Abstract
Background
A phase II trial was performed to evaluate the efficacy of a dose-dense, 7 days on/7 days off schedule of temozolomide for patients with recurrent high-grade gliomas (HGG).
Methods
Sixty patients with recurrent HGG received temozolomide at 150 mg/m2/day on days 1–7 and days 15–21 during each 4-week cycle. The primary endpoint was 6-month progression-free survival (PFS-6), with a secondary endpoint of overall survival (OS). A further exploratory objective included the investigation of whether methylation status of the O(6)-methylguanine-DNA methyltransferase (MGMT) promoter within tumor tissue predicted outcomes.
Results
Among patients with glioblastoma (n = 40), PFS-6 was 10% (95% CI, 3%–24%) with median OS of 21.6 weeks (95% CI, 16.9–30.6 weeks). PFS-6 for grade III glioma patients (n = 20) was 50% (95% CI, 27%–73%), and median OS was 100.6 weeks (95% CI, 67 weeks to not reached). There were trends towards longer PFS and OS with MGMT promoter methylation (log-rank test; P = .06 for PFS; P = .07 for OS). Additionally, bevacizumab-naïve glioblastoma patients had significantly longer PFS and OS (median PFS was 8.07 weeks [95% CI, 8 weeks to not reached] vs 7.57 weeks [95% CI, 7.29–8.29 weeks], log-rank test, P < .001; median OS was 62 weeks [26.1 weeks to not reached] vs 18.2 weeks [13.9–27.3 weeks], log-rank test, P < .001).
Conclusions
The dose-dense temozolomide regimen was well tolerated, although it has no significant activity in this population. Clinical trials.gov identified. NCT00619112 (available at http://clinicaltrials.gov/ct2/show/NCT00619112).
Keywords: bevacizumab, chemotherapy, glioblastoma, glioma, temozolomide
See the editorial by van den Bent and Taal, on pages 1161–1163.
The current management paradigm for high-grade glioma (HGG) includes maximum safe surgical resection followed by external beam radiotherapy at 60.0 Gy with concurrent daily oral temozolomide (TMZ), followed by adjuvant TMZ given 5 days out of every 28-day cycle, for a total of 6 cycles.1 The optimal treatment regimen for recurrent disease, particularly with multiple recurrences, remains unknown. While clinical trials in this setting have yielded little success, phase II trials of bevacizumab have shown an increase in response rates that resulted in its accelerated approval by the FDA in 2009,2,3 although with no clear improvement in overall survival (OS).
Alternative dosing regimens of TMZ have been developed to enhance the inhibitory action upon O6-alkylguanine-DNA alkyltransferase (the DNA repair enzyme encoded by O(6)-methylguanine-DNA methyltransferase [MGMT] gene and a major mechanism of resistance to TMZ). A more protracted exposure to temozolomide results in MGMT consumption and depletes the cell repair mechanism, preventing recovery from DNA mutations introduced by alkylating agents.4,5 Progressive depletion of MGMT following prolonged exposure to temozolomide has been demonstrated in preclinical settings6,7; metronomic or continuous TMZ appears to have anti-angiogenic effects via targeting vascular endothelial cells as well.4,8,9 A number of dose-dense regimens of TMZ have been developed and studied, including (i) continuous daily dosing at 75 mg/m2; (ii) 150 mg/m2 at days 1–5 and days15–19 for every 28 days; (iii) 150 mg/m2 at 7 days on/7 days off; and (iv) 100 mg/m2 at 21 days on/7 days off. All of these schedules appear to be well tolerated by HGG patients.10–24 Of these regiments, the last two (7 days on/7 days off and 21 days on/7days off) have the highest dose intensity, allowing for twice as much drug exposure then the conventional schedule of 5 days out of every 28 days.23
A number of phase II studies have evaluated the role of the 7 days on/7 days off schedule in both the upfront and recurrent settings.11,13,14,24–27 However, in the recurrent population, there is limited experience with the 7 days on/7 days off schedule, especially for patients who have had prior treatment with TMZ at initial diagnosis. More specifically, the activity of the 7 days on/7 days off schedule in a heavily pretreated cohort, typical of patients currently enrolled in clinical trials for recurrent high-grade gliomas, remains unclear. Therefore, we designed this phase II study to examine the efficacy and toxicity of TMZ when given on a dose-dense regimen of 7 days on at 150 mg/m2/day and 7 days off schedule to patients with recurrent high-grade glioma who have recurred despite previous treatment with the standard 5 days monthly schedule of adjuvant TMZ. We also performed an exploratory analysis to determine whether the methylation status of MGMT within the patients' tumors predicted a greater efficacy in those who were treated with this dose-intense TMZ schedule. It should be noted that this study was opened to accrual prior to the approval of bevacizumab for recurrent glioblastoma.
Materials and Methods
The study was a prospective, single-arm phase II trial conducted at the University of California, San Francisco (UCSF); participants were enrolled from November, 2007, to January, 2012. Patients with recurrent, intracranial high-grade glioma (HGG) were eligible. HGG included glioblastoma multiforme (GB), gliosarcoma (GS); HGG also included anaplastic glioma (AG), more specifically anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), or anaplastic mixed oligoastrocytoma (AMO). All pathology was centrally reviewed at UCSF. Participants were at least 18 years of age, and Karnofsky performance status (KPS) was ≥60%. All participants had undergone previous external beam radiotherapy and had been previously treated with at least one cycle of the standard adjuvant 5-day dose schedule of TMZ at 150–200 mg/m2/day. There was no limit to the number of prior treatments the participants had undergone, but they must have recovered from the prior treatment and met eligibility guidelines. Patients with active concurrent malignancy were not eligible. Evidence of adequate baseline bone marrow, liver, and renal function were required by the following laboratory tests: white blood cell count ≥ 3000/µL, absolute neutrophil count ≥1500/mm3, platelet count ≥100 000/mm3, hemoglobin ≥10 gm/dL, aspartate aminotransferase and bilirubin <2 times upper limit of normal, and creatinine <1.5 mg/dL.
