Abstract
Background
Adipocyte-secreted apelin contributes to decreased adiposity and to improved insulin resistance, but the mechanisms remain unknown. The present study aimed to assess if apelin-13 is an upstream signal regulation factor of aquaporin 7 (AQP7), a water-glycerol transporter present in the plasma membrane of adipocytes that plays a key role in the regulation of lipid accumulation.
Material/Methods
3T3-L1 pre-adipocytes were induced to fully differentiated adipocytes; hypertrophic adipocytes were then induced using palmitate. The effects of apelin-13 on AQP7 expression in hypertrophic adipocytes were investigated before and after treatment with LY249002, a PI3K inhibitor. Accumulation of cytoplasmic triglycerides (TG) in hypertrophic adipocytes was also determined.
Results
We found that 0.1 mM of palmitate induced a model of hypertrophic adipocytes with a lower AQP7 expression (0.26±0.07 vs. 0.46±0.04, P<0.05). Apelin-13 100 nM or 1000 nM upregulated AQP7 mRNA expression (100 nM: 0.54±0.06 and 1000 nM: 0.58±0.09 vs. control: 0.33±0.04, both P<0.05), and decreased accumulation of cytoplasmic triglycerides in hypertrophic adipocytes. Pretreatment using 10 μM LY294002 prevented the increase in AQP7 expression observed when using apelin-13 alone (apelin-13 + LY49002: 0.38±0.03 vs. apelin-13: 0.54±0.06, P<0.05), as well as the decreased cytoplasmic TG accumulation (apelin-13 + LY294002: 3.79±0.04 μM per μg/ml vs. apelin-13: 3.32±0.08 μM per μg/ml, P<0.05).
Conclusions
Apelin-13 decreases lipid storage in hypertrophic adipocytes in vitro, possibly through the upregulation of AQP7 expression by the PI3K signaling pathway. Treatment using apelin-13 and AQP modulators might represent novel treatment strategies against obesity and its related complications.
MeSH Keywords: Adipocytes, Apelin-13, Phosphatidylinositol 3-Kinases, 3T3-L1 Cells, Aquaporins
Background
Adipose tissue is a major endocrine organ producing a variety of adipokines affecting energy balance [1]. Obesity is characterized by increases in the number (hyperplasia) and in the size (hypertrophy) of adipocytes through mitosis and differentiation [2], and through increased triglycerides (TG) storage in mature adipocytes [3]. Obesity is intimately associated with many metabolic diseases, such as lipid disorders, coronary heart diseases, hypertension, and diabetes mellitus. Therefore, investigating safe, effective, and long-term ways to decrease body weight is an important issue in preventing a number of chronic diseases.
Aquaporin-7 (AQP7) is expressed in adipose tissue, testis, kidney, and heart [4–7], and is regulated through fasting/feeding, insulin, dexamethasone, glucocorticoids, cytokines, epinephrine, and peroxisome proliferator-activated receptor (PPAR)-γ agonists, including rosiglitazone, which activates adipogenesis and lipid accumulation in tissues, as well as increasing insulin sensitivity [8]. AQP7 plays an important role in glycerol secretion from adipose tissue [4] and facilitates glycerol transport, leading to a reduction in TG levels [9,10].
Apelin is a small peptide found in a number of tissues, including adipose tissue, and has emerged as a new factor with potent effects on energy metabolism and on the improvement of insulin sensitivity [11–13]. Previous studies reported that apelin was associated with obesity [14–16], but the mechanisms are still largely unknown.
A study reported that long-term (2 weeks) peripheral injection of apelin decreased the TG content in adipose tissue and the weight of different fat tissues in chow-fed mice and in obese mice [17]. Plasma TG levels were also decreased in normal and obese apelin-treated mice. Among insulin-responsive tissues, the apelin receptor is expressed in adipose tissue, skeletal muscles, and the liver [18].
However, upstream signal regulatory factors leading to apelin-induced effects are still not completely elucidated. A previous study showed that apelin-13 increased vascular smooth muscle cell proliferation through the PI3K pathway [19], but the involvement of PI3K in adipocyte metabolism is unknown. We selected apelin-13 because it was previously investigated in obese mice and was shown to influence adiposity in these mice [17], as well as being one of the most active isoforms [12,18]. We hypothesized that apelin decreases lipid storage, at least in part, through an autocrine pathway involving AQP7. Therefore, the purpose of this study was to assess the effects of apelin-13 on decreased lipid storage and on AQP7 expression in hypertrophic adipocytes, and the involvement of the PI3K signaling pathway.
