Abstract
Introduction
We hypothesized that the degree of preserved functional connectivity within the DMN during the first week after cardiopulmonary arrest (CPA) would be associated with functional outcome at hospital discharge.
Methods
Initially comatose CPA survivors with indeterminate prognosis at 72 hours were enrolled. Seventeen CPA subjects between 4–7 days after CPA and 17 matched controls were studied with task-free fMRI. Independent component analysis was performed to delineate the DMN. Connectivity strength in the DMN was compared between CPA subjects and controls, as well as between CPA subjects with good outcome (discharge Cerebral Performance Category or CPC 1–2) and those with bad outcome (CPC 3–5). The relationship between connectivity strength in the posterior cingulate cortex (PCC) and precuneus (PC) within the DMN with discharge CPC was evaluated using linear regression.
Results
Compared to controls, CPA subjects had significantly lower connectivity strength in subregions of the DMN, the PCC and PC (p <0.0001). Furthermore, connectivity strength in the PCC and PC was greater in CPA subjects with good outcome (n=8) than those with bad outcome (n=9) (p <0.003). Among CPA subjects, the connectivity strength in the PCC and PC showed strong linear correlations with the discharge CPC (p <0.005).
Conclusion
Among initially comatose CPA survivors with indeterminate prognosis, task-free fMRI demonstrated graded disruption of DMN connectivity, especially in those with bad outcomes. If confirmed, connectivity strength in the PC/PCC may provide a clinically useful prognostic marker for functional recovery after CPA.
INTRODUCTION
Outcomes among cardiopulmonary arrest (CPA) survivors are typically poor, with neurological deficits representing the leading cause of disability (1–5). Although specific prognostic markers of bad outcome (death or chronic disorders of consciousness) have been identified, at least half of initially comatose CPA survivors fall into an indeterminate prognostic category (5, 6). Significant healthcare resources are expended on life-sustaining therapies in this indeterminate group, many of whom will survive with disorders of consciousness (5). While some CPA survivors regain functional independence, nearly half of those who recover consciousness have chronic neurological and cognitive deficits known as the “post-cardiac arrest syndrome (5, 7–9). An early prognostic indicator of neurological outcome is needed to guide treatment of patients who currently fall into an indeterminate prognostic category.
Because injury related to CPA occurs largely on a neuronal scale, standard radiographic techniques often demonstrate few abnormalities (10, 11). Since chronic disorders of consciousness and cognitive dysfunction may be caused by disruption of cortical networks (12–14), task-free fMRI, which measures functional connectivity by quantifying the coherence of spontaneous fluctuations of blood oxygen level dependent (BOLD) signal between specific regions (15–20), may better prognosticate among CPA survivors. Task-free fMRI (also called resting state fMRI) has characterized a “default mode” network (DMN) comprising the posterior cingulate cortex (PCC), precuneus (PC), medial prefrontal cortex, and bilateral temporal-parietal cortices (20–22). There is a growing body of evidence that coherent activity within this network plays a significant role in consciousness and cognition (21–23).
We hypothesized that the degree of preserved functional connectivity within the DMN during the first week after CPA would be associated with functional outcome at hospital discharge.
METHODS
Regulatory Methods and Patient Consents
The study was approved by the Queen's Medical Center (QMC) Institutional Review Committee and the University of Hawaii Institutional Review Board. Written informed consent was obtained from the surrogate decision maker of all CPA subjects and directly from all control subjects participating in the study.
Participants
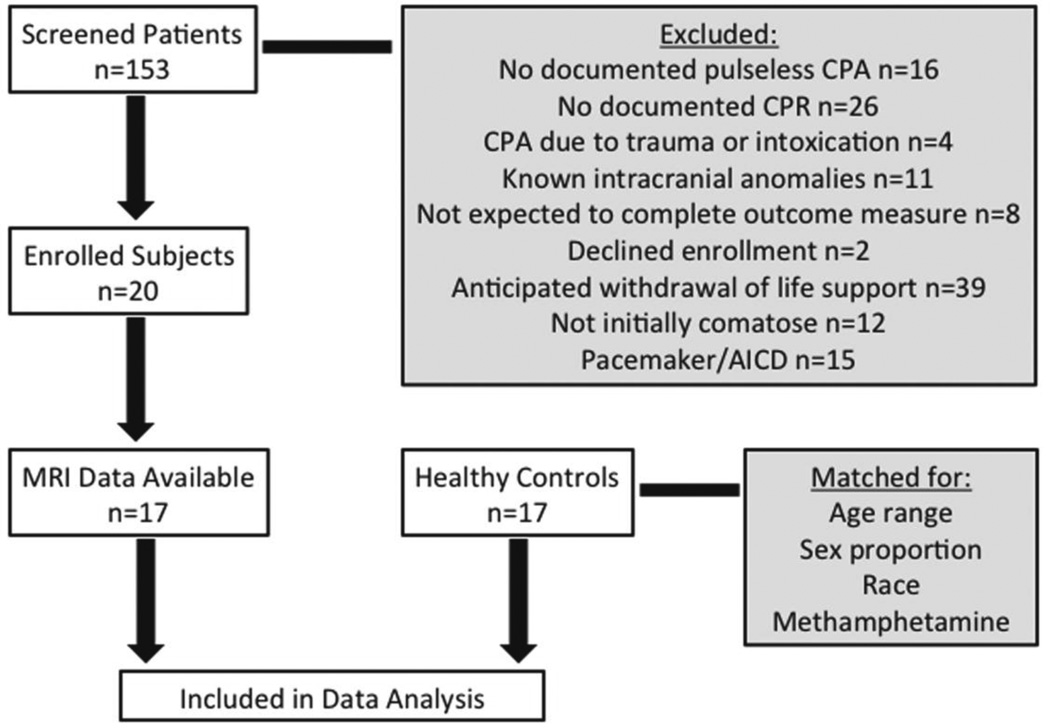
In-hospital and out-of-hospital CPA patients were identified by provider referral, screening the hospital census, and review of the hospital Code Blue record. Between July 2010 and May 2012, we screened 153 patients and enrolled 20 CPA subjects. Reasons for screen failure are reported below (Figure 1). Two studies were terminated early due to respiratory symptoms concerning for congestive heart failure exacerbated by flat positioning and one study could not be completed because of scanner malfunction, therefore, only 17 CPA subjects were included in the analysis. Seventeen healthy controls who had enrolled in other ongoing task-free fMRI studies and consented to data sharing were matched to the CPA subjects by age range, sex proportion, race, and positive urine toxicology for methamphetamine.
