Abstract
Objectives
We aimed to disentangle the effects of obesity and mobility limitation on cervical and breast cancer screening among community dwelling women.
Methods
The data source was the French national Health and Disability Survey - Household Section, 2008. The Body Mass Index (BMI) was used to categorize obesity status. We constructed a continuous score of mobility limitations to assess the severity of disability (Cronbach's alpha = 0.84). Logistic regressions were performed to examine the association between obesity, mobility limitations and the use of Pap test (n = 8 133) and the use of mammography (n = 7 561). Adjusted odds ratios were calculated (AOR). Interaction terms between obesity and the disability score were included in models testing for effect modifications.
Results
Compared with non-obese women, the odds of having a Pap test in the past 3 years was 24% lower in obese women (AOR = 0.76; 95% CI: 0.65 to 0.89), the odds of having a mammogram in the past 2 years was 23% lower (AOR = 0.77; 95% CI: 0.66 to 0.91). Each time the disability score was 5 points higher, the odds of having a Pap test decreases by 20% (AOR = 0.96; 95% CI: 0.94 to 0.98), the odds of having a mammogram decreases by 25% (AOR = 0.95; 95% CI: 0.94 to 0.97). There was no significant interaction between obesity and disability score.
Conclusion
Obesity and mobility limitation are independently associated with a lower likelihood of cervical and breast cancer screening. Protective outreach and follow-up are necessary to reduce inequalities and thus to reduce health disparities in these vulnerable and high-risk populations of obese women with disabilities.
Introduction
Cancer is a leading cause of death worldwide and accounted for 8.2 million deaths in 2012 [1]. Breast and cervical cancers are among the most common cancers in women and are curable if detected early [2]. For this reason, the mammogram and Pap test are widely offered as part of national cancer screening programs in developed countries. Unfortunately, participation in screening is variable [3], [4], even in health systems with adequate resources [5]. Disadvantages related to socio-economic factors and health variables are typically associated with inequalities in access to preventive care [6]–[9].
Emerging evidence links both obesity and disability with a relatively low likelihood of cancer screening [10]–[12]. Fontaine and colleagues were among the first researchers to provide evidence that obese women were less frequently screened for cervical and breast cancer at the recommended intervals [13], [14]. In addition, the disabled population is well known as a particularly vulnerable population that is consistently underserved: they present substantial health disparities and are often under-screened [15]–[17]. Numerous studies have shown that screening rates are strongly influenced by the severity of the disability, with a gradient observed in the use of screening programs, including Pap tests and mammograms [18], [19].
Obesity and disability are often associated with each other [20]. There is a major disparity in weight between women with and without disabilities [15], [21]. Persons who have a preexisting physical impairment have been shown to be predisposed to being obese for a variety of reasons, including a low activity level [22], [23]. Increased weight may be more problematic among people living with disabilities than among the nondisabled population [24]. The combination of mobility disability and weight gain can result in a vicious cycle, posing additional health problems and disability-related limitations, thereby increasing the severity of the disability [24], [25]. Indeed, obesity can lead to mobility disability and personal care disability, most often from arthritic conditions [26], [27]. The relationship between obesity and disability is clearly complex; obesity is causal in some cases, whereas the disability may be the primary disorder in other cases.
Given the increasing prevalence of obesity and disabling conditions [28], [29] as well as their strong association, linking these two health priorities is essential. This study contributes to the literature by exploring the joint and separate effects of obesity and mobility limitation on participation in cervical and breast cancer screening programs among community-dwelling women in France.
Materials and Methods
Data Source
The source of the data used in this study was the Health and Disability Survey – Ordinary Household Section (Enquête Handicap Santé-Ménage, HSM, available at http://www.sante.gouv.fr/handicap-sante.html), which was performed from April to September of 2008 by the French National Institute of Statistics and Economic Studies (INSEE), and the French Head Office of Research, Studies, Evaluation and Statistics of the Social Affairs Ministry.
The HSM is a national cross-sectional survey that aims to measure the prevalence of various forms of disabling situations, applying concepts developed by the World Health Organization, to assess the need for aid and to measure the social disadvantages of disabled people. The new concepts of disability listed in the International Classification of Functioning, Disability and Health [30] (ICF) place the notions of ‘health’ and ‘disability’ in a new light. The ICF identifies three levels of human function: functioning at the levels of body parts (body level), the whole person (individual level) and the whole person in their complete environment (societal level). These levels, in turn, contain three domains of human function: body functions and structures, activities and participation. The term ‘disability’ is used to denote a decrement at each level, i.e., an impairment, functional limitation or restriction in participation. Thus, in the HSM survey, individuals were asked about their impairments (physical, sensory and/or cognitive), their functional limitations (mobility, sensory and/or cognitive) and social participation restrictions (access to the labor market, educational opportunities and leisure as well as aspects of their standards of living, familial or social network, general accessibility and/or experience with discrimination). They were also asked about their diseases, their use of healthcare and the different forms of aid they received or needed. Information about their socio-demographic characteristics was also collected.
The HSM sample was taken from the database of respondents to the filter survey Everyday Life and Health Survey (VQS), which was administered to approximately 140,000 households (260,000 individuals), across French territories (mainland France and overseas territories) in 2007 by mail, by telephone or face-to-face. The VQS is a filter survey intended to prepare the sample for an in-depth survey of people with physical deficiencies, functional limitations or, more generally, difficulties in accomplishing certain activities of daily life. Because people in situations of disability or dependence are relatively rare with respect to the general population, they must be over-represented in the sample of a survey that targets their characteristics and situation to provide sufficiently robust results. The VQS achieved this outcome by sending a short questionnaire to a large number of households that contains several questions requesting a description of each member of the household. These questions concern the existence or absence of a recognized disability, difficulties in accomplishing certain tasks and other related questions. According to their answers, people were classified into four levels of presumed disability severity, from 1 (no disability) to 4 (high level of disability). These groups are then used to build the selection strata for the main survey. For sample selection, randomisation involved a high sampling rate for the most severely disabled group and a low sampling rate for people without daily living restrictions (the largest group). Each of the resulting groups was allocated a specific sampling coefficient that increased with the probability or severity of the presumed handicap. Thus, of the 260,000 individuals from the VQS filter, 39,065 were selected for the HSM survey, and 29,954 answered the questionnaire, corresponding to a response rate of 77%. In total, the HSM database contains 29,931 questionnaires considered to be ‘complete.’ Each respondent was assigned a weight reflecting the probability of being investigated (depending on presumed disability severity and geographic area of residence) and answering the questionnaire. Final weights ensured that the data were representative of the French population living in households. The design of the survey is summarized in a flow chart published by Clémence Palazzo et al. (2012) [31].
