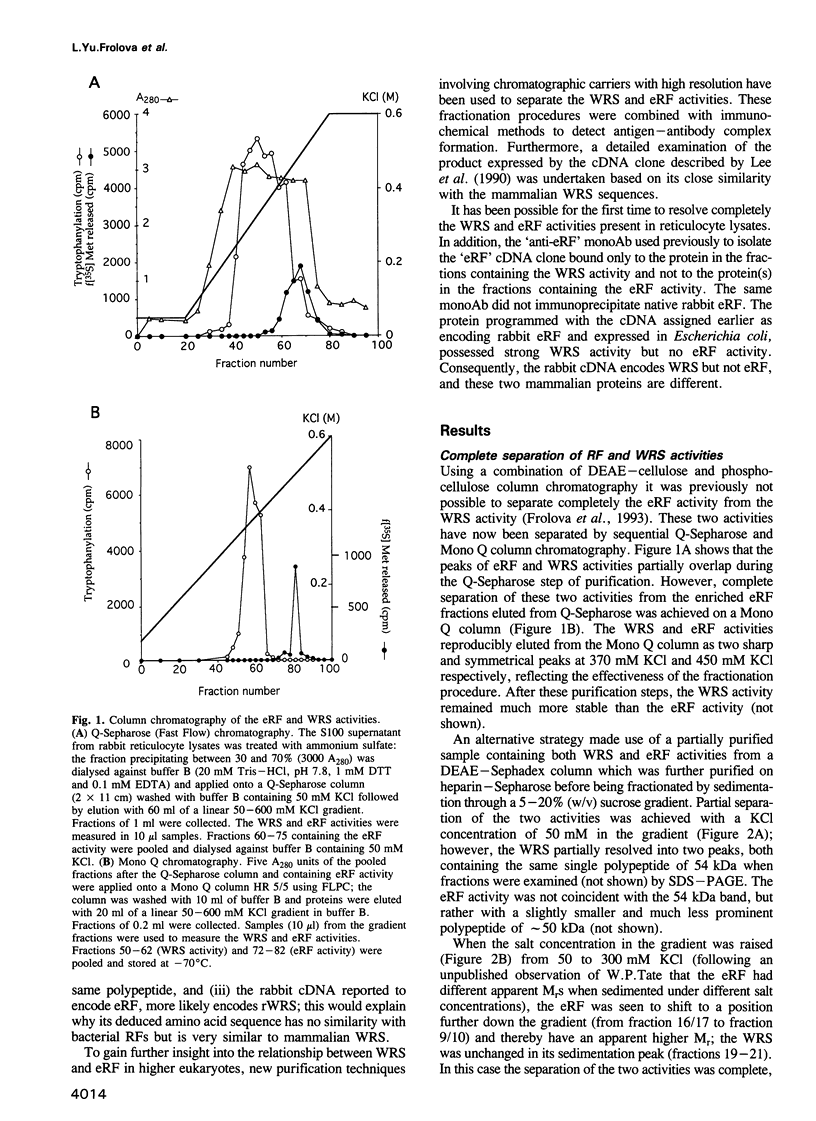
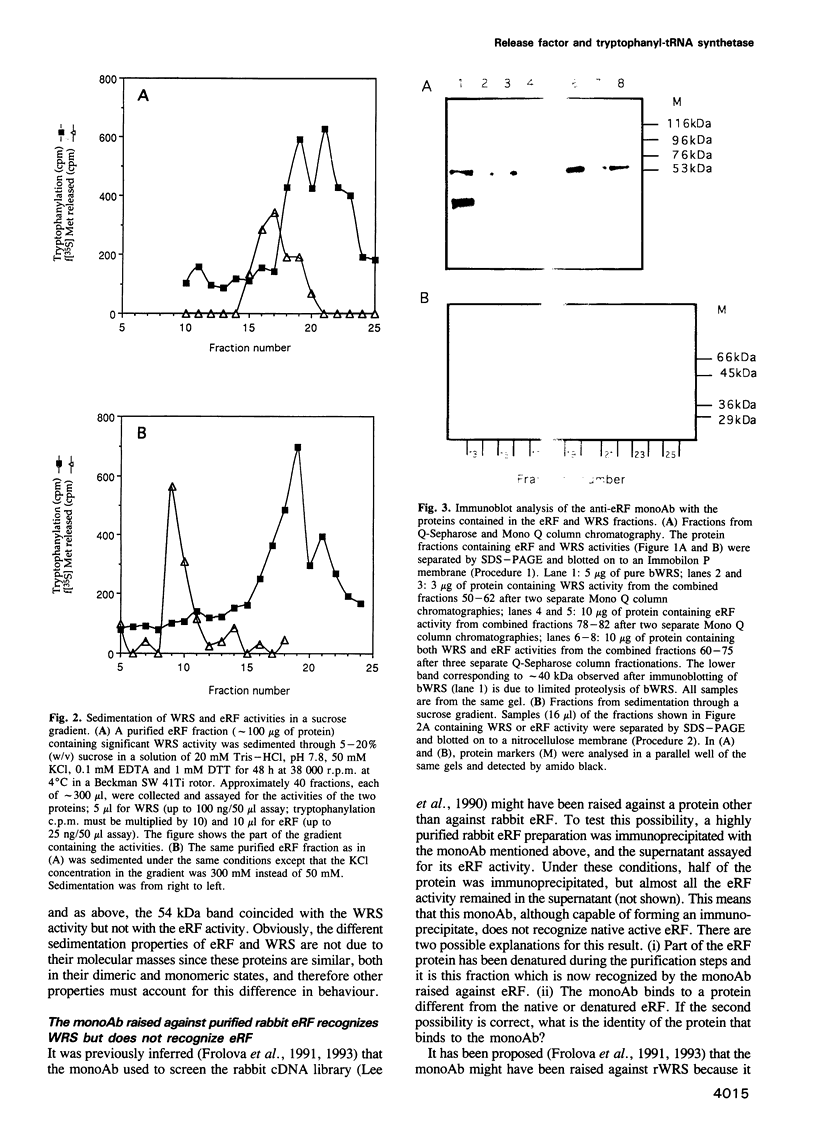
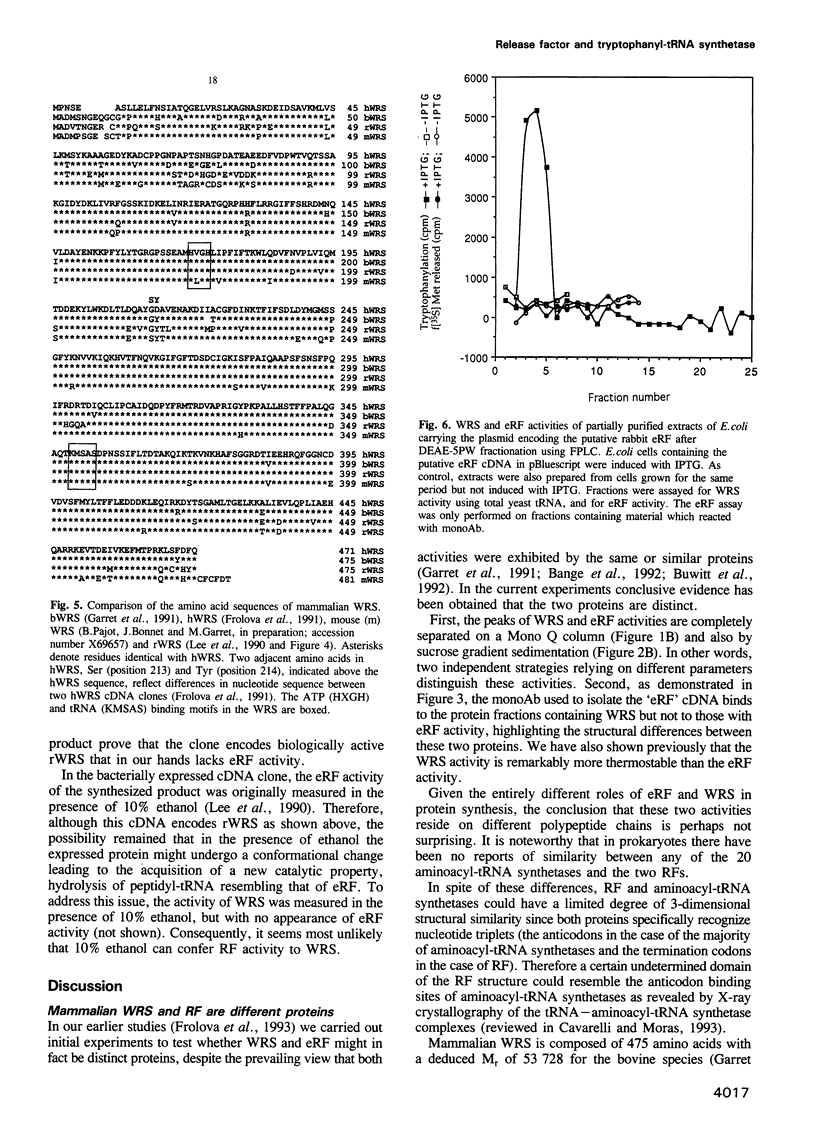
Abstract
A very high (approximately 90%) structural similarity exists between the bovine, human and murine tryptophanyl-tRNA synthetases (WRS), and quite unexpectedly the rabbit polypeptide chain release factor (eRF). This similarity may point to a very close resemblance or identity between these proteins involved in distinct steps of protein synthesis, or inadvertently to an incorrect assignment of the clone reported to encode eRF, since the structure of clones encoding WRS were confirmed by peptide sequencing. Using high resolution column chromatography and sucrose gradient centrifugation combined with assays for WRS and eRF activities, we show that functionally distinct WRS and eRF proteins can be completely separated from each other. Moreover, a putative anti-eRF monoclonal antibody appears incapable of immunoprecipitating the eRF activity or binding to protein(s) possessing eRF activity. This antibody binds to protein fractions which coincide in various separation procedures with rabbit WRS activity, and to pure bovine WRS. The protein expressed in Escherichia coli from the original cDNA clone initially reported to encode eRF, has WRS activity but not eRF activity. Resequencing of the fragment of the original rabbit cDNA demonstrates the presence of the previously overlooked HXGH motif typical of class I aminoacyl-tRNA synthetases. Consequently, mammalian WRS and eRF are different proteins, and the cDNA clone formerly assigned as encoding eRF encodes rabbit WRS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bange F. C., Flohr T., Buwitt U., Böttger E. C. An interferon-induced protein with release factor activity is a tryptophanyl-tRNA synthetase. FEBS Lett. 1992 Mar 30;300(2):162–166. doi: 10.1016/0014-5793(92)80187-l. [DOI] [PubMed] [Google Scholar]
