Abstract
Background
Glioblastoma subtypes have been defined based on transcriptional profiling, yet personalized care based on molecular classification remains unexploited. Topoisomerase II (TOP2) contributes to the transcriptional signature of the proneural glioma subtype. Thus, we targeted TOP2 pharmacologically with etoposide in proneural glioma models.
Methods
TOP2 gene expression was evaluated in mouse platelet derived growth factor (PDGF)+phosphatase and tensin homolog (PTEN)−/−p53−/− and PDGF+PTEN−/− proneural gliomas and cell lines, as well as human glioblastoma from The Cancer Genome Atlas. Correlation between TOP2 transcript levels and etoposide susceptibility was investigated in 139 human cancer cell lines from the Cancer Cell Line Encyclopedia public dataset and in mouse proneural glioma cell lines. Convection-enhanced delivery (CED) of etoposide was tested on cell-based PDGF+PTEN−/−p53−/− and retroviral-based PDGF+PTEN−/− mouse proneural glioma models.
Results
TOP2 expression was significantly higher in human proneural glioblastoma and in mouse proneural tumors at early as well as late stages of development compared with normal brain. TOP2B transcript correlated with susceptibility to etoposide in mouse proneural cell lines and in 139 human cancer cell lines from the Cancer Cell Line Encyclopedia. Intracranial etoposide CED treatment (680 μM) was well tolerated by mice and led to a significant survival benefit in the PDGF+PTEN−/−p53−/− glioma model. Moreover, etoposide CED treatment at 80 μM but not 4 μM led to a significant survival advantage in the PDGF+PTEN−/− glioma model.
Conclusions
TOP2 is highly expressed in proneural gliomas, rendering its pharmacological targeting by intratumoral administration of etoposide by CED effective on murine proneural gliomas. We provide evidence supporting clinical testing of CED of etoposide with a molecular-based patient selection approach.
Keywords: convection-enhanced delivery, etoposide, glioma, proneural, topoisomerase
Traditionally, the World Health Organization classification of gliomas is based on tumor histology. Recently, major efforts in characterizing the global expression profiles and genetic alterations in glioblastoma have led to significantly improved understanding of this disease.1–6 Of all these efforts, it is evident that one of the most robust and identifiable gene expression patterns in gliomas is the proneural (PN) subtype.7,8 Differences in glioblastoma molecular phenotypes can have differences in clinical response to therapies. The PN subgroup in particular has been found to be associated with younger patient age at diagnosis and a phenotype that is unresponsive to aggressive traditional therapies that do reduce mortality for the other subgroups.9 Therefore, there is a pressing need to identify proper therapeutic interventions that can benefit PN glioblastoma patients.
Our group previously described a murine glioma model that is phenotypically classifiable with human PN glioblastoma based on its gene expression profile.10 In this model, PN murine tumors are generated in vivo by retrovirally induced overexpression of platelet derived growth factor (PDGF) and deletion of phosphatase and tensin homolog (PTEN) with or without additional p53 deletion in glial progenitor cells using the Cre-lox system in transgenic mouse strains. More recently, we showed that cell lines derived from these murine glioma models retain their PN phenotype in vitro and can be utilized for in vitro and in vivo experimentation, providing a reasonable PN glioma model.11
We looked for potential therapeutic targets among PN-specific genes, hoping to find genes that are overexpressed in this group of patients. Topoisomerase II (TOP2) was previously identified as a PN-specific gene based on its high expression levels.9 Thus, in this current study we explored the therapeutic effects of TOP2 inhibition as a therapeutic strategy in a murine PN glioma model.
Poisons that act on TOP2, such as etoposide, have been used in combination therapies for the treatment of malignant gliomas. Unfortunately these trials have shown limited antitumoral activity and poor response rates for chemotherapy regimens involving systemic delivery of etoposide.12–14 It is likely that factors such as poor penetration across the blood–brain barrier and systemic toxicities limit the therapeutic efficacy of etoposide following systemic delivery. Moreover, as seen in expression data of The Cancer Genome Atlas (TCGA; http://tcga-data.nci.nih.gov/tcga/), TOP2 expression is variable across glioblastoma samples, suggesting that clearly defined inclusion criteria restricted to those patients with high expression of this enzyme might lead to better therapeutic results of targeting TOP2.
In this study, we explore the efficacy of etoposide in a preclinical model of PN glioma. To maximize the efficacy of this agent and overcome the challenging pharmacokinetics of systemic administration for brain tumors, we tested the use of convection-enhanced delivery (CED) for this agent. CED involves the use of catheters surgically implanted into the tumor and surrounding brain parenchyma, allowing for a continuous delivery of chemotherapy directly into the tumor through positive pressure microperfusion.15 This delivery method provides an advantage over systemic administration in bypassing the blood–brain barrier and reducing toxicities to other organs. In the context of this background, here we present preclinical data suggestive of the efficacy of etoposide treatment for the PN subgroup of glioblastoma.