Material and Methods
Cell culture
The 3T3-L1 pre-adipocytes were purchased from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). 3T3-L1 pre-adipocytes were cultured in DMEM (GIBCO, Invitrogen Inc., Carlsbad, CA, USA) supplemented with 10% calf serum (GIBCO, Invitrogen Inc., Carlsbad, CA, USA) with 5% CO2 at 37°C. Upon reaching 100% confluence, cells were incubated with DMEM supplemented with 10% calf serum for 2 days and then transferred into a differentiation-induction medium containing methylisobutylxanthine (0.5 mM), dexamethasone (1 μM) (Sigma, St Louis, MI, USA), and insulin (5 mg/L) (Sigma) in DMEM containing 10% fetal bovine serum (FBS) (Hyclone, South Logan, UT, USA). After 2 days (at day 4), the differentiation-induction medium was replaced with DMEM containing only insulin (5 mg/L) for another 2 days. Cells were then cultured in DMEM without insulin. On days 8–10, more than 90% of cells displayed an adipocyte phenotype with the accumulation of lipid droplets [20,21]. Cells were harvested in these conditions. To induce hypertrophic adipocytes, differentiated adipocytes were cultured for 12 h in serum-free DMEM to synchronize them; they were then cultured in DMEM containing 10% FBS and 0.1 mM palmitate (Sigma) for 48 h.
Hypertrophic 3T3-L1 cells were treated with 0, 1, 10, 100, and 1000 nM of apelin-13 (Phoenix Biotech, Phoenix, USA) for 0, 12, 24, 36, and 48 h. Cells were cultured with 10 μM rosiglitazone (TaiJi Group, Chongqing, China) as the positive control. To determine the involvement of PI3K in apelin-stimulated AQP7 expression, LY294002 10 μM (Sigma) [22] was preincubated 30 min before the incubation of hypertrophic 3T3-L1 cells with 100 nM of apelin-13.
Oil Red O staining
After differentiation or hypertrophy, adipocytes were stained with Oil Red O to detect lipid droplets [23]. Cells were washed 3 times with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 30 min at room temperature, and stained with filtered Oil Red O solution (60% isopropanol and 40% water) for 1 h at 37°C. After staining, Oil Red O was removed and the plates were rinsed with water and dried. Images were obtained using a microscope (ECLIPSE TE2000-S, Nikon instruments, Tokyo, Japan).
Measurement of triglyceride content
Cells were treated with palmitate at concentrations of 0, 0.1, 0.2, and 0.4 mM for 48 h in 6-well plates after adipocyte differentiation for 8 days. Hypertrophic 3T3-L1 adipocytes were cultured for 48 h with apelin-13 100 nM, with or without a 30-min preincubation with LY294002 10 μM. Cells were washed with PBS twice to remove glycerin, and were treated with 200 μl of lysis buffer on ice. Lysates (50 μl) were heated at 70°C for 10 min, and centrifuged at 2000 rpm for 5 min at room temperature; cellular TG content in supernatant was then assessed with the Triglyzyme-V commercial TG assay kit (Applygen Technologies, Beijing, China). TG content was normalized to protein concentrations. The remaining lysates were used to quantify protein concentration using a BCA Protein Assay Kit (Pierce Chemical, Dallas, TX, USA).
Semi-quantitative RT-PCR
Total cellular RNA was extracted using Trizol (Invitrogen). The ratio of the absorbance at 260 nm and 280 nm (A260/A280) was used to assess RNA purity. Reverse transcription was performed with 0.2 μg of total RNA using the High-Capacity cDNA Reverse Transcription Kit (Beijing TransGen Biotech Co. Ltd., China). PCR was performed using a MyCycler™ Thermal Cycler PCR system (Bio-Rad, Hercules, CA, USA) using: AQP7: forward 5′-GAA CAG TGA GAA AAA GAC CG-3′ and reverse 5′-CCA GAC AAT CCA GAG TTC AT-3′; 18S ribosomal RNA (18S rRNA): forward 5′-TTG GTG GAG CGA TTT GTC TG-3′ and reverse 5′-AAT GGG GTT CAA CGG GTT AC-3′). All reactions involved initial denaturation at 95 °C for 5 min followed by 36 cycles of 95°C for 30 s, 54°C for 30 s, and 72°C for 30 s. PCR products were separated by 2% agarose gel electrophoresis, stained with ethidium bromide, and analyzed using the Alpha Innotech software (Bio-Rad). A kinetic study was performed using different numbers of cycles in order to determine the optimal number of cycles for mRNA quantification during the exponential phase. We observed that 36 cycles provided the best results. AQP7 expression was normalized to the 18S rRNA expression level, and was quantified using a standard curve. There was no significant difference in baseline 18S rRNA levels between control and apelin-13- or palmitate-treated adipocytes.