Figure 1. Flow Chart of Study Enrollment.
Selection process for CPA subjects and healthy control subjects
Inclusion criteria for CPA subjects were age 18 years or older, documented CPR for spontaneous CPA with an initial rhythm of pulseless ventricular tachyarrhythmia, pulseless electrical activity, or asystole, and coma (GCS ≤8) after return of spontaneous circulation. Exclusion criteria were CPA due to trauma; known intracranial injuries such as brain tumor, stroke, or intracerebral hemorrhage; patients who could not complete the outcome measure due to anticipated transfer to another facility prior to hospital discharge; and patients with anticipated withdrawal of life support due to pre-existing orders against life-sustaining treatment. Because the goal was to select a population of patients with indeterminate prognosis, patients with highly specific negative prognostic markers were excluded. These exclusion criteria were: bilaterally absent cortical N20 responses on median nerve somatosensory evoked potential (SSEP) testing, no motor response other than reflexive posturing at 72 hours, presence of myoclonic status epilepticus, and/or absence of cranial nerve reflexes (6).
All data required for the Utstein Template for Resuscitation Research (24) were collected. Decisions regarding induced hypothermia, revascularization therapy, independent neurological consultation, additional diagnostic tests, or limitations to life-sustaining therapies were made by the clinical team and followed hospital policies for hypothermia and prognostication. Subjects treated with induced hypothermia therapy were rewarmed at least 24 hours prior to task-free fMRI. Cerebral Performance Categories (CPC) is the predominant outcome measure in CPA research, and was determined for each CPA participants on the day of discharge by one of the clinician-investigators, blinded to the task-free fMRI results. CPC was dichotomized according to standard cut points into good outcome (CPC 1–2) and bad outcome or death (CPC 3–5).
QMC has an order set for induced hypothermia after CPA, which includes standard sedation with propofol or fentanyl and midazolam infusions. After rewarming, the choice of sedative is provider-dependent, but most providers continue to use propofol infusion for moderate-to-deep sedation or bolus doses of fentanyl and midazolam for mild sedation. Sedative medications and doses within 24 hours of the fMRI scan were recorded and qualitative depth of sedation was a covariate in the analyses. We defined mild sedation as analgesic dose fentanyl (≤50 mcg IV bolus) and/or anxiolytic dose midazolam (≤2 mg IV bolus), moderate sedation as continuous infusion of propofol at procedural sedation doses (≤50 mcg/kg/min), and deep sedation as continuous infusion of propofol at general anesthetic doses (>50 mcg/kg/min) according to Micromedex. No sedation was defined as no sedative medications within 24 hours of the fMRI.
MRI Scanning
Task-free fMRI in CPA subjects was performed between hospital days 4–7 when the patient was hemodynamically stable and no longer required MRI-incompatible devices. During transport and scanning, patients were monitored by a dedicated neurological intensive care unit (ICU) team. All subjects were scanned in a 3 Tesla Siemens Tim Trio scanner (Siemens Medical Solutions, Erlangen, Germany) using a 12-channel head coil. As an overview of the MRI techniques, we obtained structural brain images and fMRI sequences at rest. The fMRI data were then superimposed upon the structural images and aggregated. The aggregate fMRI data were then processed to remove artifacts and separated into independent components so the DMN could be visually identified. Once the DMN was identified for the whole group, connectivity strength was determined for each subject and compared between groups (CPA subjects versus controls, good outcome versus bad outcome) within this network as an indicator of network integrity.
MRI included a 3-plane localizer (repetition time/echo time (TR/TE) = 20/5 ms; 1 average) and a sagittal 3-D magnetization-prepared rapid gradient-echo (MP-RAGE) scan (TR/TE inversion time = 2200/4.47/1000 ms; 1 average; 208 × 256 × 160 matrix) that was examined for structural abnormalities and used to extract spatial normalization parameters. These sequences were used to visually identify structural lesions and artifacts and to construct spatial maps for superimposition of fMRI data.