All information was directly gathered by trained investigators using the computer-assisted interview (CAPI) format to collect data from people in their homes. When individuals were unable to respond to the questionnaire by themselves, a proxy was asked to provide help; thus, all responses were self-reported or, in some cases, proxy-reported.
Ethics
This study was planned as a research project. All precautions were taken by the INSEE to ensure anonymity of the data. This study was declared of public interest by the CNIS (Conseil National d'Information Statistique) and was approved by the CNIL (Commission Nationale de l'Informatique et des Libertés, French law no. 78-17). According to the French law, written informed consent was not required for this type of study.
Study Participants
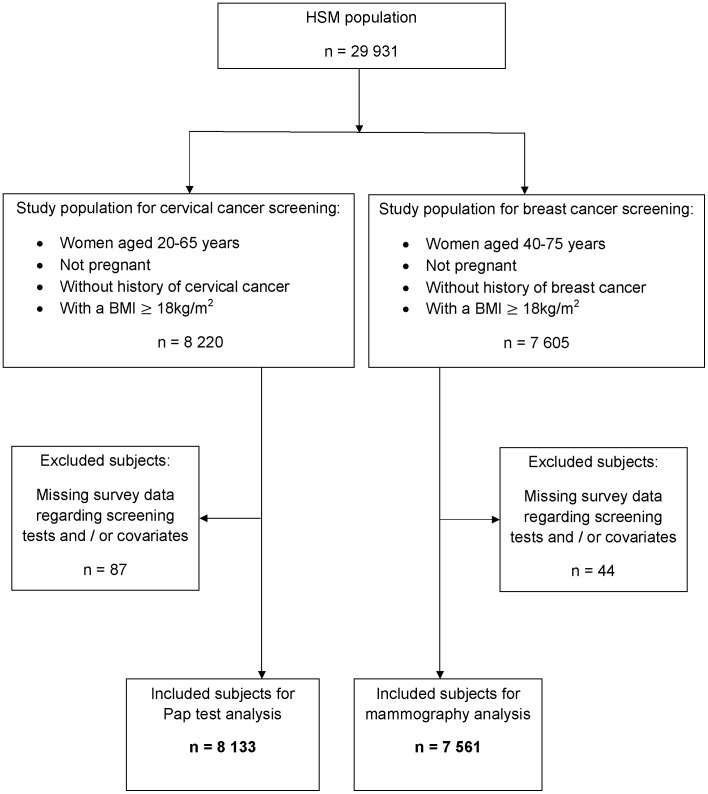
From the HSM population, we included all women aged 20–65 years for cervical cancer screening and those aged 40–75 years for breast cancer screening, as it is recommended by the European Union Council [32] and the U.S Preventive Services Task Force [33]. None of the participants were currently pregnant, and none had a history of cervical or breast cancer. Subjects with missing survey data regarding screening tests or covariates were excluded from the analysis. We also excluded underweight women (BMI<18 kg/m2). Ultimately, two groups were established: the group for cervical cancer screening analysis (n = 8,133) and the group for breast cancer screening analysis (n = 7,561) (Figure 1).
Figure 1. Flow chart illustrating the selection of study populations for cervical and breast cancer screening from the Health and Disability Survey - Household Section (HSM).
Statistical Analysis
Descriptive analyses of the two groups were performed in terms of obesity, mobility limitation level, socio-demographic status, health and health care use variables. Chi-square tests were used to compare all characteristics between those who did and did not receive the screening test in each study group, with the exception of the mobility limitation level, for which t-tests were used. Finally, t-tests were performed to determine whether the mean of the mobility limitation level differed significantly by obesity status.
Odds ratios (ORs), adjusted odds ratios (AORs) and 95% confidence intervals (95% CIs) were estimated from weighted logistic regression models to analyze the association between body weight status, severity of disability and cancer screening use. First, univariate models were used to examine obesity and severity of disability separately in association with each outcome. Second, both obesity and severity of disability were included as the main effects in the models. Next, the models were adjusted for the socio-demographic, health and health care use variables. Lastly, interaction terms between obesity and the severity of disability were included in the models. Because obesity and disability are commonly linked, testing for the interaction enabled us to examine whether the effect of obesity differed based on disability level and vice versa. All analyses were conducted in 2013 using SAS 9.3 software.
Outcome of interest
The outcomes of interest were the following: whether a Pap test was performed within the 3 years prior to the interview and whether a mammogram was performed within the 2 years prior to the interview.
Explanatory variables
Obesity
Body mass index was calculated in kg/m2 from the self-reported body weight and height of each individual and dichotomized as obese (BMI≥30 kg/m2) or not obese (BMI<30 kg/m2).