Western blot analysis
Cells were treated with the RIPA lysis buffer (Biocolor BioScience & Technology CO., Ltd., Shanghai, China) on ice for 10 min, and centrifuged at 4 °C, 12000 × g for 15 min. Protein concentrations were determined using a BCA protein assay. Cell lysates (40 μg) were separated by 12% SDS polyacrylamide gel electrophoresis, and then transferred on polyvinylidene difluoride (PVDF) membranes (0.45 μM; Millipore Corp., Billerica, MA, USA) at 4°C. Membranes were blocked with 5% skimmed milk in Tris-buffer (TBS) with 0.1% Tween 20 (TBST), for 2 h at room temperature. Membranes were blotted with rabbit polyclonal antibody against AQP7 (1:500 dilution, Abcam, Cambridge, MA, USA) and mouse monoclonal antibody against β-actin (1:1000, Boster Bioengineering Co., Ltd., Wuhan, China) overnight at 4°C. After being washed 4 times (10 min each) in TBST buffer at room temperature, membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG (Boster Bioengineering Co., Ltd.) for 1.5 h. Immunoreactive proteins were detected using a chemiluminescence ECL assay kit (GE Healthcare, Little Chanfont, UK) according to the manufacturer’s instructions. Bands were visualized using a Universal Hood II 76S/0608 image analyzer (Bio-Rad, Hercules, CA, USA). Grayscale value of each band in imaging data was analyzed using Quantity One software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All measurements were performed in triplicates during 3 or 4 independent experiments. Because results from each triplicate was averaged and because the average of the 3–4 experiments was used for analysis, data are expressed as means –SEM [24]. Differences between 2 groups were assessed using the Student’s t-test. ANOVA and the least-square difference (LSD) tests were used for multiple comparisons. Analyses were performed with SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA). A P-value <0.05 (2-tailed) was considered significant.
Results
Palmitate induced intracellular lipid accumulation in differentiated adipocytes
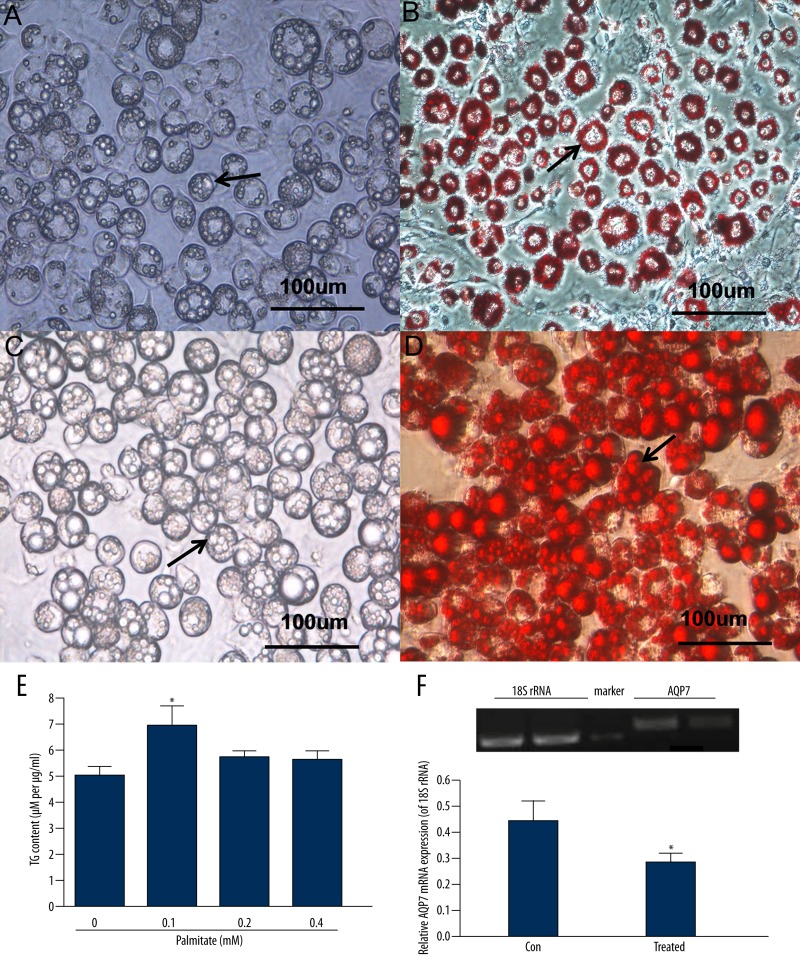
The hypertrophic 3T3-L1 adipocytes created by incubation with 0.1 mM palmitate for 48 h were identified by Oil Red O staining (Figure 1C and 1D) and they had a different appearance from fully differentiated 3T3-L1 adipocytes not exposed to palmitate, as shown by Oil Red O staining (Figure 1A and 1B). After Oil Red O staining, compared with differentiated 3T3-L1 adipocytes not exposed to palmitate, hypertrophic adipocytes were qualitatively characterized by: 1) an increased cellular volume; 2) lipid droplets increased in number and in size; and 3) decreased cellular adhesion [22,25] (Figure 1C and 1D).