Treatment consisted of TMZ given at 150 mg/m2 daily for 7 consecutive days every other week. One cycle was defined as 28 days, of which treatment with TMZ was given on days 1–7 and days 15–21 and no treatment on days 8–14 or days 22–28. The treatment continued for 12 cycles or until progression or the development of unacceptable side effects. Corticosteroids were used in the smallest dose to control symptoms of cerebral edema and mass effect. Antiepileptic and antiemetic medications were used at the treating physician's discretion, with the exception of valproic acid, which was contraindicated due to the reduced clearance of temozolomide with its concurrent use. Complete blood counts were performed every 2 weeks, liver and renal function tests every 4 weeks. Clinical assessment of disease status was performed at every cycle, and radiographic assessment was obtained every other cycle. Response was evaluated via the Macdonald criteria.28 The National Cancer Institute Common Terminology Criteria of Adverse Events, version 3.0, were used to evaluate toxicity. Due to concern over cumulative toxicity, treatment was held for a minimum of 1 week for any toxicity grade ≥3 and resumed when it resolved to grade 1. Upon resolution of toxicity, when the treatment was resumed, the TMZ dose was reduced to 125 mg/m2/day. A second dose reduction to 100 mg/m2/day was allowed. The study was approved by the institutional review board at University of California, San Francisco, and all participants provided written informed consent.
Trial Design and Statistics
The primary endpoint was progression-free survival at 6 months (PFS-6), calculated from study registration. Secondary endpoints included OS and toxicity. The trial was powered to compare the treated cohort with a historical control of recurrent glioma participants enrolled in phase II trials (PFS-6 of 10% for GB and 31% for grade III).29 Planned enrollment was 40 participants with GB and 20 participants with grade III tumors. The planned sample size was based on the GB cohort, which was powered to provide 90% probability of detecting an improvement in the PFS-6 from 10% to 25% based on a 1-sided exact test with alpha = 0.10. The proportion of participants with PFS >6 months was provided with corresponding confidence intervals. Tests of proportion were based on the exact binomial distribution. Kaplan-Meier curves and confidence intervals were provided for PFS and OS, and log-rank statistics were used for group comparisons.
MGMT Promoter Methylation Status and Evaluation
As prospectively planned, MGMT promoter methylation status was evaluated on formalin-fixed, paraffin-embedded tumor tissue samples. DNA isolation, DNA bisulfite modification, and real-time methylation-specific PCR (MSP) were performed using beta-actin (ACTB) for the reference gene, as previously described.30 The MGMT gene promoter methylation status was determined by amplification of a methylated region of the MGMT gene and methylation-neutral region of the ACTB gene using sodium bisulfite-treated genomic DNA. The copy number of methylated MGMT promoter was normalized to the ACTB gene copy number. This normalized ratio, MGMT/(ACTB*1000), was used to determine the methylation status of the sample. If the normalized ratio was higher than 2.0, the sample was considered methylated. However, if ACTB copy number did not reach 1250 copies or greater, the methylation status was not interpreted and the analysis was considered invalid, no matter what the value was of the normalized ratio.
Results
Participant Characteristics
Sixty participants were enrolled and started on dose-dense treatment (See Table 1). The cohort included 40 participants with recurrent GB and 20 participants with grade III recurrent gliomas (9 with AA, 8 with AO, and 3 with AOA. See Table 2 for further breakdown of grade III participants; please note that all but one grade III participant were originally diagnosed prior to the end of 2009, before 1P19Q and IDH testing were routinely performed at UCSF and thus made such information limited. The median age of the cohort was 53 years (standard deviation, 10.2 years), and 63% of participants were male. The median KPS score was 90%, and 72% of participants had a KPS score ≥80%. All participants had undergone radiotherapy with concurrent and adjuvant TMZ at standard dosing on initial diagnosis. Thirty-eight participants (63%) had been treated for one or more prior recurrences, and 48% of participants had failed prior bevacizumab therapy.
Table 1.
Patient characteristics
| Glioblastoma (n = 40) | Grade III glioma (n = 20) | Entire cohort (n = 60) | |
|---|---|---|---|
| Sex | |||
| Men | 28 (70%) | 10 (50%) | 38 (63%) |
| Women | 12 (30%) | 10 (50%) | 22 (37%) |
| Median age (range) | 55 (30–68) | 48 (27–70) | 53 (27–70) |
| Median KPS (range) | 85 (60%–90%) | 90 (60%–90%) | 90 (60%–90%) |
| Number of previous recurrences | |||
| 1 | 12 (30%) | 10 (50%) | 22 (37%) |
| 2 | 18 (45%) | 7 (35%) | 25 (42%) |
| ≥3 | 10 (25%) | 3 (15%) | 13 (22%) |
| Prior salvage therapy with bevacizumab | 26 (65%) | 3 (15%) | 29 (48%) |
| MGMT promoter methylation status | |||
| Methylated | 7 (18%) | 10 (50%) | 17 (28%) |
| Unmethylated | 21 (53%) | 3 (15%) | 24 (40%) |
| Invalid MGMT results | 5 (13%) | 2 (10%) | 7 (12%) |
| Tissue unavailable | 7 (18%) | 5 (25%) | 12 (20%) |
Table 2.
Grade III histology characteristics
| Grade III glioma (n = 20) | |
|---|---|
| Anaplastic pathology type | |
| Oligodendroglioma | 8 (40%) |
| Astrocytoma | 9 (45%) |
| Mixed | 3 (%) |
| 1P19Q deletion status oligodendrogliomas (n = 8) | |
| Deleted | 1 (12.5%) |
| Not deleted | 3 (37.5%) |
| Unknown | 4 (50%) |
| 1P19Q Deletion status mixed (n = 3) | |
| Not deleted | 1 (33) |
| Unknown | 2 (66) |
Response, Progression-free Survival and Overall Survival
Imaging response (defined as complete or partial response)28 was observed in 2 participants (3.3%). Among participants with GB (n = 40), PFS-6 was 10% (95% CI, 3%–24%) with median OS of 21.6 weeks (95% CI, 16.9–30.6 weeks). The PFS-6 for grade III glioma participants (n = 20) was 50% (95% CI, 27%–73%), and median OS was 100.6 weeks (95% CI, 67 to not reached). The historical control cohort had a PFS-6 of 10% for GB and 31% for grade III gliomas, whereas the median PFS was 9 weeks for GB and 13 weeks for grade III gliomas.29 Only for grade III participants was the improvement in PFS-6 over the historical control statistically significant (Table 3). See Figs 1 and 2 for PFS and OS results.
