Abstract
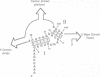
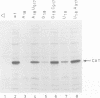
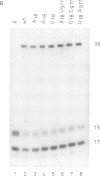
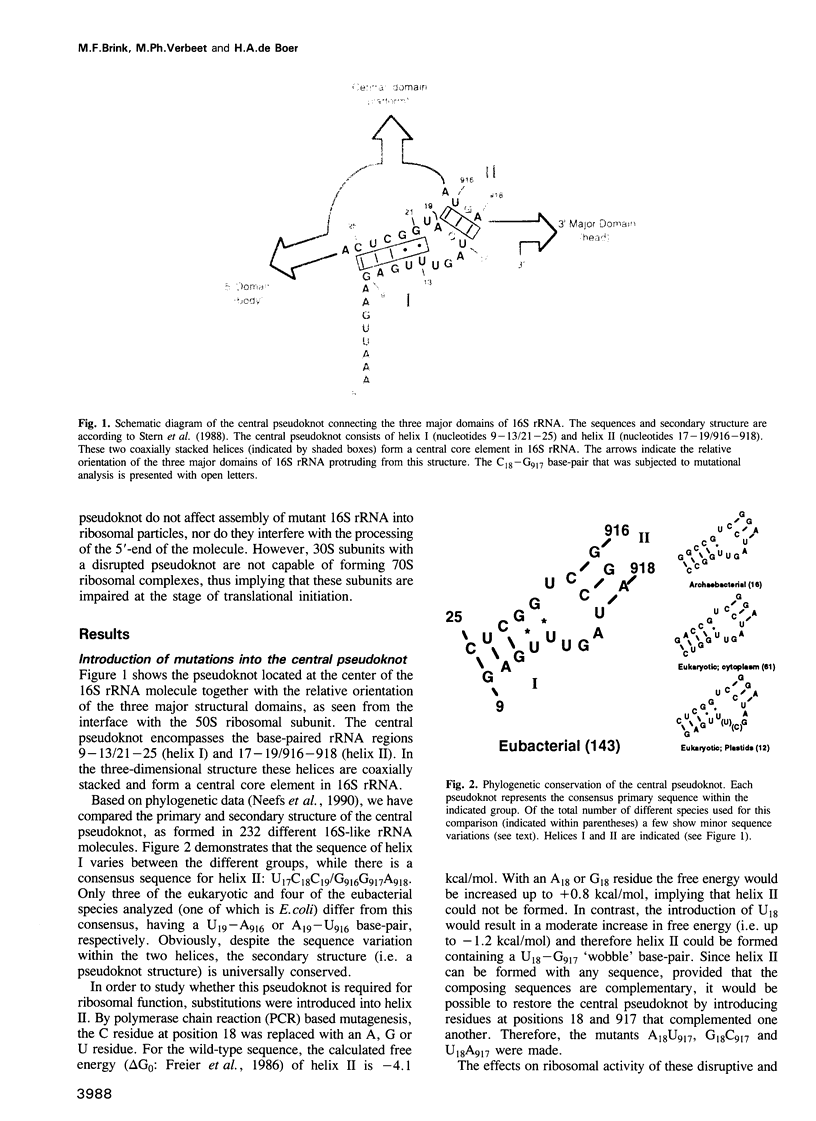
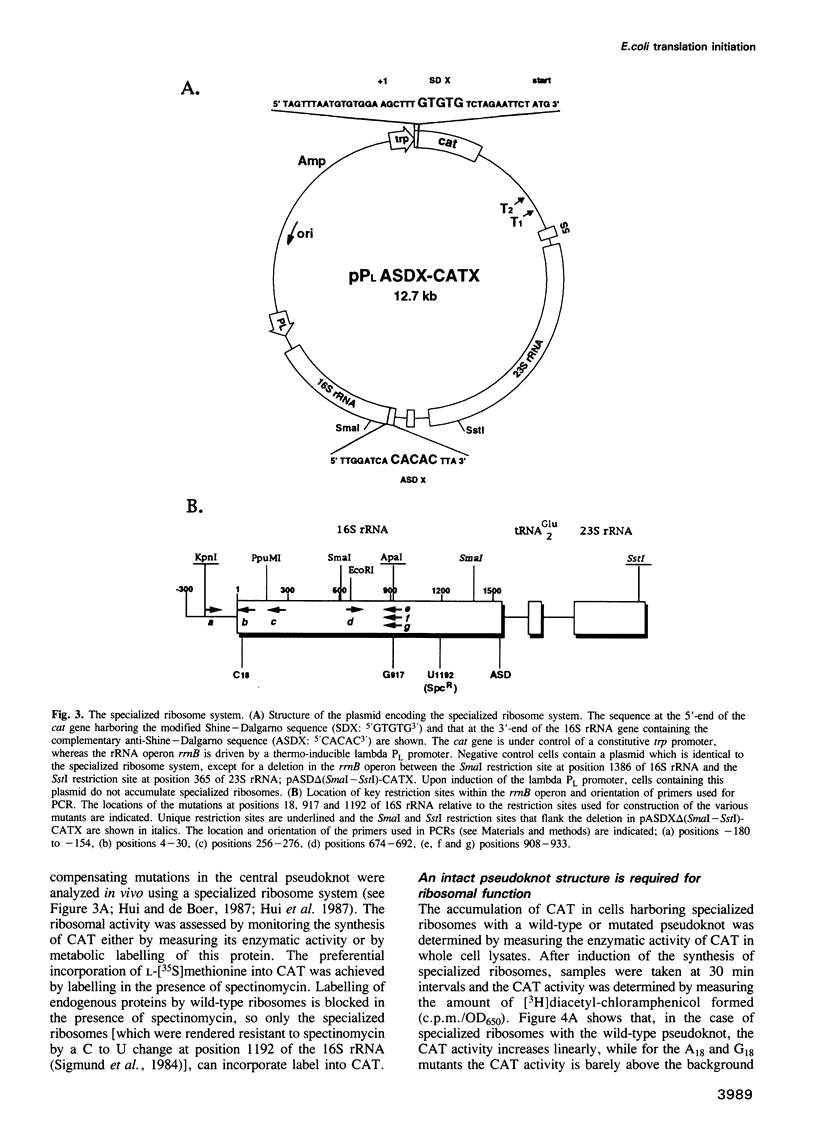
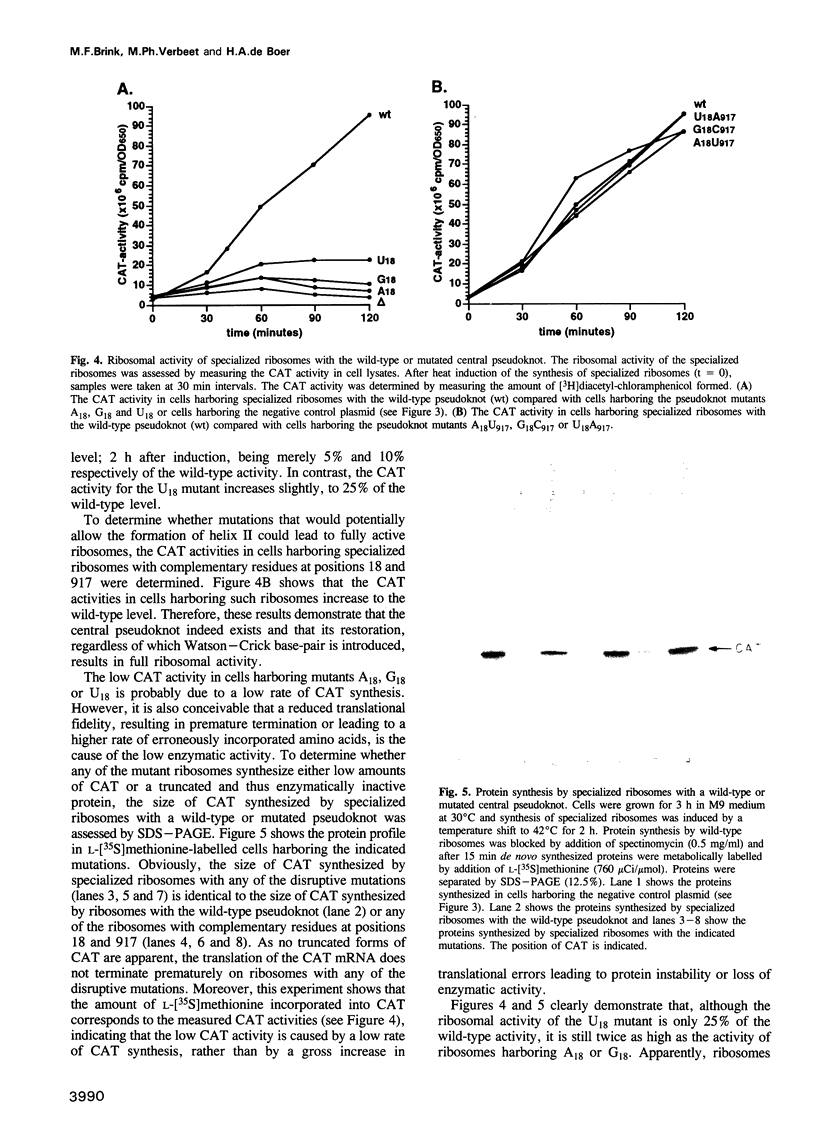
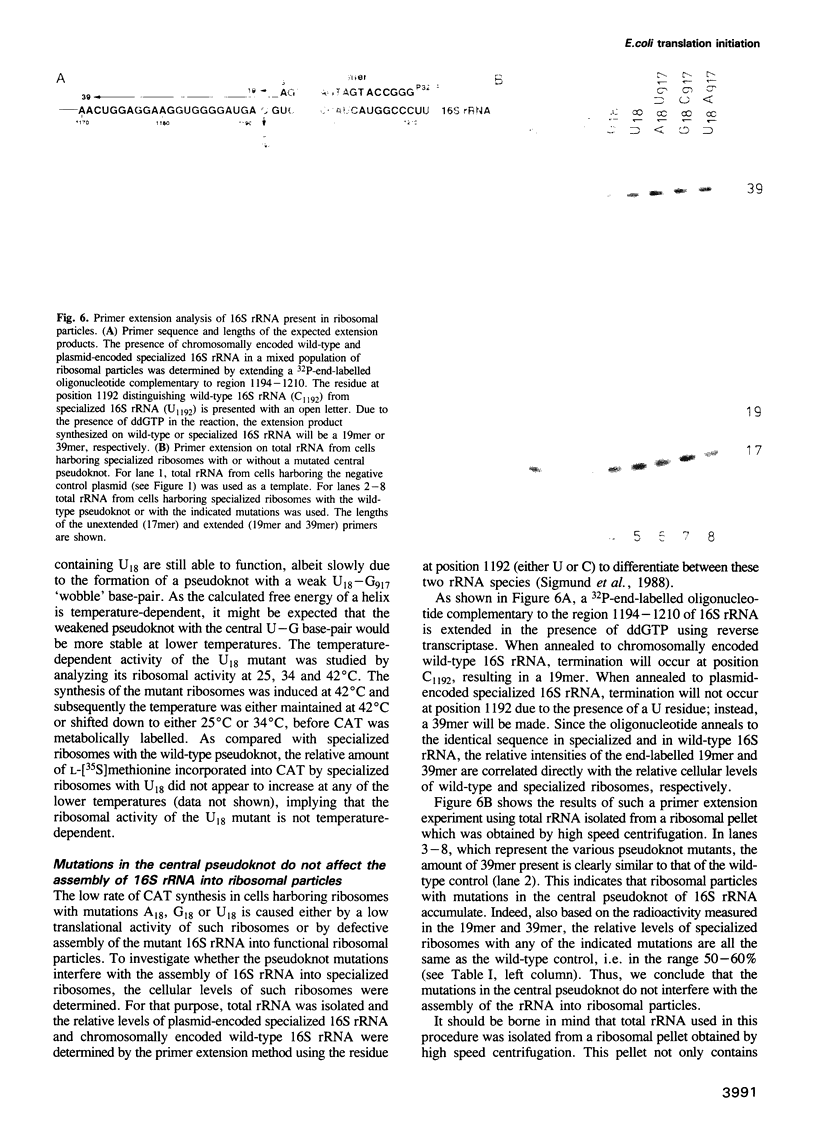
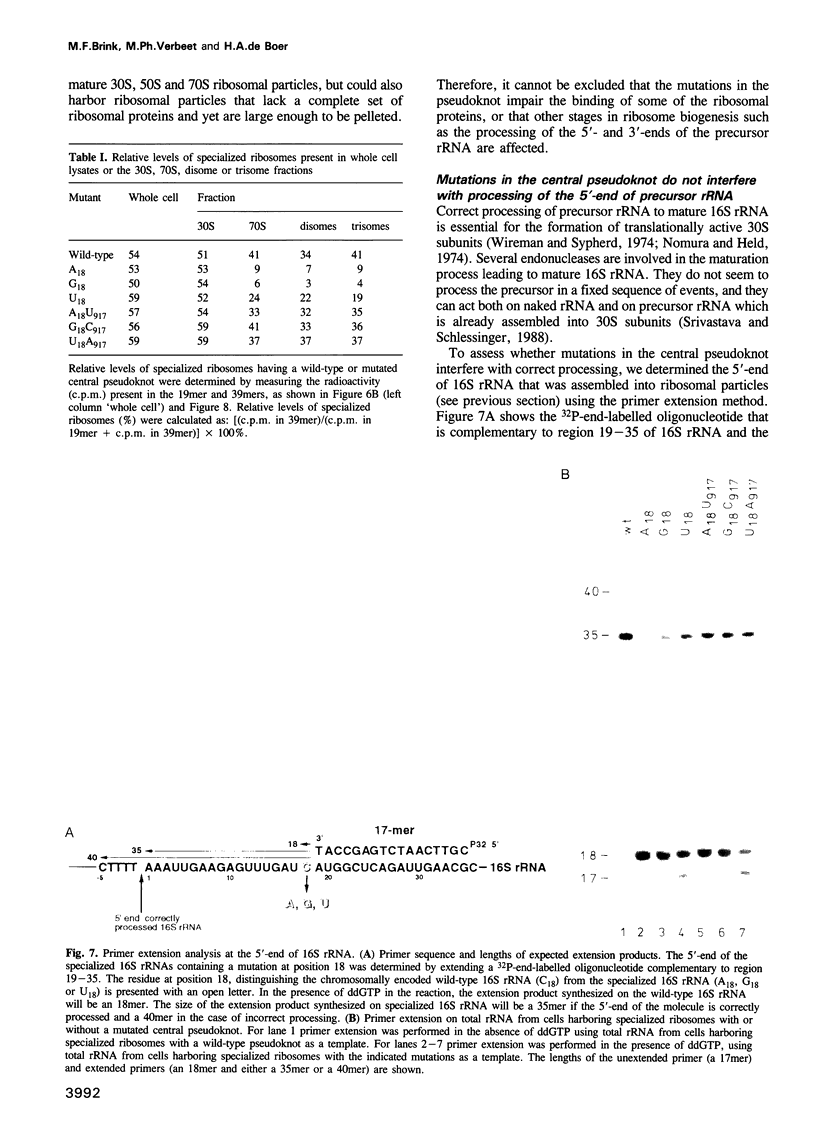
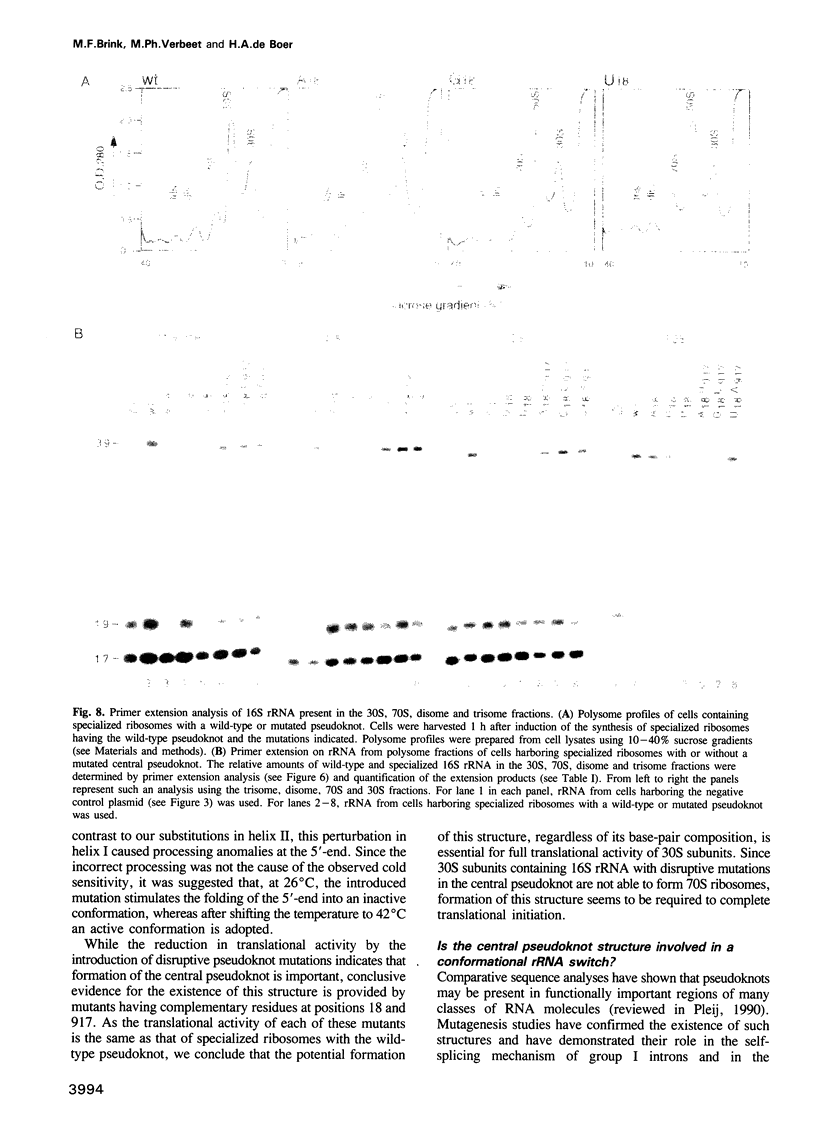
The postulated central pseudoknot formed by regions 9-13/21-25 and 17-19/916-918 of 16S rRNA of Escherichia coli is phylogenetically conserved in prokaryotic as well eukaryotic species. This pseudoknot is located at the center of the secondary structure of the 16S rRNA and connects the three major domains of this molecule. We have introduced mutations into this pseudoknot by changing the base-paired residues C18 and G917, and the effect of such mutations on the ribosomal activity was studied in vivo, using a 'specialized' ribosome system. As compared with ribosomes having the wild-type pseudoknot, the translational activity of ribosomes containing an A, G or U residue at position 18 was dramatically reduced, while the activity of mutant ribosomes having complementary bases at positions 18 and 917 was at the wild-type level. The reduced translational activity of those mutants that are incapable of forming