Figure 1.
Palmitate induced an in vitro model of hypertropic adipocytes with increased accumulation of cytoplasmic triglycerides (TG) and decreased AQP7 expression. Lipid droplet (arrows) accumulation in the fully differentiated 3T3-L1 adipocytes using 0.1 mM palmitate for 48 h. (A) Fully differentiated 3T3-L1 adipocytes not treated with palmitate (×400). (B) Fully differentiated 3T3-L1 adipocytes identified by Oil Red O staining (×400). (C) Hypertrophic 3T3-L1 adipocytes induced by palmitate (×400). (D) Hypertrophic 3T3-L1 adipocytes created using palmitate and identified by Oil Red O staining (×400). (E) Effects of different concentrations of palmitate for 48 h on intracellular TG content in fully differentiated adipocytes. (F) AQP7 mRNA levels in fully differentiated adipocytes (control) and fully differentiated adipocytes treated with 0.1mM palmitate (treated) were determined by RT-PCR. 18S rRNA was used as an internal control and results were obtained from a standard curve. Results are expressed as means ±SEM from 3 or 4 independent experiments, each performed in triplicate. * P<0.05 vs. the control group.
Levels of cytoplasmic TG adjusted for total cytoplasmic protein levels were different in adipocytes treated with palmitate. The 3T3-L1 differentiated adipocytes treated with 0.1 mM palmitate for 48 h accumulated significantly more cytoplasmic TG compared with control cells (0.1 mM: 6.93±0.80 μM per μg/ml vs. control: 5.04±0.33 μM per μg/ml, P<0.05). There were no statistically significant differences among adipocytes treated with 0.2 mM and 0.4 mM of palmitate and control cells (all P>0.05) (Figure 1E). Palmitate induced an in vitro model of hypertrophic adipocytes with decreased AQP7 mRNA expression compared with control cells (0.26±0.04 vs. 0.46±0.07, P<0.05) (Figure 1F).
Apelin-13 significantly upregulated AQP7 expression and decreased accumulation of cytoplasmic triglycerides in hypertrophic adipocytes
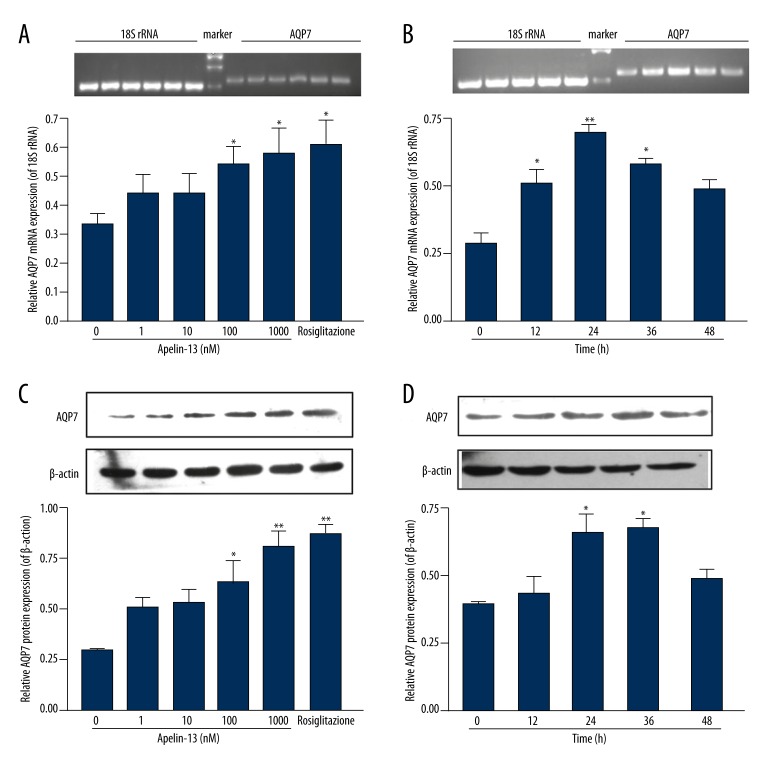
Apelin-13 significantly upregulated AQP7 mRNA levels, without having an effect on 18S rRNA. There was a significant difference compared with controls (no apelin-13 or 0 h) when hypertrophic adipocytes were treated with 100 nM and 1000 nM of apelin-13 (100 nM: 0.54±0.06 and 1000 nM: 0.58±0.09 vs. control: 0.33±0.04, both P<0.05) (Figure 2A) or with 100 nM of apelin-13 for 12, 24 and 36 h (Figure 2B). As shown in Figure 2A, upregulation of AQP7 mRNA expression by apelin-13 1000 nM was similar to upregulation by rosiglitazone 10 μM (positive control) (apelin-13 1000 mM: 0.58±0.09 vs. rosiglitazone 10 μM: 0.61±0.09, P>0.05). AQP7 protein levels followed a similar trend with mRNA expression when hypertrophic adipocytes were treated with 100 nM and 1000 nM of apelin-13 (Figure 2C) or with 100 nM of apelin-13 for 24 and 36 h (Figure 2D). Apelin-13 upregulated AQP7 protein levels (apelin-13: 0.54±0.06 vs. control: 0.28±0.06, P<0.05) (Figure 3A) while decreasing TG accumulation in hypertrophic adipocytes (apelin-13: 3.32±0.08 μM per μg/ml vs. control: 3.82±0.13 μM per μg/ml, P<0.05) (Figure 3B).