Table 3.
Survival data
| Survival | Histology |
Prior Exposure to Bevacizumab (GBM Patients only) |
Methylation Status of MGMT |
||||||
|---|---|---|---|---|---|---|---|---|---|
| GBM | Grade III | P | Bevacizumb-failed | Bevacizumab-naïve | P | Methylated | Unmethylated | P | |
| Progression-free survival | |||||||||
| Median (weeks), 95% CI | 8.0 (7.57–8.29) | 31.3 (8.43–73.9) | .007 | 7.57 (7.29–8.29) | 8.07 (8-NR) | .02 | 11.6 (8.14–49.6) | 7.57 (7.29–9.00) | .06 |
| Overall survival | |||||||||
| Median (weeks), 95% CI | 21.6 (16.9–30.6) | 100.6 (67–NR) | <.001 | 18.2 (13.9–27.3) | 62 (26.1–NR) | .001 | 65.3 (40.4–NR) | 19.4 (16.1–55.3) | .07 |
Abbreviations: CI, confidence interval; NR, not reached.
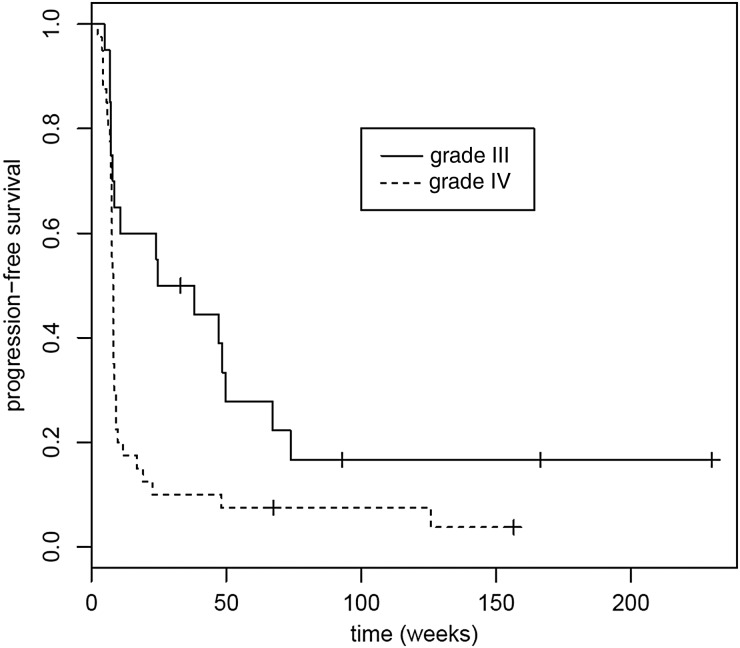
Fig. 1.
Progression-free survival (PFS) for grade III and glioblastoma cohorts. Median PFS for grade III was 31.3 weeks (95% CI, 8.43–73.9 weeks); median PFS for glioblastoma was 8.0 weeks (95% CI, 7.57–8.29 weeks). P = .007.
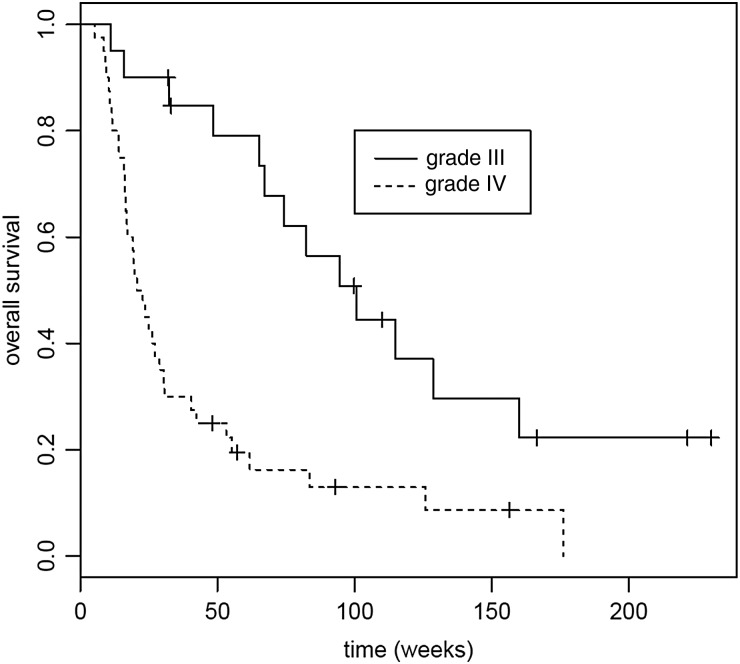
Fig. 2.
Overall survival (OS) for grade III and glioblastoma cohorts. Median OS for grade III was 100.6 weeks (95% CI, 67 weeks to not reached); median OS for glioblastoma was 21.6 weeks (95% CI, 16.9–0.6 weeks). P < .001.