Materials and Methods
Topoisomerase II Expression
The human glioblastoma dataset derived from TCGA comprised 339 patients used for comparison of TOP2A and TOP2B expression, and the class designation (PN, neural, mesenchymal, or classical) for these patients was obtained from the original publication9 and from personal communication by Dr Verhaak. These samples included 83 PN, 62 neural, 97 mesenchymal, and 97 classical TCGA glioblastoma patients.
The mouse PN glioma expression data have been previously published and are available (National Center for Biotechnology Information Gene Expression Omnibus GSE29458).10 Samples used included normal mouse brain (n = 3), PDGF+PTEN−/−p53−/− (n = 8), and PDGF+PTEN−/− mouse tumors (n = 12). The gene expression–based heatmaps were generated in Microsoft Excel 2011. For mouse and human expression array data, if 2 probes were available for a gene, the one with the highest intensity was used to summarize the value of the expression level.
For mouse TOP2A and TOP2B expression by quantitative reverse transcriptase (qRT)–PCR, cDNA was generated using the Invitrogen SuperScript VILO (variable input, linear output) cDNA synthesis kit. Expression analysis was performed using a TOP2A or TOP2B TaqMan gene expression assay (Applied Biosystems) in multiplex reactions with glyceraldehyde 3-phosphate dehydrogenase as a housekeeping gene for normalization (Applied Biosystems). TaqMan Gene Expression Master Mix was used (Applied Biosystems) according to the manufacturer’s protocol. Each group contained 7 biological replicates and 3 technical replicates per sample.
Correlation of TOP2A and TOP2B Expression With Susceptibility to Etoposide in Human Cancer Cell Lines
To compute the correlation between TOP2 expression and susceptibility to etoposide among human cancer cell lines, 139 cell lines with publicly available relevant data were used. These cell lines were the overlap between (i) 1037 human cancer cell lines for which whole-genome expression profiles have been reported in the Cancer Cell Line Encyclopedia (CCLE)16 and (ii) a list of 242 human cancer cell lines for which dose response curves to etoposide were performed by Basu et al17 as part of a larger study. Susceptibility to etoposide was derived from areas under the dose response curves in the setting of etoposide treatment. Lower AUC was interpreted as higher sensitivity to etoposide.
Mouse Glioma Models and Cell Lines
PDGF–internal ribosomal entry site (IRES)–cyclization recombination (Cre) retrovirus and the transgenic mice were generated as previously described.10 Intracranial injection was performed under stereotactic guidance (coordinates 2.0 mm lateral, 2.0 mm rostral, and 2.0 mm deep relative to bregma). For de novo tumor generation, mice were injected with 2.0 μL PDGF-IRES-Cre retrovirus into the brain with a Hamilton syringe. Tumor growth was assessed through monitoring of luciferase signaling by bioluminescence imaging as previously described.10
The isolation, propagation, and in vitro experimentation using the murine PN glioma cell lines have been previously described by our group.10,11 Briefly, tumor cells were grown in adherent conditions on polylysine-coated flasks maintained in culture using basal media supplemented with B104 conditioned media, PDGF, and fibroblast growth factor.
Automated Cell Viability Assays
The density of each cell line was first optimized to ensure linear cell growth in tissue culture–treated 384-well plates (Greiner Bio-One 781080) for the duration of the experiment. Starting with 16 000 cells per well, the cells were 2-fold serially diluted to test 10 different concentrations of growth in the microplate. CellTiter-Glo assay (Promega) was used to measure total ATP levels of the wells every 24 h for 96 h. Optimal cell density was chosen based on the linear growth of cells by graphing the total luminescence count versus time. Each cell line was then plated employing the cell::explorer automation system (PerkinElmer) at the optimal density on a PerkinElmer Janus Automated Workstation. The Janus was equipped with a 96-tip Modular Dispense Technology pipetting head, used with sterile tips (235 µL; PerkinElmer 69000045) for plating 50 µL of the cell solution into the microplates. The plates were incubated in the Liconic STX-500 ICSA for 24 h prior to drug addition. To generate a concentration response curve of each cell line against etoposide, the Hewlett-Packard D300 Digital Dispenser was used to dispense specific amounts of the drug for a titration curve. Each concentration of the drug was dispensed in triplicate by the Digital Dispenser using Hewlett-Packard inkjet technology. A maximum dimethyl sulfoxide concentration of 0.4% was normalized across all the wells, and thimerosal at 20 µM was used as the positive control, with dimethyl sulfoxide–treated wells serving as the negative control. After 48 h of incubation with the drug, the cell::explorer removed the plates from the incubator and placed them in the Liconic LPX 200 Hotel to let them equilibrate to room temperature. We added 25 µL CellTiter-Glo using the PerkinElmer Flexdrop PLUS Precision Reagent Dispenser. After shaking at 600 rpm for 5 min, the plates were read by the PerkinElmer Envision 2104 using an enhanced luminescence protocol. The viability of each well was then calculated utilizing the control wells in each plate.