Figure 2.
Apelin-13 upregulated AQP7 expression in hypertrophic adipocytes. (A) AQP7 mRNA levels in the presence of 0, 1, 10, 100, and 1000 nM of apelin-13 for 24 h. (B) AQP7 mRNA levels in the presence of 100 nM of apelin-13 for 0, 12, 24, 36, and 48 h. mRNA levels were determined by RT-PCR. 18S rRNA was used as an internal control. (C) AQP7 protein levels in the presence of 0, 1, 10, 100, and 1000 nM of apelin-13 for 24 h. (D) AQP7 protein levels in the presence of 100 nM of apelin-13 for 0, 12, 24, 36, and 48 h. Protein expression was assessed by Western blot. β-actin was used as an internal control. Results are expressed as means ±SEM from 3 or 4 independent experiments, each performed in triplicate. * P<0.05, ** P <0.01 vs. the controls (no apelin-13 or 0 h).
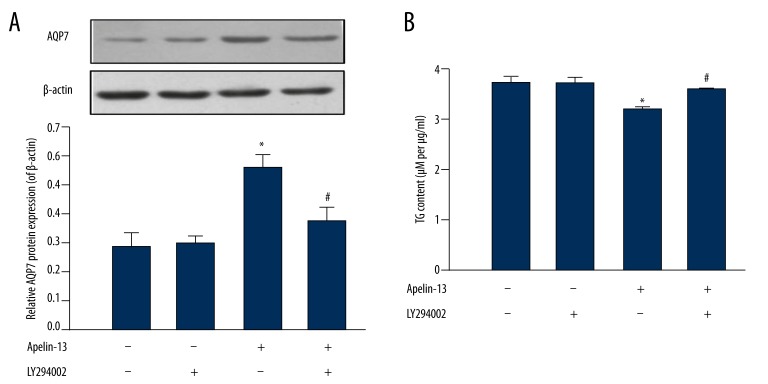
Figure 3.
Apelin-13 upregulated AQP7 protein expression by the PI3K signaling pathway in hypertrophic 3T3-L1 cells. Hypertrophic 3T3-L1 cells were treated with apelin-13 (100 nM) for 24 h and pretreated with a PI3K inhibitor, LY294002 (10 μM), for 30 min. (A) AQP7 protein levels were determined by Western blot. β-actin was used as an internal control. (B) Cytoplasmic TG content. Results are expressed as means ±SEM from 3 or 4 independent experiments, each performed in triplicate. * P<0.05 vs. the control group (both apelin-13 + LY294002 non-treated); # P<0.05 apelin-13 + LY294002 vs. apelin-13.
Apelin-13 upregulated AQP7 expression by the PI3K signaling pathway
The PI3K signaling pathway has been reported to play an important role in the apelin-mediated regulation of energy metabolism [14,22]. In the present study, pretreatment using LY294002 10 μM for 30 min prevented the increase in AQP7 expression observed when using apelin-13 alone (apelin-13+LY294002: 0.38±0.03 vs. apelin-13: 0.54±0.06, P<0.05) (Figure 3A), as well as the decreased cytoplasmic TG accumulation (apelin-13 + LY294002: 3.79±0.04 μM per μg/ml vs. apelin-13: 3.32±0.08 μM per μg/ml, P<0.05) (Figure 3B).
Discussion
In the present study, using 0.1 mM of palmitate, we implemented a model of hypertrophic adipocytes characterized by lipid droplets accumulation in the cytoplasm; higher palmitate concentrations did not induce adipocyte hypertrophy, possibly due to intracellular palmitate cytotoxicity [10,26]. We showed that apelin-13 treatment increased AQP7 expression. Finally, LY294002, a PI3K inhibitor, prevented the effects of apelin-13. These results suggest that apelin-13 decreases lipid storage through upregulating AQP7 expression in hypertrophic adipocytes by the PI3K signaling pathway.