a pseudoknot was caused by their inability to form 70S ribosomal complexes. These results demonstrate that the potential formation of a central pseudoknot in 16S rRNA with any base-paired residues at positions 18 and 917 is essential to complete the initiation process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Dam E., Pleij K., Draper D. Structural and functional aspects of RNA pseudoknots. Biochemistry. 1992 Dec 1;31(47):11665–11676. doi: 10.1021/bi00162a001. [DOI] [PubMed] [Google Scholar]
- Dammel C. S., Noller H. F. A cold-sensitive mutation in 16S rRNA provides evidence for helical switching in ribosome assembly. Genes Dev. 1993 Apr;7(4):660–670. doi: 10.1101/gad.7.4.660. [DOI] [PubMed] [Google Scholar]
- Expert-Bezançon A., Wollenzien P. L. Three-dimensional arrangement of the Escherichia coli 16 S ribosomal RNA. J Mol Biol. 1985 Jul 5;184(1):53–66. doi: 10.1016/0022-2836(85)90043-9. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell R. R., Noller H. F., Woese C. R. Higher order structure in ribosomal RNA. EMBO J. 1986 May;5(5):1111–1113. doi: 10.1002/j.1460-2075.1986.tb04330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan J. J., Noller H. F. Altered topography of 16S RNA in the inactive form of Escherichia coli 30S ribosomal subunits. Biochemistry. 1978 Feb 21;17(4):587–593. doi: 10.1021/bi00597a005. [DOI] [PubMed] [Google Scholar]