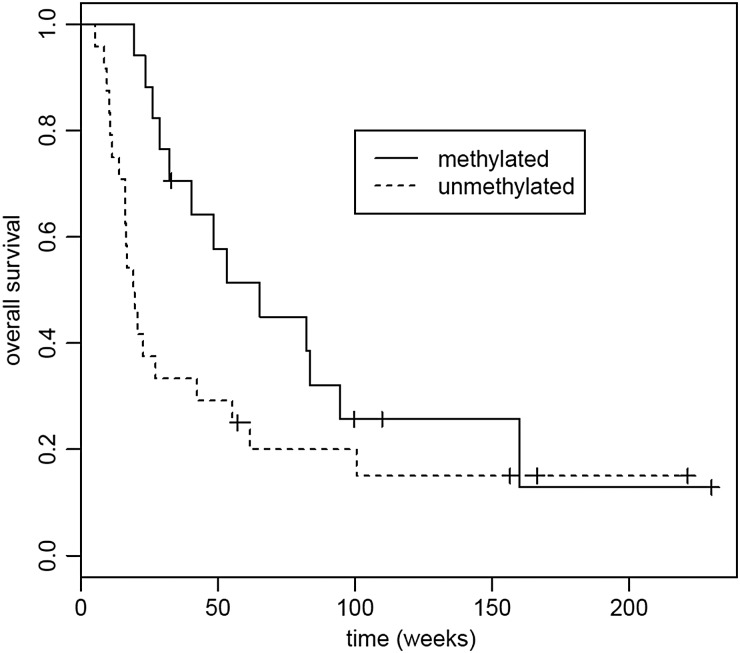
Participants with GB who progressed on bevacizumab and then were treated on this trial had a median PFS of 7.57 weeks (95% CI, 7.29–8.29 weeks), and median OS of 18.2 weeks (95% CI, 13.9–27.3 weeks), while bevacizumab-naïve GB participants had median PFS at 8.07 weeks (95% CI, 8 weeks to not reached), and median OS of 62 weeks (95% CI, 26.1 week to not reached). The differences in both PFS and OS between the 2 glioblastoma groups were statistically significant (P = .02, P =0.001, respectively). See Figs 3 and 4. In a post hoc analysis, we analyzed whether established pretreatment prognostic factors were different between the bevacizumab-failed and the bevacizumab-naïve participants. The 2 groups were similar in age and KPS (P = 0.28, Student t test and P = .22, Fisher exact test, respectively). Bevacizumab-naïve participants, however, had significantly fewer prior progressions and hence fewer prior therapies (median 1 vs 2, P = .01, Fisher exact test). Notably, all participants who were bevacizumab naïve did go on to eventually receive bevacizumab, albeit in different sequences and combinations.
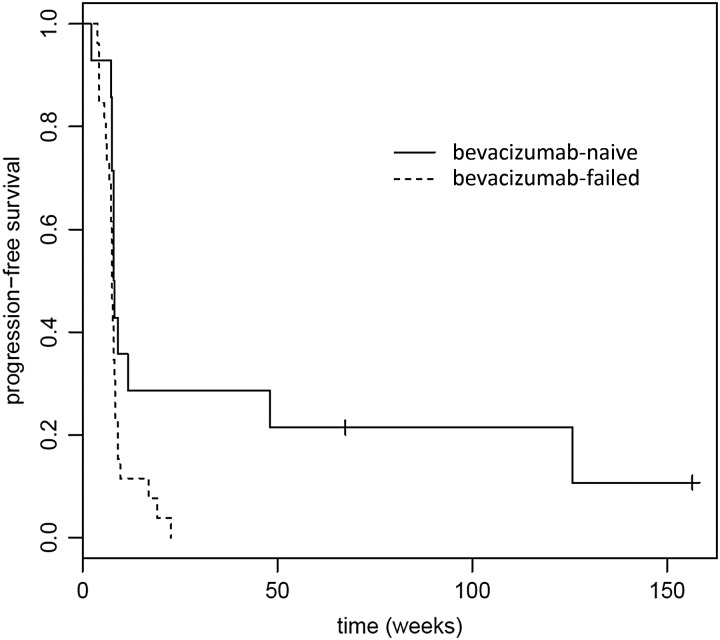
Fig. 3.
Progression-free survival (PFS) for recurrent glioblastoma patients (n = 40) who were bevacizumab naïve (n = 14) and those who had previously failed bevacizumab (n = 26). Median PFS for bevacizumab naïve was 8.07 weeks (95% CI, 8 weeks to not reached); median PFS for bevacizumab-failed was 7.57 weeks (95% CI, 7.29–8.29 weeks). P = .02.
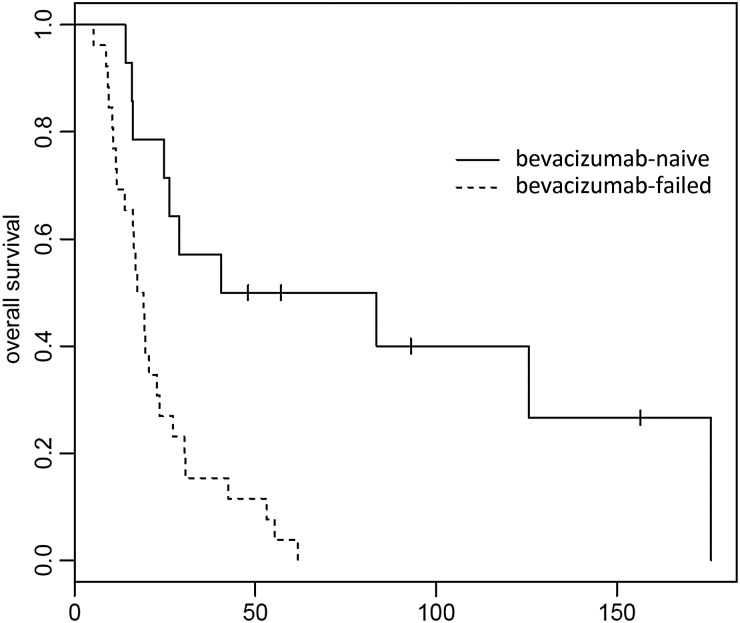
Fig. 4.
Overall survival (OS) for recurrent glioblastoma patients (n = 40) who were bevacizumab naïve (n = 14) and those who had failed bevacizumab (n = 26). Median OS for bevacizumab-failed was 18.2 weeks (95% CI, 13.9–27.3 weeks); median OS for bevacizumab-naïve was 62 weeks (95% CI, 26.1 weeks to not reached). P =.001.