Mobility limitation level
We constructed a continuous severity score, ranging from 0 to 27, by summing each respondent's self-reported level of difficulty (no difficulty = 0; some difficulty = 1; much difficulty = 2; unable to perform = 3) in performing each of the following nine tasks without any aid: (1) controlling stool and urine; (2) biting and chewing hard foods, such as an apple; (3) stooping, crouching and/or kneeling; (4) raising arms (for example, moving an object from an elevated place); (5) walking 500 meters on flat ground; (6) walking up and down a flight of stairs; (7) grasping or holding an object in their hands; (8) using hands and fingers with a normal level of dexterity (for example, turning on a faucet or using a pencil); and (9) lifting or carrying a bag as heavy as 5 kilograms for a distance of 10 meters. The choice of the items assessing the mobility limitations was based on the Nagi Disability Scale [34], and also inspired by previous studies [23], [25], [35], [36]. We verified the homogeneity of the items using Cronbach's alpha, and we obtained a value of 0.84, which corresponds to excellent internal consistency. The continuous severity score is hereafter termed the disability score. The Pearson's r correlation between this disability score and the screening use percentages were -0.76 for Pap test use and −0.87 for mammogram use (p<0.0001). This result indicates strong negative linear relationships between the disability score and the two cancer screening rates. We therefore chose to retain the score as a continuous variable in our models.
Socio-demographic, health and health care use variables
The socio-demographic variables included were age (categorized in 10-year age groups); marital status (single/married/divorced/widowed); level of education (non-high school graduate/high school graduate); and employment status (employed/retired/unemployed). The latter two variables were used as proxies for socio-economic status. Health and health care use variables included having at least one chronic disease (i.e., when the course of the disease lasted for more than 6 months) (yes/no) and visit(s) to a general practitioner in the last year (yes/no). These variables were primarily selected on the basis of earlier studies [9], [37].
Results
Characteristics of the study participants
Table 1 summarizes the characteristics of the two groups. The chi-square analysis showed that all variables were significantly associated with screening use (Table 2). Women with a lower level of education and those who were unmarried or unemployed were less likely to receive a Pap test in the past 3 years or a mammogram in the past 2 years. Women who have visited their general practitioner were more likely to receive these screening tests. The presence of a chronic disease was associated with less Pap tests but more mammograms. We also observed a significant peak in Pap test use in women between 30 and 40 years of age and a peak in mammogram use in women between 50 and 65 years of age. BMI was significantly associated with screening use: obese women reported lower rates of screening use compared with women with a BMI under 30 kg/m2. Concerning the disability score, the mean score for those who received screening tests was significantly lower than the mean score for those who did not receive screening for both cervical and breast cancer. Comparisons of disability score means by obesity status are given in table 3; these results show that the mean disability score was significantly higher in obese women than in non-obese women in the two groups studied.
Table 1. Characteristics of study participants.
| Group for cervical cancer screening analysisa | Group for breast cancer screening analysisb | ||||
| Characteristics | n | % | n | % | |
| 8,133 | 100.0 | 7,561 | 100.0 | ||
| Age | |||||
| 20–30 | 890 | 10.9 | _ | _ | |
| 30–40 | 1,341 | 16.5 | _ | _ | |
| 40–50 | 2,048 | 25.2 | 2,029 | 26.8 | |
| 50–65 | 3,854 | 47.4 | 3,732 | 49.4 | |
| 65–75 | _ | _ | 1,800 | 23.8 | |
| Educational level | |||||
| Non-high school graduate | 5,679 | 69.8 | 6,024 | 79.7 | |
| High school graduate | 2,454 | 30.2 | 1,537 | 20.3 | |
| Marital status | |||||
| Married | 4,390 | 54.0 | 4,450 | 58.9 | |
| Single | 2,354 | 28.9 | 1,171 | 15.5 | |
| Divorced | 972 | 12.0 | 1,005 | 13.3 | |
| Widowed | 417 | 5.1 | 935 | 12.4 | |
| Employment status | |||||
| Employed | 3,910 | 48.1 | 2,588 | 34.2 | |
| Retired | 984 | 12.1 | 2,530 | 33.5 | |
| Unemployed | 3,239 | 39.8 | 2,443 | 32.3 | |
| Chronic disease(s) | |||||
| Yes | 5,034 | 61.9 | 5,446 | 72.0 | |
| No | 3,099 | 38.1 | 2,115 | 28.0 | |
| Visit(s) to the GP in the last year | |||||
| Yes | 7,486 | 92.0 | 7,063 | 93.4 | |
| No | 647 | 8.0 | 498 | 6.6 | |
| Obesity | |||||
| Yes (BMI≥30) | 1,707 | 21.0 | 1,854 | 24.5 | |
| No (BMI<30) | 6,426 | 79.0 | 5,707 | 75.5 | |
| Disability score | |||||
| Mean ± S.D | 2,76±4,43 | 4,14±5,28 | |||
| = 0 | 4,137 | 50.9 | 2,727 | 36.1 | |
| ≥1 | 3,996 | 49.1 | 4,834 | 63.9 | |
Notes. GP = General Practitioner; BMI = Body Mass Index.
Women aged 20–65 years, not pregnant and without history of cervical cancer.
Women aged 40–75 years, not pregnant and without history of breast cancer.
Table 2. Comparison of characteristics of study participants between those who did and did not receive the screening testa.