Maximal Soluble Dose and Biostability of Etoposide Maintained at 37°C
Etoposide (20 mg; Sigma) was reconstituted in 80 mg modified polysorbate 80/Tween 80, 650 mg polyethylene glycol 300, and 30.5% (volume/volume) ethanol. Serial dilutions were performed to determine the maximal soluble dose of etoposide. Drug dilutions were stored in an incubator at 37°C (protected from light) and monitored daily for precipitate formation, for up to 7 days. The efficacy of etoposide maintained at 37°C was determined by treating cells from murine tumor preps as described above, with the maximal soluble dose of etoposide (680 μM) at day 0 (baseline) and after 7-day storage. Control cells at matched time points were treated in etoposide-free media. Triplicate wells were plated for each treatment condition. Following 72 h treatment, the cell viability was determined using CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer's recommendations.
Convection-enhanced Delivery of Etoposide in Animal Experiments
Viable tumor formation was defined as a luciferase signal of at least 106 bioluminescence (p/sec/cm2) for 2 consecutive imaging dates. Upon reaching this threshold, mice were randomized to either etoposide treatment or control. There were no significant differences in bioluminescence between groups at time of treatment allocation in any of the survival experiments.
A CED pump and infusion apparatus were implanted into tumor-bearing mice, and 7-day CED treatment with either etoposide or control was initiated. Changes in tumor bioluminescence were monitored using bioluminescence during and following treatment. Mice were monitored daily for signs of tumor morbidity and were sacrificed once these were observed. Survival analysis was calculated using the Kaplan–Meier method with the GraphPad Prism 5.0 statistical software package.
For CED, an Alzet micro-osmotic pump (model 1007D) and the Alzet Brain Infusion Kit 3 were used, including 3 mm plastic tubing and a polycarbonate cannula with a 3-mm, 30-gauge stainless steel delivery tube, which was set to a depth of 2 mm using depth-adjustment spacers. Treatment was delivered at a rate of 0.5 μL/h for 7 days starting upon meeting the bioluminescence threshold (see above for details). Briefly, the burrhole used for initial intracranial injection for tumor generation was reopened and the infusion cannula was stereotactically inserted 2 mm deep to the skull surface and secured onto the skull using adhesive glue (Scienceware). The osmotic pump was inserted subcutaneously into the back of the mouse. Incision was sutured over the system.
The osmotic pumps were loaded with 100 μL etoposide solution or phosphate buffered saline in the case of the control animals in the PDGF+PTEN−/−p53−/− model experiments. For the survival study with the PDGF+PTEN−/−p53−/− cell-based glioma model, mice were injected with the tumor cell line on 2 separate days, with mice allocated to treatment and control from both dates in a balanced distribution. In the case of the retrovirus-induced PDGF+PTEN−/− mouse model in vivo experiments, etoposide solution or its respective excipients were used for treatment and control groups, respectively.
Dose Escalation Toxicity Study
To determine the potential toxicity from CED of etoposide, non–tumor bearing adult mice were treated with 7-day CED of etoposide. A dose escalation scale of 200, 400, 600, and 680 μM was used, with 2 mice treated at each dosing level. Mice were monitored daily for signs of morbidity. Weights were recorded pre–pump insertion, following completion of treatment, and before sacrifice. All mice were sacrificed on 10 POD (postoperative days since CED pump implantation), perfused with paraformaldehyde, and processed for histological analysis with hematoxylin and eosin staining. All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee of Columbia University and performed in accordance with institutional policies.
Results
Topoisomerase II Is Highly Expressed in Proneural Gliomas
Gene expression profilings of 339 human glioblastoma tumors from TCGA were compared to determine the expression of TOP2 among the 4 different molecular subtypes (Fig. 1A). We found that the PN subtype demonstrated a significantly higher level of both TOP2A and TOP2B expression (P < .00001) than other groups. In fact, TOP2B is one of the classifier genes in defining PN subtype.9
Fig. 1.