Toxicity
The treatment regimen was well tolerated. The most common toxicity was myelosuppression. Common Terminology Criteria for Adverse Events grade 4 hematotoxicity was observed in 7 participants (12%). Grade 3 toxicity was observed in 33 participants (55%) and included lymphopenia (n = 22), thrombocytopenia (n = 2), leukopenia (n = 1), pain and neuropathy (n = 2), somnolence (n = 1), aphasia (n = 1), fatigue (n = 1), cellulitis (n = 1), hyperglycemia (n = 1), and nausea (n = 1). Toxicity prompted reduction of the TMZ dose in 7 participants (12%) and ultimately discontinuation of therapy for 3 participants (5%, Table 4).
Table 4.
Grade 3 or 4 toxicities
| Toxicity | Number of Cases | Outcome |
|---|---|---|
| Lymphopenia | 26 | Dose reduced in 4 cases |
| Thrombocytopenia | 3 | Treatment stopped in 1 case, dose reduced in 2 cases |
| Leukopenia | 3 | Treatment stopped in 1 case, dose reduced in 1 case |
| Pain/neuropathy | 2 | Resolved |
| Somnolence | 1 | Resolved |
| Aphasia | 1 | Resolved |
| Fatigue | 1 | Dose reduced |
| Cellulitis | 1 | Resolved |
| Hyperglycemia | 1 | Resolved |
| Nausea | 1 | Treatment stopped |
Exploratory MGMT Analysis
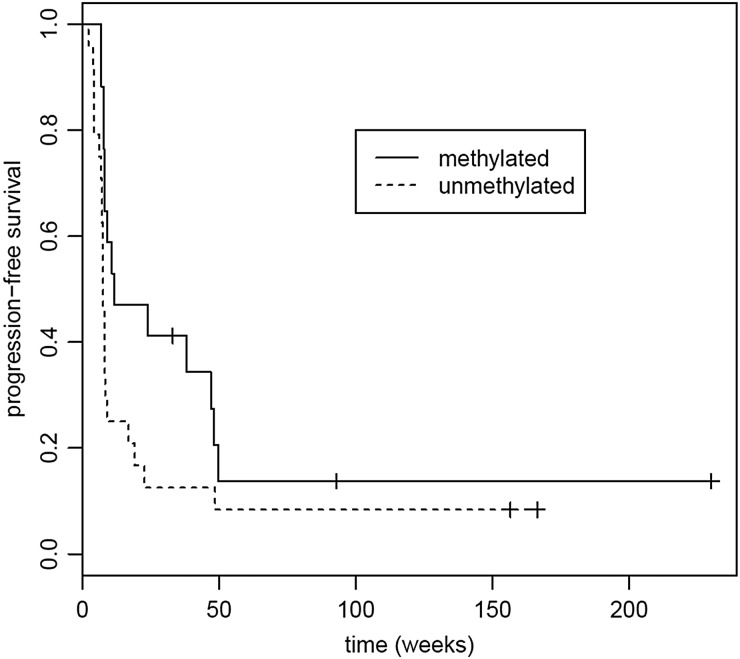
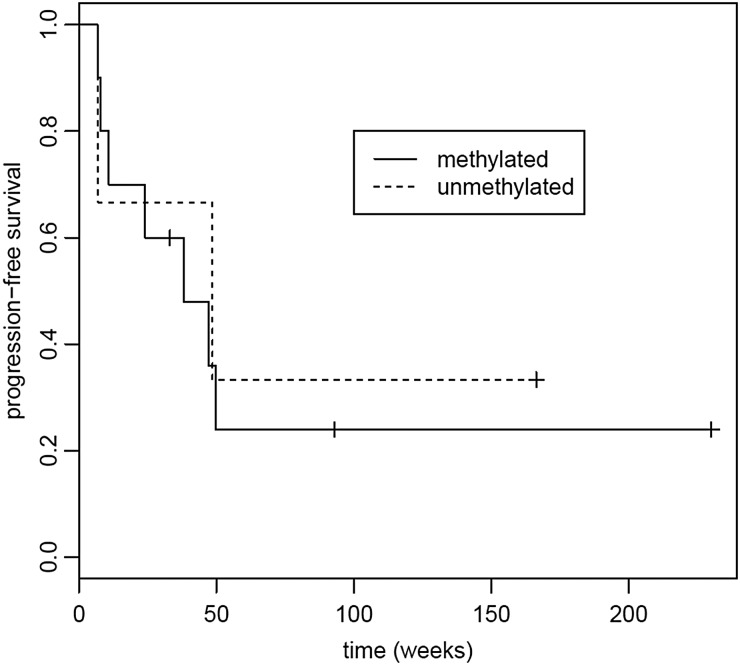
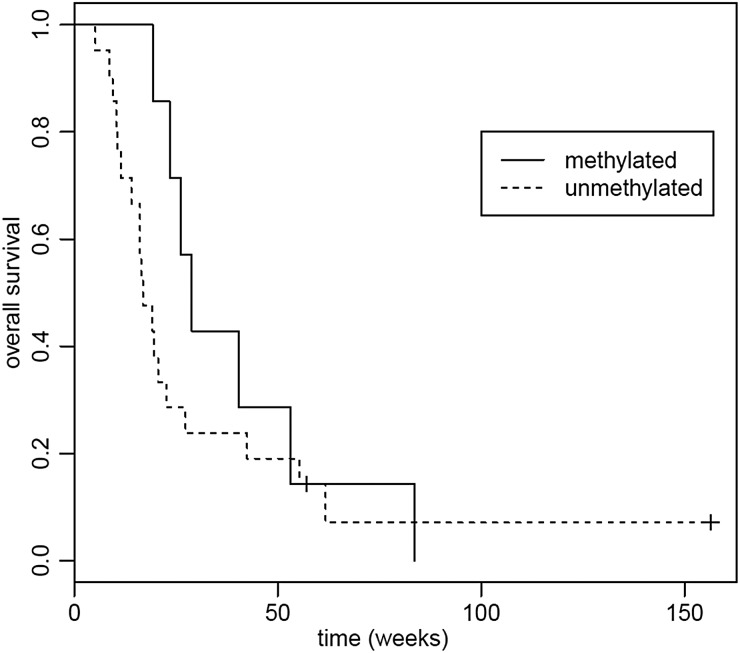
Tumor tissue was available for the exploratory MGMT methylation analysis in 48 participants. Seventeen (28%) tumors had methylated MGMT, and 24 (40%) had unmethylated MGMT. In 7 samples (12%), the tissue was inadequate for analysis. Median PFS in participants with unmethylated MGMT was 7.57 weeks (95% CI, 7.29–9.00 weeks), and median OS was 19.4 weeks (95% CI, 16.1–55.3 weeks) compared with median PFS of 11.57 weeks (95% CI, 8.14–49.6 weeks) and median OS of 65.3 weeks (95% CI, 40.4 weeks to not reached) in participants with methylated MGMT. In both the PFS and survival analyses, there were trends towards longer PFS and OS with MGMT promoter methylation (log-rank test, P = .06, P = .07, respectively). See Figs 5 and 6.