| Individuals who received a Pap test within 3-yearsb | Individuals who received a mammogram within 2-yearsc | ||||
| Characteristics | n | (row) % | n | (row) % | |
| 5,795 | 71.3 | 5,140 | 68.0 | ||
| Age | |||||
| 20–30 | 600 | 67.4 | _ | _ | |
| 30–40 | 1,075 | 80.2 | _ | _ | |
| 40–50 | 1,582 | 77.3 | 1,073 | 52.9 | |
| 50–65 | 2,538 | 65.9 | 2,866 | 76.8 | |
| 65–75 | _ | _ | 1,201 | 66.7 | |
| p<0.0001 | p<0.0001 | ||||
| Educational level | |||||
| Non-high school graduate | 3,791 | 66.8 | 3,997 | 66.4 | |
| High school graduate | 2,004 | 81.7 | 1,143 | 74.4 | |
| p<0.0001 | p<0.0001 | ||||
| Marital status | |||||
| Married | 3,299 | 75.2 | 3,216 | 72.3 | |
| Single | 1,560 | 66.3 | 661 | 56.5 | |
| Divorced | 693 | 71.3 | 694 | 69.1 | |
| Widowed | 243 | 58.3 | 569 | 60.9 | |
| p<0.0001 | p<0.0001 | ||||
| Employment status | |||||
| Employed | 3,168 | 81.0 | 1,809 | 69.9 | |
| Retired | 607 | 61.7 | 1,810 | 71.5 | |
| Unemployed | 2,020 | 62.4 | 1,521 | 62.3 | |
| p<0.0001 | p<0.0001 | ||||
| Chronic disease(s) | |||||
| Yes | 3,436 | 68.3 | 3,737 | 68.6 | |
| No | 2,359 | 76.1 | 1,403 | 66.3 | |
| p<0.0001 | p = 0.0461 | ||||
| Visit(s) to the GP in the last year | |||||
| Yes | 5,389 | 72.0 | 4,880 | 69.1 | |
| No | 406 | 62.8 | 260 | 52.2 | |
| p<0.0001 | p<0.0001 | ||||
| Obesity | |||||
| Yes (BMI≥30) | 1,041 | 61.0 | 1,183 | 63.3 | |
| No (BMI<30) | 4,754 | 74.0 | 3,957 | 69.8 | |
| p<0.0001 | p<0.0001 | ||||
| Disability score | |||||
| Mean ± S.D | 2,29±3,98 | 3,81±4,94 | |||
| p<0.0001 d | p<0.0001 d | ||||
| = 0 | 3,211 | 77.6 | 1,908 | 70.0 | |
| ≥1 | 2,584 | 64.7 | 3,232 | 66.3 | |
| p<0.0001 | p = 0.0054 | ||||
Notes. GP = General Practitioner; BMI = Body Mass Index.
Bivariate analyses were performed by chi-square testing comparing characteristics between those who did and did not receive the screening test.
Group for cervical cancer screening analysis: women aged 20–65 years, not pregnant and without history of cervical cancer.
Group for breast cancer screening analysis: women aged 40–75 years, not pregnant and without history of breast cancer.
A t-test was performed to determine whether the mean score for those who received the screening test differed significantly from the mean score for those who didn't receive it, in each study group.
Table 3. Comparison of disability score means by obesity statusa.
| Not obese | Obese | |
| (BMI<30) | (BMI≥30) | |
| Group for cervical cancer screening analysis | 2,24±4,01 | 4,82±5,32 |
| Disability score | ||
| Mean ± S.D | ||
| Group for breast cancer screening analysis | 3,47±4,91 | 6,19±5,75 |
| Disability score | ||
| Mean ± S.D |
Notes. BMI = Body Mass Index.
T-tests were performed to determine whether or the mean score differed significantly by BMI categories, in each study group. All tests were significant - P<.0001.
Logistic Regression Models
Univariate and bivariate models
Table 4 summarizes the results of the logistic regressions for the univariate and bivariate models. The results were very similar for the two models. A significant gradient in screening use with respect to obesity and disability score was observed. Relative to the non-obese women, obese women were less likely to report a Pap test in the past 3 years or a mammogram in the past 2 years. Additionally, as the disability score increased, the likelihood of reporting a Pap test or a mammogram decreased.
Table 4. Weighted logistic regressionsa on the use of Pap tests and mammograms: Univariate and Bivariate models.
| Pap test within 3 yearsb | Mammogram within 2 yearsc | |||
| n = 8 133 | n = 7 561 | |||
| Variables | OR | 95% CI | OR | 95% CI |
| Univariate Model | ||||
| Obesity | ||||
| Yes (BMI≥30) | 0,59*** | 0,51–0,59 | 0,75*** | 0,65–0,87 |
| No (BMI<30) | Referent | Referent | ||
| Disability score | 0,91*** | 0,89–0,93 | 0,97** | 0,95–0,98 |
| Bivariate Model | ||||
| Obesity | ||||
| Yes (BMI≥30) | Referent | Referent | ||
| No (BMI<30) | 0,68*** | 0,58–0,78 | 0,79** | 0,68–0,91 |
| Disability score | 0,92*** | 0,90–0,94 | 0,97** | 0,96–0,99 |
Notes. OR = Odds Ratios; CI = Confidence Intervals; BMI = Body Mass Index.
Analysis were performed on probability-weighted sample data with SAS 9.3 software.
Women aged 20–65 years, not pregnant and without history of cervical cancer.
Women aged 40–75 years, not pregnant and without history of breast cancer.
*P<.1;
**P<.05;
***P<.0001.
Multivariate model
In the model that was adjusted for covariates, we found significant negative associations between obesity and Pap testing (AOR = 0.76; 95% CI: 0.65 to 0.89), between obesity and mammograms (AOR = 0.77; 95% CI: 0.66 to 0.91), between disability score and Pap testing (AOR = 0.96; 95% CI: 0.94 to 0.98) and between disability score and mammograms (AOR = 0.95; 95% CI: 0.94 to 0.97). To more meaningfully interpret the latter two AORs, each time the disability score increased by 5 points, the odds of having a Pap test in the past 3 years decreased by 20% ((1–0.96)×100×5) and the odds of having a mammogram in the past 2 years decreased by 25% ((1–0.95)×100×5) (Table 5).