Adipocyte differentiation and growth is highly controlled, and a number of crucial genes are involved. A number of studies showed that AQP7, as no other aquaporin in adipose tissue, has been linked to regulation of fat tissue metabolism during periods of feast and famine, and that it is regulated by known regulators of fatty acids metabolism, such as insulin, dexamethasone, and epinephrine [8,27]. AQP7 is barely expressed in pre-adipocytes, while it is markedly expressed in mature adipocytes [28]. AQP7 facilitates glycerol transport from the cells, leading to the decrease of cytoplasmic TG levels through a decreased TG synthesis due to a lack of substrate [29]. An AQP7-knockout mice model showed that increased adipose glycerol kinase activity accelerates TG synthesis in adipocytes, finally inducing obesity [9,30]. Indeed, AQP7 expression in adipose tissue is different in lean individuals compared with obese patients [31] and the delicate balance in AQP7 expression is perturbed in obese subjects [32]. Moreover, some patients with a mutated or parafunctional AQP7 gene are at increased risk of developing obesity and/or type 2 diabetes [33,34]. A downregulated AQP7 expression could then be involved in obesity susceptibility by reducing glycerol release and by promoting the accumulation of lipids in subcutaneous adipose tissue [31]. Studies also suggested that AQP7 expression is higher in visceral adipose tissue from obese subjects, compared with subcutaneous, in agreement with the fact that visceral adipose tissue is more metabolically active (higher lipolytic capability) than subcutaneous (higher fat accumulation) [35,36]. Therefore, in the present study, palmitate-induced TG accumulation in adipocytes may be associated with a decreased AQP7 expression. Designing new modulators of AQP7 expression might be a way to treat obesity and its related complications [27].
Adipose tissue is a major endocrine organ, producing a variety of adipokines affecting energy balance. Previous studies reported that apelin secreted by adipocytes contributes to decreased adiposity and to improved insulin resistance [22,37], but the mechanisms are still unknown. In the present study, we observed that apelin-13 upregulated AQP7 expression while decreasing TG accumulation. Rosiglitazone prevents lipid accumulation by increasing AQP7 expression in adipocytes [38]. The present study showed that the levels of AQP7 mRNA were not significantly different in adipocytes treated with 1000 nM of apelin-13 or 10 μM of rosiglitazone. Since AQP7 is known to increase glycerol transport out of the adipocytes, leading to a reduction in TG synthesis, we hypothesize that apelin downregulated TG accumulation in adipocytes by upregulating AQP7 expression. However, the effects of apelin-13 on the expression levels of key lipolytic enzymes still need to be assessed. Nevertheless, since apelin is effective in inducing AQP7 expression in hypertrophic adipocytes, its use might be beneficial in treating obesity, as previously shown in mice [17]. The effects of apelin-13 on AQP7 expression vanished after 48 h, which may be associated with apelin-13 degradation.
A major finding of our study is that the PI3K pathway is involved in AQP7 upregulation by apelin-13 in adipocytes. Apelin and its receptor are expressed in many tissues, and previous studies observed that the PI3K/Akt pathway is downstream of the apelin receptor [22]. The present study showed that LY294002, a PI3K inhibitor, prevented the effects of apelin-13 in apelin-treated hypertrophic adipocytes. These results suggest that AQP7 is a new factor involved in apelin-mediated decreased lipid storage and attenuated insulin resistance, and that the PI3K pathway is involved. Our results are in agreement with a previous study suggesting that insulin and leptin modulate AQP7 expression through the PI3K pathway [39]: inhibiting the PI3K also decreased the effects of insulin and leptin on AQP7 expression. A study also showed that apelin-13 is involved in vascular smooth muscle cell proliferation through the PI3K pathway [19], suggesting that apelin-13 might also be involved in the proliferation of other cell types, such as in the hyperplasia observed in obesity. However, other transduction signal pathways (such as ERK, p38 MAPK, and protein C kinase) may be involved, and future research should also assess their effects on AQP7 expression in adipocytes and their role in lipid accumulation [40–44].
Conclusions
In conclusion, our results suggest that apelin-13 decreases lipid storage in hypertrophic adipocytes through an AQP7 upregulation, and that the PI3K signaling pathway is involved in these effects, suggesting potential therapeutic targets against obesity and related health problems.