Eyes-closed task-free fMRI data were acquired using an in/out spiral acquisition sequence (TR/TE=2000/30 ms, 5 mm thickness, 30 axial slices, 642 matrix, 22 cm FOV, 156 time points, 5.2 minutes per scan) (25). Subjects and controls were instructed to remain awake with their eyes closed during the duration of the scan. To ensure usable scanning data, each patient had 2 task-free fMRI scans during the same session. Images were reconstructed using in-house software and then submitted to FMRI of the brain Software Laboratory (FSL, www.fmrib.ox.ac.uk/fsl) for movement assessment and conversion to Neuroimaging Informatics Technology Initiative (NIFTI) format (26). Scans with movement exceeding 1 mm or 1 degree were rejected from further analysis. Additionally, the first 5 volumes of images submitted to subsequent analysis were removed to ensure MR signals were stable. Of the 2 task-free fMRI scans collected per patient, we selected the scan with less movement for subsequent analyses. Within Statistical Parametric Mapping 8 (SPM8, www.fil.ion.ucl.ac.uk/spm), each subject’s task-free fMRI data were co-registered to their structural image. Then, parameters for spatial normalization to the Montreal Neurological Institute 152 (MNI152) atlas were extracted from each subject’s anatomical image and applied to the task-free fMRI images which were then smoothed with an 8 mm full width at half maximum (FWHM) isotropic kernel.
Subjects’ smoothed and normalized images were submitted to group independent component analysis (ICA) within the Group ICA of fMRI Toolbox (GIFT) (27). ICA allows identification of independent, low frequency brain networks for visual identification of the DMN within the entire group of controls and CPA subjects. The number of independent components (IC) was constrained to 20, which is the default setting for GIFT and is consistent with prior publications for the DMN (28, 29). We chose 20 ICs since no difference in the DMN was found when the number of ICs was increased to 32 (30), and fewer ICs would avoid separating networks into subnetworks.
Following identification of the DMN, component images for the control and CPA subject groups were analyzed independently with a within-group 1-sample t-test in SPM8. Between-group comparisons were performed using a 2-sample t-test and an additional analysis with subject age as a covariate was performed to account for potential age effects between the 2 groups. For the good versus bad outcome contrasts, 2-sample t-test analyses were performed both with and without the level of sedation as a covariate. All analyses were constrained with a DMN mask, which was first visually identified by comparison to previously defined DMN maps (28, 29). Subsequent analyses of the DMN were constrained by an SPM8-generated mask that included only contiguous voxels within the DMN that were above threshold (t=10).
The connectivity strength within the identified DMN was then calculated. Connectivity strength is a measure of coherence of spontaneous fluctuations in BOLD signal within a time series. Since brain regions with highly correlated time series are assumed to belong to the same network, connectivity strength represents a measure of functional network integrity. Mean DMN connectivity strength was extracted from a 27 voxel (3 mm3 voxels) region of interest (ROI) centered on 3 local maxima that showed the most significant group differences in the CPA subjects with good outcome versus bad outcome. Connectivity strength from these 3 regions was regressed against the discharge CPC score in each CPA subject.
RESULTS
Subjects
For demographic information, see Table 1. The CPA subjects were 55±17.9 years old and included 14 (82%) men, 7 (41%) Asians, 5 (29%) whites, 3 (18%) Filipinos, and 2 (12%) Native Hawaiians. Thirteen (76%) of the CPA subjects had out-of-hospital CPA, and three (18%) had positive urine toxicology for methamphetamine. The initial rhythm was ventricular fibrillation or ventricular tachycardia in 9 subjects (52.9%), asystole in 5 subjects (29.4%), and pulseless electrical activity (PEA) in 3 subjects (17.6%). Fifteen (88%) subjects received induced hypothermia for 24 hours. Median admission Glasgow Coma Scale (GCS) score was 4 and median admission Full Outline of UnResponsiveness (FOUR) score (31) was 6. The 17 control subjects had similar characteristics (Table 2).
Table 1.
Characteristics of the Study Population
| Age | Gender | Race | Initial rhythm | Hypothermia | fMRI GCS | Discharge CPC |
|---|---|---|---|---|---|---|
| 28 | Male | Asian | Ventricular Fib/Tach | Yes | 14 | 1 (day 8) |
| 24 | Female | White | PEA | Yes | 14 | 1 (day 8) |
| 69 | Male | White | PEA | Yes | 4 | 5 (day 65) |
| 38 | Male | White | Asystole | Yes | 9 | 2 (day 32) |
| 73 | Male | Asian | Asystole | Yes | 6 | 3 (day 28) |
| 63 | Female | White | Ventricular Fib/Tach | Yes | 11 | 2 (day 18) |
| 47 | Male | Asian | Ventricular Fib/Tach | Yes | 9 | 2 (day 11) |
| 54 | Male | Filipino | Ventricular Fib/Tach | Yes | 3 | 5 (day 24) |
| 81 | Male | Asian | Ventricular Fib/Tach | Yes | 7 | 3 (day 15) |
| 64 | Male | Asian | Ventricular Fib/Tach | Yes | 9 | 3 (day 20) |
| 74 | Male | Asian | Ventricular Fib/Tach | Yes | 10 | 2 (day 15) |
| 46 | Male | Hawaiian | Asystole | Yes | 9 | 5 (day 13) |
| 29 | Male | Filipino | Asystole | Yes | 6 | 4 (day 156) |
| 59 | Female | Asian | PEA | Yes | 7 | 3 (day 90) |
| 73 | Male | Filipino | Asystole | No | 9 | 3 (day 97) |
| 56 | Male | Hawaiian | Ventricular Fib/Tach | No | 14 | 1 (day 7) |
| 72 | Male | White | Ventricular Fib/Tach | Yes | 10 | 1 (day 10) |
Table 2.