Table 5. Weighted logistic regressionsa on the use of Pap tests and mammograms: Multivariable model.
| Pap test within 3 yearsb | Mammogram within 2 yearsc | |||
| n = 8 133 | n = 7 561 | |||
| Variables | AOR | 95% CI | AOR | 95% CI |
| Age | ||||
| 20–30 | 0.77** | 0.64–0.94 | _ | _ |
| 30–40 | 1.47*** | 1.22–1.78 | _ | _ |
| 40–50 | Referent | Referent | ||
| 50–65 | 0.70*** | 0.60–0.82 | 3.78*** | 3.25–4.40 |
| 65–75 | _ | _ | 2.78*** | 2.20–3.51 |
| Educational level | ||||
| Non-high school graduate | 0.62*** | 0.55–0.70 | 0.66*** | 0.57–0.75 |
| High school graduate | Referent | Referent | ||
| Marital status | ||||
| Married | Referent | Referent | ||
| Single | 0.67*** | 0.58–0.78 | 0.51*** | 0.43–0.60 |
| Divorced | 0.78** | 0.65–0.95 | 0.84* | 0.70–1.01 |
| Widowed | 0.79* | 0.60–1.02 | 0.55*** | 0.46–0.67 |
| Employment status | ||||
| Employed | Referent | Referent | ||
| Retired | 0.52*** | 0.43–0.64 | 0.99 | 0.78–1.26 |
| Unemployed | 0.46*** | 0.41–0.52 | 0.70*** | 0.59–0.82 |
| Chronic disease(s) | ||||
| Yes | 1.07 | 0.94–1.21 | 1.24** | 1.09–1.41 |
| No | Referent | Referent | ||
| Visit(s) to the GP in the last year | ||||
| Yes | Referent | Referent | ||
| No | 0.50*** | 0.43–0.59 | 0.44*** | 0.36–0.53 |
| Obesity | ||||
| Yes (BMI≥30) | 0.76** | 0.65–0.89 | 0.77** | 0.66–0.91 |
| No (BMI<30) | Referent | Referent | ||
| Disability score | 0.96** | 0.94–0.98 | 0.95*** | 0.94–0.97 |
Notes. AOR = Adjusted Odds Ratios; CI = Confidence Intervals; GP = General Practitioner; BMI = Body Mass Index.
Analysis were performed on probability-weighted sample data adjusted for potential confounders with SAS 9.3 software.
Women aged 20–65 years, not pregnant and without history of cervical cancer.
Women aged 40–75 years, not pregnant and without history of breast cancer.
*P<.1;
**P<.05;
***P<.0001.
Models with interactions
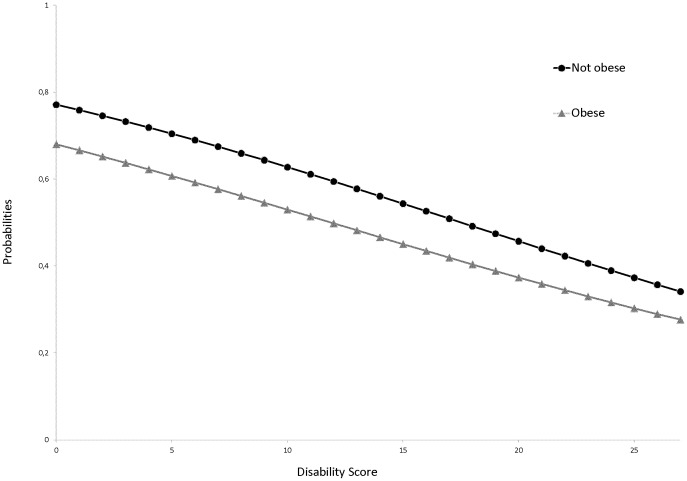
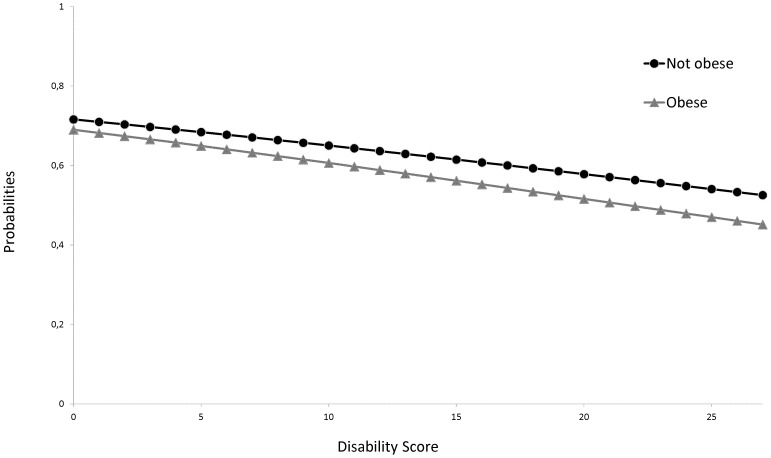
We did not find any significant interaction between obesity and disability score. The slopes of the logistic curves did not differ based on obesity status. The probability of having received a Pap test or a mammogram decreased similarly with the disability score in obese and non-obese women (Figures 2 and 3).
Figure 2. Predicted probabilities of having received a Pap test within the past 3 years: model with an interaction term between obesity and disability score.
Figure 3. Predicted probabilities of having received a mammogram within the past 2 years: model with an interaction term between obesity and disability score.
Discussion
To the best of our knowledge, this study is the first to investigate the relative contributions of obesity and mobility disability to both cervical and breast cancer screening. Using a large population-based sample from the French national Health and Disability Survey, our analysis showed that obesity and mobility limitation are independently associated with a lower likelihood of cervical and breast cancer screening. The higher the BMI or disability score is, the lower the likelihood is of Pap test and mammogram use. There were no significant interaction effects.
Our results also confirm several significant associations usually found in the literature [6], [8], [9] between socio-demographic, health and health care use-related characteristics and cancer screening use. Indeed, according to regression models, having a higher educational level, being married, being employed, having at least one chronic disease and having visited a GP in the past year were associated with a higher likelihood of screening.
To reduce cancer-related mortality, the political focal point of recent decades has been to improve access to routine screening. To shift the focus in this manner and to plan appropriate public health programs, it is important to clearly identify existing inequalities and the reasons for these inequalities.