- Buwitt U., Flohr T., Böttger E. C. Molecular cloning and characterization of an interferon induced human cDNA with sequence homology to a mammalian peptide chain release factor. EMBO J. 1992 Feb;11(2):489–496. doi: 10.1002/j.1460-2075.1992.tb05079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. T., Beaudet A. L., Tate W. P. Mammalian release factor; in vitro assay and purification. Methods Enzymol. 1974;30:293–303. doi: 10.1016/0076-6879(74)30032-8. [DOI] [PubMed] [Google Scholar]
- Cavarelli J., Moras D. Recognition of tRNAs by aminoacyl-tRNA synthetases. FASEB J. 1993 Jan;7(1):79–86. doi: 10.1096/fasebj.7.1.8422978. [DOI] [PubMed] [Google Scholar]
- Cerini C., Kerjan P., Astier M., Gratecos D., Mirande M., Sémériva M. A component of the multisynthetase complex is a multifunctional aminoacyl-tRNA synthetase. EMBO J. 1991 Dec;10(13):4267–4277. doi: 10.1002/j.1460-2075.1991.tb05005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Fleckner J., Rasmussen H. H., Justesen J. Human interferon gamma potently induces the synthesis of a 55-kDa protein (gamma 2) highly homologous to rabbit peptide chain release factor and bovine tryptophanyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11520–11524. doi: 10.1073/pnas.88.24.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova LYu, Fleckner J., Justesen J., Timms K. M., Tate W. P., Kisselev L. L., Haenni A. L. Are the tryptophanyl-tRNA synthetase and the peptide-chain-release factor from higher eukaryotes one and the same protein? Eur J Biochem. 1993 Mar 1;212(2):457–466. doi: 10.1111/j.1432-1033.1993.tb17682.x. [DOI] [PubMed] [Google Scholar]