Fig. 5.
Progression-free survival (PFS) based on tumor MGMT promoter methylation status. Median PFS for methylated MGMT:was 11.6 weeks (95% CI, 8.14–49.6 weeks); median PFS for unmethylated MGMT was 7.57 weeks (95% CI, 7.29–9.00 weeks). P = .06.
Fig. 6.
Overall survival (OS) based on tumor MGMT promoter methylation status. Median OS for methylated MGMT was 65.3 weeks (95% CI, 40.4 weeks to not reached); median OS for unmethylated MGMT was 19.4 weeks (95% CI, 16.1–55.3 weeks). P = .07.
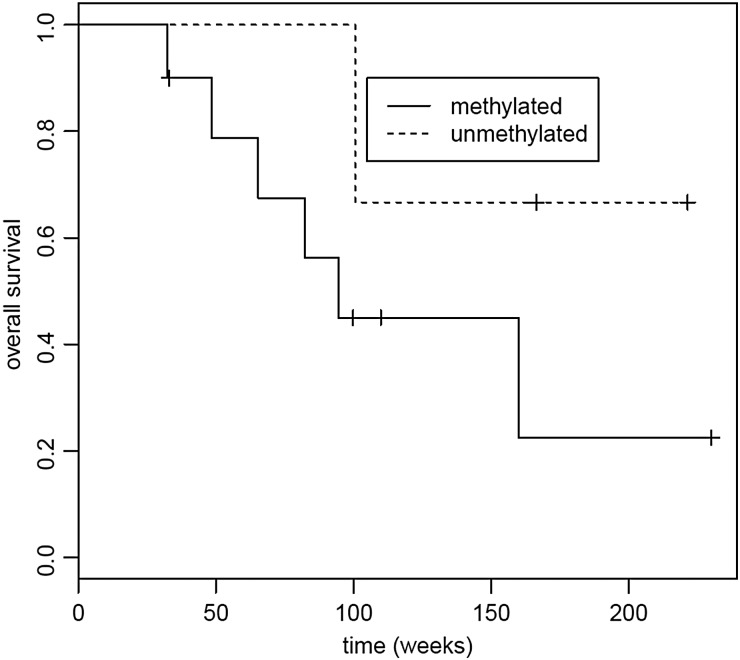
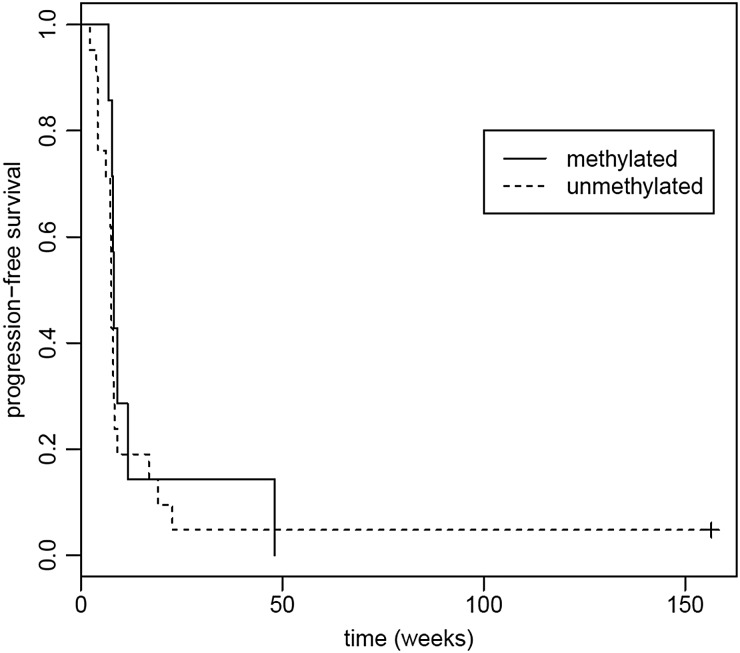
Among the participants with grade III tumors, 10 tumors (50%) had methylated MGMT, while 3 tumors (15%) had unmethylated MGMT. Median PFS in grade III tumor participants with methylated MGMT was 38.1 weeks (95% CI, 10.71 weeks to not reached) and median OS was 94.6 weeks (95% CI, 65.3 weeks to not reached), compared with median PFS of 48.6 weeks (95% CI, 6.86 weeks to not reached) and median OS of 100.6 weeks (no CI due to small sample size) in participants with unmethylated MGMT. Among GB participants, 7 tumors (18%) had methylated MGMT, while 21 tumors (53%) had unmethylated MGMT. Median PFS in GB participants with methylated MGMT was 8.14 weeks (95% CI, 7.71 weeks to not reached), and median OS was 28.9 weeks (95% CI, 2.34 weeks to not reached), compared with median PFS of 7.57 weeks (95% CI, 7.29–9 weeks) and median OS of 16.9 weeks (95% CI, 14.0–42.4 weeks) in participants with unmethylated MGMT. Among individual histological types, comparisons of PFS and OS based on MGMT methylation status did not yield significant differences. See Figs 7–10.