- Hubbard J. M., Hearst J. E. Computer modeling 16 S ribosomal RNA. J Mol Biol. 1991 Oct 5;221(3):889–907. doi: 10.1016/0022-2836(91)80182-t. [DOI] [PubMed] [Google Scholar]
- Hui A., Jhurani P., de Boer H. A. Directing ribosomes to a single mRNA species: a method to study ribosomal RNA mutations and their effects on translation of a single messenger in Escherichia coli. Methods Enzymol. 1987;153:432–452. doi: 10.1016/0076-6879(87)53070-1. [DOI] [PubMed] [Google Scholar]
- Hui A., de Boer H. A. Specialized ribosome system: preferential translation of a single mRNA species by a subpopulation of mutated ribosomes in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4762–4766. doi: 10.1073/pnas.84.14.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob W. F., Santer M., Dahlberg A. E. A single base change in the Shine-Dalgarno region of 16S rRNA of Escherichia coli affects translation of many proteins. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4757–4761. doi: 10.1073/pnas.84.14.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kössel H., Hoch B., Zeltz P. Alternative base pairing between 5'- and 3'-terminal sequences of small subunit RNA may provide the basis of a conformational switch of the small ribosomal subunit. Nucleic Acids Res. 1990 Jul 25;18(14):4083–4088. doi: 10.1093/nar/18.14.