Our results first highlight the differences in access to routine screening. Compared with 74% of non-obese women, only 61% of obese women reported having a Pap test within the past 3 years. Among women with a disability score of 0, 78% received a Pap test within the past 3 years, whereas only 65% of those with a disability score greater than or equal to 1 received a Pap test. The differences were not as pronounced for breast cancer screening but remained significant. These results are highly important from a public health perspective, mostly as we know now that cancer incidence and mortality can be significantly reduced if cases are detected and treated early [2]. For example, it has been shown that mammography can reduce breast cancer mortality by 20 to 30% when the screening coverage is over 70% [38]. A Pap testing coverage of 80% would decrease the cervical cancer incidence by 2.5% per year [39].
Second, in addition to strengthening the already well-known links between the level of limitation, obesity and the lower rate of screening, these findings also help us to better understand the complex relationships among these factors. According to our results, obesity and disability appear to have similar but independent effects on the rate of screening test participation. It is already well known that functional limitations as such constitute a barrier to cancer screening use [11], [16]. People with mobility limitations encounter logistic and/or architectural obstacles such as access to buildings, machines and examination tables [11], [40]. A telephone survey [41] recently conducted among 256 physicians in the US confirmed a lack of accessibility to care—especially gynecological care—among people with a mobility impairment. In the current study, we also showed that obesity as such constitutes a barrier to cancer screening use that is independent of the functional limitations commonly found in obese people, which are usually mentioned in the literature to explain the well-known association between obesity and decreased rate of cancer screening [42], [43]. The literature provides some interpretations of these results. According to Fontaine and colleagues [13], [14], apart from the equipment- or facility-related barriers due to mobility limitations, attitudinal obstacles are also given as reasons for the lower frequency of preventive care among people with obesity. These obstacles include competing demands for the management of other obesity-related chronic conditions; the fear of discomfort and pain from procedures; anxiety regarding physical privacy, ridicule or reproach due to excess weight; and a low perceived risk. Findings from these early studies have been confirmed by a number of subsequent studies [44], [45]. For example, vaginal speculum examinations and mammograms have been reported to potentially be more difficult, painful or time-consuming for obese women, which may lead to deferral of the exam. Another recent study [46] based on qualitative data reported that disrespectful treatment, embarrassment and negative attitudes of providers as well as insensitive comments about weight are all barriers to routine gynecologic cancer screening.
Finally, we showed that there was no interaction effect between obesity and disability score. These findings suggest that, although essential, addressing limitation-related barriers by modifying environmental factors is not sufficient to achieve a high level of participation in screening programs among individuals in these vulnerable populations. Governments from developed countries have enacted laws and established norms that require adaptation of infrastructure and medical equipment to allow access for people with functional limitations [47]. There are also international standards that address the accessibility of buildings, such as ISO/TR 9527:1994, Building construction — Needs of disabled people in buildings — Design guidelines. At a national level, many European Union member states have regulations and standards concerning buildings and transport. They have accessibility provisions in their legislation (generally anti-discrimination legislation), such as the Disability Discrimination Act (DDA) in the UK, the Liondau law in Spain (law 51/2003 on equal opportunities, non-discrimination and universal accessibility for people with disabilities) and the law of 11 February 2005 in France (law on equal rights and opportunities, participation and citizenship of people with disabilities).
However, current health policies do not view obesity alone as a barrier to screening participation. Our finding reveals the importance of raising awareness among medical personnel who provide screening tests and the importance of fostering self-confidence among obese individuals. Doctors or other health care professionals may need to explicitly talk to their obese patients about the importance of following screening recommendations, just as they do with their non-obese patients, if not to a greater extent. This is especially important because obese women are at increased risk of developing these cancers [48].
This study has limitations that are common to this type of survey. The main limitation is that data were self-reported and not physician-confirmed, which is likely to be accurate for disability assessment but may lack accuracy for screening. Self-reported screening behaviors from national surveys often overestimate screening use [49]. If this was the case, it would strengthen our results. Second, height and weight, which are the metrics used to calculate BMI, were not clinically evaluated. Previous studies have shown that weight is often under-reported, especially in overweight female populations, whereas height is generally over-reported, leading to an underestimation of BMI and a misclassification in BMI categories [50]. This phenomenon is partially explained by social desirability, which can be further influenced by the method of data collection. Indeed, a Canadian study [51] showed that obesity prevalence in a group that was surveyed face-to-face was significantly higher than that in a group that was surveyed by phone. We can assume that this weight and height reporting bias was attenuated in our present study. Furthermore, an underestimation of BMI could also work in favor of the results of the current study in the sense that it most likely led to an underestimation of the highlighted association between obesity and cancer screening. Another limitation of the present study concerns individuals who were excluded because they did not answer the screening questions or other questions related to the independent variables. This exclusion could constitute a selection bias; however, it affected a very small number of individuals compared with the overall sample.
Conclusions
Our findings are especially intriguing because they highlight a lack of preventive care among women included in a healthcare system in which women are widely encouraged to be screened through national screening programs, recommendations, reminders, ease of payment and reimbursements. Policies should provide comprehensive strategies with a focus on improving the attitudes of providers, support for women and encouragement to follow cancer screening interval recommendations, particularly in these vulnerable and high-risk populations of obese women with disabilities. Protective outreach and follow-up are necessary to reduce inequalities and thus to reduce health disparities.
Acknowledgments
We ensure that each author has provided 1) substantial contributions to conception and design, or analysis and interpretation of data 2) draft or revision of the article and 3) final approval of the version to be published. Moreover, each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. The source of the data used in this study was the Health and Disability Survey – Ordinary Household Section, available at http://www.drees.sante.gouv.fr/les-enquetes-handicap-sante,4267.html
Funding Statement
These authors have no support or funding to report.
References
- 1.Fact Sheets by Cancer (n.d.). GLOBOCAN 2012 IARC Sect Cancer Inf. Available: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 2014 Mar 3.
- 2.WHO (2007) Early detection of cancer. WHO. Available: http://www.who.int/cancer/detection/en/. Accessed 2013 Apr 26.