Fig. 8.
Overall survival (OS) based on grade III tumors MGMT promoter methylation status. Median OS for methylated MGMT was 94.6 weeks (95% CI, 65.3 weeks to not reached); median OS for unmethylated MGMT was 100.6 weeks (No CI due to small sample size). P = .46.
Fig. 9.
Progression-free survival (PFS) based on GB tumor MGMT promoter methylation status. Median PFS for methylated MGMT was 8.14 weeks (95% CI, 7.71 weeks to not reached); median PFS for unmethylated MGMT was 7.57 weeks (95% CI, 7.29–9 weeks). P = .22.
Fig. 7.
Progression-free survival (PFS) based on grade III tumor MGMT promoter methylation status. Median PFS for methylated MGMT was 38.1 weeks (95% CI, 10.71 weeks to not reached); median PFS for unmethylated MGMT was 48.6 weeks (95% CI, 6.86 weeks to not reached). P = .79.
Fig. 10.
Overall survival (OS) based on GB tumors MGMT promoter methylation status. Median OS for methylated MGMT was 28.9 weeks (95% CI, 2.34 weeks to not reached); median OS for unmethylated MGMT was 16.9 weeks (95% CI, 14.0–42.4 weeks). P = .33.
Discussion
Our results demonstrate the feasibility of the dose-dense 7 days on/7 days off schedule of TMZ in the typical, heavily pretreated population with recurrent HGG enrolled in clinical trials. The primary efficacy endpoint, based on historical controls, was not met in the GB cohort, with PFS-6 of only 10% and is therefore not a treatment option for such patients. However, it is feasible that the regimen may have some activity in the recurrent grade III population, in whom PFS-6 was 50% and median OS was 100.6 weeks when all grade III participants were combined. However, the authors strongly caution that this was a very small study with a mixed population including several participants with AO who may, at baseline and regardless of therapy, possess a more favorable prognosis or favorable response to therapy. As such, conclusions about the grade III population in general cannot be made at present without larger ongoing trials that separate the different types of grade III histologies.
A wide range of alternative dosing schedules for TMZ administration has been developed and studied in clinical trials.10,12,14–24 Intensified, dose-dense regimens were designed with the goal of improving the therapeutic index in hopes of counteracting the mechanisms of resistance to alkylating agents such as TMZ. Although direct comparisons of these intensified dose regimens are difficult among different clinical trials, administration of all the regimens seems to be feasible because they are relatively well tolerated and show signs of activity. For example, a previous randomized phase II trial evaluated the same dose schedule as the present study (7 days on/7 days off at 150 mg/m2) versus daily continuous TMZ at 50 mg/m2 following the standard concurrent TMZ for newly diagnosed GB and revealed that only the 7 days on/7 days off schedule was superior in OS.11 However, toxicities appear to be more frequent with the 7 days on/7 days off regimen compared with trials utilizing the daily regimen, which may be due to due the higher dose exposure achieved.12,13,19,21 A recent multicenter, randomized phase III trial evaluated the 21 days on/7 days off regimen versus the standard regimen of TMZ in newly diagnosed GB and found that the dose-dense 21 days on/7 days off regimen was not superior to the standard dosing, regardless of MGMT status.31 The authors did confirm the prognostic benefit of MGMT methylation in both cohorts.
Previous prospective early phase studies evaluating the activity of the 7 days on/7 days off schedule have been conducted only in the setting of either newly diagnosed patients with malignant glioma or patients with recurrent disease who had not universally been exposed to the standard TMZ regimen. To our knowledge, this is the first prospective study reporting the efficacy of 7 days on/7 days off dosing in a heavily pretreated population, including universal prior treatment with the standard concurrent and adjuvant TMZ and nearly half with a history of recurrence after bevacizumab.
A recent phase II trial tested daily continuous dosing of TMZ for recurrent HGG in the era of bevacizumab.12 In their cohort of recurrent GB patients (n = 37) who were similar in their treatment histories to our cohort, the results were more promising, with PFS-6 of 19% and a median OS of 7 months (95% CI, 5–12 months). The outcomes observed in their exploratory cohort of grade III HGG were not as favorable as those seen in our cohort (PFS-6 of 30% vs 50%, median OS of 18 months vs 23.4 months). However, caution must be exercised in interpreting these results, given the small number of participants included.
The aforementioned trial also demonstrated that among participants with recurrent GB, those who were bevacizumab naïve fared much more favorably after the daily continuous TMZ than those who had failed on bevacizumab.12 The authors noted in a post hoc multivariate analysis, however, that the bevacizumab-naïve participants had been enrolled after significantly fewer prior recurrences, which was also true and represented a significant confounding factor in our cohort of GB participants. Similar results are found within the study presented here; however, whether previous exposure to bevacizumab serves as an independent predictor of response to an intensified TMZ regimen at a future recurrence remains unclear.
An ongoing question surrounding the utilization TMZ for HGGs is whether a more intensified TMZ dosing regimen would abrogate the differential responses and survival outcomes seen in the upfront setting from standard TMZ dosing based on the tumor's MGMT methylation status. In the upfront setting, the benefit of TMZ correlated with epigenetic silencing of DNA repair enzyme MGMT and activity of enzyme predictor of response to TMZ.32 Interestingly, even just among trials testing the 7 days on/7 days off regimen, some studies have found more favorable outcomes in participants with tumors with methylated MGMT,27,33 while others report no such difference.11,13 The current results are suggestive of improved outcomes when MGMT methylation is present in both recurrent GB and recurrent grade 3 HGGs participants after the 7 days on/7 days off regimen. A large phase III trial in a homogeneous population would be better suited for addressing this question, although it is not likely to be done due to only isolated possible activity.
In summary, the regimen of temozolomide given at 7 days on/7 days off was well tolerated and appeared to have no significant activity in this heavily pretreated population, including prior treatment with temozolomide and bevacizumab, and did not meet the predefined endpoint for patients with glioblastoma. Results were more favorable for bevacizumab-naïve and recurrent grade III participants; however; due to limited numbers and inherent patient heterogeneity, a confident conclusion cannot be made.