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc D., Brakier-Gingras L. A conformational switch involving the 915 region of Escherichia coli 16 S ribosomal RNA. FEBS Lett. 1991 Feb 25;279(2):171–174. doi: 10.1016/0014-5793(91)80141-o. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989 Nov 9;342(6246):142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Neefs J. M., Van de Peer Y., Hendriks L., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2237–2317. doi: 10.1093/nar/18.suppl.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen S. K., Green P. P., 3rd, Fowlkes D. M. A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA. 1987 Apr;6(2):173–178. doi: 10.1089/dna.1987.6.173. [DOI] [PubMed] [Google Scholar]
- Pleij C. W. Pseudoknots: a new motif in the RNA game. Trends Biochem Sci. 1990 Apr;15(4):143–147. doi: 10.1016/0968-0004(90)90214-v. [DOI] [PubMed] [Google Scholar]
- Pleij C. W., Rietveld K., Bosch L. A new principle of RNA folding based on pseudoknotting. Nucleic Acids Res. 1985 Mar 11;13(5):1717–1731. doi: 10.1093/nar/13.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T., Noller H. F. A functional pseudoknot in 16S ribosomal RNA. EMBO J. 1991 Aug;10(8):2203–2214. doi: 10.1002/j.1460-2075.1991.tb07756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T., Noller H. F. Dominant lethal mutations in a conserved loop in 16S rRNA. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1042–1046. doi: 10.1073/pnas.87.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi J. D., Wyatt J. R., Tinoco I., Jr A pseudoknotted RNA oligonucleotide. Nature. 1988 Jan 21;331(6153):283–286. doi: 10.1038/331283a0. [DOI] [PubMed] [Google Scholar]
- Schimmel P. RNA pseudoknots that interact with components of the translation apparatus. Cell. 1989 Jul 14;58(1):9–12. doi: 10.1016/0092-8674(89)90395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Borden A., Morgan E. A. Antibiotic resistance mutations in ribosomal RNA genes of Escherichia coli. Methods Enzymol. 1988;164:673–690. doi: 10.1016/s0076-6879(88)64077-8. [DOI] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Morgan E. A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984 Jun 11;12(11):4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. K., Schlessinger D. Coregulation of processing and translation: mature 5' termini of Escherichia coli 23S ribosomal RNA form in polysomes. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7144–7148. doi: 10.1073/pnas.85.19.