- Frolova LYu, Sudomoina M. A., Grigorieva AYu, Zinovieva O. L., Kisselev L. L. Cloning and nucleotide sequence of the structural gene encoding for human tryptophanyl-tRNA synthetase. Gene. 1991 Dec 30;109(2):291–296. doi: 10.1016/0378-1119(91)90624-k. [DOI] [PubMed] [Google Scholar]
- Garret M., Pajot B., Trézéguet V., Labouesse J., Merle M., Gandar J. C., Benedetto J. P., Sallafranque M. L., Alterio J., Gueguen M. A mammalian tryptophanyl-tRNA synthetase shows little homology to prokaryotic synthetases but near identity with mammalian peptide chain release factor. Biochemistry. 1991 Aug 6;30(31):7809–7817. doi: 10.1021/bi00245a021. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Beaudet A. L., Caskey C. T. Peptide chain termination with mammalian release factor. Proc Natl Acad Sci U S A. 1970 Sep;67(1):99–106. doi: 10.1073/pnas.67.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev L. L., Favorova O. O., Kovaleva G. K. Tryptophanyl-tRNA synthetase from beef pancreas. Methods Enzymol. 1979;59:234–257. doi: 10.1016/0076-6879(79)59087-9. [DOI] [PubMed] [Google Scholar]
- Kisselev L. L., Favorova O. O., Nurbekov M. K., Dmitriyenko S. G., Engelhardt W. A. Bovine tryptophanyl-tRNA synthetase. A zinc metalloenzyme. Eur J Biochem. 1981 Dec;120(3):511–517. doi: 10.1111/j.1432-1033.1981.tb05729.x. [DOI] [PubMed] [Google Scholar]
- Konecki D. S., Aune K. C., Tate W., Caskey C. T. Characterization of reticulocyte release factor. J Biol Chem. 1977 Jul 10;252(13):4514–4520. [PubMed] [Google Scholar]