Topoisomerase II gene expression in human glioblastoma and mouse glioma samples. (A) Bar plot representation of expression array data for TOP2A and TOP2B for samples from different human glioblastoma subgroups according to Verhaak et al21 (data obtained from TCGA). *Significant difference in comparison with other groups, ANOVA P < .00001. (B) TOP2A and TOP2B expression determined by microarray data for samples of PDGF+PTEN−/− as well as PDGF+PTEN−/−p53−/− mouse PN glioma tumor specimens and normal mouse brain. Red color indicates higher expression, black intermediate expression, and green lower expression. (C) TOP2A (left) and TOP2B (right) expressions were longitudinally characterized by qRT-PCR on PDGF+PTEN−/− mouse PN gliomas. Days post i.c. injection for retroviral-mediated tumor induction (dpi). *Significant difference in expression in comparison with mouse brain (P < .05). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Next, we characterized the expression of TOP2 in 2 distinct murine transgenic PN glioma models previously described by our group.10 Consistent with the finding in human PN gliomas, both PDGF+PTEN−/− and PDGF+PTEN−/−p53−/− murine PN glioma models exhibited significantly higher levels of TOP2 in comparison with normal mouse brain (P < .001; Fig. 1B). To investigate whether TOP2 expression is upregulated in early stages of PN glioma development, we performed qRT-PCR for TOP2A and TOP2B on PDGF+PTEN−/− mouse PN gliomas and found that their expression was significantly higher than that seen in normal mouse brain at all time points of tumor development, including 21 days postinjection of PDGF-IRES-Cre retrovirus for tumor induction—the earliest time evaluated (P < .05)—and a stage of tumor formation in which lesions lack palisading necrosis or vascular proliferation but in which the PN phenotype is already present10,18 (Fig. 1C).
Topoisomerase IIB Transcript Levels Correlate With Susceptibility to Etoposide in Human Cancer Cell Lines
TOP2 protein level is known to correlate with susceptibility to etoposide treatment in cancer19 and specifically in glioma cell lines,20 yet it is not known whether TOP2 expression defined by its transcript levels also correlates with susceptibility to etoposide. This is important given that the characteristic expression of this gene in PN glioblastoma in the database of TCGA was determined based on RNA expression array.21 To directly investigate this question, we computed the correlation between TOP2A and TOP2B transcript levels from 139 human cancer cell lines with their sensitivity to etoposide obtained from previously published data.16,17 We found a significant negative correlation (P < .05) between TOP2B expression and AUC derived from dose response curves for etoposide treatment, showing that higher expression of TOP2B is associated with higher sensitivity to etoposide (P < .05; Fig. 2). No correlation was found between AUC for etoposide and TOP2A expression among these cell lines.
Fig. 2.
Topoisomerase IIB transcript levels correlate with susceptibility to etoposide among human cancer cell lines. One hundred and thirty-nine human cancer cell lines from CCLE (columns) were sorted from high to low AUC derived from dose response curves to etoposide performed by Basu et al17 (higher AUC signifies resistance, whereas lower AUC signifies susceptibility to etoposide) represented in the top row. TOP2B transcript levels for each of the cell lines obtained from CCLE16 are represented in the bottom row. A significant inverse correlation was found between AUC for etoposide and TOP2B expression among these cell lines (P < .05). To plot gene expression for TOP2B and AUC for etoposide, both of these data were normalized independently using z-score transformation. Yellow represents higher values and black lower values.
High Sensitivity of Murine Proneural Glioma Cell Lines to Etoposide Correlates With Transcript Levels of Topoisomerase IIB
We investigated the susceptibility of mouse PN cell lines to etoposide. For this experiment, we employed PDGF+PTEN−/− and PDGF+PTEN−/−p53−/− glioma cell lines that had been previously classified as PN based on their gene expression profile.11 In vitro treatment of 3 separate PDGF+PTEN−/− and 1 PDGF+PTEN−/−p53−/− mouse PN glioma cell lines inhibited cell proliferation in a dose-dependent manner (Fig. 3A). In addition, inhibition of cell proliferation was increased as length of etoposide treatment increased, as demonstrated by a more pronounced effect at 72 h in comparison with 48 h (Fig. 3A). There was no difference in the susceptibility to etoposide between PDGF+PTEN−/−p53−/− and PDGF+PTEN−/− PN glioma cell lines, but all of these were significantly more susceptible than the GL261 murine glioma cell line at 48 and 72 h (Fig. 3A). To evaluate whether the susceptibility to etoposide had any relationship to the transcript levels of TOP2, the expression levels of TOP2A and TOP2B were evaluated in these cell lines by qRT-PCR. Consistent with our findings in the dataset of 139 human cancer cell lines, we did not find significant differences in TOP2A transcript levels between the PN cell lines compared with GL261. Nevertheless, similar to the case of human cancer cell lines, there was significantly higher transcript levels of TOP2B in the PDGF+PTEN−/−p53−/− and PDGF+PTEN−/− PN glioma cell lines versus GL261 (Fig. 3B), which correlated with the susceptibility to etoposide.
Fig. 3.
Proneural glioma cell lines are highly sensitive to etoposide, and this sensitivity correlates with transcript levels of TOP2B. (A) Cell viability was determined on dose response curves for etoposide in murine glioma cell line GL261, in 3 separate PDGF+PTEN−/− murine PN glioma cell lines (JF, JM3, PTENYB) and in a PDGF+PTEN−/−p53−/− PN glioma cell line 48 h (top) and 72 h (bottom). (B) TOP2A (left) and TOP2B (right) transcript levels were compared between GL261, PDGF+PTEN−/− (JF cell line from panel A), and a PDGF+PTEN−/−p53−/− mouse glioma cell line, evaluated by qRT-PCR. PDGF+PTEN−/− and PDGF+PTEN−/−p53−/− glioma cell lines were previously classified as PN based on their gene expression profile.11 *Represents significant difference in expression with GL261 as a reference (P < .05). IC50, half-maximal inhibitory concentration.