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Weiser B., Noller H. F. Model for the three-dimensional folding of 16 S ribosomal RNA. J Mol Biol. 1988 Nov 20;204(2):447–481. doi: 10.1016/0022-2836(88)90588-8. [DOI] [PubMed] [Google Scholar]
- Triman K., Becker E., Dammel C., Katz J., Mori H., Douthwaite S., Yapijakis C., Yoast S., Noller H. F. Isolation of temperature-sensitive mutants of 16 S rRNA in Escherichia coli. J Mol Biol. 1989 Oct 20;209(4):645–653. doi: 10.1016/0022-2836(89)92000-7. [DOI] [PubMed] [Google Scholar]
- Wireman J. W., Sypherd P. S. In vitro assembly of 30S ribosomal particles from precursor 16S RNA of Escherichia coli. Nature. 1974 Feb 22;247(5442):552–554. doi: 10.1038/247552a0. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Gutell R. R. Evidence for several higher order structural elements in ribosomal RNA. Proc Natl Acad Sci U S A. 1989 May;86(9):3119–3122. doi: 10.1073/pnas.86.9.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenzien P. L., Cantor C. R. Gel electrophoretic technique for separating crosslinked RNAs. Application to improved electron microscopic analysis of psoralen crosslinked 16 S ribosomal RNA. J Mol Biol. 1982 Jul 25;159(1):151–166. doi: 10.1016/0022-2836(82)90036-5. [DOI] [PubMed] [Google Scholar]