- 3. Bigby J, Holmes MD (2005) Disparities across the breast cancer continuum. Cancer Causes Control CCC 16: 35–44 10.1007/s10552-004-1263-1 [DOI] [PubMed] [Google Scholar]
- 4. Newmann SJ, Garner EO (2005) Social inequities along the cervical cancer continuum: a structured review. Cancer Causes Control CCC 16: 63–70 10.1007/s10552-004-1290-y [DOI] [PubMed] [Google Scholar]
- 5. Blackwell DL, Martinez ME, Gentleman JF (2008) Women's compliance with public health guidelines for mammograms and pap tests in Canada and the United States: an analysis of data from the Joint Canada/United States Survey Of Health. Womens Health Issues Off Publ Jacobs Inst Womens Health 18: 85–99 10.1016/j.whi.2007.10.006 [DOI] [PubMed] [Google Scholar]
- 6. Lin S-J (2008) Factors influencing the uptake of screening services for breast and cervical cancer in Taiwan. J R Soc Promot Health 128: 327–334. [DOI] [PubMed] [Google Scholar]
- 7. Hsia J, Kemper E, Kiefe C, Zapka J, Sofaer S, et al. (2000) The Importance of Health Insurance as a Determinant of Cancer Screening: Evidence from the Women's Health Initiative. Prev Med 31: 261–270 10.1006/pmed.2000.0697 [DOI] [PubMed] [Google Scholar]
- 8. Selvin E, Brett KM (2003) Breast and Cervical Cancer Screening: Sociodemographic Predictors Among White, Black, and Hispanic Women. Am J Public Health 93: 618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hewitt M, Devesa SS, Breen N (2004) Cervical cancer screening among U.S. women: analyses of the 2000 National Health Interview Survey. Prev Med 39: 270–278 10.1016/j.ypmed.2004.03.035 [DOI] [PubMed] [Google Scholar]
- 10. Cohen SS, Palmieri RT, Nyante SJ, Koralek DO, Kim S, et al. (2008) Obesity and screening for breast, cervical, and colorectal cancer in women: a review. Cancer 112: 1892–1904 10.1002/cncr.23408 [DOI] [PubMed] [Google Scholar]
- 11. Angus J, Seto L, Barry N, Cechetto N, Chandani S, et al. (2012) Access to cancer screening for women with mobility disabilities. J Cancer Educ Off J Am Assoc Cancer Educ 27: 75–82 10.1007/s13187-011-0273-4 [DOI] [PubMed] [Google Scholar]
- 12. Aldrich T, Hackley B (2010) The impact of obesity on gynecologic cancer screening: an integrative literature review. J Midwifery Womens Health 55: 344–356 10.1016/j.jmwh.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 13. Fontaine KR, Faith MS, Allison DB, Cheskin LJ (1998) Body weight and health care among women in the general population. Arch Fam Med 7: 381–384. [DOI] [PubMed] [Google Scholar]
- 14. Fontaine KR, Heo M, Allison DB (2001) Body weight and cancer screening among women. J Womens Health Gend Based Med 10: 463–470 10.1089/152460901300233939 [DOI] [PubMed] [Google Scholar]
- 15. Wisdom JP, McGee MG, Horner-Johnson W, Michael YL, Adams E, et al. (2010) Health disparities between women with and without disabilities: a review of the research. Soc Work Public Health 25: 368–386 10.1080/19371910903240969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iezzoni LI, McCarthy EP, Davis RB, Siebens H (2000) Mobility impairments and use of screening and preventive services. Am J Public Health 90: 955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramirez A (2005) Disability and Preventive Cancer Screening: Results from the 2001 California Health Interview Survey. Am J Public Health 95: 2057–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diab ME, Johnston MV (2004) Relationships between level of disability and receipt of preventive health services1. Arch Phys Med Rehabil 85: 749–757 10.1016/j.apmr.2003.06.028 [DOI] [PubMed] [Google Scholar]
- 19. Carney CP, Jones LE (2006) The Influence of Type and Severity of Mental Illness on Receipt of Screening Mammography. J Gen Intern Med 21: 1097–1104 10.1111/j.1525-1497.2006.00565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ells LJ, Lang R, Shield JPH, Wilkinson JR, Lidstone JSM, et al. (2006) Obesity and disability – a short review. Obes Rev 7: 341–345 10.1111/j.1467-789X.2006.00233.x [DOI] [PubMed] [Google Scholar]
- 21. Froehlich-Grobe K, Lee J, Washburn RA (2013) Disparities in Obesity and Related Conditions Among Americans with Disabilities. Am J Prev Med 45: 83–90 10.1016/j.amepre.2013.02.021 [DOI] [PubMed] [Google Scholar]
- 22. Rimmer JH, Riley B, Wang E, Rauworth A, Jurkowski J (2004) Physical activity participation among persons with disabilities: barriers and facilitators. Am J Prev Med 26: 419–425 10.1016/j.amepre.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 23. Vincent HK, Vincent KR, Lamb KM (2010) Obesity and mobility disability in the older adult. Obes Rev Off J Int Assoc Study Obes 11: 568–579 10.1111/j.1467-789X.2009.00703.x [DOI] [PubMed] [Google Scholar]
- 24. Froehlich-Grobe K, Lollar D (2011) Obesity and disability: time to act. Am J Prev Med 41: 541–545 10.1016/j.amepre.2011.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Forhan M, Gill SV (2013) Obesity, functional mobility and quality of life. Best Pract Res Clin Endocrinol Metab 27: 129–137 10.1016/j.beem.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 26. Armour BS, Courtney-Long EA, Campbell VA, Wethington HR (2013) Disability prevalence among healthy weight, overweight, and obese adults. Obes Silver Spring Md 21: 852–855 10.1002/oby.20312 [DOI] [PubMed] [Google Scholar]
- 27. Tukker A, Visscher TLS, Picavet HSJ (2009) Overweight and health problems of the lower extremities: osteoarthritis, pain and disability. Public Health Nutr 12: 359–368 10.1017/S1368980008002103 [DOI] [PubMed] [Google Scholar]
- 28.Brault MW (2008) Americans with disabilities: 2005. Current Population Reports. Wash DCUS Census Bur: P70–117.