To explore the feasibility of prolonged CED of etoposide, we tested the cytotoxic effect of this agent following an incubation period at physiological temperature. For this, etoposide solution was prepared and stored in an incubator at 37°C for 7 days to assess drug solubility and bioactivity. Following this period of incubation—a physiological temperature—the maximal dose of etoposide that did not precipitate was 680 μM. The cytotoxic activity of 680 μM etoposide solution incubated at 37°C for 7 days was compared with that of a fresh preparation, and there was no statistical difference in cell viability between the 2 drug preparations (2.40 ± 1.2% vs 2.70 ± 1.4%, P = .875) following 72 h of treatment of PDGF+PTEN−/−p53−/− luciferase+ mouse glioma cells (Supplementary Fig. 1).
Seven-day Convection-enhanced Delivery of Etoposide Is Safe and Well Tolerated
A dose escalation toxicity study of 7-day CED was performed on non–tumor bearing mice, with etoposide dosing ranging from 200 μM to 680 μM (maximal soluble dose) and 2 mice per dosing level. All animals survived with no significant neurological deficits and no observed systemic toxicities at any dosing level. Histological analysis showed that only focal tissue damage limited the site of catheter placement.
Convection-enhanced Delivery of Etoposide Reduces Tumor Burden and Prolongs Survival in Murine Proneural Glioma Models
The therapeutic effect of etoposide was investigated in vivo on murine PN gliomas generated by i.c. injection of PDGF+PTEN−/−p53−/− luciferase+ mouse glioma cells. Upon reaching a bioluminescence signal of at least 106 (p/sec/cm2) for 2 consecutive dates (15–25 d following i.c. tumor cell injection), mice were randomized into CED of 680 μM etoposide (n = 12) or control treatment (n = 9) for 7 days. Convection-enhanced delivery of 680 μM etoposide led to a significant survival advantage over control animals (log-rank test P < .001; Fig. 4A). Consistent with this, we noticed lower bioluminescence signal for PDGF+PTEN−/−p53−/− tumors treated with etoposide as opposed to controls (Fig. 4B).
Fig. 4.

In vivo survival study of mice treated with 680 μM etoposide or phosphate buffered saline (controls) for 7 days utilizing CED. Pumps were implanted and treatment was initiated when animals reached 106 luciferase bioluminescent (p/sec/cm2) signaling. (A) Kaplan–Meier plot shows that survival of mice bearing PDGF+PTEN−/−p53−/− cell-based tumors treated with etoposide (n = 12) is significantly prolonged compared with the control group (n = 9) (log-rank test P < .001). (B) PDGF+PTEN−/−p53−/− tumors treated with etoposide showed lower bioluminescence signal than that seen in control tumors. Survival was measured starting at day of treatment with i.c. implantation of CED pump.
The experiment performed using cell-based PDGF+PTEN−/−p53−/− PN gliomas relied on the use of fully transformed cells. Given that TOP2A and TOP2B are highly expressed in PDGF+PTEN−/− PN gliomas at early stages of tumor progression (Fig. 1C), we hypothesized that etoposide might be effective on de novo PN gliomas treated during tumor development. To investigate this and test the efficacy of etoposide in that context and in a different experimental PN model, we performed survival experiments using the retrovirally induced PDGF+PTEN−/− murine PN gliomas. In this case, PDGF-IRES-Cre retrovirus was injected into the subcortical white matter of PTEN lox−lox luciferasestop-flox adult mice, leading to glial progenitor cell transformation and formation of intracranial tumors with histological appearance of glioblastoma upon reaching end stage.10 This tumor model has a PN phenotype and spontaneously develops genetic alterations during progression that are specifically found in human PN glioblastoma.18
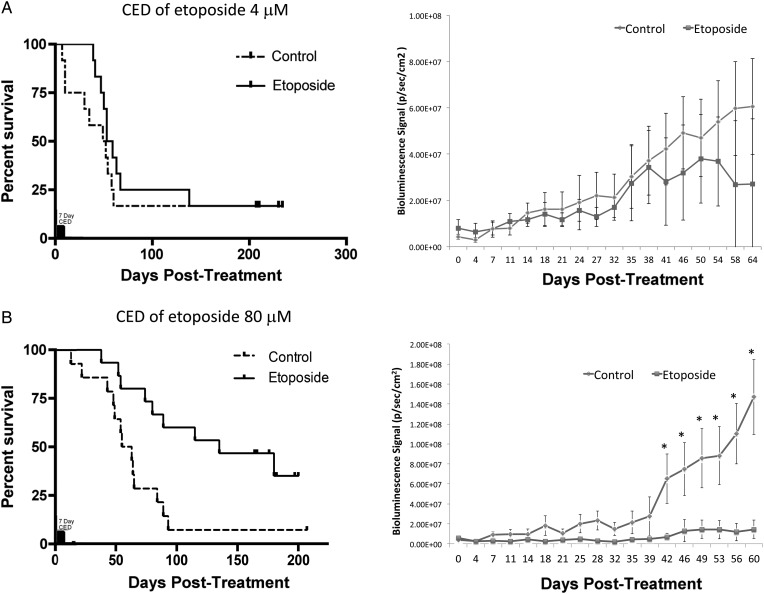