Acknowledgements
We thank the Laboratory of Molecular Cardiology, First Affiliated Hospital, Shantou University Medical College, for supplying the laboratory. We also thank Libiao W and Bozhi C for their technical assistance.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
Source of support: This work was supported by grants from the National Natural Science Foundation of China (81072177), the special financial grant from the China Postdoctoral Science Foundation (201104379), the Guangdong Natural Science Foundation (10151503102000019), the Educational Commission of Guangdong Province (2013kjcx0077), the Science and Technology Planning Project of Shantou City (2012-133, 2013-63), and the Guangdong provincial undergraduates’ training programs of innovation and entrepreneurship (201229)
References
- 1.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon HS, Lee HG, Choi YJ, et al. Proposed mechanisms of (−)-epigallocatechin-3-gallate for anti-obesity. Chem Biol Interact. 2007;167:85–98. doi: 10.1016/j.cbi.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–87. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 4.Kondo H, Shimomura I, Kishida K, et al. Human aquaporin adipose (AQPap) gene. Genomic structure, promoter analysis and functional mutation. Eur J Biochem. 2002;269:1814–26. doi: 10.1046/j.1432-1033.2002.02821.x. [DOI] [PubMed] [Google Scholar]
- 5.Skowronski MT, Lebeck J, Rojek A, et al. AQP7 is localized in capillaries of adipose tissue, cardiac and striated muscle: implications in glycerol metabolism. Am J Physiol Renal Physiol. 2007;292:F956–65. doi: 10.1152/ajprenal.00314.2006. [DOI] [PubMed] [Google Scholar]
- 6.Ishibashi K, Kuwahara M, Gu Y, et al. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J Biol Chem. 1997;272:20782–86. doi: 10.1074/jbc.272.33.20782. [DOI] [PubMed] [Google Scholar]
- 7.Nejsum LN, Elkjaer M, Hager H, et al. Localization of aquaporin-7 in rat and mouse kidney using RT-PCR, immunoblotting, and immunocytochemistry. Biochem Biophys Res Commun. 2000;277:164–70. doi: 10.1006/bbrc.2000.3638. [DOI] [PubMed] [Google Scholar]
- 8.Fruhbeck G, Catalan V, Gomez-Ambrosi J, Rodriguez A. Aquaporin-7 and glycerol permeability as novel obesity drug-target pathways. Trends Pharmacol Sci. 2006;27:345–47. doi: 10.1016/j.tips.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Maeda N, Funahashi T, Hibuse T, et al. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc Natl Acad Sci USA. 2004;101:17801–6. doi: 10.1073/pnas.0406230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishida K, Shimomura I, Kondo H, et al. Genomic structure and insulin-mediated repression of the aquaporin adipose (AQPap), adipose-specific glycerol channel. J Biol Chem. 2001;276:36251–60. doi: 10.1074/jbc.M106040200. [DOI] [PubMed] [Google Scholar]
- 11.O’Dowd BF, Heiber M, Chan A, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–60. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 12.Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–76. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 13.Dray C, Debard C, Jager J, et al. Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humans. Am J Physiol Endocrinol Metab. 2010;298:E1161–69. doi: 10.1152/ajpendo.00598.2009. [DOI] [PubMed] [Google Scholar]
- 14.Boucher J, Masri B, Daviaud D, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–71. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 15.Heinonen MV, Purhonen AK, Miettinen P, et al. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept. 2005;130:7–13. doi: 10.1016/j.regpep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Erdem G, Dogru T, Tasci I, et al. Low plasma apelin levels in newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116:289–92. doi: 10.1055/s-2007-1004564. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi K, Masaki T, Gotoh K, et al. Apelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology. 2007;148:2690–97. doi: 10.1210/en.2006-1270. [DOI] [PubMed] [Google Scholar]
- 18.Carpene C, Dray C, Attane C, et al. Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem. 2007;63:359–73. [PubMed] [Google Scholar]
- 19.Liu C, Su T, Li F, et al. PI3K/Akt signaling transduction pathway is involved in rat vascular smooth muscle cell proliferation induced by apelin-13. Acta Biochim Biophys Sin (Shanghai) 2010;42:396–402. doi: 10.1093/abbs/gmq035. [DOI] [PubMed] [Google Scholar]
- 20.Arai T, Kawakami Y, Matsushima T, et al. Intracellular fatty acid downregulates ob gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;297:1291–96. doi: 10.1016/s0006-291x(02)02376-8. [DOI] [PubMed] [Google Scholar]
- 21.Shintani M, Nishimura H, Yonemitsu S, et al. Downregulation of leptin by free fatty acids in rat adipocytes: effects of triacsin C, palmitate, and 2-bromopalmitate. Metabolism. 2000;49:326–30. doi: 10.1016/s0026-0495(00)90154-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhu S, Sun F, Li W, et al. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol Cell Biochem. 2011;353:305–13. doi: 10.1007/s11010-011-0799-0. [DOI] [PubMed] [Google Scholar]