Comparison between CPA Subjects and Controls
| CPA Subjects | Controls | p-value | |
|---|---|---|---|
| Age in Years (mean±sd) | 55±17.9 | 46.8±9.8 | 0.107 |
| Male Gender | 14/17 | 15/17 | 1.000 |
| Methamphetamine Use | 3/17 | 3/17 | 1.000 |
| Asian | 7/17 | 6/17 | 0.864 |
| White | 5/17 | 7/17 | |
| Filipino | 3/17 | 3/17 | |
| Native Hawaiian | 2/17 | 1/17 |
Although all CPA subjects were comatose (GCS ≤8) at the time of enrollment, 11 had improved to GCS >8 at the time of the MRI scans (Table 1). The interval between enrollment and task-free fMRI ranged from 1–6 days (mean 3). At the time of task-free fMRI, the GCS was 8.9 ±3.2 (median 9, range 3–14). No sedation was given within 24 hours of task-free fMRI in 10/17 subjects (58.8%). Mild sedation (total doses of midazolam 2–5 mg IV or fentanyl 50 mcg IV) was given within 24 hours of task-free fMRI in 5/17 subjects (29.5%) and moderate sedation (propofol 25–35 mcg/kg/min) in 2/17 subjects (11.8%).
Three subjects (18%) died prior to hospital discharge. In all cases, death occurred due to withdrawal of life sustaining measures because of prolonged unconsciousness. Among the 14 survivors, 9 (64%) were discharged home, 1 (7%) was discharged to an acute rehabilitation hospital, and 4 (29%) were discharged to skilled nursing facilities. Among survivors, discharge CPC 1 occurred in 4 subjects, CPC 2 in 4 subjects, CPC 3 in 5 subjects, and CPC 4 in 1 subject. Using the dichotomized CPC cut point, good outcome (CPC 1–2) occurred in 8 subjects and bad outcome (CPC 3–5) occurred in 9 subjects.
MRI Studies
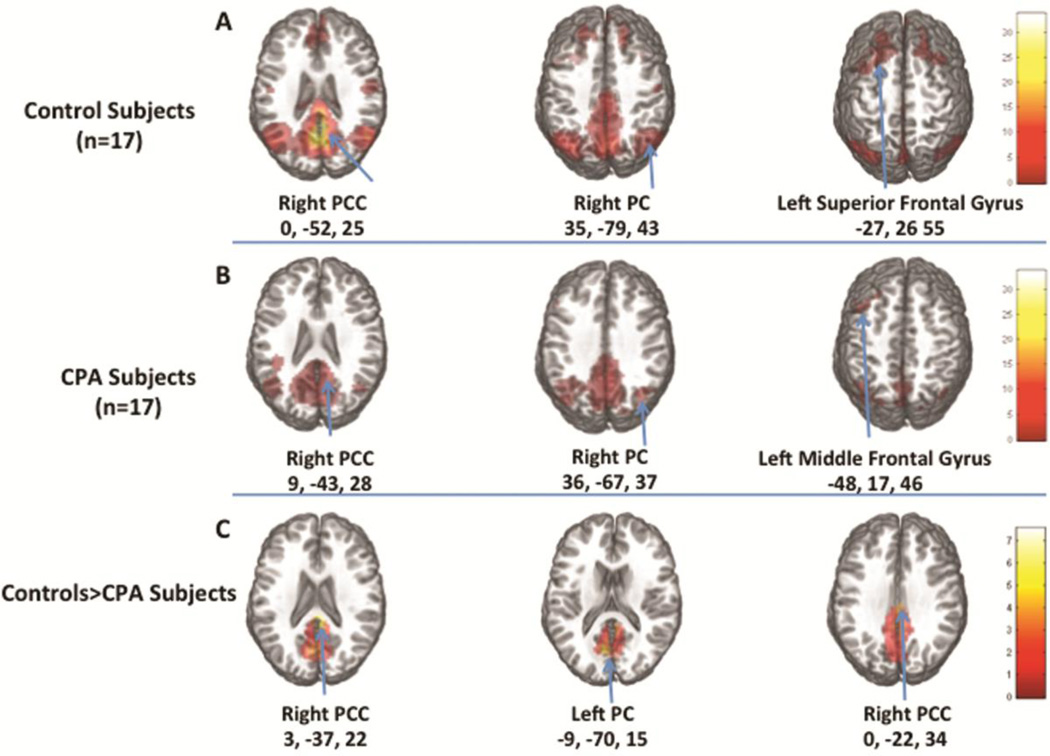
Structural MRI (MP-RAGE) scans were examined and none of the subjects had structural lesions. Both controls and CPA subjects showed the typical DMN including bilateral PCC (p <0.00001), PC (p <0.00001), middle temporal gyrus (controls: p<0.00001, CPA subjects: p<0.001), and superior and medial frontal gyri (controls: p<0.0001, CPA subjects: p<0.003). Connectivity was significantly lower in CPA subjects in a 1293 voxel cluster within the PCC (p<0.008) (Table 3). Due to a trend for younger age in the control group (46.8±9.8 vs. 55±17.9 years, p= 0.075), an additional between-group analysis was performed including age as a covariate (Figure 2). The effect of age was minimal, with the CPA subjects showing lower connectivity than controls in the same brain region (cluster size: 1265 voxels, p <0.0001) (Table 3, Figure 2).
Table 3.