To evaluate the effects of different therapeutic doses, upon reaching 106 bioluminescence (p/sec/cm2) for 2 consecutive imaging dates, we performed CED treatment with etoposide 4 μM for 7 days on a cohort of mice bearing retrovirally generated PDGF+PTEN−/− PN gliomas (n = 30), which is a 140-fold lower dose than the maximal soluble dose. At this concentration, there were no significant survival or bioluminescence differences between mice treated with etoposide and those treated with the excipient (log-rank test P > .05; Fig. 5A). A second cohort of mice bearing retrovirally generated PDGF+PTEN−/− PN gliomas (n = 28) underwent CED treatment with 80 μM etoposide, in which case there was a significant survival advantage over the control mice (log-rank test P < .01; Fig. 5B). Prior to the difference in survival being evident, mice bearing PDGF+PTEN−/− PN gliomas treated with CED with 80 μM etoposide showed tumor growth arrest, as suggested by a stable bioluminescence that was significantly less than that of control mice by 42 POD and remained less for the remainder of the survival study (P < .05). Etoposide-treated mice that died had evidence of tumor burden based on bioluminescence and histological analysis (data not shown).
Fig. 5.
Survival experiments for retrovirally induced PDGF+PTEN−/− PN gliomas treated with a 7-day course of etoposide CED shows a dose response relationship. (A) Retrovirally induced PDGF+PTEN−/− PN gliomas treated with 4 μM etoposide for 7 days upon reaching 106 bioluminescence (p/sec/cm2) for 2 consecutive imaging dates had no significant survival benefit over control mice (n = 30) (log-rank test P > .05), as demonstrated by the Kaplan–Meier plot (left), and no significant difference in luminescence (right). (B) Retrovirally induced PDGF+PTEN−/− PN gliomas treated with 80 μM etoposide for 7 days had a significant survival benefit over control mice (n = 28) (log-rank test P < .01), as demonstrated by the Kaplan–Meier plot (left), and a significantly lower luminescence signal following treatment. *Represents significant difference in bioluminescence between groups (P < .05).
Discussion
The major molecular intertumoral heterogeneity found within glioblastoma22 contrasts with the homogeneous treatment paradigm of resection, radiation therapy, and chemotherapy with temozolomide that is established as the standard of care for patients bearing this disease. Advances in the characterization of this tumor and the organization of its genetic and phenotypic variations have elucidated clinically meaningful subgroups that present distinct phenotype, genotype, and susceptibility to treatments.21 PN glioblastomas in particular are noticeable for being resistant to aggressive therapeutic interventions.21 In an effort to exploit the knowledge derived from glioblastoma subtyping for personalizing treatment, in this study we present preclinical data that suggest that PN glioblastoma is susceptible to intratumoral delivery of etoposide. The rationale for testing this particular agent for PN gliomas derives from data showing that TOP2, the target of etoposide, is highly and specifically expressed in this subtype of tumors.
Topoisomerases play key roles in DNA replication, transcriptional regulation, and chromosome segregation.23 Different isoforms of topoisomerase vary in their function and are differentially expressed during development and in cancer (reviewed by Nitiss23). In particular, TOP2 has an important role in transcriptional regulation and plays a role in neural development.23 Verhaak et al9 identified TOP2B as a gene with highly variable expression among human glioblastomas, and thus used it as one of the classifier genes for defining the PN subtype signature. In our study, we provide data to show that TOP2A and TOP2B are highly expressed in 2 separate PN tumor models and that tumoral expression is significantly higher than that seen in normal brain. Consistent with this, we showed a relatively selective effect of intratumoral CED of etoposide, with high efficacy and no detectable toxicity in 2 PN murine glioma models. This is of particular therapeutic relevance given that intratumoral CED of etoposide might result in high levels of this agent in the brain parenchyma. We also found that both TOP2A and TOP2B transcripts were significantly elevated in PDGF+PTEN−/− murine PN gliomas at early stages of development, in which these tumors have the histological appearance of low-grade gliomas.10 This finding is important given that low-grade gliomas have a strong tendency for malignant progression and are mainly classified as PN tumors.24,25 The possibility of etoposide being effective for treating PN low-grade gliomas should be further investigated in future studies.