- 23.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–47. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 24.Curran-Everett D. Explorations in statistics: standard deviations and standard errors. Adv Physiol Educ. 2008;32:203–8. doi: 10.1152/advan.90123.2008. [DOI] [PubMed] [Google Scholar]
- 25.Perrini S, Natalicchio A, Laviola L, et al. Dehydroepiandrosterone stimulates glucose uptake in human and murine adipocytes by inducing GLUT1 and GLUT4 translocation to the plasma membrane. Diabetes. 2004;53:41–52. doi: 10.2337/diabetes.53.1.41. [DOI] [PubMed] [Google Scholar]
- 26.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–95. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 27.Fruhbeck G. Obesity: aquaporin enters the picture. Nature. 2005;438:436–37. doi: 10.1038/438436b. [DOI] [PubMed] [Google Scholar]
- 28.MacDougald OA, Burant CF. Obesity and metabolic perturbations after loss of aquaporin 7, the adipose glycerol transporter. Proc Natl Acad Sci USA. 2005;102:10759–60. doi: 10.1073/pnas.0504965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez A, Catalan V, Gomez-Ambrosi J, Fruhbeck G. Role of aquaporin-7 in the pathophysiological control of fat accumulation in mice. FEBS Lett. 2006;580:4771–76. doi: 10.1016/j.febslet.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 30.Hibuse T, Maeda N, Funahashi T, et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci USA. 2005;102:10993–98. doi: 10.1073/pnas.0503291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrades MP, Milagro FI, Martinez JA, Moreno-Aliaga MJ. Differential expression of aquaporin 7 in adipose tissue of lean and obese high fat consumers. Biochem Biophys Res Commun. 2006;339:785–89. doi: 10.1016/j.bbrc.2005.11.080. [DOI] [PubMed] [Google Scholar]
- 32.Miranda M, Escote X, Ceperuelo-Mallafre V, et al. Paired subcutaneous and visceral adipose tissue aquaporin-7 expression in human obesity and type 2 diabetes: differences and similarities between depots. J Clin Endocrinol Metab. 2010;95:3470–79. doi: 10.1210/jc.2009-2655. [DOI] [PubMed] [Google Scholar]
- 33.Guan HP, Li Y, Jensen MV, et al. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat Med. 2002;8:1122–28. doi: 10.1038/nm780. [DOI] [PubMed] [Google Scholar]
- 34.Prudente S, Flex E, Morini E, et al. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes. 2007;56:1468–74. doi: 10.2337/db06-1389. [DOI] [PubMed] [Google Scholar]
- 35.Miranda M, Ceperuelo-Mallafre V, Lecube A, et al. Gene expression of paired abdominal adipose AQP7 and liver AQP9 in patients with morbid obesity: relationship with glucose abnormalities. Metabolism. 2009;58:1762–68. doi: 10.1016/j.metabol.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Catalan V, Gomez-Ambrosi J, Pastor C, et al. Influence of morbid obesity and insulin resistance on gene expression levels of AQP7 in visceral adipose tissue and AQP9 in liver. Obes Surg. 2008;18:695–701. doi: 10.1007/s11695-008-9453-7. [DOI] [PubMed] [Google Scholar]
- 37.Attane C, Foussal C, Le Gonidec S, et al. Apelin treatment increases complete Fatty Acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes. 2012;61:310–20. doi: 10.2337/db11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee DH, Park DB, Lee YK, et al. The effects of thiazolidinedione treatment on the regulations of aquaglyceroporins and glycerol kinase in OLETF rats. Metabolism. 2005;54:1282–89. doi: 10.1016/j.metabol.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez A, Catalan V, Gomez-Ambrosi J, et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J Clin Endocrinol Metab. 2011;96:E586–97. doi: 10.1210/jc.2010-1408. [DOI] [PubMed] [Google Scholar]
- 40.Cao C, Sun Y, Healey S, et al. EGFR-mediated expression of aquaporin-3 is involved in human skin fibroblast migration. Biochem J. 2006;400:225–34. doi: 10.1042/BJ20060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell CE, Lariviere NM, Watson PH, Watson AJ. Mitogen-activated protein kinase (MAPK) pathways mediate embryonic responses to culture medium osmolarity by regulating Aquaporin 3 and 9 expression and localization, as well as embryonic apoptosis. Hum Reprod. 2009;24:1373–86. doi: 10.1093/humrep/dep010. [DOI] [PubMed] [Google Scholar]
- 42.Yang H, Li F, Kong X, et al. Molecular cloning, tissue distribution and ontogenetic expression of Xiang pig chemerin and its involvement in regulating energy metabolism through Akt and ERK1/2 signaling pathways. Mol Biol Rep. 2012;39:1887–94. doi: 10.1007/s11033-011-0934-8. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki Y, Song SH, Sato K, et al. Chemerin analog regulates energy metabolism in sheep. Anim Sci J. 2012;83:263–67. doi: 10.1111/j.1740-0929.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 44.Karmazyn M, Purdham DM, Rajapurohitam V, Zeidan A. Signalling mechanisms underlying the metabolic and other effects of adipokines on the heart. Cardiovasc Res. 2008;79:279–86. doi: 10.1093/cvr/cvn115. [DOI] [PubMed] [Google Scholar]