Brain Regions Showing Significant Group Differences
| Brain region(s) in cluster | Talairach coordinates at local maxima of the clusters |
Cluster level | Voxel level |
|
|---|---|---|---|---|
| x,y,z (mm) | Correcte d p-value |
# of voxels |
T-score | |
| Controls > Subjects | ||||
| Right Posterior Cingulate (BA 23) | 3, −37, 22 | < 0.008 | 1293 | 7.50 |
| Left Posterior Cingulate (BA 23) | −9, −57, 13 | 5.40 | ||
| Cingulate (BA 24) | 0, −22, 34 | 4.92 | ||
| Controls > Subjects, co-varying age | ||||
| Right Posterior Cingulate (BA 23) | 3, −37, 22 | < 0.005 | 1265 | 7.41 |
| Left Cuneus (BA 18) | −9, −70, 15 | 5.95 | ||
| Posterior Cingulate (BA 24) | 0, −22, 34 | 5.17 | ||
| Good Outcome > Bad Outcome, co-varying sedation level | ||||
| Left Precuneus, (BA 31) | −21, −67, 25 | < 0.001 | 2540 | 6.18 |
| Right Precuneus (BA 7) | 9, −58, 31 | 5.59 | ||
| Cuneus (BA 18) | 0, −70, 19 | 5.28 | ||
Figure 2. DMN Connectivity in CPA Subjects Compared to Controls.
A) average ICA maps of the DMN in 17 healthy controls, B) average ICA maps of the DMN in 17 CPA subjects, and C) areas where the connectivity strength is significantly greater in controls than CPA subjects, co-varying for age.
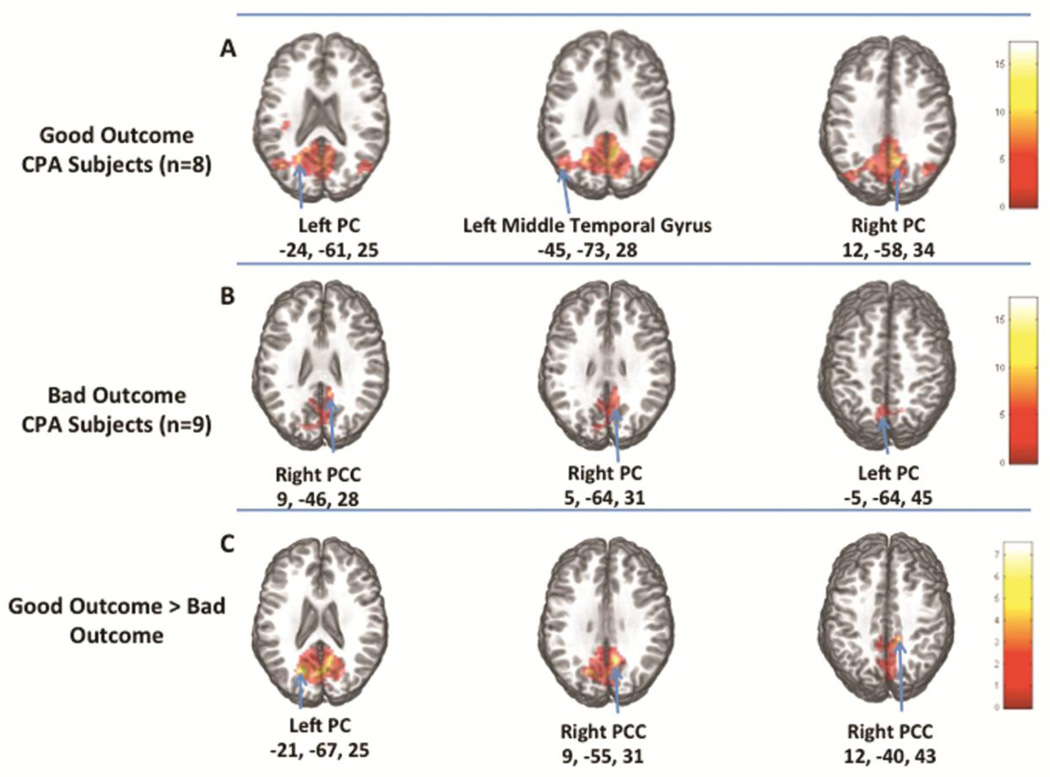
CPA subjects with good outcome (CPC 1–2) had an intact DMN that included PCC and PC (p<0.00001), temporal and parietal regions (p<0.00001), left insula (p<0.0002), and left superior frontal gyrus (p<0.003) (Table 3, Figure 3A). In contrast, CPA subjects with bad outcome (CPC 3–5) showed above threshold connectivity only in the PC and PCC regions (p<0.00001) (Table 3, Figure 3B). Subjects with bad outcome showed lesser connectivity than those with good outcome in the PCC region (cluster size 1449 voxels, p<0.001). When sedative medication was included as a covariate in the analysis, subjects with bad outcome showed even lower connectivity than those with good outcome (cluster size: 2540 voxels, p<0.0001) (Table 3, Figure 3C).
Figure 3. DMN Connectivity in Good Outcome Compared to Bad Outcome.
A) average maps of the 8 CPA subjects with "good outcome" (CPC 1–2), B) average maps of the 9 CPA subjects with "bad outcome" (CPC 3–5), C) DMN areas where good outcome subjects had greater connectivity than bad outcome subjects.