Etoposide is a poison that interacts with TOP2 to induce double-strand DNA breaks, with subsequent induction of apoptosis (reviewed by Nitiss26). Consistent with this mechanism of action, multiple groups have previously shown that TOP2 protein levels play a role in determining susceptibility to etoposide on different cancers, including gliomas.19,20 We found significant differences in transcript levels of TOP2 within glioblastomas and between PN gliomas and the surrounding brain parenchyma. Interestingly, mouse and human cancer cell line–derived data showed a clear correlation between transcript levels of TOP2B and susceptibility to etoposide, but no correlation was found in the case of TOP2A. Previous reports correlating the TOP2A expression with etoposide susceptibility in glioma cell lines have evaluated its expression at protein levels.27 It is possible that the lack of association of TOP2A transcript levels with etoposide susceptibility encountered in both mouse glioma and human cancer cell lines relates to the fact that TOP2A is subject to translational regulation.28
Previous studies have evaluated the efficacy and pharmacokinetics of systemic etoposide in the context of gliomas.12,29 Overall, a lack of efficacy and systemic toxicities temper the use of etoposide in this context. We attribute the lack of efficacy to the variable levels of TOP2 among malignant gliomas and to the relatively low concentrations of etoposide in the tumor tissue, which have been shown to range between 12% and 36% of the blood concentration.30–32 Our strategy of delivering etoposide by intratumoral CED can achieve tumoral drug concentrations multiple orders of magnitude higher than that previously tested. Our preclinical data suggest that such an approach might lead to a significant efficacy with limited systemic toxicity.
Several studies have examined CED chemotherapeutics in animal models and in clinical trials.33–35 Work by our group has focused on CED of topoisomerase inhibitors. Using a rat glioma model, we have shown that CED of topotecan, a TOP1 inhibitor, results in a significant survival advantage.36,37 Our phase I trial of topotecan CED for malignant gliomas demonstrated significant antitumoral activity with limited toxicity.36 In addition, we have shown the safety and feasibility of chronic treatment by CED by implantation of the infusion pump subcutaneously in a large animal model.38
In conclusion, this study has demonstrated that 2 separate murine PN glioma models are highly responsive to inhibition of TOP2 by etoposide. This constitutes a step toward bridging the apparent gap in translating the glioblastoma molecular classification into clinical applications. Selecting therapies most likely to be responsive in a subgroup of glioma patients may be more effective than treating all glioma patients as a whole. This study provides preclinical evidence supporting the clinical trial of CED of etoposide in a subset of glioblastoma patients with molecular-based inclusion criteria.
Supplementary Material
Funding
This study was supported by NIH/NCI grant R01 CA161404 (J.N.B.), NIH/NINDS grant 1R01NS066955 (P.C.), an NREF award by the AANS (A.M.S.), MAGNet grant 5U54CA121852-09 (M.B. and A.C.), and HHGG grant 5R01NS061776-05 (A.C.).
Supplementary Material
References
- 1.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Rich JN, Hans C, Jones B, et al. Gene expression profiling and genetic markers in glioblastoma survival. Cancer Res. 2005;65(10):4051–4058. doi: 10.1158/0008-5472.CAN-04-3936. [DOI] [PubMed] [Google Scholar]
- 5.Nigro JM, Misra A, Zhang L, et al. Integrated array-comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res. 2005;65(5):1678–1686. doi: 10.1158/0008-5472.CAN-04-2921. [DOI] [PubMed] [Google Scholar]
- 6.Liang Y, Diehn M, Watson N, et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci U S A. 2005;102(16):5814–5819. doi: 10.1073/pnas.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhat KP, Salazar KL, Balasubramaniyan V, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev. 2011;25(24):2594–2609. doi: 10.1101/gad.176800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10(5):319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 9.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 0000;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei L, Sonabend AM, Guarnieri P, et al. Glioblastoma models reveal the connection between adult glial progenitors and the proneural phenotype. PLoS One. 2011;6(5):e20041. doi: 10.1371/journal.pone.0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonabend AM, Yun J, Lei L, et al. Murine cell line model of proneural glioma for evaluation of anti-tumor therapies. J Neurooncol. 2013;112(3):375–382. doi: 10.1007/s11060-013-1082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer. 2009;101(12):1986–1994. doi: 10.1038/sj.bjc.6605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki T, Mizutani T, Nojima K, et al. Phase II study of ifosfamide, carboplatin, and etoposide in patients with a first recurrence of glioblastoma multiforme. J Neurosurg. 2010;112(1):50–56. doi: 10.3171/2009.5.JNS081738. [DOI] [PubMed] [Google Scholar]