Funding
This study was funded by Merck.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 3.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tolcher AW, Gerson SL, Denis L, et al. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88(7):1004–1011. doi: 10.1038/sj.bjc.6600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SM, Thatcher N, Crowther D, et al. Inactivation of O6-alkylguanine-DNA alkyltransferase in human peripheral blood mononuclear cells by temozolomide. Br J Cancer. 1994;69(3):452–456. doi: 10.1038/bjc.1994.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wedge SR, Porteus JK, May BL, et al. Potentiation of temozolomide and BCNU cytotoxicity by O(6)-benzylguanine: a comparative study in vitro. Br J Cancer. 1996;73(4):482–490. doi: 10.1038/bjc.1996.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weller M, Steinbach JP, Wick W. Temozolomide: a milestone in the pharmacotherapy of brain tumors. Fut Oncol. 2005;1(6):747–754. doi: 10.2217/14796694.1.6.747. [DOI] [PubMed] [Google Scholar]
- 8.Kim JT, Kim JS, Ko KW, et al. Metronomic treatment of temozolomide inhibits tumor cell growth through reduction of angiogenesis and augmentation of apoptosis in orthotopic models of gliomas. Oncol Rep. 2006;16(1):33–39. [PubMed] [Google Scholar]
- 9.Kurzen H, Schmitt S, Naher H, et al. Inhibition of angiogenesis by non-toxic doses of temozolomide. Anticancer Drugs. 2003;14(7):515–522. doi: 10.1097/00001813-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Abacioglu U, Caglar HB, Yumuk PF, et al. Efficacy of protracted dose-dense temozolomide in patients with recurrent high-grade glioma. J Neurooncol. 2011;103(3):585–593. doi: 10.1007/s11060-010-0423-2. [DOI] [PubMed] [Google Scholar]
- 11.Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27(23):3861–3867. doi: 10.1200/JCO.2008.20.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omuro A, Chan TA, Abrey LE, et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol. 2013;15(2):242–250. doi: 10.1093/neuonc/nos295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wick A, Felsberg J, Steinbach JP, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25(22):3357–3361. doi: 10.1200/JCO.2007.10.7722. [DOI] [PubMed] [Google Scholar]
- 14.Wick W, Steinbach JP, Kuker WM, et al. One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma. Neurology. 2004;62(11):2113–2115. doi: 10.1212/01.wnl.0000127617.89363.84. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong TS, Wefel JS, Wang M, et al. Net Clinical Benefit Analysis of Radiation Therapy Oncology Group 0525: A Phase III Trial Comparing Conventional Adjuvant Temozolomide With Dose-Intensive Temozolomide in Patients With Newly Diagnosed Glioblastoma. J Clin Oncol. 2013;31(32):4076–4084. doi: 10.1200/JCO.2013.49.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brock CS, Newlands ES, Wedge SR, et al. Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res. 1998;58(19):4363–4367. [PubMed] [Google Scholar]
- 17.Kesari S, Schiff D, Drappatz J, et al. Phase II study of protracted daily temozolomide for low-grade gliomas in adults. Clin Cancer Res. 2009;15(1):330–337. doi: 10.1158/1078-0432.CCR-08-0888. [DOI] [PubMed] [Google Scholar]
- 18.Khan RB, Raizer JJ, Malkin MG, et al. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro Oncol. 2002;4(1):39–43. doi: 10.1215/15228517-4-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong DS, Lee JI, Kim JH, et al. Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro Oncol. 2010;12(3):289–296. doi: 10.1093/neuonc/nop030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norden AD, Lesser GJ, Drappatz J, et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol. 2013;15(7):930–935. doi: 10.1093/neuonc/not040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28(12):2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 22.Verhoeff JJ, Lavini C, van Linde ME, et al. Bevacizumab and dose-intense temozolomide in recurrent high-grade glioma. Ann Oncol. 2010;21(8):1723–1727. doi: 10.1093/annonc/mdp591. [DOI] [PubMed] [Google Scholar]
- 23.Wick W, Platten M, Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009;11(1):69–79. doi: 10.1215/15228517-2008-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong S, Rosenthal MA, Dowling A, et al. Phase II study of two-weekly temozolomide in patients with high-grade gliomas. J Clin Neurosci. 2006;13(1):18–22. doi: 10.1016/j.jocn.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Dall'oglio S, D'Amico A, Pioli F, et al. Dose-intensity temozolomide after concurrent chemoradiotherapy in operated high-grade gliomas. J Neurooncol. 2008;90(3):315–319. doi: 10.1007/s11060-008-9663-9. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert MR, Gonzalez J, Hunter K, et al. A phase I factorial design study of dose-dense temozolomide alone and in combination with thalidomide, isotretinoin, and/or celecoxib as postchemoradiation adjuvant therapy for newly diagnosed glioblastoma. Neuro Oncol. 2010;12(11):1167–1172. doi: 10.1093/neuonc/noq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiler M, Hartmann C, Wiewrodt D, et al. Chemoradiotherapy of newly diagnosed glioblastoma with intensified temozolomide. Int J Radiat Oncol Biol Phys. 2010;77(3):670–676. doi: 10.1016/j.ijrobp.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald DR, Cascino TL, Schold SC, Jr., et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 29.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 30.Vlassenbroeck I, Califice S, Diserens AC, et al. Validation of real-time methylation-specific PCR to determine O6-methylguanine-DNA methyltransferase gene promoter methylation in glioma. J Mol Diagn. 2008;10(4):332–337. doi: 10.2353/jmoldx.2008.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert MR, Wang M, Aldape KD, et al. Dose-Dense Temozolomide for Newly Diagnosed Glioblastoma: A Randomized Phase III Clinical Trial. J Clin Oncol. 2013;31(32):4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 33.Chinot OL, Barrie M, Fuentes S, et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. J Clin Oncol. 2007;25(12):1470–1475. doi: 10.1200/JCO.2006.07.4807. [DOI] [PubMed] [Google Scholar]