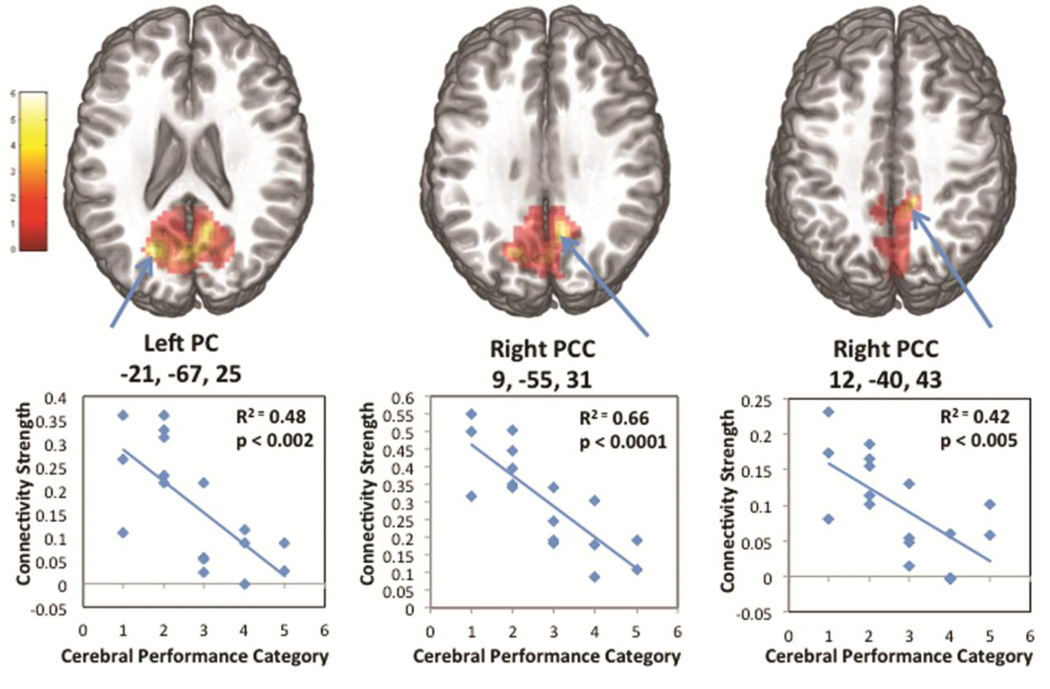
Regression analyses of connectivity strength in the PC/PCC on non-dichotomized discharge CPC score demonstrated a strong linear association (Figure 4). Linear regression of mean connectivity strength in selected ROIs against the discharge CPC accounted for 48% of the variance in the left PC (p <0.002) and 66% and 42% of the variance in the 2 right PCC ROIs (p<0.0001 and p<0.005 respectively) (Figure 4).
Figure 4. DMN Connectivity in PC/PCC Correlated with Outcome.
A) 3 local maxima of greatest difference between the 2 outcome groups, and B) regression plots of CPC category and connectivity strength within each local maxima.
DISCUSSION
These data demonstrate disruption of functional connectivity within the DMN in CPA survivors who were initially comatose and had indeterminate prognosis at the time of enrollment. Taken as a group, CPA survivors had diminished connectivity within the DMN compared to controls with similar age, sex proportion, race, and methamphetamine use. Among CPA subjects, those who survived with severe neurological deficits or who died from withdrawal of life-sustaining therapies after prolonged unconsciousness had severely disrupted or absent connectivity within the DMN compared to CPA survivors with good outcomes. In brain regions that showed the greatest group differences, PCC and PC components of the DMN, connectivity strength was associated with discharge functional outcomes.
We enrolled too few subjects to calculate sensitivity and specificity and the association between connectivity strength and functional outcome was imperfect. These findings require validation in larger cohorts. If confirmed, task-free fMRI may ultimately have a complementary role in prognostication for the significant number of CPA survivors with indeterminate prognosis according to current algorithms. A specific threshold of DMN connectivity may further classify which patients will have good functional outcomes.
These results are consistent with observational studies of task-free fMRI in patients with chronic disorders of consciousness (23, 32, 33). In these series, patients with preserved consciousness could often be distinguished from those in a persistent vegetative state by the preservation of DMN connectivity (23, 33). One study found absence of DMN activity in 3 subjects in a persistent vegetative state (32). A larger series of non-communicative subjects with chronic brain injuries (ranging from locked-in state to persistent vegetative state) found an association between DMN connectivity and severity of neurological impairment (33). They also found the strongest association between the extent of neurological injury and functional connectivity in the PC/PCC (33).
To date, only a single task-free fMRI study has been undertaken in 13 acutely comatose patients with severe brain injuries after CPA (34). Similar to the present study, 11 subjects with bad outcomes demonstrated absent or diminished DMN connectivity and 2 subjects who recovered consciousness demonstrated relative preservation of DMN connectivity. This prior study, however, included subjects with severe brain injuries who already had specific prognostic markers of bad outcome – including burst-suppression EEG patterns and absent cortical N20 responses on SSEP (34). The number of subjects with preserved DMN connectivity and good functional outcomes was small, hence the additive prognostic value of task-free fMRI was difficult to discern.
