed] [Google Scholar]
- 69. López DE, Saldaña E, Nodal FR, Merchán MA, Warr WB (1999) Projections of cochlear root neurons, sentinels of the rat auditory pathway. J Comp Neurol 415: 160–74. [PubMed] [Google Scholar]
- 70. Gómez-Nieto R, Sinex DG, C Horta-Júnior JD, Castellano O, Herrero-Turrión JM, et al. (2014) A fast cholinergic modulation of the primary acoustic startle circuit in rats. Brain Struct Funct in press. [DOI] [PubMed] [Google Scholar]
- 71. McNaughton N, Corr PJ (2004) A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev 28: 285–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting figures. Figure S1, Table summary of the effects of D2 drugs on the complementary categories of the elevated plus-maze. Effects of intra-IC injections of vehicle, quinpirole 1.0 µg/0.2 µL or 2.0 µg/0.2 µL and vehicle and 1.0, 2.0 or 4.0 µg/0.2 µL sulpiride on the complementary ethological categories of rats submitted to the elevated plus-maze. Figure S2, Table summary of the effects of D1 drugs on the complementary categories of the elevated plus-maze. Effects of intra-IC injections of vehicle, 1.0 or 2.0 µg/0.2 µL SKF-38393, and vehicle or 1.0, 2.0 or 4.0 µg/0.2 µL SCH-23390 on the complementary ethological categories of rats submitted to the elevated plus-maze. Figure S3, D1 drugs in the elevated plus-maze (EPM). Effects of intra-IC injections of vehicle, 1.0 and 2.0 µg/0.2 µL SKF-38393 or vehicle, 1.0, 2.0 or 4.0 µg/0.2 µL SCH-23390 on exploratory behavior of rats submitted to the elevated plus-maze. (A and D) Number of entries in the closed arms of the maze. (B and E) Number of entries in the open arms of the maze. (C and F). % of time spent into the open arms in relation to total. Figure S4, Fos-positive immunohistochemistry in response to aversive acoustic stimuli (AAS). Number of Fos-positive cells in midbrain (A) and telencephalic (B) structures in rats exposed to testing sessions with or without (Control) presentation of AASs. Figure S5, Fos-positive immunohistochemistry in midbrain structures in response to aversive acoustic stimuli (AAS). Photomicrographs of Fos-positive cells (dark dots) in the dmPAG, dlPAG, vlPAG, and IC in rats exposed to testing sessions with and without (Control) AAS presentation. Figure S6, Fos positive immunohistochemistry in telencephalic structures in response to aversive acoustic stimuli (AAS). Photomicrographs of Fos-positive cells (dark dots) in the Cg1, CPu, NAcC and NAcSh of rats exposed to testing sessions with (AAS) or without (Control) AAS presentation.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.