- 29. Flegal KM, Carroll MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA J Am Med Assoc 303: 235–241 10.1001/jama.2009.2014 [DOI] [PubMed] [Google Scholar]
- 30.WHO (2011) International Classification of Functioning, Disability and Health (ICF). WHO. Available: http://www.who.int/classifications/icf/en/. Accessed 2013 Sep 3.
- 31. Palazzo C, Ravaud J-F, Trinquart L, Dalichampt M, Ravaud P, et al. (2012) Respective Contribution of Chronic Conditions to Disability in France: Results from the National Disability-Health Survey. PLoS ONE 7: e44994 10.1371/journal.pone.0044994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Union Council (n.d.) Council recommendation of 2 December 2003 on cancer screening. Official Journal of European Union 878 (EC: L327), 34–35.
- 33.U.S Preventive Services Task Force. (n.d.) Recommendations for Adults (USPSTF). Cervical cancer screening. Available: http://www.uspreventiveservicestaskforce.org/adultrec.htm. Accessed 2013 May 3.
- 34. Nagi SZ (1976) An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc 54: 439–467. [PubMed] [Google Scholar]
- 35. Bannerman E, Miller MD, Daniels LA, Cobiac L, Giles LC, et al. (2002) Anthropometric indices predict physical function and mobility in older Australians: the Australian Longitudinal Study of Ageing. Public Health Nutr 5: 655–662 10.1079/PHN2002336 [DOI] [PubMed] [Google Scholar]
- 36. Rasch EK, Hochberg MC, Magder L, Magaziner J, Altman BM (2008) Health of community-dwelling adults with mobility limitations in the United States: prevalent health conditions. Part I. Arch Phys Med Rehabil 89: 210–218 10.1016/j.apmr.2007.08.146 [DOI] [PubMed] [Google Scholar]
- 37. Schootman M, Jeffe DB (2003) Identifying factors associated with disability-related differences in breast cancer screening (United States). Cancer Causes Control CCC 14: 97–107. [DOI] [PubMed] [Google Scholar]
- 38.WHO (2007) Breast cancer: prevention and control WHO. Available: http://www.who.int/cancer/detection/breastcancer/en/index3.html. Accessed 2014 Jan 7.
- 39.Duport N, Haguenoer K, Ancelle-Park R, Bloch J (2007) Dépistage organisé du cancer du col de l'utérus: évaluation épidémiologique des quatre départements “pilotes.” Institut de Veille Sanitaire. Available: http://www.invs.sante.fr/publications/2007/cancer_col_uterus%20evaluation/col_uterus.pdf. Accessed 2014 Apr 23.
- 40. Barr JK, Giannotti TE, Van Hoof TJ, Mongoven J, Curry M (2008) Understanding barriers to participation in mammography by women with disabilities. Am J Health Promot AJHP 22: 381–385 10.4278/ajhp.22.6.381 [DOI] [PubMed] [Google Scholar]
- 41. Lagu T, Hannon NS, Rothberg MB, Wells AS, Green KL, et al. (2013) Access to Subspecialty Care for Patients With Mobility ImpairmentA Survey. Ann Intern Med 158: 441–446 10.7326/0003-4819-158-6-201303190-00003 [DOI] [PubMed] [Google Scholar]
- 42. Mitchell RS, Padwal RS, Chuck AW, Klarenbach SW (2008) Cancer Screening Among the Overweight and Obese in Canada. Am J Prev Med 35: 127–132 10.1016/j.amepre.2008.03.031 [DOI] [PubMed] [Google Scholar]
- 43. Park JK, Park HA, Park JJ, Cho YG (2012) Obesity and screening compliance for breast and cervical cancer in Korean women. Asian Pac J Cancer Prev APJCP 13: 3271–3274. [DOI] [PubMed] [Google Scholar]
- 44. Maruthur NM, Bolen SD, Brancati FL, Clark JM (2009) Obesity and Mammography: A Systematic Review and Meta-Analysis. J Gen Intern Med 24: 665–677 10.1007/s11606-009-0939-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maruthur NM, Bolen SD, Brancati FL, Clark JM (2009) The association of obesity and cervical cancer screening: a systematic review and meta-analysis. Obes Silver Spring Md 17: 375–381 10.1038/oby.2008.480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amy NK, Aalborg A, Lyons P, Keranen L (2006) Barriers to routine gynecological cancer screening for White and African-American obese women. Int J Obes 2005 30: 147–155 10.1038/sj.ijo.0803105 [DOI] [PubMed] [Google Scholar]
- 47.European Commission (2009) Ensuring accessibility and non-discrimination of people with disabilities Toolkit for using EU Structural and Cohesion Funds. Available: ISBN: 978-92-79-11741-1.
- 48. Calle EE, Thun MJ (2004) Obesity and cancer. Oncogene 23: 6365–6378 10.1038/sj.onc.1207751 [DOI] [PubMed] [Google Scholar]
- 49. Cronin KA, Miglioretti DL, Krapcho M, Yu B, Geller BM, et al. (2009) Bias associated with self-report of prior screening mammography. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 18: 1699–1705 10.1158/1055-9965.EPI-09-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Connor Gorber S, Tremblay M, Moher D, Gorber B (2007) A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev Off J Int Assoc Study Obes 8: 307–326 10.1111/j.1467-789X.2007.00347.x [DOI] [PubMed] [Google Scholar]
- 51. Elgar FJ, Stewart JM (2008) Validity of self-report screening for overweight and obesity. Evidence from the Canadian Community Health Survey. Can J Public Health Rev Can Santé Publique 99: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. The source of the data used in this study was the Health and Disability Survey – Ordinary Household Section, available at http://www.drees.sante.gouv.fr/les-enquetes-handicap-sante,4267.html