Our study was designed to specifically examine DMN integrity within a select population of CPA survivors who would be expected to have equal odds of good functional recovery or survival with disorders of consciousness based on current guidelines (6). These guidelines were based on studies prior to therapeutic hypothermia. More recent studies show conflicting data on whether or not hypothermia alters the prognostic accuracy of clinical findings, such as the motor exam (35–37). All but 2 of our subjects were treated with hypothermia (Table 1), so the extent to which hypothermia altered the prognostic value of task-free fMRI cannot be discerned. Amongst the 17 CPA subjects, 8 had significant neurological recovery at the time of hospital discharge and the remaining 9 either died from withdrawal of life sustaining measures after prolonged unconsciousness or survived with severe neurological deficits. We excluded patients with highly specific prognostic markers of bad neurological outcomes since established prognostic algorithms (6) already exist for such patients. The majority of these patients ultimately die from withdrawal of life sustaining measures. By excluding this population of CPA survivors, the study was designed to largely avoid the problem of self-fulfilling prophecies that taint many studies of prognostication in CPA survivors. Although 3 subjects ultimately died from withdrawal of life sustaining therapies, transition to palliative care was only undertaken after prolonged unconsciousness (13, 24, and 65 days).
One of the major limitations of this study was that, although all subjects remained comatose (GCS ≤8) at the time of enrollment, 11 had further neurological recovery at the time task-free fMRI was performed. The delay between enrollment and the MR scans was unavoidable because we waited until the subjects were hemodynamically stable and all MRI-incompatible devices were removed prior to MRI. Ideally, all patients would remain comatose at the time of the task-free fMRI, and therefore would continue to have equal chances of good or bad outcome. None of the 6 patients with GCS ≤8 at the time of fMRI had a good outcome (CPC 1–2) at discharge, so we could not test whether the association between DMN connectivity strength and outcome was independent of the GCS. For this reason, we cannot determine the additive value of fMRI to current prognostic algorithms with this study alone. This important question will be evaluated by future studies.
Second, 7 CPA subjects required sedation before or during task-free fMRI. Although deep sedation has been shown to reduce DMN connectivity, the dose of sedatives received by the CPA subjects would not be expected to cause the observed degree of DMN disruption (38, 39). In fact, the subjects with lower GCS and greater disruption of DMN connectivity required less sedatives during the task-free fMRI scans. Furthermore, when the category of sedation was added to the multivariable logistic regression model, the association between DMN connectivity and discharge CPC strengthened.
Third, reduced DMN connectivity may be affected by a global hypoperfusion, which would result in a proportionate reduction in BOLD signal. We did not perform perfusion or arterial spin labeling sequences as part of the study protocol. PET scans of cerebral perfusion in the subacute period after CPA, however, suggest that perfusion is more likely to be increased rather than reduced in patients with severe hypoxic-ischemic encephalopathy due to loss of cerebral vasomotor control (40).
Fourth, the timing of the discharge CPC assessment varied significantly between subjects because of variable length of stay (Table 1). As expected, subjects with prolonged length of stay all had bad outcomes so it is unlikely that the association between DMN connectivity and outcome was significantly affected by length of stay. We cannot fully exclude, however, that a subject discharged early in the bad outcome group might later improve to the good outcome group. In future studies, using a standard outcome assessment interval would address this problem.
Lastly, because of the high ratio of screened patients to enrolled subjects, the generalizability of these results may be limited.
Currently, aside from the clinical exam, sensitive and specific markers of good functional outcome after CPA are lacking. A larger independent cohort of comatose patients with indeterminate prognosis after CPA will be needed to determine whether task-free fMRI is able to accurately predict which patients will recover consciousness. If these results are confirmed with a high degree of prognostic accuracy, task-free fMRI could become an important addition to established prognostic algorithms.
Although the structural MRI did not show visible lesions, functional disruption of network activity within the DMN may be caused by microstructural injury associated with CPA. Future studies to evaluate microstructural integrity of the DMN of these patients using complementary techniques, including MR spectroscopy and diffusion tensor imaging are also needed.
ACKNOWLEDGEMENTS
The authors thank Denise Dittrich, RN and Tracy Stern, RN from the Queen’s Medical Center Neuroscience Institute for study coordination; Todd Seto, MD from the Queen’s Medical Center Department of Cardiology for assistance with database design; and Ahnate Lim from the University of Hawaii MRI Research Center for assistance with data processing. Funding support for this study was provided by the Hawaii Community Foundation grant 11ADVC-49231, Queen Emma Research Fund, and NIH grants 1P30GM103341-01, U54NS56883, and K24DA16170.
Footnotes
CONFLICT OF INTEREST
Matthew Koenig declares that he has no conflict of interest. John Holt declares that he has no conflict of interest. Thomas Ernst consults for and holds patents through Kineticor, Inc. Steven Buchthal declares that he has no conflict of interest. Kazuma Nakagawa declares that he has no conflict of interest. Victor Stenger declares that he has no conflict of interest. Linda Chang declares that she has no conflict of interest.
