Abstract
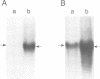
Ribonucleotide reductase R1 gene expression is elevated in BALB/c 3T3 fibroblasts treated with the tumor promoter, 12-O-tetradecanoylphorbol-13-acetate (TPA). We show that TPA treatment increased the half-life of R1 mRNA by 5-fold, indicating that TPA regulates R1 gene expression by a post-transcriptional mechanism. We investigated the possibility that the 3' untranslated region (3'UTR) of R1 mRNA contains regulatory information for TPA-mediated message stability. Our studies demonstrated that a 49 nucleotide (nt) TPA-responsive region existed within the R1 mRNA 3'UTR. Deletion of the 49 nt region led to the abolishment of TPA-induced stability of R1 and hybrid CAT mRNAs. Further deletions of the 3'UTR did not significantly affect mRNA turnover rates. In addition, we detected by cross-linking a novel 52-57 kDa R1 mRNA-binding protein (R1BP) that bound selectively to the 49 nt region of the R1 mRNA 3'UTR and did not bind to the 5'UTR, the coding region or other mRNA 3'UTRs. The R1BP-RNA binding activity observed in unstimulated cells was rapidly and markedly down-regulated after TPA treatment, suggesting a role for R1BP in mRNA metabolism, and in the mechanism of action of TPA-induced R1 message stabilization. These results support a novel model of R1 gene regulation in which a cis-element(s) within the 49 nt region of the R1 mRNA 3'UTR interacts with R1BP in a mechanism that regulates R1 message stability. We propose that this model accounts for the TPA-mediated stability alteration of R1 message, through down-regulation of R1BP-RNA binding activity linked to a reduction in the rate of R1 mRNA degradation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Blosmanis R., Wright J. A., Goldenberg G. J. Sensitivity to melphalan as a function of transport activity and proliferative rate in BALB/c 3T3 fibroblasts. Cancer Res. 1987 Mar 1;47(5):1273–1277. [PubMed] [Google Scholar]
- Bohjanen P. R., Petryniak B., June C. H., Thompson C. B., Lindsten T. An inducible cytoplasmic factor (AU-B) binds selectively to AUUUA multimers in the 3' untranslated region of lymphokine mRNA. Mol Cell Biol. 1991 Jun;11(6):3288–3295. doi: 10.1128/mcb.11.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. An A + U-rich element RNA-binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991 May;11(5):2460–2466. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Figge J., Hansen U., Wright C., Jeang K. T., Khoury G., Livingston D. M., Roberts T. M. lac repressor can regulate expression from a hybrid SV40 early promoter containing a lac operator in animal cells. Cell. 1987 Jun 5;49(5):603–612. doi: 10.1016/0092-8674(87)90536-8. [DOI] [PubMed] [Google Scholar]
- Carter B. Z., Malter J. S. Regulation of mRNA stability and its relevance to disease. Lab Invest. 1991 Dec;65(6):610–621. [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy B. K., McClarty G. A., Wright J. A. Transient elevation of ribonucleotide reductase activity, M2 mRNA and M2 protein in BALB/c 3T3 fibroblasts in the presence of 12-O-tetradecanoylphorbol-13-acetate. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1417–1424. doi: 10.1016/0006-291x(89)90832-2. [DOI] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Sassone-Corsi P. More is better: activators and repressors from the same gene. Cell. 1992 Feb 7;68(3):411–414. doi: 10.1016/0092-8674(92)90178-f. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hargrove J. L., Schmidt F. H. The role of mRNA and protein stability in gene expression. FASEB J. 1989 Oct;3(12):2360–2370. doi: 10.1096/fasebj.3.12.2676679. [DOI] [PubMed] [Google Scholar]
- Hurta R. A., Samuel S. K., Greenberg A. H., Wright J. A. Early induction of ribonucleotide reductase gene expression by transforming growth factor beta 1 in malignant H-ras transformed cell lines. J Biol Chem. 1991 Dec 15;266(35):24097–24100. [PubMed] [Google Scholar]
- Hurta R. A., Wright J. A. Alterations in the activity and regulation of mammalian ribonucleotide reductase by chlorambucil, a DNA damaging agent. J Biol Chem. 1992 Apr 5;267(10):7066–7071. [PubMed] [Google Scholar]
- Hurta R. A., Wright J. A. Regulation of mammalian ribonucleotide reductase by the tumor promoters and protein phosphatase inhibitors okadaic acid and calyculin A. Biochem Cell Biol. 1992 Oct-Nov;70(10-11):1081–1087. doi: 10.1139/o92-153. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990 Jul 13;62(1):15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstein T., June C. H., Ledbetter J. A., Stella G., Thompson C. B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989 Apr 21;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Malter J. S., Hong Y. A redox switch and phosphorylation are involved in the post-translational up-regulation of the adenosine-uridine binding factor by phorbol ester and ionophore. J Biol Chem. 1991 Feb 15;266(5):3167–3171. [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- Neupert B., Thompson N. A., Meyer C., Kühn L. C. A high yield affinity purification method for specific RNA-binding proteins: isolation of the iron regulatory factor from human placenta. Nucleic Acids Res. 1990 Jan 11;18(1):51–55. doi: 10.1093/nar/18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondon I. J., MacMillan L. A., Beckman B. S., Goldberg M. A., Schneider T., Bunn H. F., Malter J. S. Hypoxia up-regulates the activity of a novel erythropoietin mRNA binding protein. J Biol Chem. 1991 Sep 5;266(25):16594–16598. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Thelander L., Berg P. Isolation and characterization of expressible cDNA clones encoding the M1 and M2 subunits of mouse ribonucleotide reductase. Mol Cell Biol. 1986 Oct;6(10):3433–3442. doi: 10.1128/mcb.6.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. The reprogramming of transcriptional competence. Cell. 1992 May 15;69(4):573–575. doi: 10.1016/0092-8674(92)90218-2. [DOI] [PubMed] [Google Scholar]
- Volloch V., Housman D. Stability of globin mRNA in terminally differentiating murine erythroleukemia cells. Cell. 1981 Feb;23(2):509–514. doi: 10.1016/0092-8674(81)90146-x. [DOI] [PubMed] [Google Scholar]
- Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983 Aug;43(8):3466–3492. [PubMed] [Google Scholar]
- Wisdom R., Lee W. The protein-coding region of c-myc mRNA contains a sequence that specifies rapid mRNA turnover and induction by protein synthesis inhibitors. Genes Dev. 1991 Feb;5(2):232–243. doi: 10.1101/gad.5.2.232. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Alam T. G., McClarty G. A., Tagger A. Y., Thelander L. Altered expression of ribonucleotide reductase and role of M2 gene amplification in hydroxyurea-resistant hamster, mouse, rat, and human cell lines. Somat Cell Mol Genet. 1987 Mar;13(2):155–165. doi: 10.1007/BF01534695. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Chan A. K., Choy B. K., Hurta R. A., McClarty G. A., Tagger A. Y. Regulation and drug resistance mechanisms of mammalian ribonucleotide reductase, and the significance to DNA synthesis. Biochem Cell Biol. 1990 Dec;68(12):1364–1371. doi: 10.1139/o90-199. [DOI] [PubMed] [Google Scholar]