- Kovaleva G. K., Zheltova A. O., Nikitushkina T. V., Egorov T. A., Musoljamov A. Ch, Kisselev L. L. Carbohydrates in mammalian tryptophanyl-tRNA synthetase. FEBS Lett. 1992 Sep 14;309(3):337–339. doi: 10.1016/0014-5793(92)80802-n. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Craigen W. J., Muzny D. M., Harlow E., Caskey C. T. Cloning and expression of a mammalian peptide chain release factor with sequence similarity to tryptophanyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1990 May;87(9):3508–3512. doi: 10.1073/pnas.87.9.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire G., Gros C., Epely S., Kaminski M., Labouesse B. Multiple forms of tryptophanyl-tRNA synthetase from beef pancreas. Eur J Biochem. 1975 Feb 3;51(1):237–252. doi: 10.1111/j.1432-1033.1975.tb03924.x. [DOI] [PubMed] [Google Scholar]
- Moras D. Structural and functional relationships between aminoacyl-tRNA synthetases. Trends Biochem Sci. 1992 Apr;17(4):159–164. doi: 10.1016/0968-0004(92)90326-5. [DOI] [PubMed] [Google Scholar]
- Prasolov V. S., Favorova O. O., Margulis G. V., Kisselev L. L. Limited proteolysis of the tryptophanyl-tRNA synthetase. Biochim Biophys Acta. 1975 Jan 6;378(1):92–106. doi: 10.1016/0005-2787(75)90140-9. [DOI] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Xing L., Powell R. J., Tate W. P. Interferon induces tryptophanyl-tRNA synthetase expression in human fibroblasts. J Biol Chem. 1991 Dec 25;266(36):24245–24248. [PubMed] [Google Scholar]
- Schimmel P. Classes of aminoacyl-tRNA synthetases and the establishment of the genetic code. Trends Biochem Sci. 1991 Jan;16(1):1–3. doi: 10.1016/0968-0004(91)90002-d. [DOI] [PubMed] [Google Scholar]
- Tate W. P., Brown C. M. Translational termination: "stop" for protein synthesis or "pause" for regulation of gene expression. Biochemistry. 1992 Mar 10;31(9):2443–2450. doi: 10.1021/bi00124a001. [DOI] [PubMed] [Google Scholar]