REFERENCES
- 1.Bedell SE, Delbanco TL, Cook EF, Epstein EF. Survival after cardiopulmonary resuscitation in the hospital. N Engl J Med. 1983;309:569–576. doi: 10.1056/NEJM198309083091001. [DOI] [PubMed] [Google Scholar]
- 2.Edgren E, Kelsey S, Sutton K, Safar P. The presenting ECG pattern in survivors of cardiac arrest and its relation to the subsequent long-term survival. Brain Resuscitation Clinical Trial I Study Group. Acta Aneasthesiol Scand. 1989;33:265–271. doi: 10.1111/j.1399-6576.1989.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 3.Berek K, Jeschow M, Aichner F. The prognostication of cerebral hypoxia after out-of-hospital cardiac arrest in adults. Eur Neurol. 1997;37:135–145. doi: 10.1159/000117426. [DOI] [PubMed] [Google Scholar]
- 4.Nichol G, Stiell IG, Herbert P, Wells GA, Vandemheen K, Laupacis A. What is the quality of life for survivors of cardiac arrest? A prospective study. Acad Emerg Med. 1999;6:95–102. doi: 10.1111/j.1553-2712.1999.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 5.Neumar RW, Nolan JP, Adrie C, et al. Post-Cardiac Arrest Syndrome. Epidemiology, Pathophysiology, Treatment, and Prognostication. A Consensus Statement from the International Liaison Committee on Resuscitation. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 6.Wijdicks EFM, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review) Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 7.Bunch TJ, White RD, Gersh BJ, et al. Long-term outcomes of out-of-hospital cardiac arrest after successful early defibrillation. N Engl J Med. 2003;348:2626–2633. doi: 10.1056/NEJMoa023053. [DOI] [PubMed] [Google Scholar]
- 8.van Alem AP, de Vos R, Schmand B, Koster RW. Cognitive impairment in survivors of out-of-hospital cardiac arrest. Am Heart J. 2004;148:416–421. doi: 10.1016/j.ahj.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Moulaert VRMP, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2009;80:297–305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Wijdicks EF, Campeau NG, Miller GM. MR imaging in comatose survivors of cardiac resuscitation. Am J Neuroradiol. 2001;22:1561–1565. [PMC free article] [PubMed] [Google Scholar]
- 11.Els T, Kassubek J, Kubalek R, Klisch J. Diffusion-weighted MRI during early global cerebral hypoxia: a predictor for clinical outcome? Acta Neurol Scand. 2004;110:361–367. doi: 10.1111/j.1600-0404.2004.00342.x. [DOI] [PubMed] [Google Scholar]
- 12.Llinas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laureys S, Faymonville ME, Peigneux P, et al. Cortical processing of noxious somatosensory stimuli in the persistent vegetative state. Neuroimage. 2002;17:732–741. [PubMed] [Google Scholar]
- 14.Schiff ND, Rodriguez-Moreno D, Kamel A, et al. fMRI reveals large-scale network activation in minimally conscious patients. Neurology. 2005;64:514–523. doi: 10.1212/01.WNL.0000150883.10285.44. [DOI] [PubMed] [Google Scholar]
- 15.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 16.Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:513–519. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiff ND. Multimodal neuroimaging approaches to disorders of consciousness. J Head Trauma Rehabil. 2006;21:388–397. doi: 10.1097/00001199-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 18.He BJ, Shulman GL, Snyder AZ, Corbetta M. The role of impaired neuronal communication in neurological disorders. Curr Opin Neurol. 2007;20:655–660. doi: 10.1097/WCO.0b013e3282f1c720. [DOI] [PubMed] [Google Scholar]
- 19.Ioannides AA. Dynamic functional connectivity. Curr Opin Neurobiol. 2007;17:161–170. doi: 10.1016/j.conb.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 21.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Espejo D, Soddu A, Cruse D, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol. 2012;72:335–343. doi: 10.1002/ana.23635. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs I, Nadkarni V the ILCOR Task Force on Cardiac Arrest and Cardiopulmonary Resuscitation Outcomes. Update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation. Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 25.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 26.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45(Suppl 1):S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Foryt P, Ochs R, et al. Abnormalities in resting-state functional connectivity in early human immunodeficiency virus infection. Brain Connect. 2011;1:207–217. doi: 10.1089/brain.2011.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wijdicks EF, Bamlet WR, Maramatton BV, Manno EM, McClelland RL. Validation of a new coma scale: the FOUR score. Ann Neurol. 2005;58:585–593. doi: 10.1002/ana.20611. [DOI] [PubMed] [Google Scholar]
- 32.Cauda F, Micon BM, Sacco K, et al. Disrupted intrinsic functional connectivity in the vegetative state. J Neurol Neurosurg Psychiatry. 2009;80:429–431. doi: 10.1136/jnnp.2007.142349. [DOI] [PubMed] [Google Scholar]
- 33.Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133:161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norton L, Hutchison RM, Young GB, Lee DH, Sharpe MD, Mirsattari SM. Disruptions of functional connectivity in the default mode network of comatose patients. Neurology. 2012;78:175–181. doi: 10.1212/WNL.0b013e31823fcd61. [DOI] [PubMed] [Google Scholar]
- 35.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67:301–307. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
- 36.Fugate JE, Wijdicks EF, White RD, Rabinstein AA. Does therapeutic hypothermia affect time to awakening in cardiac arrest survivors? Neurology. 2011;77:1346–1350. doi: 10.1212/WNL.0b013e318231527d. [DOI] [PubMed] [Google Scholar]
- 37.Al Thenayan E, Savard M, Sharpe M, Norton L, Young B. Predictors of poor neurologic outcome after induced mild hypothermia following cardiac arrest. Neurology. 2008;71:1535–1537. doi: 10.1212/01.wnl.0000334205.81148.31. [DOI] [PubMed] [Google Scholar]
- 38.Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, Jbabdi S, Rogers R, Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boveroux P, Vanhaudenhuyse A, Bruno MA, et al. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- 40.Schaafsma A, de Jong BM, Bams JL, Haaxma-Reiche H, Pruim J, Zijlstra JG. Cerebral perfusion and metabolism in resuscitated patients with severe post-hypoxic encephalopathy. J Neurol Sci. 2003;210:23–30. doi: 10.1016/s0022-510x(03)00063-7. [DOI] [PubMed] [Google Scholar]