- 14.Franceschi E, Cavallo G, Scopece L, et al. Phase II trial of carboplatin and etoposide for patients with recurrent high-grade glioma. Br J Cancer. 2004;91(6):1038–1044. doi: 10.1038/sj.bjc.6602105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bobo RH, Laske DW, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barretina J, Caponigro G, Stransky N, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–607. doi: 10.1038/nature11003. Erratum in Nature. 2012; 492(7428):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu A, Bodycombe NE, Cheah JH, et al. An interactive resource to identify cancer genetic and lineage dependencies targeted by small molecules. Cell. 2013;154(5):1151–1161. doi: 10.1016/j.cell.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonabend AM, Bansal M, Guarnieri P, et al. The transcriptional regulatory network of proneural glioma determines the genetic alterations selected during tumor progression. Cancer Res. 2014;74(5):1440–1451. doi: 10.1158/0008-5472.CAN-13-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker JV, Nitiss JL. DNA topoisomerase II as a target for cancer chemotherapy. Cancer Invest. 2002;20(4):570–589. doi: 10.1081/cnv-120002156. [DOI] [PubMed] [Google Scholar]
- 20.Sevim H, Parkinson JF, McDonald KL. Etoposide-mediated glioblastoma cell death: dependent or independent on the expression of its target, topoisomerase II alpha? J Cancer Res Clin Oncol. 2011;137(11):1705–1712. doi: 10.1007/s00432-011-1046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonavia R, Inda MM, Cavenee WK, et al. Heterogeneity maintenance in glioblastoma: a social network. Cancer Res. 2011;71(12):4055–4060. doi: 10.1158/0008-5472.CAN-11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 2009;9(5):327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper LA, Gutman DA, Long Q, et al. The proneural molecular signature is enriched in oligodendrogliomas and predicts improved survival among diffuse gliomas. PloS one. 2010;5(9):e12548. doi: 10.1371/journal.pone.0012548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9(5):338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das CM, Aguilera D, Vasquez H, et al. Valproic acid induces p21 and topoisomerase-II (alpha/beta) expression and synergistically enhances etoposide cytotoxicity in human glioblastoma cell lines. J Neurooncol. 2007;85(2):159–170. doi: 10.1007/s11060-007-9402-7. [DOI] [PubMed] [Google Scholar]
- 28.Srikantan S, Abdelmohsen K, Lee EK, et al. Translational control of TOP2A influences doxorubicin efficacy. Mol Cell Biol. 2011;31(18):3790–3801. doi: 10.1128/MCB.05639-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitz MW, Desai A, Grossman SA, et al. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011;104(3):629–638. doi: 10.1007/s11060-011-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiya K, Uozumi T, Ogasawara H, et al. Penetration of etoposide into human malignant brain tumors after intravenous and oral administration. Cancer Chemother Pharmacol. 1992;29(5):339–342. doi: 10.1007/BF00686001. [DOI] [PubMed] [Google Scholar]
- 31.Zucchetti M, Rossi C, Knerich R, et al. Concentrations of VP16 and VM26 in human brain tumors. Ann Oncol. 1991;2(1):63–66. doi: 10.1093/oxfordjournals.annonc.a057826. [DOI] [PubMed] [Google Scholar]
- 32.Stewart DJ, Richard MT, Hugenholtz H, et al. Penetration of VP-16 (etoposide) into human intracerebral and extracerebral tumors. J Neurooncol. 1984;2(2):133–139. doi: 10.1007/BF00177899. [DOI] [PubMed] [Google Scholar]
- 33.Lopez KA, Waziri AE, Canoll PD, et al. Convection-enhanced delivery in the treatment of malignant glioma. Neurol Res. 2006;28(5):542–548. doi: 10.1179/016164106X116836. [DOI] [PubMed] [Google Scholar]
- 34.White E, Bienemann A, Pugh J, et al. An evaluation of the safety and feasibility of convection-enhanced delivery of carboplatin into the white matter as a potential treatment for high-grade glioma. J Neurooncol. 2012;108(1):77–88. doi: 10.1007/s11060-012-0833-4. [DOI] [PubMed] [Google Scholar]
- 35.Sawyer AJ, Saucier-Sawyer JK, Booth CJ, et al. Convection-enhanced delivery of camptothecin-loaded polymer nanoparticles for treatment of intracranial tumors. Drug Deliv Transl Res. 2011;1(1):34–42. doi: 10.1007/s13346-010-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruce JN, Fine RL, Canoll P, et al. Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery. 2011;69(6):1272–1279. doi: 10.1227/NEU.0b013e3182233e24. discussion 1279–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez KA, Tannenbaum AM, Assanah MC, et al. Convection-enhanced delivery of topotecan into a PDGF-driven model of glioblastoma prolongs survival and ablates both tumor-initiating cells and recruited glial progenitors. Cancer Res. 2011;71(11):3963–3971. doi: 10.1158/0008-5472.CAN-10-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonabend AM, Stuart RM, Yun J, et al. Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump. Neuro Oncol. 2011;13(8):886–893. doi: 10.1093/neuonc/nor051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.